Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion
Abstract
1. Introduction
2. Results
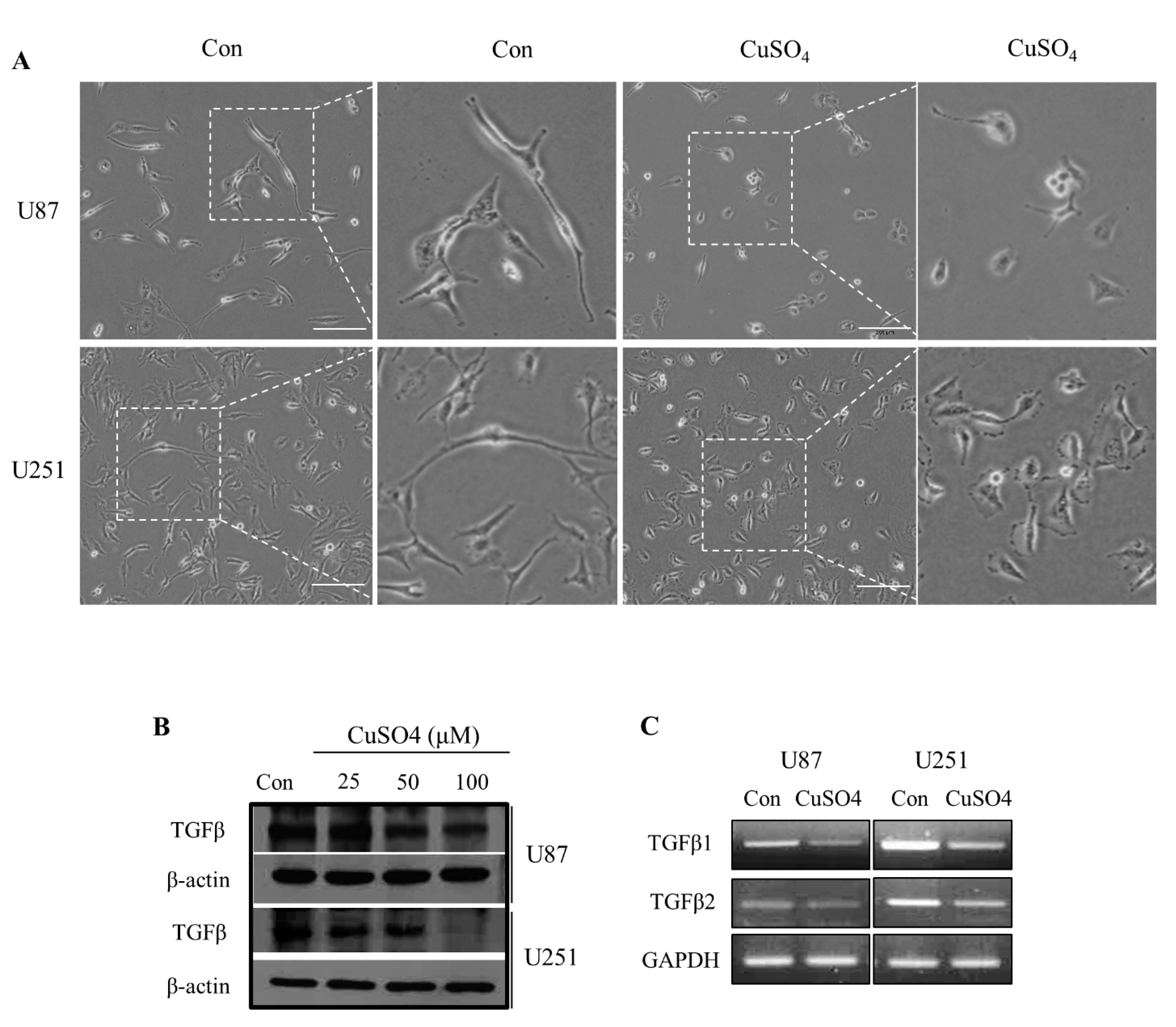
2.1. TGF-β Expressions and Morphological Changes with CuSO4 in GBM Cells
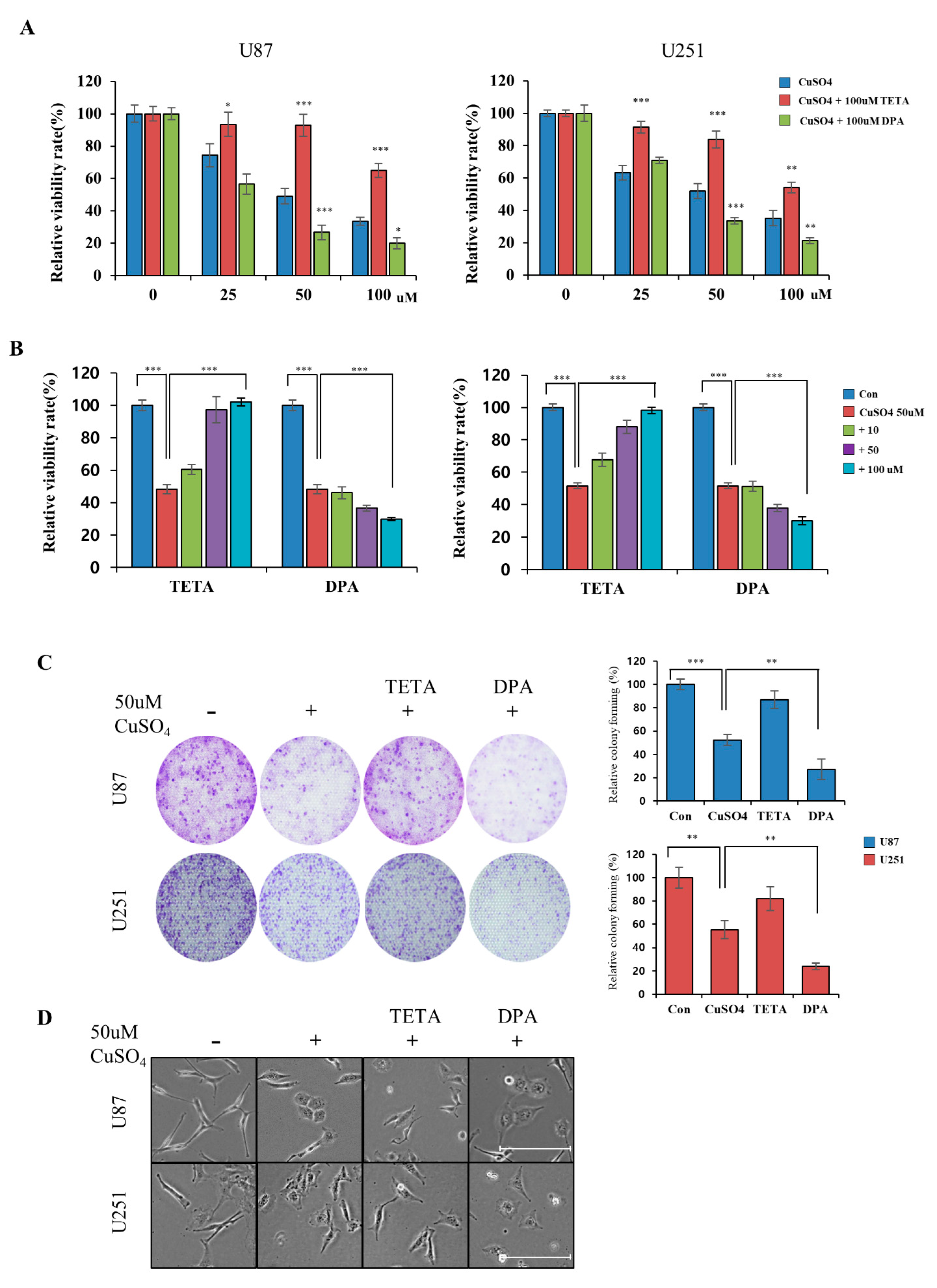
2.2. Regulation of Cellular Viability by CuSO4 and Chelators
2.3. Effect of CuSO4 and Chelators on EMT
2.4. Correlations between TGF-β and EMT Markers in GBM Cells
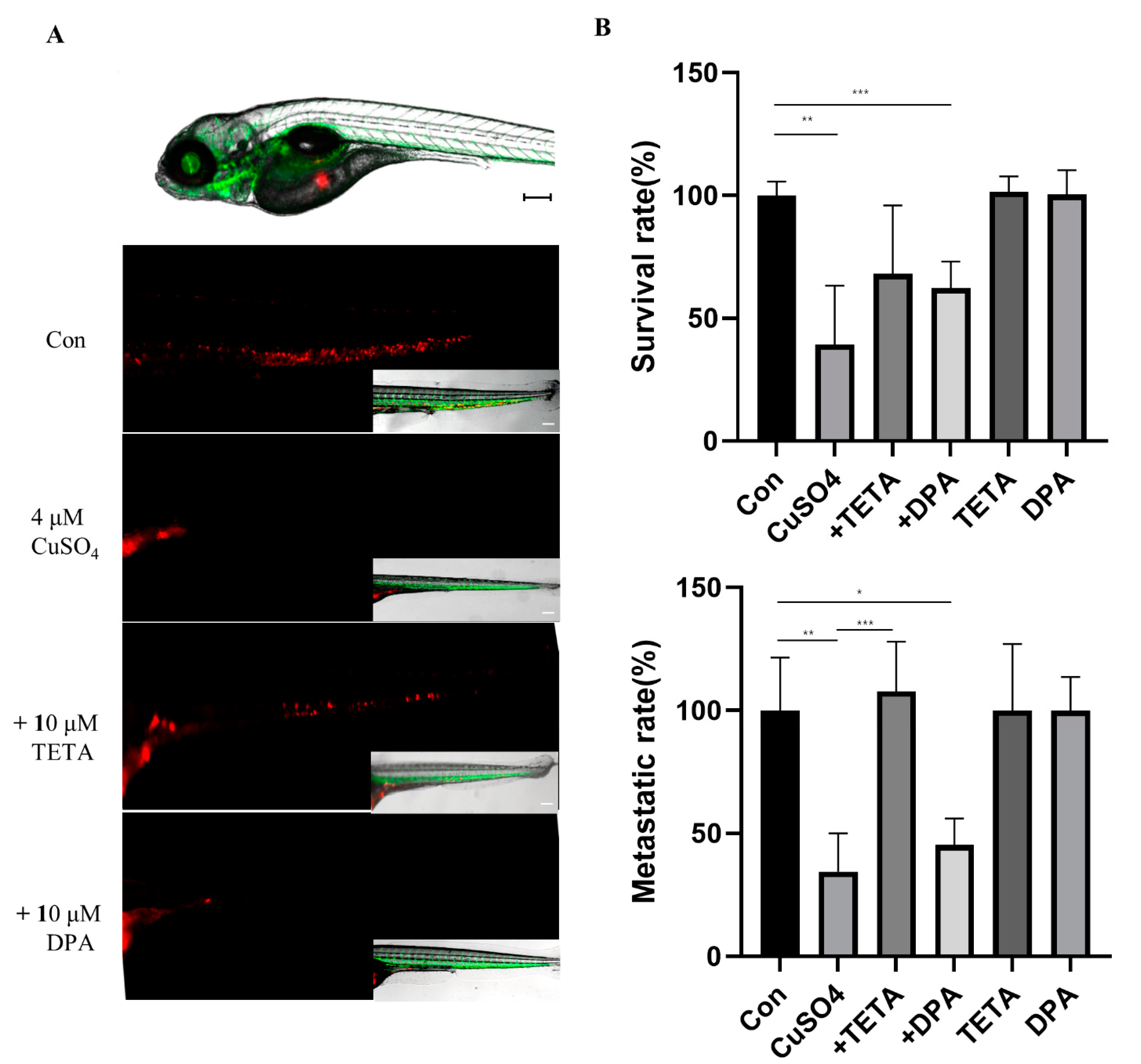
2.5. Invasion Capability of U87 Cells with CuSO4 and/or Chelators in Zebrafish Embryos
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reverse Transcription-PCR (RT-PCR)
4.3. Reagents and Antibodies
4.4. Western Blot Analysis
4.5. Wound Healing Assay
4.6. Invasion and Migration Assay
4.7. Colony Forming Assay
4.8. Fluorescence Microscopy
4.9. Copper Assay Kit
4.10. Zebrafish Xenograft
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Khasraw, M.; Ameratunga, M.S.; Grant, R.; Wheeler, H.; Pavlakis, N. Antiangiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Su, H.-K.; Zhao, H.-F.; Chen, Z.-P.; To, S.-S.T. Progress in the application of molecular biomarkers in gliomas. Biochem. Biophys. Res. Commun. 2015, 465, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Iwadate, Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016, 11, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Ganesan, S.; Kang, Y. Emerging strategies for treating metastasis. Nat. Cancer 2021, 2, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming growth factor-β signaling in immunity and cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.V.; Conroy, S.; Tomar, T.; Eggens-Meijer, E.; Bhat, K.; Copray, S.; E Walenkamp, A.M.; Boddeke, E.; Balasubramanyian, V.; Wagemakers, M.; et al. TGF-β is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 2014, 5, e1443. [Google Scholar] [CrossRef]
