Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis
Abstract
:1. Introduction
2. Ionic Liquids and Whole-Cell Biocatalysis
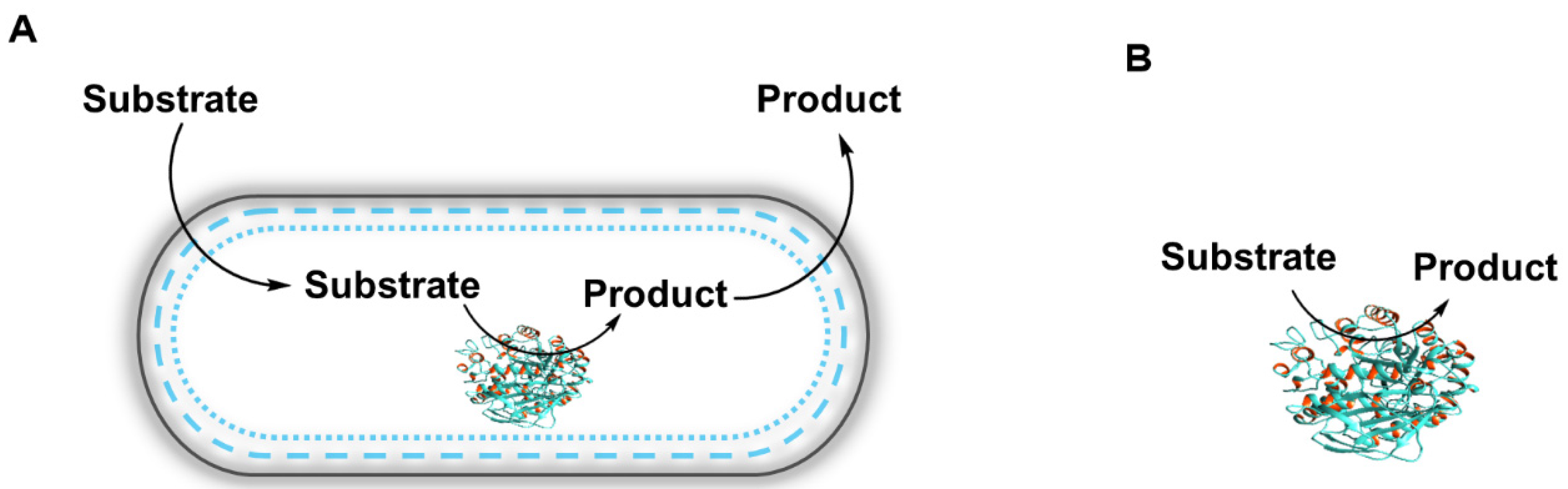
2.1. Introduction to Whole-Cell Biocatalysis
2.2. Ionic Liquids as Additives in Whole-Cell Biocatalytic Processes
2.3. Literature on the Effects of Ionic Liquids on Whole-Cell Biocatalysis
| Reaction | IL System | MO | Ref |
|---|---|---|---|
| Helicid + vinyl benzoate → 6′-O-benzoyl-helicid | Acetone/[BMIM][PF6] (5%, v/v) | Aspergillus oryzae | [29] |
| o-chloromandelonitrile → (R)-o-chloromandelic acid | [BMIM][PF6]-based biphasic system | Escherichia coli BL21 (DE3) nitrilase mutant F189T/T132A | [30] |
| caffeic acid + 2-phenyl ethanol → caffeic acid phenethyl ester | [EMIM][NTf2] | Aspergillus niger EXF 4321 | [31] |
| Phytosterols → Androst-4-ene-3,17-dione | 1% (v/v) [Ch][Asp] | Mycobacterium sp. MB 3683 | [32] |
| 3,5-bis(trifluoromethyl) acetophenone → (R)-[3,5-bis(trifluoromethyl)phenyl] ethanol | [N1,1,1,1][PF6] + distilled water reaction system | Trichoderma asperellum ZJPH0810 | [33] |
| benzaldehyde + glucose → (R)-phenylacetylcarbinol | [BMIM][PF6]-based biphasic system | Saccharomyces cerevisiae BY4741 | [34] |
| (4-chlorophenyl)-(pyridin-2-yl) methanone → (S)-(4-chlorophenyl)-(pyridin-2-yl) methanol | [EMIM][(MeO)HPO2] | Cryptococcus sp. M9-3 | [35] |
| Geraniol → geranyl glucoside | [HPYR][NTf2] | Escherichia coli expressing VvGT14a | [36] |
| (R)-carvone → (2R,5R)-dihydrocarvone | 20% (v/v) [HMP][NTf2] | Escherichia coli overexpressing ene-reductase | [37] |
| N-ethyl-methyl-carbamic acid → N-ethyl-methyl-carbamic acid-3-[(1S)-hydroxy-ethyl]-phenyl ester | [BMIM][BF4] | Escherichia coli BL21 (DE3) | [38] |
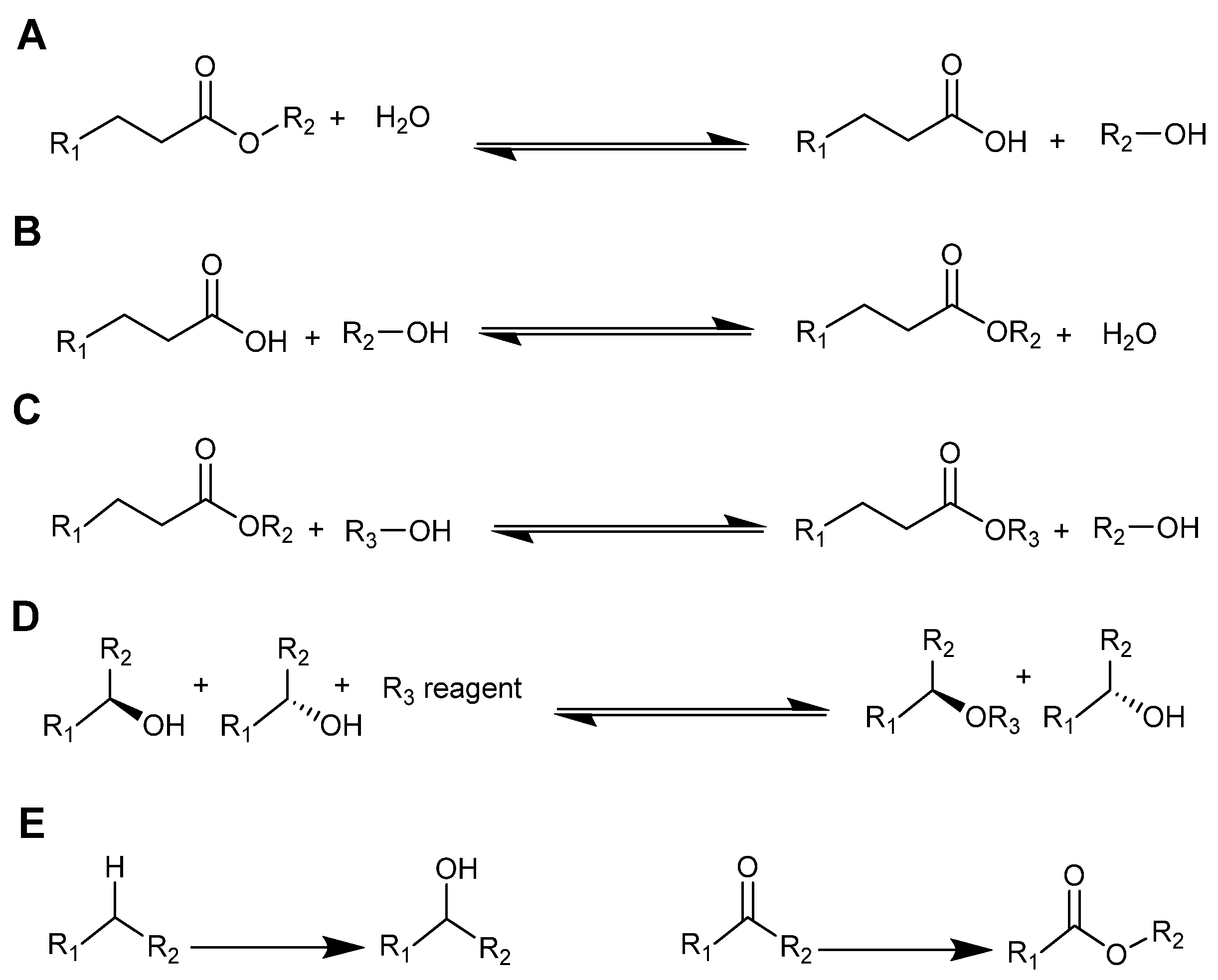
3. Ionic Liquids and Isolated Enzymes
3.1. Studying Enzymes in Ionic Liquids: Spectroscopic and Analytical Methods
3.2. Enzyme Activity in Neat Ionic Liquids
3.3. Enzymes in Aqueous/Ionic Liquid Mixtures
3.3.1. Enzymes in Aqueous/Ionic Liquid Mixtures with at Least 50% IL
3.3.2. Enzymes in Aqueous/Ionic Liquid Mixtures with Less than 50% IL
3.4. Coating Enzymes with Ionic Liquids
3.5. Protein Modification to Manipulate Solubility in Ionic Liquids
3.6. Enzyme Recycling Using Ionic Liquids
3.6.1. Ionic Liquids in Enzyme Entrapment
3.6.2. Ionic Liquid Tethering for Flow Biocatalysis
3.6.3. Ionic Liquid Sponges
4. Summary and Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Woodley, J.M. Role of Biocatalysis in Sustainable Chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef]
- Schmidt, S.; Scherkus, C.; Muschiol, J.; Menyes, U.; Winkler, T.; Hummel, W.; Gröger, H.; Liese, A.; Herz, H.-G.; Bornscheuer, U.T. An Enzyme Cascade Synthesis of ε-Caprolactone and its Oligomers. Angew. Chemie Int. Ed. 2015, 54, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Scholz, K.E.; Okrob, D.; Kopka, B.; Grünberger, A.; Pohl, M.; Jaeger, K.E.; Krauss, U. Synthesis of chiral cyanohydrins by recombinant Escherichia coli cells in a micro-aqueous reaction system. Appl. Environ. Microbiol. 2012, 78, 5025–5027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Zheng, G.W.; Du, P.X.; Zong, M.H.; Lou, W.Y. Whole-Cell Biocatalytic Processes with Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 371–386. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E.; Holtmann, D. Enzymatic reductions for the chemist. Green Chem. 2011, 13, 2285–2314. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.C.E.; Buehler, K.; Schallmey, A.; Bühler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011, 13, 226–265. [Google Scholar] [CrossRef]
- Vitale, P.; Perna, F.M.; Agrimi, G.; Pisano, I.; Mirizzi, F.; Capobianco, R.V.; Capriati, V. Whole-cell biocatalyst for chemoenzymatic total synthesis of rivastigmine. Catalysts 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Webb, C. Cellular Systems. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2, pp. 11–23. [Google Scholar]
- Muñoz Solano, D.; Hoyos, P.; Hernáiz, M.J.; Alcántara, A.R.; Sánchez-Montero, J.M. Industrial biotransformations in the synthesis of building blocks leading to enantiopure drugs. Bioresour. Technol. 2012, 115, 196–207. [Google Scholar] [CrossRef]
