Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-ray Diffraction, and Electrical Properties for Photovoltaic Cells
Abstract
1. Introduction
2. Experimental
2.1. Cyclic Voltammetry Experiments
2.2. The Catalytic Degradation
2.3. Synthetic Procedures
2.3.1. Synthesis of H2TClPP (1)
2.3.2. Synthesis of [CoII(TClPP)] (2)
2.3.3. Synthesis of [CoIII(TClPP)Cl] (3)
2.3.4. Synthesis of [CoIII(TClPP)Cl(NTC)]·CH2Cl2 (4)
3. Results and Discussion
3.1. IR and Proton NMR Spectroscopic Data
3.2. Photophysical Properties
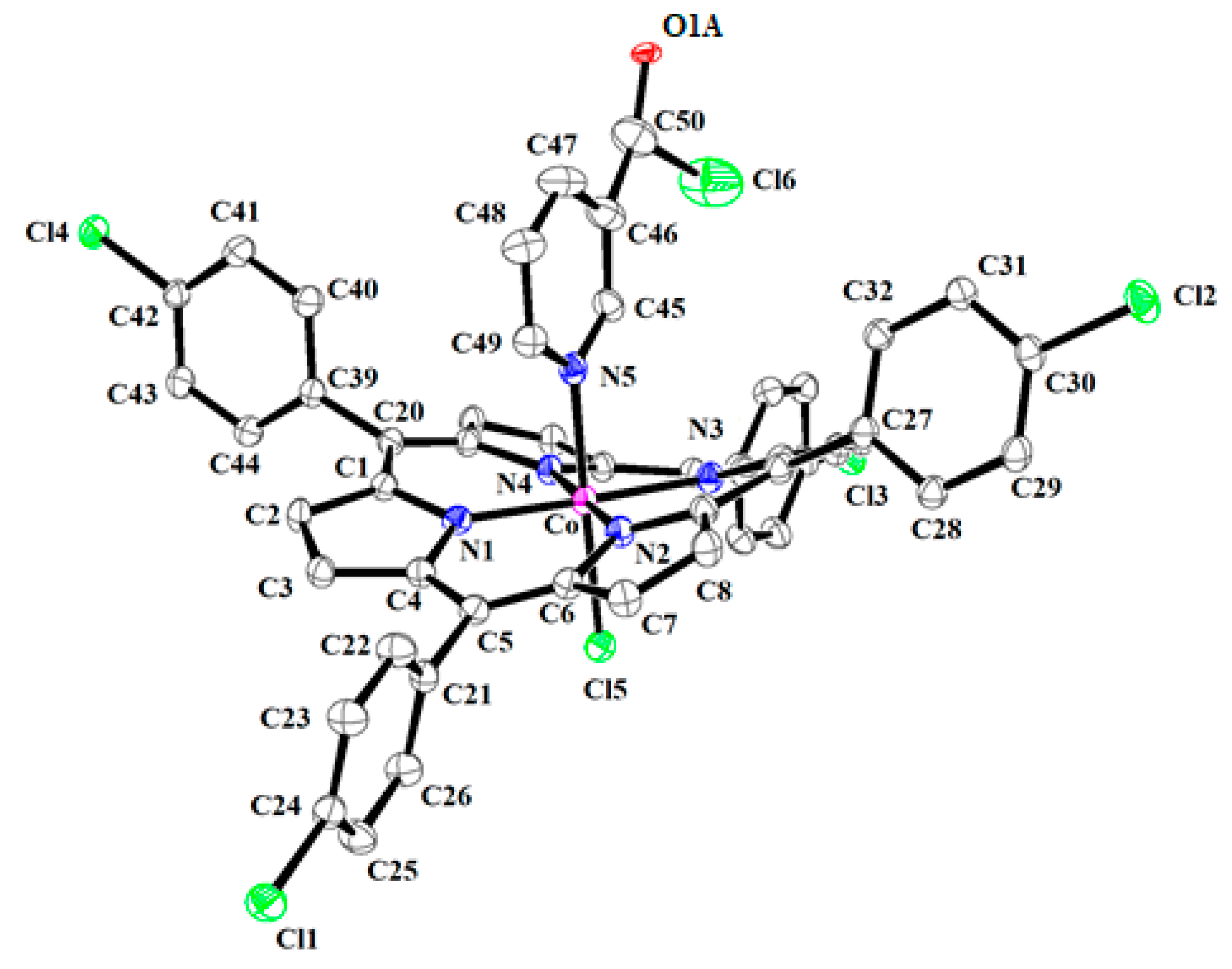
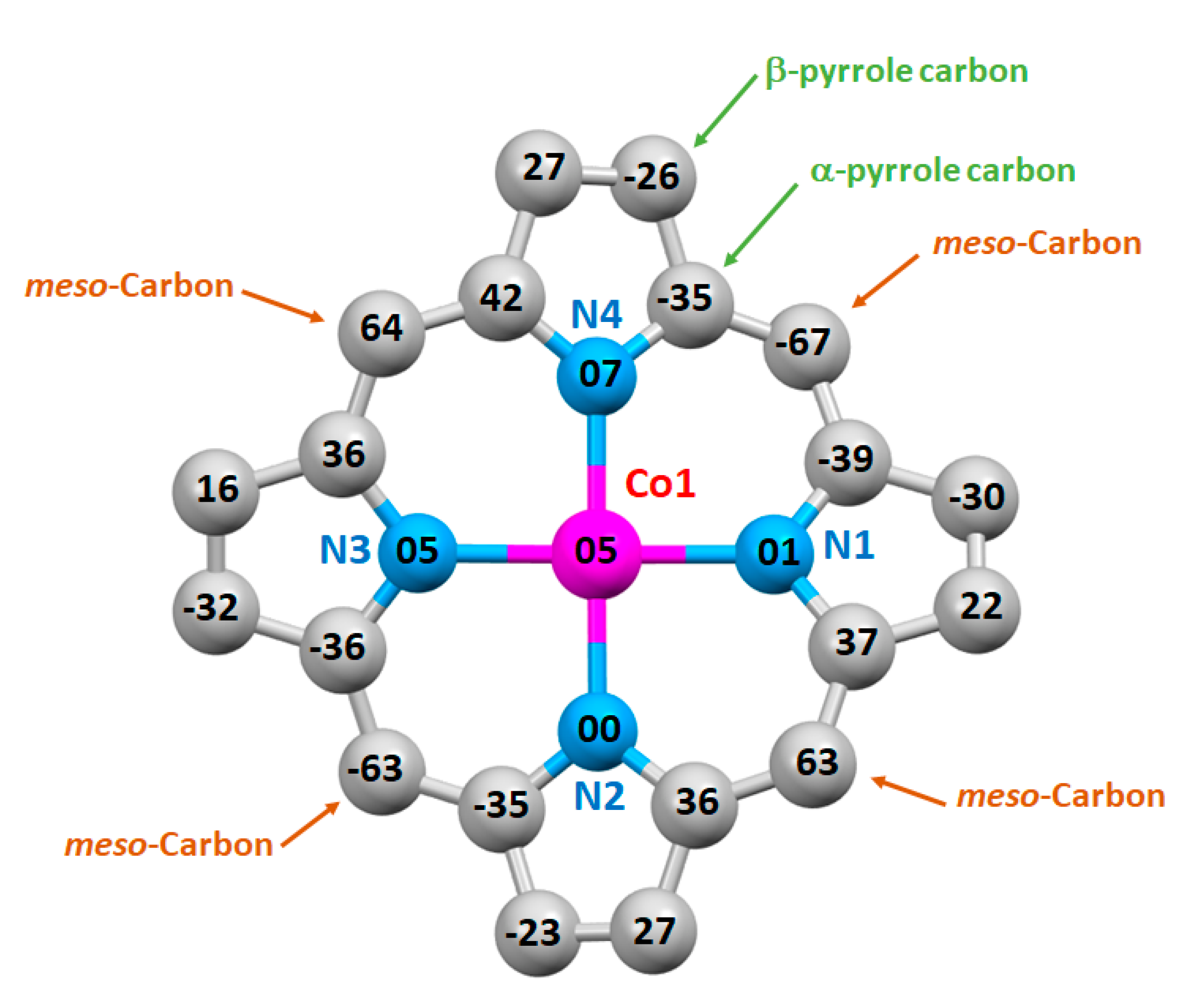
3.3. X-ray Molecular Structure of Complex 4
3.4. Hirshfeld Surface Analysis
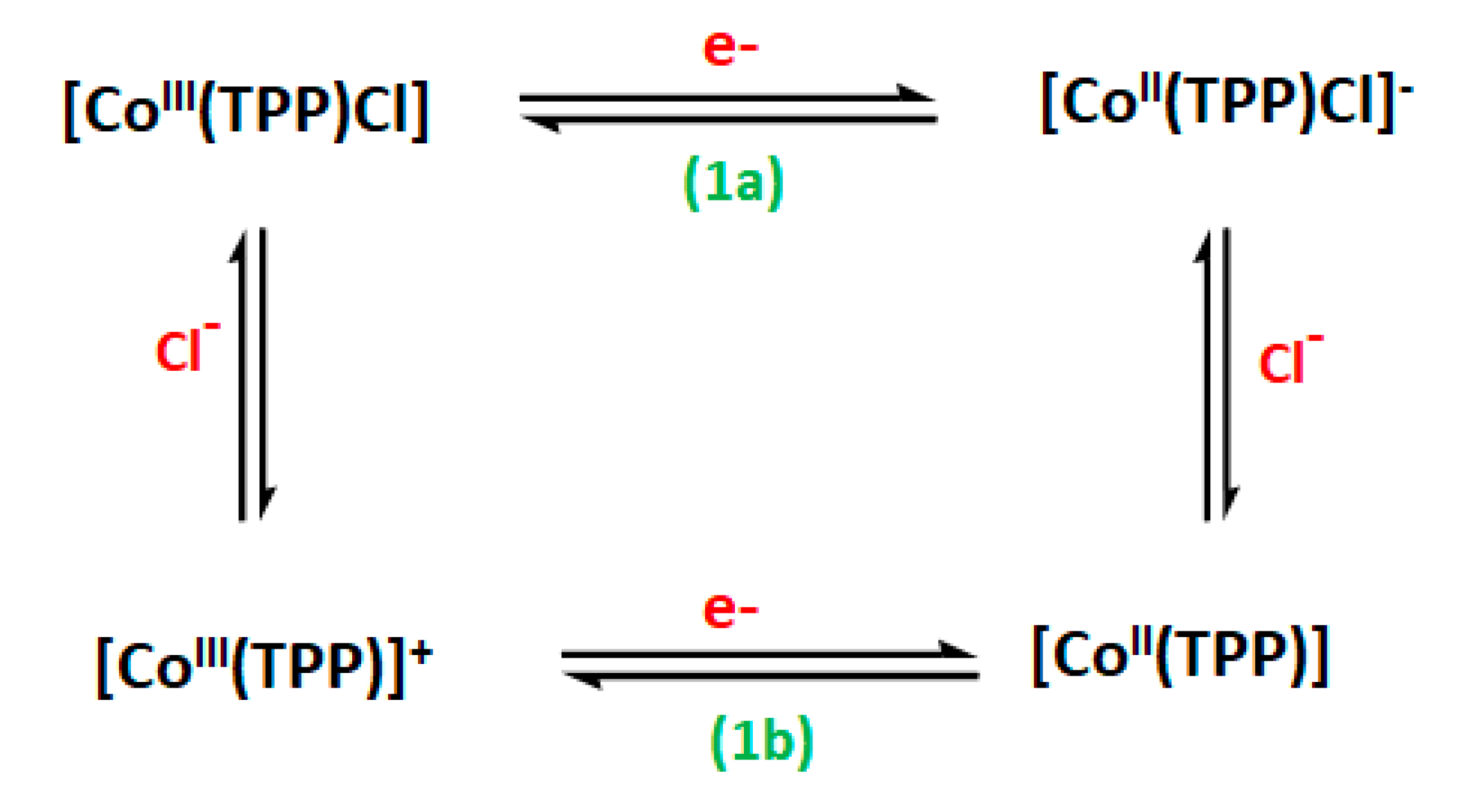
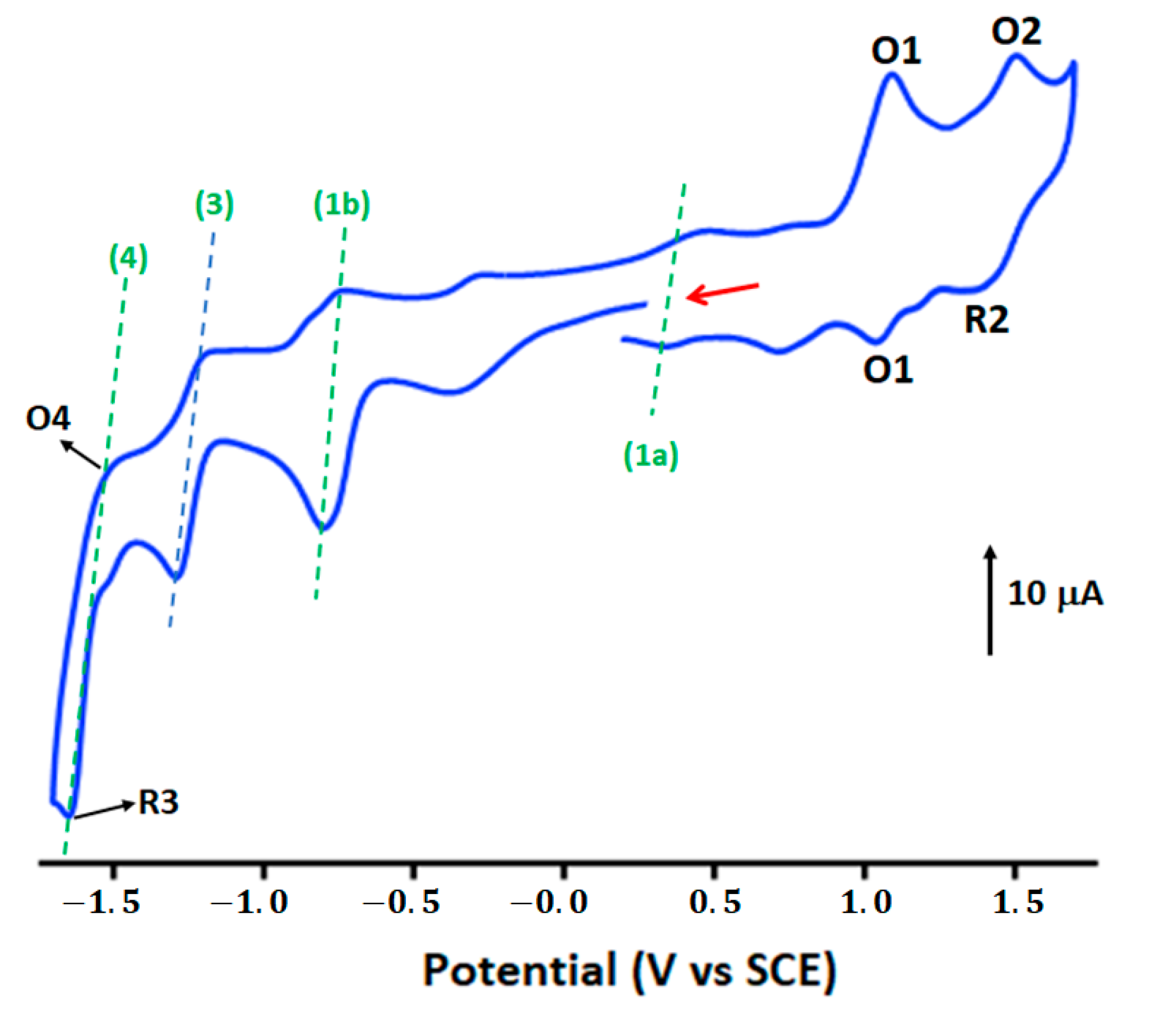
3.5. Cyclic Voltammetry Investigation on [CoIII(TClPP)Cl(NTC)]·CH2Cl2 (4)
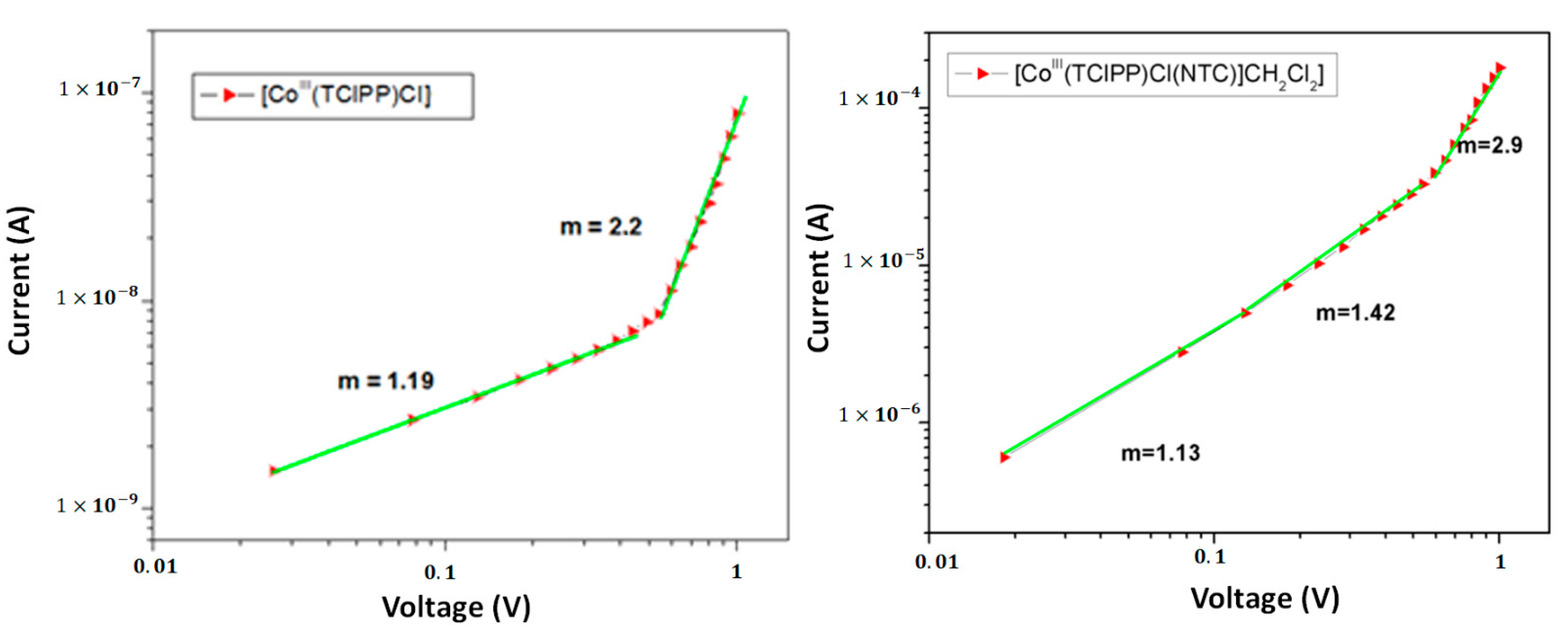
4. Photovoltaic Performance of DSSCs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maki, A.H.; Edelstein, N.; Davison, A.; Holm, R.H. Electron Paramagnetic Resonance Studies of the Electronic Structures of Bis(maleonitriledithiolato)copper(II), -nickel(III), -cobalt(II), and -rhodium(II) Complexes. J. Am. Chem. Soc. 1964, 86, 4580–4587. [Google Scholar] [CrossRef]
- Walker, F.A. An Electron Spin Resonance Study of Coordination to the Fifth and Sixth Positions of α,ß,γ,δ-Tetra (p-methoxyphenyl)porphinato cobalt(II). J. Am. Chem. Soc. 1970, 92, 4235–4244. [Google Scholar] [CrossRef]
- Lexa, D.; Savéant, J.; Soufflet, J. Chemical catalysis of the electrochemical reduction of alkyl halides Comparison between cobalt-tetraphenyl porphin and vitamin B12 derivatives. J. Electroanal. Chem. 1979, 100, 159–172. [Google Scholar] [CrossRef]
- Lyaskovskyy, V.; Suarez, A.I.O.; Lu, H.; Jiang, H.; Zhang, X.P.; de Bruin, B. Mechanism of Cobalt(II) Porphyrin-Catalyzed C-H Amination withOrganic Azides: Radical Nature and H-Atom Abstraction Ability of the Key Cobalt(III)-Nitrene Intermediates. J. Am. Chem. Soc. 2011, 133, 12264–12273. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, S.-H.; Yoon, Y.; Thangadurai, T.D.; Yoon, S. A fluorescent ammonia sensor based on a porphyrin cobalt(II)–dansyl complex. Tetrahedron Lett. 2011, 52, 2645–2648. [Google Scholar] [CrossRef]
- Karimipour, G.; Kowkabi, S.; Naghiha, A. New Amino porphyrins Bearing Urea Derivative Substituents: Synthesis, Characterization, Antibacterial and Antifungal Activity. Braz. Arch. Biol. Technol. 2015, 58, 431–442. [Google Scholar] [CrossRef]