- Kahlert, U.; Nikkhah, G.; Maciaczyk, J. Epithelial-to-mesenchymal (-like) transition as a relevant molecular event in malignant gliomas. Cancer Lett. 2013, 331, 131–138. [Google Scholar] [CrossRef]
- Joseph, J.V.; Balasubramaniyan, V.; Walenkamp, A.; Kruyt, F.A. TGF-β as a therapeutic target in high grade gliomas–promises and challenges. Biochem. Pharmacol. 2013, 85, 478–485. [Google Scholar] [CrossRef]
- Kim, Y. Regulation of cell proliferation and migration in glioblastoma: New therapeutic approach. Front. Oncol. 2013, 3, 53. [Google Scholar] [CrossRef]
- Bruna, A.; Darken, R.S.; Rojo, F.; Ocaña, A.; Peñuelas, S.; Arias, A.; Seoane, J. High TGFβ-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell 2007, 11, 147–160. [Google Scholar] [CrossRef]
- Guo, S.-K.; Shen, M.-F.; Yao, H.-W.; Liu, Y.-S. Enhanced expression of TGFBI promotes the proliferation and migration of glioma cells. Cell. Physiol. Biochem. 2018, 49, 1138–1150. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef]
- Balsano, C.; Porcu, C.; Sideri, S. Is copper a new target to counteract the progression of chronic diseases? Metallomics 2018, 10, 1712–1722. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. Cancer’s Copper Connections; American Association for the Advancement of Science: Washington, DC, USA, 2015. [Google Scholar]
- Gutteridge, J.M.; Halliwell, B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem. Sci. 1990, 15, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D. Cellular copper transport and metabolism. Annu. Rev. Nutr. 2000, 20, 291. [Google Scholar] [CrossRef]