- Zajkoska, P.; Rosenberg, M.; Heath, R.; Malone, K.J.; Stloukal, R.; Turner, N.J.; Rebroš, M. Immobilised whole-cell recombinant monoamine oxidase biocatalysis. Appl. Microbiol. Biotechnol. 2015, 99, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Dolejš, I.; Líšková, M.; Krasňan, V.; Markošová, K.; Rosenberg, M.; Lorenzini, F.; Marr, A.C.; Rebroš, M. Production of 1,3-propanediol from pure and crude glycerol using immobilized clostridium butyricum. Catalysts 2019, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Tao, Y. Whole-cell biocatalysts by design. Microb. Cell Fact. 2017, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zajkoska, P.; Rebroš, M.; Rosenberg, M. Biocatalysis with immobilized Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 1441–1455. [Google Scholar] [CrossRef]
- Kell, D.B.; Swainston, N.; Pir, P.; Oliver, S.G. Membrane transporter engineering in industrial biotechnology and whole cell biocatalysis. Trends Biotechnol. 2015, 33, 237–246. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, C.C.C.R. Whole cell biocatalysts: Essential workers from Nature to the industry. Microb. Biotechnol. 2017, 10, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Cull, S.G.; Holbrey, J.D.; Vargas-Mora, V.; Seddon, K.R.; Lye, G.J. Room-Temperature Ionic Liquids as Multiphase Bioprocess Operations. Biotechnol. Bioeng. 2000, 69, 227–233. [Google Scholar] [CrossRef]
- Egorova, K.S.; Ananikov, V.P. Ionic liquids in whole-cell biocatalysis: A compromise between toxicity and efficiency. Biophys. Rev. 2018, 10, 881–900. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Rebros, M.; Gunaratne, H.Q.N.; Ferguson, J.; Seddon, K.R.; Stephens, G. A high throughput screen to test the biocompatibility of water-miscible ionic liquids. Green Chem. 2009, 11, 402–440. [Google Scholar] [CrossRef]
- Wood, N.; Ferguson, J.L.; Gunaratne, H.Q.N.; Seddon, K.R.; Goodacre, R.; Stephens, G.M. Screening ionic liquids for use in biotransformations with whole microbial cells. Green Chem. 2011, 13, 1843–1851. [Google Scholar] [CrossRef]
- Liu, X.H.; Rebroš, M.; Dolejš, I.; Marr, A.C. Designing Ionic Liquids for the Extraction of Alcohols from Fermentation Broth: Phosphonium Alkanesulfonates, Solvents for Diol Extraction. ACS Sustain. Chem. Eng. 2017, 5, 8260–8268. [Google Scholar] [CrossRef]
- Liu, S.; Rebros, M.; Stephens, G.; Marr, A.C. Adding value to renewables: A one pot process combining microbial cells and hydrogen transfer catalysis to utilise waste glycerol from biodiesel production. Chem. Commun. 2009, 17, 2308–2310. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.C.; Liu, S. Combining bio- and chemo-catalysis: From enzymes to cells, from petroleum to biomass. Trends Biotechnol. 2011, 29, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Lorenzini, F.; Rebros, M.; Saunders, G.C.; Marr, A.C. Combining bio- and chemo-catalysis for the conversion of bio-renewable alcohols: Homogeneous iridium catalysed hydrogen transfer initiated dehydration of 1,3-propanediol to aldehydes. Green Chem. 2016, 18, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Mai, N.L.; Koo, Y.-M. Whole-Cell Biocatalysis in Ionic Liquids. In Application of Ionic Liquids in Biotechnology; Itoh, T., Koo, Y.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 105–132. ISBN 978-3-030-23081-4. [Google Scholar]
- Dennewald, D.; Weuster-Botz, D. Ionic Liquids and Whole-Cell–Catalyzed Processes. In Ionic Liquids in Biotransformations and Organocatalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 261–314. ISBN 9781118158753. [Google Scholar]
- Yang, R.; Wu, T.; Xu, N.; Zhao, X.; Wang, Z.; Luo, H.; Bilal, M.; Nie, Z.; Song, Y. Improving whole-cell biocatalysis for helicid benzoylation by the addition of ionic liquids. Biochem. Eng. J. 2020, 161, 107695. [Google Scholar] [CrossRef]
- Zou, S.; Hua, D.; Jiang, Z.; Han, X.; Xue, Y.; Zheng, Y. A integrated process for nitrilase-catalyzed asymmetric hydrolysis and easy biocatalyst recycling by introducing biocompatible biphasic system. Bioresour. Technol. 2021, 320, 124392. [Google Scholar] [CrossRef]