- Beyene, B.B.; Wassie, G.A. Antibacterial activity of Cu(II) and Co(II) porphyrins: Role of ligand modification. BMC Chem. 2020, 14, 51. [Google Scholar] [CrossRef]
- Crawley, M.R.; Zhang, D.; Oldacre, A.N.; Beavers, C.M.; Friedman, A.E.; Cook, T.R. Tuning the Reactivity of Cofacial Porphyrin Prisms for Oxygen Reduction Using Modular Building Blocks. J. Am. Chem. Soc. 2021, 143, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Cheng, N.; Kemna, C.; Goubert-Renaudin, S.; Wieckowski, A. Reduction Reaction by Porphyrin-Based Catalysts for Fuel Cells. Electrocatalysis 2012, 3, 238–251. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, Q.; Zheng, R.; Qiu, C.; Qin, J.; Li, J.; Xie, Y.; Wang, A.; Huang, J.; Song, Y. Controlled pyrolysis of ionically self-assembled metalloporphyrins on carbon as cathodic electrocatalysts of polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 11041–11050. [Google Scholar] [CrossRef]
- Alvarez, I.B.; Wu, Y.; Sanchez, J.; Ge, Y.; Ramos-Garcés, M.V.; Chu, T.; Jaramillo, T.F.; Colón, J.L.; Villagrán, D. Cobalt porphyrin intercalation into zirconium phosphate layers for electrochemical water oxidation. Sustain. Energy Fuels 2021, 5, 430–437. [Google Scholar] [CrossRef]
- Wirojsaengthong, S.; Aryuwananon, D.; Aeungmaitrepirom, W.; Pulpoka, B.; Tuntulani, T. A colorimetric paper-based optode sensor for highly sensitive and selective determination of thiocyanate in urine sample using cobalt porphyrin derivative. Talanta 2021, 231, 122371. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, B.; Wu, S.; Wang, H.; Cao, T.; Zhu, T.; Zhang, X.; Liu, L.; Tong, Z. Construction of cobalt porphyrin/tantalum molybdatenanocomposite for simultaneous electrochemical detection of ascorbic acid and dopamine. J. Mater. Sci. Res. 2021, 36, 916–924. [Google Scholar] [CrossRef]
- Benkhaya, S.; El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Yamjala, K.; Nainar, M.S.; Ramisetti, N.R. Methods for the analysis of azo dyes employed in food industry—A review. Food Chem. 2016, 192, 813–824. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; MAsiri, A.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Birhanlı, A.; Ozmen, M. Evaluation of the Toxicity and Teratogenity of Six Commercial Textile Dyes Using the Frog Embryo Teratogenesis Assay–Xenopus. Drug Chem. Toxicol. 2005, 28, 51–65. [Google Scholar] [PubMed]
- Feng, M.; Wu, L.; Wang, X.; Wang, J.; Wang, D.; Li, C. A strategy of designed anionic metal–organic framework adsorbent based on reticular chemistry for rapid selective capture of carcinogenic dyes. Appl. Organomet. Chem. 2022, 35, e6546. [Google Scholar] [CrossRef]