- Prohaska, J.R.; Gybina, A.A. Intracellular copper transport in mammals. J. Nutr. 2004, 134, 1003–1006. [Google Scholar] [CrossRef]
- Martin, F.; Linden, T.; Katschinski, D.M.; Oehme, F.; Flamme, I.; Mukhopadhyay, C.K.; Eckhardt, K.; Tröger, J.; Barth, S.; Camenisch, G.; et al. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood 2005, 105, 4613–4619. [Google Scholar] [CrossRef]
- Turecký, L.; Kalina, P.; Uhlíková, E.; Námerová, Š.; Križko, J. Serum ceruloplasmin and copper levels in patients with primary brain tumors. Klin. Wochenschr. 1984, 62, 187–189. [Google Scholar] [CrossRef]
- Singh, G.; Fries, J.F.; A Williams, C.; Zatarain, E.; Spitz, P.; A Bloch, D. Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis. J. Rheumatol. 1991, 18, 188–194. [Google Scholar]
- Lu, J. Triethylenetetramine Pharmacology and Its Clinical ApplicationsTETA Pharmacology and Clinical Applications. Mol. Cancer Ther. 2010, 9, 2458–2467. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef]
- Hamilton, L.; Astell, K.R.; Velikova, G.; Sieger, D. A zebrafish live imaging model reveals differential responses of microglia toward glioblastoma cells in vivo. Zebrafish 2016, 13, 523–534. [Google Scholar] [CrossRef]
- Yang, X.-J.; Cui, W.; Gu, A.; Xu, C.; Yu, S.-C.; Li, T.-T.; Cui, Y.-H.; Zhang, X.; Bian, X.-W. A novel zebrafish xenotransplantation model for study of glioma stem cell invasion. PLoS ONE 2013, 8, e61801. [Google Scholar] [CrossRef]
- Gamble, J.; Elson, D.; Greenwood, J.; Tanguay, R.; Kolluri, S. The zebrafish xenograft models for investigating cancer and cancer therapeutics. Biology 2021, 10, 252. [Google Scholar] [CrossRef]