- Rajapriya, G.; Morya, V.K.; Mai, N.L.; Koo, Y.M. Aspergillus niger whole-cell catalyzed synthesis of caffeic acid phenethyl ester in ionic liquids. Enzyme Microb. Technol. 2018, 111, 67–73. [Google Scholar] [CrossRef]
- Xiao, X.; He, J.K.; Guan, Y.X.; Yao, S.J. Effect of cholinium amino acids ionic liquids as cosolvents on the bioconversion of phytosterols by mycobacterium sp. Resting cells. ACS Sustain. Chem. Eng. 2020, 8, 17124–17132. [Google Scholar] [CrossRef]
- Li, J.; Qian, F.; Wang, P. Exploiting benign ionic liquids to effectively synthesize chiral intermediate of NK-1 receptor antagonists catalysed by Trichoderma asperellum cells. Biocatal. Biotransform. 2021, 39, 124–129. [Google Scholar] [CrossRef]
- Kandar, S.; Suresh, A.K.; Noronha, S.B. (R)-PAC Biosynthesis in [BMIM][PF6]/Aqueous Biphasic System Using Saccharomyces cerevisiae BY4741 Cells. Appl. Biochem. Biotechnol. 2015, 175, 1771–1788. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, S.; Zhao, Y.; Xia, J.; Liu, X.; Xu, J.M.; He, B.; Wu, B.; Zhang, J. Asymmetric whole-cell bioreduction of sterically bulky 2-benzoylpyridine derivatives in aqueous hydrophilic ionic liquid media. Chem. Eng. J. 2017, 316, 919–927. [Google Scholar] [CrossRef]
- Schmideder, A.; Priebe, X.; Rubenbauer, M.; Hoffmann, T.; Huang, F.C.; Schwab, W.; Weuster-Botz, D. Non-water miscible ionic liquid improves biocatalytic production of geranyl glucoside with Escherichia coli overexpressing a glucosyltransferase. Bioprocess Biosyst. Eng. 2016, 39, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, K.; Fu, Y.; Polte, I.; Leupold, S.; Meo, A.; Weuster-Botz, D. Asymmetric whole-cell bioreduction of (R)-carvone by recombinant Escherichia coli with in situ substrate supply and product removal. Biochem. Eng. J. 2017, 117, 102–111. [Google Scholar] [CrossRef]
- Xie, P.; Zhou, X.; Zheng, L. Stereoselective synthesis of a key chiral intermediate of (S)-Rivastigmine by AKR-GDH recombinant whole cells. J. Biotechnol. 2019, 289, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Elgharbawy, A.A.M.; Moniruzzaman, M.; Goto, M. Facilitating enzymatic reactions by using ionic liquids: A mini review. Curr. Opin. Green Sustain. Chem. 2021, 27, 100406. [Google Scholar] [CrossRef]
- Zhao, H. What do we learn from enzyme behaviors in organic solvents?—Structural functionalization of ionic liquids for enzyme activation and stabilization. Biotechnol. Adv. 2020, 45, 107638. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Moniruzzaman, M.; Goto, M. Recent advances of enzymatic reactions in ionic liquids: Part II. Biochem. Eng. J. 2020, 154, 107426. [Google Scholar] [CrossRef]
- Meyer, L.E.; von Langermann, J.; Kragl, U. Recent developments in biocatalysis in multiphasic ionic liquid reaction systems. Biophys. Rev. 2018, 10, 901–910. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of ionic liquids towards protein stability: A comprehensive overview on the current understanding and their implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef]
- Lozano, P.; Alvarez, E.; Bernal, J.M.; Nieto, S.; Gomez, C.; Sanchez-Gomez, G. Ionic Liquids for Clean Biocatalytic Processes. Curr. Green Chem. 2017, 4, 116–129. [Google Scholar] [CrossRef]
- Itoh, T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef] [PubMed]
- Schindl, A.; Hagen, M.L.; Muzammal, S.; Gunasekera, H.A.D.; Croft, A.K. Proteins in ionic liquids: Reactions, applications, and futures. Front. Chem. 2019, 7, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Biocatalysis in ionic liquids. RSC Catal. Ser. 2014, 2014, 20–43. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Lau, R.M.; Sorgedrager, M.J.; van Rantwijk, F.; Seddon, K.R. Biocatalysis in ionic liquids. Green Chem. 2002, 4, 147–151. [Google Scholar] [CrossRef]