- Chen, H.; Liu, P.; Liu, J.; Feng, X.; Zhou, S. Mechanochemical in-situ incorporation of Ni on MgO/MgH2 surface for the selective O-/C-terminal catalytic hydrogenation of CO2 to CH4. J. Catal. 2021, 394, 397–405. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, K.; Hu, W.; Cheng, Z.; Chen, H.; Feng, X.; Peng, T.; Kou, Z. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 306, 121095. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Feng, X.; Zhu, L.; Fang, Q.; Li, S.; Wang, L.; Li, Z.; Kou, Z. A chainmail effect of ultrathin N-doped carbon shell on Ni2P nanorod arrays for efficient hydrogen evolution reaction catalysis. Colloid Interface Sci. 2022, 607, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J.-Q. A new 3D 8-connected Cd(ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal Organic Framework as an Efficient Adsorbent for Drugs from Wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen, T.A.; Nguyen, X.S.; Truong, T.N.; Ninh, H.D.; Vo, H.T.; Bhosale, S.V.; Chang, S.W.; Rene, E.R.; Nguyen, T.H.; et al. Self-assembly of porphyrin on the surface of a novel composite high performance photocatalyst for the degradation of organic dye from water: Characterization and performance evaluation. J. Environ. Chem. Eng. 2021, 9, 106034. [Google Scholar] [CrossRef]
- Li, M.; Zhao, H.; Lu, Z.-Y. Porphyrin-based porous organic polymer, Py-POP, as a multifunctional platform for efficient selective adsorption and photocatalytic degradation of cationic dyes. Microporous Mesoporous Mater. 2020, 292, 109774. [Google Scholar] [CrossRef]
- Silvestri, S.; Fajardo, A.R.; Iglesias, B.A. Supported porphyrins for the photocatalytic degradation of organic contaminants in water: A review. Environ. Chem. Lett. 2022, 20, 731–771. [Google Scholar] [CrossRef]
- Kechiche, A.; Fradi, T.; Noureddine, O.; Guergueb, M.; Loiseau, F.; Guerineau, V.; Issoui, N.; Lemeune, A.; Nasri, H. Synthesis, characterization and catalytic studies of chromium(III) porphyrin complex with axial cyanate ligands. J. Mol. Struct. 2022, 1250, 131801. [Google Scholar] [CrossRef]
- Amiri, N.; Taheur, F.B.; Chevreux, S.; Rodrigues, C.M.; Dorcet, V.; Lemercier, G.; Nasri, H. Syntheses, crystal structures, photo-physical properties, antioxidant and antifungal activities of Mg(II) 4,4′-bipyridine and Mg(II) pyrazine complexes of the 5,10,15,20 tetrakis(4–bromophenyl) porphyrin. Inorg. Chim. Acta 2021, 525, 120466. [Google Scholar] [CrossRef]