- Kardos, J.; Kovács, I.; Hajós, F.; Kálmán, M.; Simonyi, M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989, 103, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Barada, K.; Jomaa, M.; Kobeissy, F.; Makkawi, A.-K.; Abou-Kheir, W.; Usta, J. Chemosensitivity of U251 cells to the co-treatment of D-penicillamine and copper: Possible implications on Wilson disease patients. Front. Mol. Neurosci. 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current biomedical use of copper chelation therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef]
- Brem, S.; Grossman, S.A.; Carson, K.A.; New, P.; Phuphanich, S.; Alavi, J.B.; Mikkelsen, T.; Fisher, J.D. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro-Oncology 2005, 7, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Kuo, M.T.; Liu, Y.-S.; Cheng, Y.-M.; Wu, P.-Y.; Chou, C.-Y. A dose escalation study of trientine plus carboplatin and pegylated liposomal doxorubicin in women with a first relapse of epithelial ovarian, tubal, and peritoneal cancer within 12 months after platinum-based chemotherapy. Front. Oncol. 2019, 9, 437. [Google Scholar] [CrossRef]
- Ye, X.-Z.; Xu, S.-L.; Xin, Y.-H.; Yu, S.-C.; Ping, Y.-F.; Chen, L.; Xiao, H.-L.; Wang, B.; Yi, L.; Wang, Q.-L.; et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, J.; Xie, G.; Feng, H.; Li, X.; Liu, L.; Wang, X.; Li, D.; Liu, Z.; Qian, J.; et al. Effect of copper on the expression of TGF-β in incubated chondrocytes of newborn pigs. Biol. Trace Elem. Res. 2011, 143, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Jian, Z.; Liu, H.; Cui, H.; Deng, H.; Fang, J.; Zuo, Z.; Wang, X.; Zhao, L.; Geng, Y.; et al. TGF-β1-induced EMT activation via both Smad-dependent and MAPK signaling pathways in Cu-induced pulmonary fibrosis. Toxicol. Appl. Pharmacol. 