- Sivapragasam, M.; Moniruzzaman, M.; Goto, M. Recent advances in exploiting ionic liquids for biomolecules: Solubility, stability and applications. Biotechnol. J. 2016, 11, 1000–1013. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, 1–12. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Wang, J. Improvement on the crystallization of lysozyme in the presence of hydrophilic ionic liquid. Analyst 2010, 135, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Smith, K.M.; Darmanin, C.; Ryan, T.M.; Drummond, C.J.; Greaves, T.L. Lysozyme conformational changes with ionic liquids: Spectroscopic, small angle x-ray scattering and crystallographic study. J. Colloid Interface Sci. 2021, 585, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Nordwald, E.M.; Plaks, J.G.; Snell, J.R.; Sousa, M.C.; Kaar, J.L. Crystallographic Investigation of Imidazolium Ionic Liquid Effects on Enzyme Structure. ChemBioChem 2015, 16, 2456–2459. [Google Scholar] [CrossRef] [Green Version]
- Warner, L.; Gjersing, E.; Follett, S.E.; Elliott, K.W.; Dzyuba, S.V.; Varga, K. The effects of high concentrations of ionic liquid on GB1 protein structure and dynamics probed by high-resolution magic-angle-spinning NMR spectroscopy. Biochem. Biophys. Rep. 2016, 8, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Figueiredo, A.M.; Cabrita, E.J. Epitope mapping of imidazolium cations in ionic liquid-protein interactions unveils the balance between hydrophobicity and electrostatics towards protein destabilisation. Phys. Chem. Chem. Phys. 2014, 16, 23394–23403. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, Q.; Jiang, L.; Liu, M.; Li, C. Protein stability analysis in ionic liquids by 19F NMR. Anal. Bioanal. Chem. 2019, 411, 4929–4935. [Google Scholar] [CrossRef] [PubMed]
- Summers, S.R.; Sprenger, K.G.; Pfaendtner, J.; Marchant, J.; Summers, M.F.; Kaar, J.L. Mechanism of Competitive Inhibition and Destabilization of Acidothermus cellulolyticus Endoglucanase 1 by Ionic Liquids. J. Phys. Chem. B 2017, 121, 10793–10803. [Google Scholar] [CrossRef] [PubMed]
- Jha, I.; Bisht, M.; Mogha, N.K.; Venkatesu, P. Effect of Imidazolium-Based Ionic Liquids on the Structure and Stability of Stem Bromelain: Concentration and Alkyl Chain Length Effect. J. Phys. Chem. B 2018, 122, 7522–7529. [Google Scholar] [CrossRef]
- Arunkumar, R.; Drummond, C.J.; Greaves, T.L. FTIR spectroscopic study of the secondary structure of globular proteins in aqueous protic ionic liquids. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bui-Le, L.; Clarke, C.J.; Bröhl, A.; Brogan, A.P.S.; Arpino, J.A.J.; Polizzi, K.M.; Hallett, J.P. Revealing the complexity of ionic liquid–protein interactions through a multi-technique investigation. Commun. Chem. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Lau, R.M.; Sorgedrager, M.J.; Carrea, G.; Van Rantwijk, F.; Secundo, F.; Sheldon, R.A. Dissolution of Candida antarctica lipase B in ionic liquids: Effects on structure and activity. Green Chem. 2004, 6, 483–487. [Google Scholar] [CrossRef]
- Bihari, M.; Russell, T.P.; Hoagland, D.A. Dissolution and dissolved state of cytochrome c in a neat, hydrophilic ionic liquid. Biomacromolecules 2010, 11, 2944–2948. [Google Scholar] [CrossRef]
- Strassburg, S.; Bermudez, H.; Hoagland, D. Lysozyme Solubility and Conformation in Neat Ionic Liquids and Their Mixtures with Water. Biomacromolecules 2016, 17, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, C.; He, Q.; Qin, H.; Liang, G.; Liu, W. Efficient resolution of (R,S)-1-(1-naphthyl)ethylamine by Candida antarctica lipase B in ionic liquids. Mol. Catal. 2018, 448, 116–121. [Google Scholar] [CrossRef]
- Zhao, H.; Kanpadee, N.; Jindarat, C. Ether-functionalized ionic liquids for nonaqueous biocatalysis: Effect of different cation cores. Process Biochem. 2019, 81, 104–112. [Google Scholar] [CrossRef]
- Zhao, H.; Toe, C. “Water-like” ammonium-based ionic liquids for lipase activation and enzymatic polymerization. Process Biochem. 2020, 98, 59–64. [Google Scholar] [CrossRef]
- De Diego, T.; Lozano, P.; Gmouh, S.; Vaultier, M.; Iborra, J.L. Understanding structure—Stability relationships of Candida antartica lipase B in ionic liquids. Biomacromolecules 2005, 6, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nakamura, N.; Igarashi, K.; Samejima, M.; Ohno, H. Biocatalytic oxidation of cellobiose in an hydrated ionic liquid. Green Chem. 2009, 11, 351–354. [Google Scholar] [CrossRef]
- Fujita, K.; Sanada, M.; Ohno, H. Sugar chain-binding specificity and native folding state of lectins preserved in hydrated ionic liquids. Chem. Commun. 2015, 51, 10883–10886. [Google Scholar] [CrossRef]