- Amiri, N.; Guergueb, M.; Al-Fakeh, M.S.; Bourguiba, M.; Nasri, H. A new cobalt(ii) meso-porphyrin: Synthesis, characterization, electric properties and application in the catalytic degradation of dyes. RSC Adv. 2020, 10, 44920–44932. [Google Scholar] [CrossRef]
- Mansour, A.; Belghith, Y.; Belkhiria, M.S.; Bujacz, A.; Guérineau, V.; Nasri, H. Synthesis, crystal structures and spectroscopic characterization of Co(II) bis(4,4′-bipyridine) with meso-porphyrins α,β,α,β-tetrakis(o-pivalamidophenyl) porphyrin (α,β,α,β-TpivPP) and tetraphenylporphyrin (TPP). J. Porphyr. Phthalocyanines 2013, 17, 1094–1103. [Google Scholar] [CrossRef]
- Guergueb, M.; Nasri, S.; Brahmi, J.; Loiseau, F.; Molton, F.; Roisnel, T.; Guerineau, V.; Turowska-Tyrk, I.; Aouadi, K.; Nasri, H. Effect of the coordination of p-acceptor 4-cyanopyridine ligand on the structural and electronic properties of meso-tetra(para-methoxy)and meso-tetra(para-chlorophenyl) porphyrincobalt(II) coordination compounds. Application in the catalytic degradation of methylene blue dye. RSC Adv. 2020, 10, 6900–6918. [Google Scholar] [PubMed]
- Guergueb, M.; Nasri, S.; Brahmi, J.; Al-Ghamdi, Y.O.; Loiseau, F.; Molton, F.; Roisnel, T.; Guerineau, V.; Nasri, H. Spectroscopic characterization, X-ray molecular structures and cyclic voltammetry study of two (piperazine) cobalt(II) meso-arylporphyincomplexes. Application as a catalyst for the degradation of 4-nitrophenol. Polyhedron 2021, 209, 115468. [Google Scholar] [CrossRef]
- Sugimoto, H.; Ueda, N.; Mori, M. Preparation and Physicochemical Properties of Tervalent Cobalt Complexes of Porphyrins. Bull. Chem. Soc. Jpn. 1981, 54, 3425–3432. [Google Scholar] [CrossRef]
- Albrecht, M.; Maji, P.; Häusl, C.; Monney, A.; Müller-Bunz, H. N-Heterocyclic carbene bonding to cobalt porphyrin complexes. Inorg. Chim. Acta 2012, 380, 90–95. [Google Scholar] [CrossRef]
- Weiss, R.; Fischer, J.; Bulach, V.; Schünemann, V.; Gerdan, M.; Trautwein, A.X.; Shelnutt, J.A.; Gros, C.P.; Tabard, A.; Guilard, R. Structure and mixed spin state of the chloro iron(III) complex of 2,3,7,8,12,13,17,18-octaphenyl-5,10,15,20-tetraphenylporphyrin, Fe(dpp)Cl. Inorg. Chim. Acta 2002, 337, 223–232. [Google Scholar] [CrossRef]
- Owens, J.W.; Smith, R.; Robinson, R.; Robins, M. Photophysical properties of porphyrins, phthalocyanines, and benzochlorins. Inorg. Chim. Acta 1998, 279, 226–231. [Google Scholar] [CrossRef]
- Amiri, N.; Nouir, S.; Hajji, M.; Roisnel, T.; Guerfel, T.; Simonneaux, G.; Nasri, H. Synthesis, structure, photophysical properties and biological activity of a cobalt(II) coordination complex with 4,4′-bipyridine and porphyrin chelating ligands. J. Saudi Chem. Soc. 2019, 23, 781–794. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Senge, M.O. The shape of porphyrins. Coord. Chem. Rev. 2021, 431, 213760. [Google Scholar] [CrossRef]