2021, 418, 115500. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Chen, X.; Zhu, Y. The role of TGF-β1/Smad3 signaling pathway and oxidative stress in the inhibition of osteoblast mineralization by copper chloride. Environ. Toxicol. Pharmacol. 2021, 84, 103613. [Google Scholar] [CrossRef]
- Hernandez, P.P.; Undurraga, C.; Gallardo, V.E.; Mackenzie, N.; Allende, M.L.; Reyes, A.E. Sublethal concentrations of waterborne copper induce cellular stress and cell death in zebrafish embryos and larvae. Biol. Res. 2011, 44, 7–15. [Google Scholar] [CrossRef][Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jo, S.; Kim, I.-G.; Kim, R.-K.; Kahm, Y.-J.; Jung, S.-H.; Lee, J.H. Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion. Molecules 2022, 27, 8851. https://doi.org/10.3390/molecules27248851
Kim H, Jo S, Kim I-G, Kim R-K, Kahm Y-J, Jung S-H, Lee JH. Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion. Molecules. 2022; 27(24):8851. https://doi.org/10.3390/molecules27248851
Chicago/Turabian StyleKim, Heabin, Seonmi Jo, In-Gyu Kim, Rae-Kwon Kim, Yeon-Jee Kahm, Seung-Hyun Jung, and Jei Ha Lee. 2022. "Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion" Molecules 27, no. 24: 8851. https://doi.org/10.3390/molecules27248851
APA StyleKim, H., Jo, S., Kim, I.-G., Kim, R.-K., Kahm, Y.-J., Jung, S.-H., & Lee, J. H. (2022). Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion. Molecules, 27(24), 8851. https://doi.org/10.3390/molecules27248851