- Mazid, R.R.; Vijayaraghavan, R.; MacFarlane, D.R.; Cortez-Jugo, C.; Cheng, W. Inhibited fragmentation of mAbs in buffered ionic liquids. Chem. Commun. 2015, 51, 8089–8092. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, X.; Zhang, L.; Li, J.; Yang, L.; Gao, P.; Zhou, Z. Lipase-catalyzed synthesis of biodiesel in a hydroxyl-functionalized ionic liquid. Chem. Eng. Res. Des. 2018, 132, 199–207. [Google Scholar] [CrossRef]
- Bisht, M.; Mondal, D.; Pereira, M.M.; Freire, M.G.; Venkatesu, P.; Coutinho, J.A.P. Long-term protein packaging in cholinium-based ionic liquids: Improved catalytic activity and enhanced stability of cytochrome c against multiple stresses. Green Chem. 2017, 19, 4900–4911. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, H.; Singh, M.; Singh, G. Biamphiphilic ionic liquid based aqueous microemulsions as an efficient catalytic medium for cytochrome c †. Phys. Chem. Chem. Phys. 2021, 23, 320–328. [Google Scholar] [CrossRef]
- Fujita, K.; MacFarlene, D.R.; Forsyth, M.; Yoshizawa-Fujita, M.; Murata, K.; Nakamura, N.; Ohno, H. Solubility and stability of cytochrome c in hydrated ionic liquids: Effect of oxo acid residues and kosmotropicity. Biomacromolecules 2007, 8, 2080–2086. [Google Scholar] [CrossRef]
- Weaver, K.D.; Vrikkis, R.M.; Van Vorst, M.P.; Trullinger, J.; Vijayaraghavan, R.; Foureau, D.M.; McKillop, I.H.; MacFarlane, D.R.; Krueger, J.K.; Elliott, G.D. Structure and function of proteins in hydrated choline dihydrogen phosphate ionic liquid. Phys. Chem. Chem. Phys. 2012, 14, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; de Gonzalo, G.; Fraaije, M.W.; Gotor, V. Ionic liquids for enhancing the enantioselectivity of isolated BVMO-catalysed oxidations. Green Chem. 2010, 12, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Zou, X.; Lv, S.; Jin, Q.; Wang, X. Influence of ionic liquids on lipase activity and stability in alcoholysis reactions. RSC Adv. 2016, 6, 87703–87709. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Freire, C.C.C.; Souza, R.L.; Cabrera-Padilla, R.Y.; Pereira, M.M.; Freire, M.G.; Lima, Á.S.; Soares, C.M.F. Effects of phosphonium-based ionic liquids on the lipase activity evaluated by experimental results and molecular docking. Biotechnol. Prog. 2019, 35, 1–10. [Google Scholar] [CrossRef]
- Ghanbari-Ardestani, S.; Khojasteh-Band, S.; Zaboli, M.; Hassani, Z.; Mortezavi, M.; Mahani, M.; Torkzadeh-Mahani, M. The effect of different percentages of triethanolammonium butyrate ionic liquid on the structure and activity of urate oxidase: Molecular docking, molecular dynamics simulation, and experimental study. J. Mol. Liq. 2019, 292, 111318. [Google Scholar] [CrossRef]
- Bhakuni, K.; Sindhu, A.; Bisht, M.; Venkatesu, P. Exploring the Counteracting and Refolding Ability of Choline-Based Ionic Liquids toward Crowding Environment-Induced Changes in HSA Structure. ACS Sustain. Chem. Eng. 2021, 9, 422–437. [Google Scholar] [CrossRef]
- Ramos-Martín, J.; Khiari, O.; Alcántara, A.R.; Sánchez-Montero, J.M. Biocatalysis at extreme temperatures: Enantioselective synthesis of both enantiomers of mandelic acid by transesterification catalyzed by a thermophilic lipase in ionic liquids at 120 °C. Catalysts 2020, 10, 1055. [Google Scholar] [CrossRef]
- Pedersen, J.N.; Liu, S.; Zhou, Y.; Balle, T.; Xu, X.; Guo, Z. Synergistic effects of binary ionic liquid-solvent systems on enzymatic esterification of esculin. Food Chem. 2020, 310, 125858. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kim, M. Ionic Liquid-Coated Enzyme for Biocatalysis in Organic Solvent of methodology in organic synthesis. 1 For example, lipase catalysis in organic solvents is of great use for the synthesis of optically active compounds such as chiral limitations, many app. J. Org. Chem. 2002, 67, 6845–6847. [Google Scholar] [CrossRef]
- Itoh, T.; Matsushita, Y.; Abe, Y.; Han, S.H.; Wada, S.; Hayase, S.; Kawatsura, M.; Takai, S.; Morimoto, M.; Hirose, Y. Increased enantioselectivity and remarkable acceleration of lipase-catalyzed transesterification by using an imidazolium PEG-Alkyl sulfate ionic liquid. Chem. A Eur. J. 2006, 12, 9228–9237. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Han, S.; Matsushita, Y.; Hayase, S. Enhanced enantioselectivity and remarkable acceleration on the lipase-catalyzed transesterification using novel ionic liquids. Green Chem. 2004, 6, 437–439. [Google Scholar] [CrossRef]