- Chen, L.; Fox, J.J.B.; Yi, G.-B.; Khan, M.A.; Richter-Addo, G.B. Synthesis and molecular structures of N,N-dialkyl-4-nitrosoaniline adducts of formally d6 metalloporphyrins of ruthenium and cobalt. J. Porphyr. Phthalocyanines 2001, 5, 702–707. [Google Scholar] [CrossRef]
- Li, J.; Noll, B.; Oliver, A.; Ferraudi, G.; Lappin, A.G.; Scheidt, W.R. Oxygenation of Cobalt Porphyrinates: Coordination or Oxidation? Inorg. Chem. 2010, 49, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Belghith, Y.; Daran, J.-C.; Nasri, H. Chlorido(pyridine-jN)(5,10,15,20-tetraphenylporphyrinato-j4 N)cobalt(III)chloroform hemisolvate. Acta Cryst. 2012, 68, m1104–m1105. [Google Scholar]
- Kaduk, J.A.; Scheidt, W.R. Stereochemistry of low-spin cobalt porphyrins. V. Molecular stereochemistry of nitro-.alpha.,.beta.,.gamma.,.delta.-tetraphenylporphinato(3,5-lutidine)cobalt(III). Inorg. Chem. 1974, 13, 1875–1880. [Google Scholar] [CrossRef]
- Goodwin, J.; Bailey, R.; Pennington, W.; Rasberry, R.; Green, T.; Shasho, S.; Yongsavanh, M.; Echevarria, V.; Tiedeken, J.; Brown, C.; et al. Structural and Oxo-Transfer Reactivity Differences of Hexacoordinate and Pentacoordinate (Nitro)(tetraphenylporphinato)cobalt(III) Derivatives. Inorg. Chem. 2001, 40, 4217–4225. [Google Scholar] [CrossRef] [PubMed]
- Doppelt, P.; Fischer, J.; Weiss, R. Synthesis and structure of bis(mercapto)cobalt(III) porphyrins. Models for the active site of cytochromes P 450. J. Am. Chem. Soc. 1984, 106, 5188–5193. [Google Scholar] [CrossRef]
- Sakurai, T.; Yamamoto, K.; Naito, H.; Nakamoto, N. The Crystal and Molecular Structure of Chloro-α,β,γ,δ-tetraphenylporphinatocobalt(III). Bull. Chem. Soc. Jpn. 1976, 49, 3042–3046. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; The University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- Venkatesan, P.; Thamotharan, S.; Ilangovan, A.; Liang, H.; Sundius, T. Crystal structure, Hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino] prop-2-enoic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 153, 625–636. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta. Cryst C. 2015, 71, 9–18. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 7, 3814–3816. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; SemiChem Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Kadish, K.M.; Lin, X.Q.; Han, B.C. Chloride-binding reactions, and electrochemistry of (tetraphenylporphyrinato)cobalt and chloro(tetraphenylporphyrinato)cobalt in dichloromethane. Inorg. Chem. 1987, 26, 4161–4167. [Google Scholar] [CrossRef]