- Nishihara, T.; Shiomi, A.; Kadotani, S.; Nokami, T.; Itoh, T. Remarkably improved stability and enhanced activity of a: Burkholderia cepacia lipase by coating with a triazolium alkyl-PEG sulfate ionic liquid. Green Chem. 2017, 19, 5250–5256. [Google Scholar] [CrossRef]
- Abe, Y.; Yagi, Y.; Hayase, S.; Kawatsura, M.; Itoh, T. Ionic liquid engineering for lipase-mediated optical resolution of secondary alcohols: Design of ionic liquids applicable to ionic liquid coated-lipase catalyzed reaction. Ind. Eng. Chem. Res. 2012, 51, 9952–9958. [Google Scholar] [CrossRef]
- Abe, Y.; Yoshiyama, K.; Yagi, Y.; Hayase, S.; Kawatsura, M.; Itoh, T. A rational design of phosphonium salt type ionic liquids for ionic liquid coated-lipase catalyzed reaction. Green Chem. 2010, 12, 1976–1980. [Google Scholar] [CrossRef]
- Bekhouche, M.; Doumèche, B.; Blum, L.J. Chemical modifications by ionic liquid-inspired cations improve the activity and the stability of formate dehydrogenase in [MMIm][Me2PO4]. J. Mol. Catal. B Enzym. 2010, 65, 73–78. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Kaar, J.L. Stabilization of enzymes in ionic liquids via modification of enzyme charge. Biotechnol. Bioeng. 2013, 110, 2352–2360. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Kaar, J.L. Mediating electrostatic binding of 1-butyl-3-methylimidazolium chloride to enzyme surfaces improves conformational stability. J. Phys. Chem. B 2013, 117, 8977–8986. [Google Scholar] [CrossRef] [PubMed]
- Brogan, A.P.S.; Hallett, J.P. Solubilizing and Stabilizing Proteins in Anhydrous Ionic Liquids through Formation of Protein-Polymer Surfactant Nanoconstructs. J. Am. Chem. Soc. 2016, 138, 4494–4501. [Google Scholar] [CrossRef] [Green Version]
- Brogan, A.P.S.; Bui-Le, L.; Hallett, J.P. Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase. Nat. Chem. 2018, 10, 859–865. [Google Scholar] [CrossRef]
- Mukhopadhayay, A.; Singh, D.; Sharma, K.P. Neat Ionic liquid and α-Chymotrypsin-Polymer Surfactant Conjugate-Based Biocatalytic Solvent. Biomacromolecules 2020, 21, 867–877. [Google Scholar] [CrossRef]
- Chado, G.R.; Holland, E.N.; Tice, A.K.; Stoykovich, M.P.; Kaar, J.L. Modification of Lipase with Poly(4-acryloylmorpholine) Enhances Solubility and Transesterification Activity in Anhydrous Ionic Liquids. Biomacromolecules 2018, 19, 1324–1332. [Google Scholar] [CrossRef]
- Dotsenko, A.S.; Rozhkova, A.M.; Zorov, I.N.; Sinitsyn, A.P. Protein surface engineering of endoglucanase Penicillium verruculosum for improvement in thermostability and stability in the presence of 1-butyl-3-methylimidazolium chloride ionic liquid. Bioresour. Technol. 2020, 296, 122370. [Google Scholar] [CrossRef]
- Frauenkron-Machedjou, V.J.; Fulton, A.; Zhu, L.; Anker, C.; Bocola, M.; Jaeger, K.E.; Schwaneberg, U. Towards Understanding Directed Evolution: More than Half of All Amino Acid Positions Contribute to Ionic Liquid Resistance of Bacillus subtilis Lipase A. ChemBioChem 2015, 16, 937–945. [Google Scholar] [CrossRef]
- Pramanik, S.; Dhoke, G.V.; Jaeger, K.E.; Schwaneberg, U.; Davari, M.D. How to Engineer Ionic Liquids Resistant Enzymes: Insights from Combined Molecular Dynamics and Directed Evolution Study. ACS Sustain. Chem. Eng. 2019, 7, 11293–11302. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: Applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Alnoch, R.C.; Alves dos Santos, L.; de Almeida, J.M.; Krieger, N.; Mateo, C. Recent trends in biomaterials for immobilization of lipases for application in non-conventional media. Catalysts 2020, 10, 697. [Google Scholar] [CrossRef]
- Cottone, G.; Giu, S.; Bettati, S.; Bruno, S.; Campanini, B.; Marchetti, M.; Abbruzzetti, S.; Viappiani, C.; Cupane, A.; Mozzarelli, A.; et al. More than a Confinement: ”Soft” and “Hard” Enzyme Entrapment Modulates Biological Catalyst Function. Catalysts 2019, 9, 1024. [Google Scholar] [CrossRef] [Green Version]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Doan, T.T.N.; Ha, S.H.; Koo, Y.M. Using ionic liquids to stabilize lipase within sol-gel derived silica. J. Mol. Catal. B Enzym. 2007, 45, 57–61. [Google Scholar] [CrossRef]
- Tamborini, L.; Fernandes, P.; Paradisi, F.; Molinari, F. Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol. 2018, 36, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernández, M.; Paradisi, F. Protein immobilization technology for flow biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 1–8. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.E.; Kara, S. The rise of continuous flow biocatalysis-fundamentals, very recent developments and future perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Santi, M.; Sancineto, L.; Nascimento, V.; Azeredo, J.B.; Orozco, E.V.M.; Andrade, L.H.; Gröger, H.; Santi, C. Flow biocatalysis: A challenging alternative for the synthesis of APIs and natural compounds. Int. J. Mol. Sci. 2021, 22, 990. [Google Scholar] [CrossRef]
- Reetz, M.T.; Wiesenhöfer, W.; Franciò, G.; Leitner, W. Biocatalysis in ionic liquids: Batchwise and continuous flow processes using supercritical carbon dioxide as the mobile phase. Chem. Commun. 2002, 2, 992–993. [Google Scholar] [CrossRef]
- Lozano, P.; de Diego, T.; Carrié, D.; Vaultier, M.; Iborra, J.L. Continuous green biocatalytic processes using ionic liquids and supercritical carbon dioxide. Chem. Commun. 2002, 7, 692–693. [Google Scholar] [CrossRef]
- Reetz, M.T.; Wiesenhöfer, W.; Franciò, G.; Leitner, W. Continuous Flow Enzymatic Kinetic Resolution and Enantiomer Separation using Ionic Liquid/Supercritical Carbon Dioxide Media. Adv. Synth. Catal. 2003, 345, 1221–1228. [Google Scholar] [CrossRef]
- Lozano, P.; De Diego, T.; Larnicol, M.; Vaultier, M.; Iborra, J.L. Chemoenzymatic dynamic kinetic resolution of rac-1-phenylethanol in ionic liquids and ionic liquids/supercritical carbon dioxide systems. Biotechnol. Lett. 2006, 28, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; De Diego, T.; Sauer, T.; Vaultier, M.; Gmouh, S.; Iborra, J.L. On the importance of the supporting material for activity of immobilized Candida antarctica lipase B in ionic liquid/hexane and ionic liquid/supercritical carbon dioxide biphasic media. J. Supercrit. Fluids 2007, 40, 93–100. [Google Scholar] [CrossRef]
- Lozano, P.; De Diego, T.; Mira, C.; Montague, K.; Vaultier, M.; Iborra, J.L. Long term continuous chemoenzymatic dynamic kinetic resolution of rac-1-phenylethanol using ionic liquids and supercritical carbon dioxide. Green Chem. 2009, 11, 538–554. [Google Scholar] [CrossRef]
- García-Verdugo, E.; Altava, B.; Burguete, M.I.; Lozano, P.; Luis, S.V. Ionic liquids and continuous flow processes: A good marriage to design sustainable processes. Green Chem. 2015, 17, 2693–2713. [Google Scholar] [CrossRef] [Green Version]
- Lozano, P.; Nieto, S.; Serrano, J.L.; Perez, J.; Sanchez-Gomez, G.; Garcia-Verdugo, E.; Luis, S.V. Flow Biocatalytic Processes in Ionic Liquids and Supercritical Fluids. Mini. Rev. Org. Chem. 2017, 14, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Sandig, B.; Michalek, L.; Vlahovic, S.; Antonovici, M.; Hauer, B.; Buchmeiser, M.R. A Monolithic Hybrid Cellulose-2.5-Acetate/Polymer Bioreactor for Biocatalysis under Continuous Liquid-Liquid Conditions Using a Supported Ionic Liquid Phase. Chem. A Eur. J. 2015, 21, 15835–15842. [Google Scholar] [CrossRef] [PubMed]
- Sandig, B.; Buchmeiser, M.R. Highly Productive and Enantioselective Enzyme Catalysis under Continuous Supported Liquid–Liquid Conditions Using a Hybrid Monolithic Bioreactor. ChemSusChem 2016, 9, 2917–2921. [Google Scholar] [CrossRef]
- Zhang, M.; Ettelaie, R.; Yan, T.; Zhang, S.; Cheng, F.; Binks, B.P.; Yang, H. Ionic Liquid Droplet Microreactor for Catalysis Reactions Not at Equilibrium. J. Am. Chem. Soc. 2017, 139, 17387–17396. [Google Scholar] [CrossRef]
- Deng, Q.; Tran, N.N.; Razi Asrami, M.; Schober, L.; Gröger, H.; Hessel, V. Ionic Liquid/Water Continuous-Flow System with Compartmentalized Spaces for Automatic Product Purification of Biotransformation with Enzyme Recycling. Ind. Eng. Chem. Res. 2020, 59, 21001–21011. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Garcia-Verdugo, E.; Sanchez-Gomez, G.; Vaultier, M.; Burguete, M.I.; Luis, S.V. Sponge-like ionic liquids: A new platform for green biocatalytic chemical processes. Green Chem. 2015, 17, 3706–3717. [Google Scholar] [CrossRef]