- Rauf, M.A.; Bukallah, S.B.; Hammadi, A.; Soliman, A.; Hammadi, F. The effect of operational parameters on the photoinduced decoloration of dyes using a hybrid catalyst V2O5/TiO2. Chem. Eng. J. 2007, 129, 167–172. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Sweii, F.B.S.; Bouazizi, A. Effect of Thermal Annealing on the Electrical Properties of Inverted Organic Solar Cells Based on PCDTBT: PC70BM Nanocomposites. J. Electron. Mater. 2019, 48, 352–357. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.-L.; Chao, T.-S.; Sze, S. A new parallel adaptive finite volume method for the numerical simulation of semiconductor devices. Comput. Phys. Commun. 2001, 142, 285–289. [Google Scholar] [CrossRef]
- Hamza, S.; Mhamdi, A.; Aloui, W.; Bouazizi, A.; Khirouni, K.; Saidi, H. Effect of illumination on the dielectrical properties of P3HT:PC70BM nanocomposites. Mater. Res. Express 2017, 4, 055003. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Ferenczi, T.A.M.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer:fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar] [CrossRef]
- Brahmi, J.; Nasri, S.; Saidi, H.; Nasri, H. Aouadi. K. Synthesis of new porphyrin complexes: Evaluations on optical, electrochemical, electronic properties and application as an optical sensor. Chem. Select. 2019, 4, 31–37. [Google Scholar]
- Ezhov, A.V.; Aleksandrov, A.E.; Zhdanova, K.A.; Zhdanov, A.P.; Klyukin, I.N.; Zhizhin, K.Y.; Bragina, N.A.; Mironov, A.F.; Tameev, A.R. Synthesis of Zn(II) porphyrin dyes and revealing an influence of their alkyl substituents on performance of dye-sensitized solar cells. Synth. Met. 2020, 269, 116567. [Google Scholar] [CrossRef]
- Rashmi; Kappor, A.K.; Annapoorni, S.; Kumar, V. Conduction mechanisms in poly(3-hexylthiophene) thin-film sandwiched structures. Semicond. Sci. Technol. 2008, 23, 035008. [Google Scholar] [CrossRef]
- Murgatroyd, P.N. Theory of space-charge-limited current enhanced by Frenkel effect. J. Phys. D: Appl. Phys. 1970, 3, 151–156. [Google Scholar] [CrossRef]
- Amorim, C.; Cavallari, M.; Santos, G.; Fonseca, F.; Andrade, A.; Mergulhão, S. Determination of carrier mobility in MEH-PPV thin-films by stationary and transient current techniques. J. Non-Cryst. Solids 2012, 358, 484–491. [Google Scholar] [CrossRef]


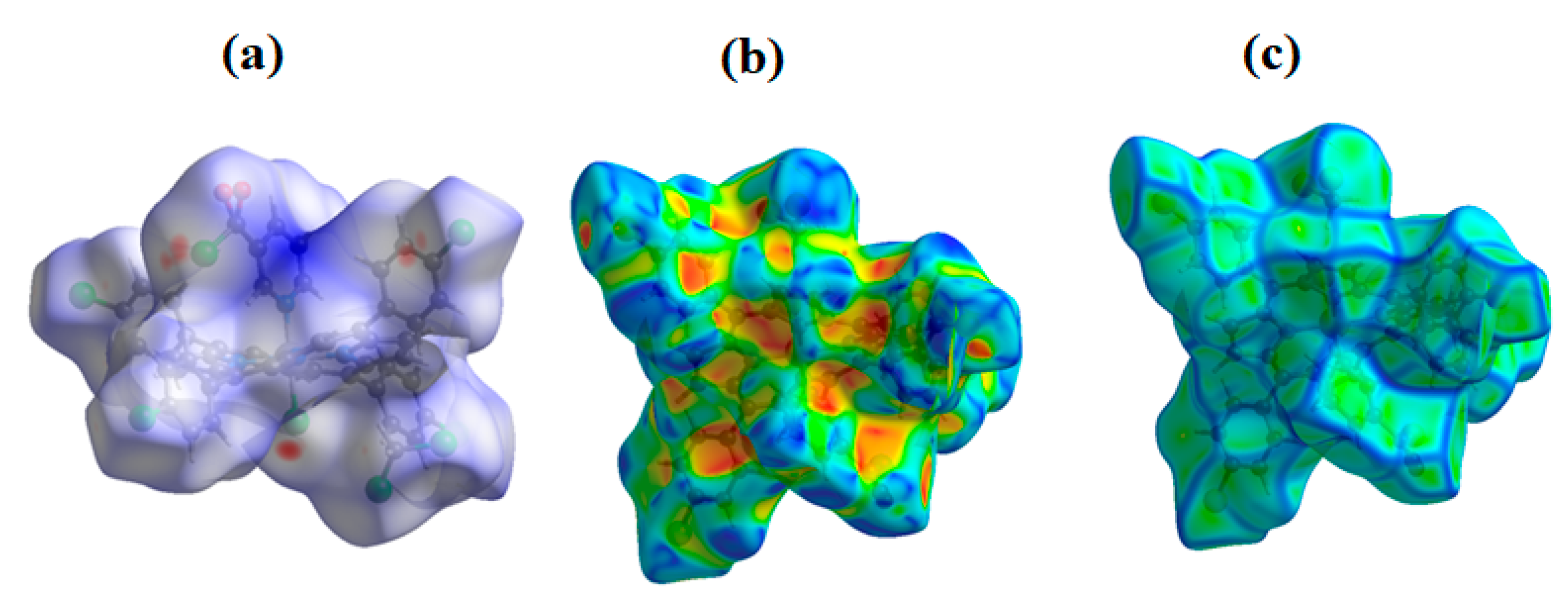