- Villa, R.; Alvarez, E.; Porcar, R.; Garcia-Verdugo, E.; Luis, S.V.; Lozano, P. Ionic liquids as an enabling tool to integrate reaction and separation processes. Green Chem. 2019, 21, 6527–6544. [Google Scholar] [CrossRef]
- Lozano, P.; Bernal, J.M.; Piamtongkam, R.; Fetzer, D.; Vaultier, M. One-phase ionic liquid reaction medium for biocatalytic production of biodiesel. ChemSusChem 2010, 3, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Bernal, J.M.; Navarro, A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012, 14, 3026–3033. [Google Scholar] [CrossRef]
- Alvarez, E.; Rodriguez, J.; Villa, R.; Gomez, C.; Nieto, S.; Donaire, A.; Lozano, P. Clean enzymatic production of flavor esters in spongelike ionic liquids. ACS Sustain. Chem. Eng. 2019, 7, 13307–13314. [Google Scholar] [CrossRef]
- Lozano, P.; Gomez, C.; Nicolas, A.; Polo, R.; Nieto, S.; Bernal, J.M.; García-Verdugo, E.; Luis, S.V. Clean enzymatic preparation of oxygenated biofuels from vegetable and waste cooking oils by using spongelike ionic liquids technology. ACS Sustain. Chem. Eng. 2016, 4, 6125–6132. [Google Scholar] [CrossRef]
- Lozano, P.; Gomez, C.; Nieto, S.; Sanchez-Gomez, G.; García-Verdugo, E.; Luis, S.V. Highly selective biocatalytic synthesis of monoacylglycerides in sponge-like ionic liquids. Green Chem. 2017, 19, 390–396. [Google Scholar] [CrossRef]
- Ohno, H.; Fukumoto, K. Amino acid ionic liquids. Acc. Chem. Res. 2007, 40, 1122–1129. [Google Scholar] [CrossRef]
- Mohajeri, A.; Ashrafi, A. Structure and electronic properties of amino acid ionic liquids. J. Phys. Chem. A 2011, 115, 6589–6593. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, D.; Zhang, J.; Zhu, Y.; Zhang, Z.; Qian, C.; Ren, Q.; Xing, H. Long-chain fatty acid-based phosphonium ionic liquids with strong hydrogen-bond basicity and good lipophilicity: Synthesis, characterization, and application in extraction. ACS Sustain. Chem. Eng. 2015, 3, 309–316. [Google Scholar] [CrossRef]
- Gusain, R.; Khan, A.; Khatri, O.P. Fatty acid-derived ionic liquids as renewable lubricant additives: Effect of chain length and unsaturation. J. Mol. Liq. 2020, 301, 112322. [Google Scholar] [CrossRef]
- Gontrani, L. Choline-amino acid ionic liquids: Past and recent achievements about the structure and properties of these really “green” chemicals. Biophys. Rev. 2018, 10, 873–880. [Google Scholar] [CrossRef]
- Pagar, A.D.; Patil, M.D.; Flood, D.T.; Yoo, T.H.; Dawson, P.E.; Yun, H. Recent Advances in Biocatalysis with Chemical Modification and Expanded Amino Acid Alphabet. Chem. Rev. 2021, 121, 6173–6245. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.R.; Mangas-Sanchez, J.; Turner, N.J. Expanding the synthetic scope of biocatalysis by enzyme discovery and protein engineering. Tetrahedron 2021, 82, 131926. [Google Scholar] [CrossRef]
- Gargiulo, S.; Soumillion, P. Directed evolution for enzyme development in biocatalysis. Curr. Opin. Chem. Biol. 2021, 61, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Seo, J.H.; Oh, D.K.; Bornscheuer, U.T.; Park, J.B. Design and engineering of whole-cell biocatalytic cascades for the valorization of fatty acids. Catal. Sci. Technol. 2020, 10, 46–64. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imam, H.T.; Krasňan, V.; Rebroš, M.; Marr, A.C. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules 2021, 26, 4791. https://doi.org/10.3390/molecules26164791
Imam HT, Krasňan V, Rebroš M, Marr AC. Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules. 2021; 26(16):4791. https://doi.org/10.3390/molecules26164791
Chicago/Turabian StyleImam, Hasan Tanvir, Vladimír Krasňan, Martin Rebroš, and Andrew Craig Marr. 2021. "Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis" Molecules 26, no. 16: 4791. https://doi.org/10.3390/molecules26164791
APA StyleImam, H. T., Krasňan, V., Rebroš, M., & Marr, A. C. (2021). Applications of Ionic Liquids in Whole-Cell and Isolated Enzyme Biocatalysis. Molecules, 26(16), 4791. https://doi.org/10.3390/molecules26164791