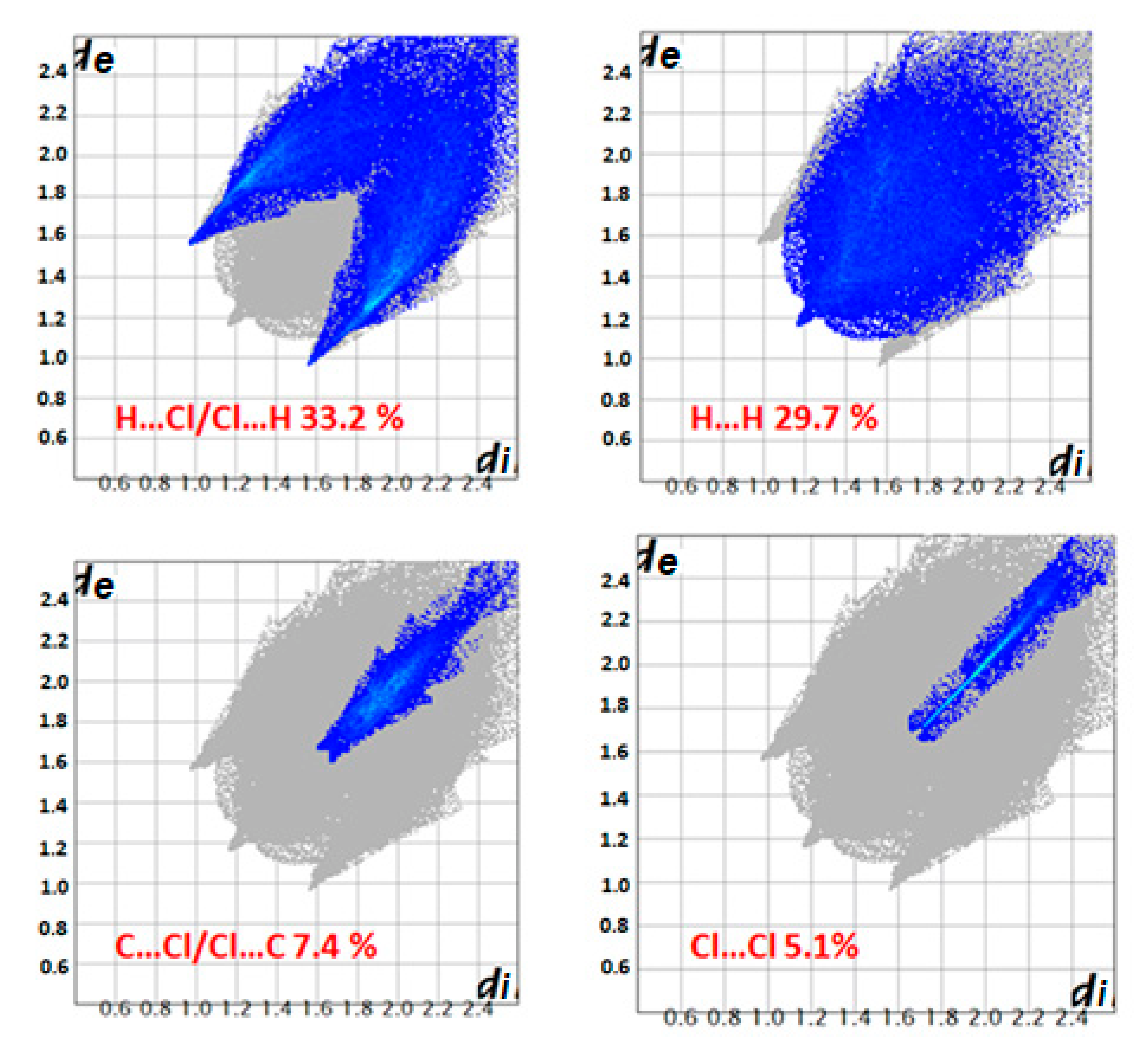
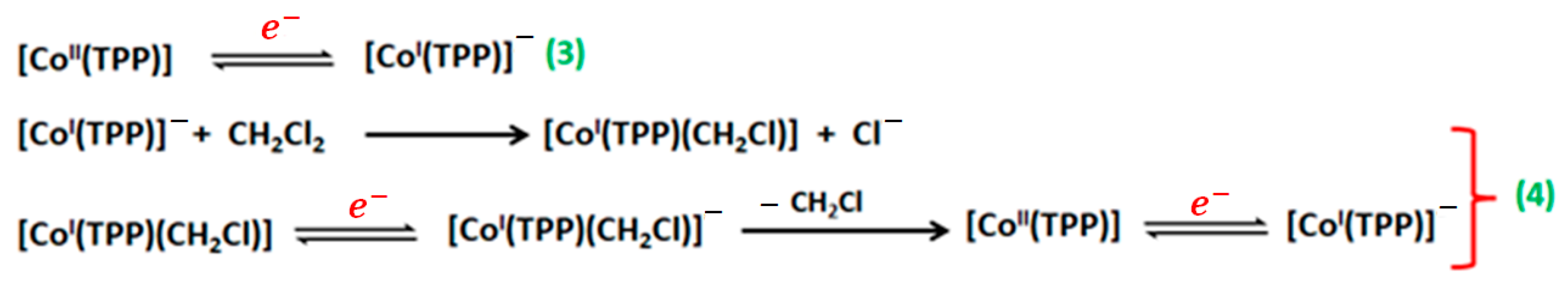

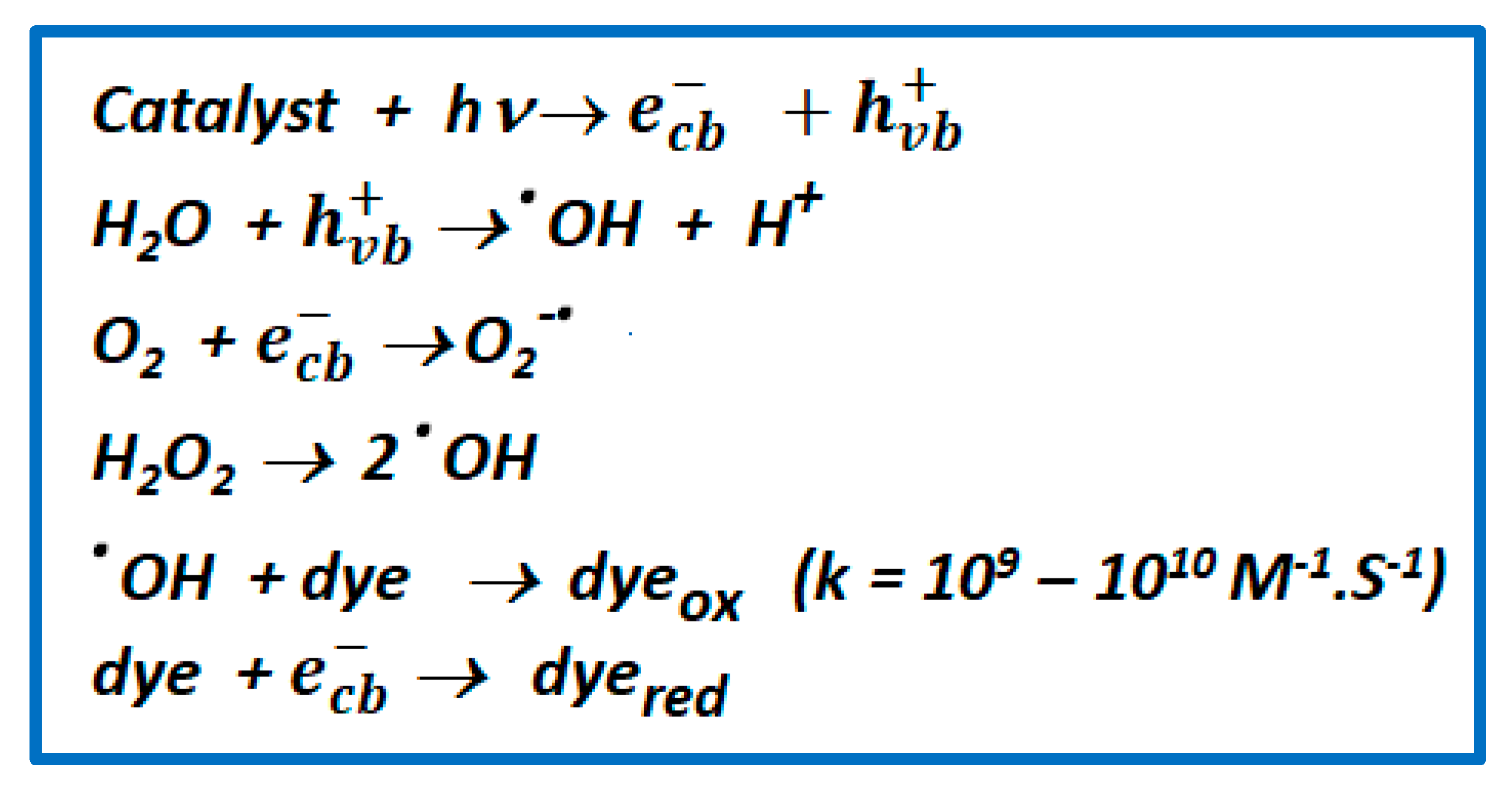
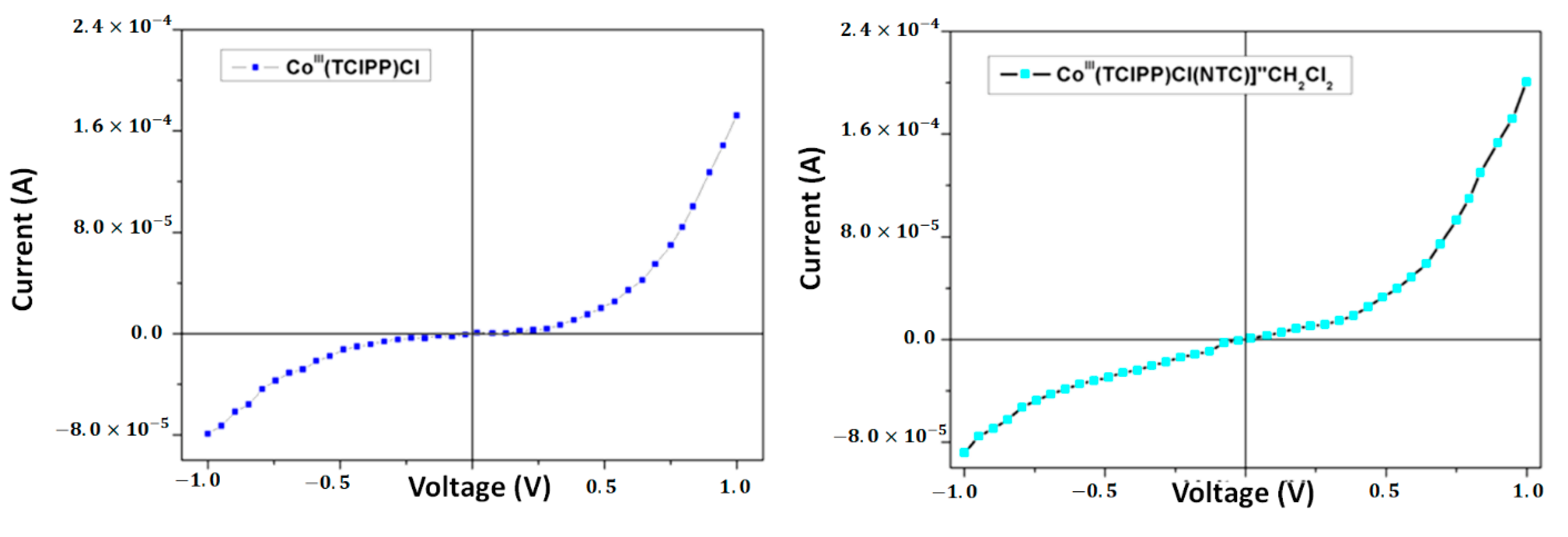
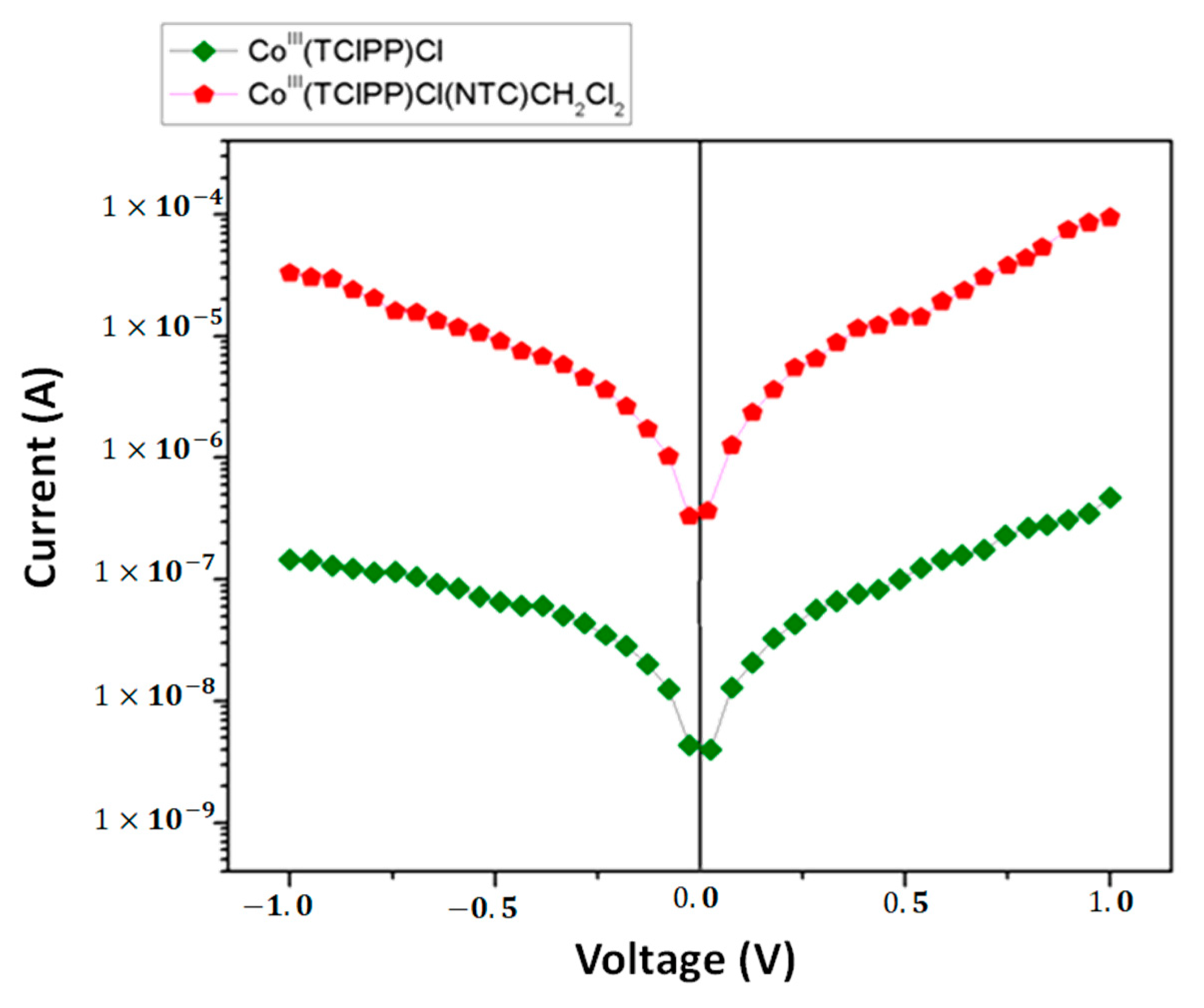
| Compound | Hβ-Pyrrolic Protons (ppm) | H-Phenyl Protons (ppm) | Ref. |
|---|---|---|---|
| Meso-arylporphyrins | |||
| H2TPP a | 8.84 | 8.23; 7.91; 7.67; 7.26 | [31] |
| H2TMPP b | 8.86 | 8.08; 7.27 | [32] |
| H2ClTPP (1) | 8.89 | 8.18; 7.74 | t.w. |
| [CoII(TPP)] a | 15.75 | 13.10; 9.80; 7.95 | [31] |
| [CoII(TMPP)] b | 15.90 | 13.10; 9.43 | [33] |
| [CoII(TClPP)] (2) | 15.75 | 12.93; 9.9 | t.w. |
| [CoIII(TPP)(Cl)(py)] | 9.00 | 8.80; 7.70 | [34] |
| [CoIII(TPP)(DMI)]+,a,c | 8.95 | 7.86; 7.71 | [35] |
| [CoIII(TPP)Cl(DMI)] a,c | 8.83 | 7.87; 7.65 | [35] |
| [CoIII(TClPP)(Cl)] (3) | 8.95 | 7.95; 7.78 | t.w. |
| [CoIII(TClPP)(Cl)(NTC)] (4) | 9.08 | 8.09; 7.75 | t.w. |
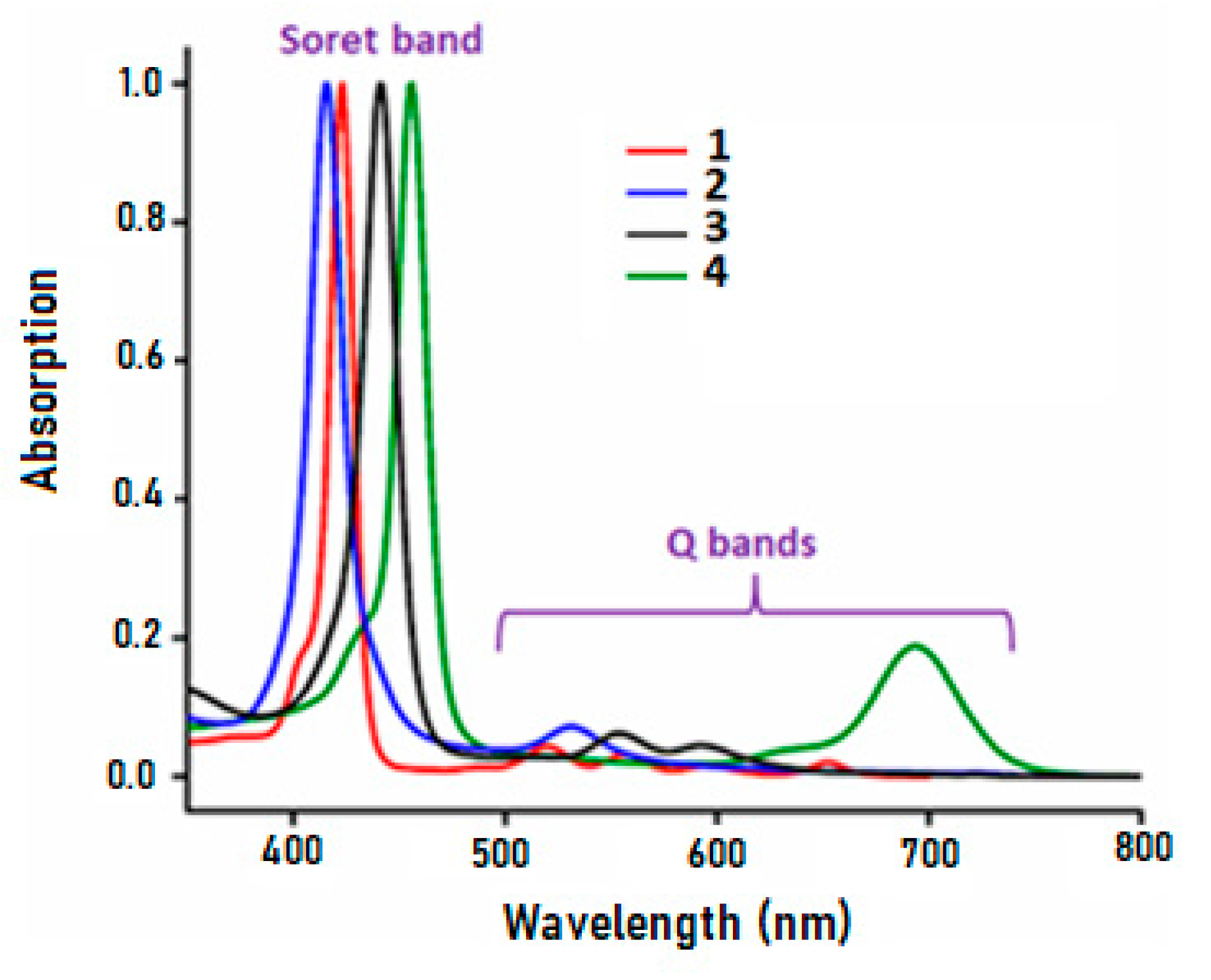
| Compound | λmax (nm) (ε × 10−3 M−1.cm−1) | Eg (eV) | Ref. | |
|---|---|---|---|---|
| Soret Band | Q Bands | |||
| Free-base meso-arylporphyrins | ||||
| H2TClPP (1) | 421(335) | 522(85) 557(56) 599(29) 651(36) | 1.88 | t.w. |
| H2TMPP a | 423(344) | 521(24) 558(20) 597(16) 650(15) | 1.76 | [21] |
| Cobalt meso-arylporphyrins | ||||
| [CoII(TClPP)] (2) | 414 (340) | 532(56) | 2.01 | t.w. |
| [CoII(TPP)] b | 412 | 528 | [21] | |
| [CoIII(TClPP)(Cl)] (3) | 442(296) | 557(50) 596(36) | 1.96 | t.w. |
| [CoIII(TClPP)Cl(NTC)] (4) | 455(335) | 560 (sh) 696(59) | 1.73 | t.w. |
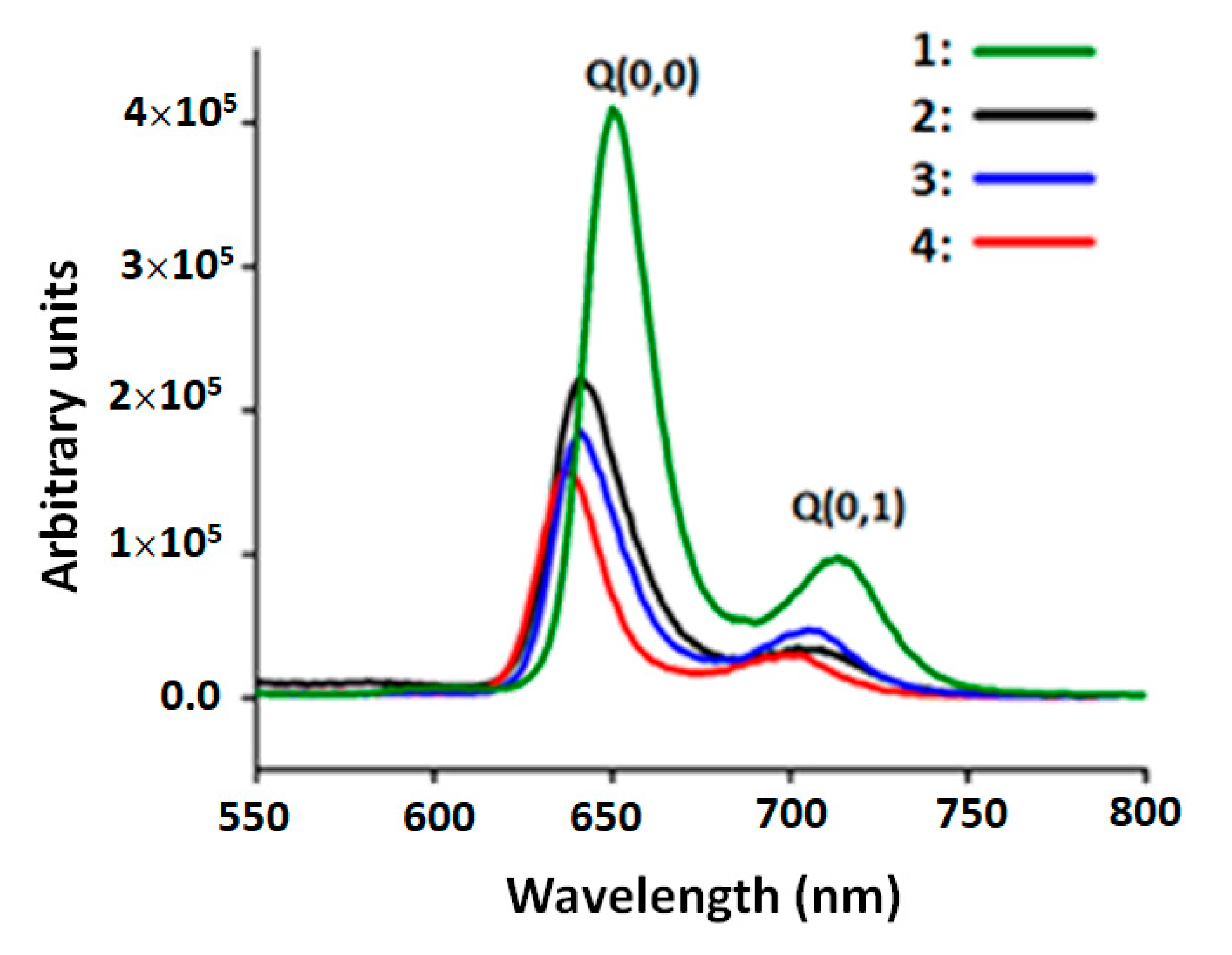
| Compound | λmax (nm) | φf a | τf b (in ns) | Solvent | Ref. | |
|---|---|---|---|---|---|---|
| O(0,0) | Q(0,1) | |||||
| Free-base meso-arylporphyrins | ||||||
| H2TPP c | 653 | 722 | 0.120 | 9.60 | DMF | [37] |
| H2TMPP d | 656 | 719 | 0.080 | 7.16 | CH2Cl2 | [32] |
| H2TClPP (1) | 650 | 714 | 0.089 | 7.40 | CH2Cl2 | this work |
| Cobalt(II) meso-arylporphyrins | ||||||
| [CoII(TMPP)] d | 655 | 719 | 0.035 | 6.02 | CH2Cl2 | [32] |
| [CoII(TClPP)] (2) | 641 | 709 | 0.04 | 6.10 | CH2Cl2 | this work |
| [CoII(TClPP)(4-CNpy)] | 653 | 714 | 0.06 | 2.00 | CH2Cl2 | [32] |
| [CoII(TPBP)(4,4′-bipy)2] e | 652 | 718 | 0.036 | CH2Cl2 | [38] | |
| Cobalt(III) meso-arylporphyrins | ||||||
| [CoIII(TClPP)Cl] (3) | 641 | 705 | 0.051 | 2.30 | CH2Cl2 | this work |
| [CoIII(TClPP)Cl(NTC)] (4) | 637 | 699 | 0.065 | 2.50 | CH2Cl2 | this work |
| Compound | Porphyrin Core Deformation Type a | Cp–Np b | Co–XL c | Ref. |
|---|---|---|---|---|
| [CoIII(TPP)(ONC6H4NMe2)2] d,e | Ruf ++,Wav ++ | 1.957(1) | 1.94(2)/1.98(2) | [40] |
| [CoIII(TpivPP)(2-MeIm)(2-MeHIm)] f,g,i | Ruf +,Wav +++ | 1.938(3) | 1.972(4)/1.953(3) | [41] |
| [CoIII(TPP)Cl(py)] d,j | Ruf +,Wav + | 1.938(3) | 2.234(1) 1/1.999(2) 2 | [42] |
| [CoIII(TPP)(NO2)(2,5-Lut)] d,k | Ruf ++,Wav ++ | 1.953 | 1.948 3/2.037 4 | [43] |
| [CoIII(TPP)(NO2)(Cl2py)] d,l | Ruf ++,Wav ++ | 1.955(3) | 1.912(3) 3/2.044(3) 4 | [44] |
| [CoIII(TPP)(DMIC)(MeOH)]BF4 m | Ruf +++,Wav +++ | 1.927(3) | 2.059(2) 5/1.929(3) 6 | [40] |
| [CoIII(TPP)(SPhF4)2] d,n | Planar | 1.978 | 2.347 | [45] |
| [CoIII(TPP)Cl] d | Planar | 1.984 | 2.150 | [46] |
| [CoIII(TClPP)Cl(NTC)]·CH2Cl2 (4) | Ruf +++,Wav +++ | 1.939(4) | 2.230(1) 1/1.991(4) 7 | this work |
| Oxidation | Reduction | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Complex | 1st Metal Oxd | 2nd Metal Oxd | 1st Metal Red | 2nd Metal Red | 1st Metal Red | ||||||
| (O1, R1) | (O2, R2) | (1a) | (1b) | (3) | (4) (R4, O4) | ||||||
| E1/2 a | E1/2 a | Eap b | Ecp c | Eap b | Ecp c | Eap b | Ecp c | Eap b | Ecp c | ||
| [CoIII(TPP)Cl] d | 0.90 | 1.15 | - | 0.10 | - | 0.57 | - | −0.90 | - | −1.42 | [53] |
| [CoIII(TClPP)Cl(NTC)] | 1.06 | 1.43 | - | 0.37 | - | −0.71 | - | −1.29 | - | −1.65 | this work |
| Complex | ||
|---|---|---|
| (3) | ||
| (4) | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, S.; Guergueb, M.; Brahmi, J.; Al-Ghamdi, Y.O.; Molton, F.; Loiseau, F.; Turowska-Tyrk, I.; Nasri, H. Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-ray Diffraction, and Electrical Properties for Photovoltaic Cells. Molecules 2022, 27, 8866. https://doi.org/10.3390/molecules27248866
Nasri S, Guergueb M, Brahmi J, Al-Ghamdi YO, Molton F, Loiseau F, Turowska-Tyrk I, Nasri H. Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-ray Diffraction, and Electrical Properties for Photovoltaic Cells. Molecules. 2022; 27(24):8866. https://doi.org/10.3390/molecules27248866
Chicago/Turabian StyleNasri, Soumaya, Mouhieddinne Guergueb, Jihed Brahmi, Youssef O. Al-Ghamdi, Florian Molton, Frédérique Loiseau, Ilona Turowska-Tyrk, and Habib Nasri. 2022. "Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-ray Diffraction, and Electrical Properties for Photovoltaic Cells" Molecules 27, no. 24: 8866. https://doi.org/10.3390/molecules27248866
APA StyleNasri, S., Guergueb, M., Brahmi, J., Al-Ghamdi, Y. O., Molton, F., Loiseau, F., Turowska-Tyrk, I., & Nasri, H. (2022). Synthesis of New Cobalt(III) Meso-Porphyrin Complex, Photochemical, X-ray Diffraction, and Electrical Properties for Photovoltaic Cells. Molecules, 27(24), 8866. https://doi.org/10.3390/molecules27248866






