Changes in Gel Characteristics, Rheological Properties, and Water Migration of PSE Meat Myofibrillar Proteins with Different Amounts of Sodium Bicarbonate
Abstract
1. Introduction
2. Result and Discussion
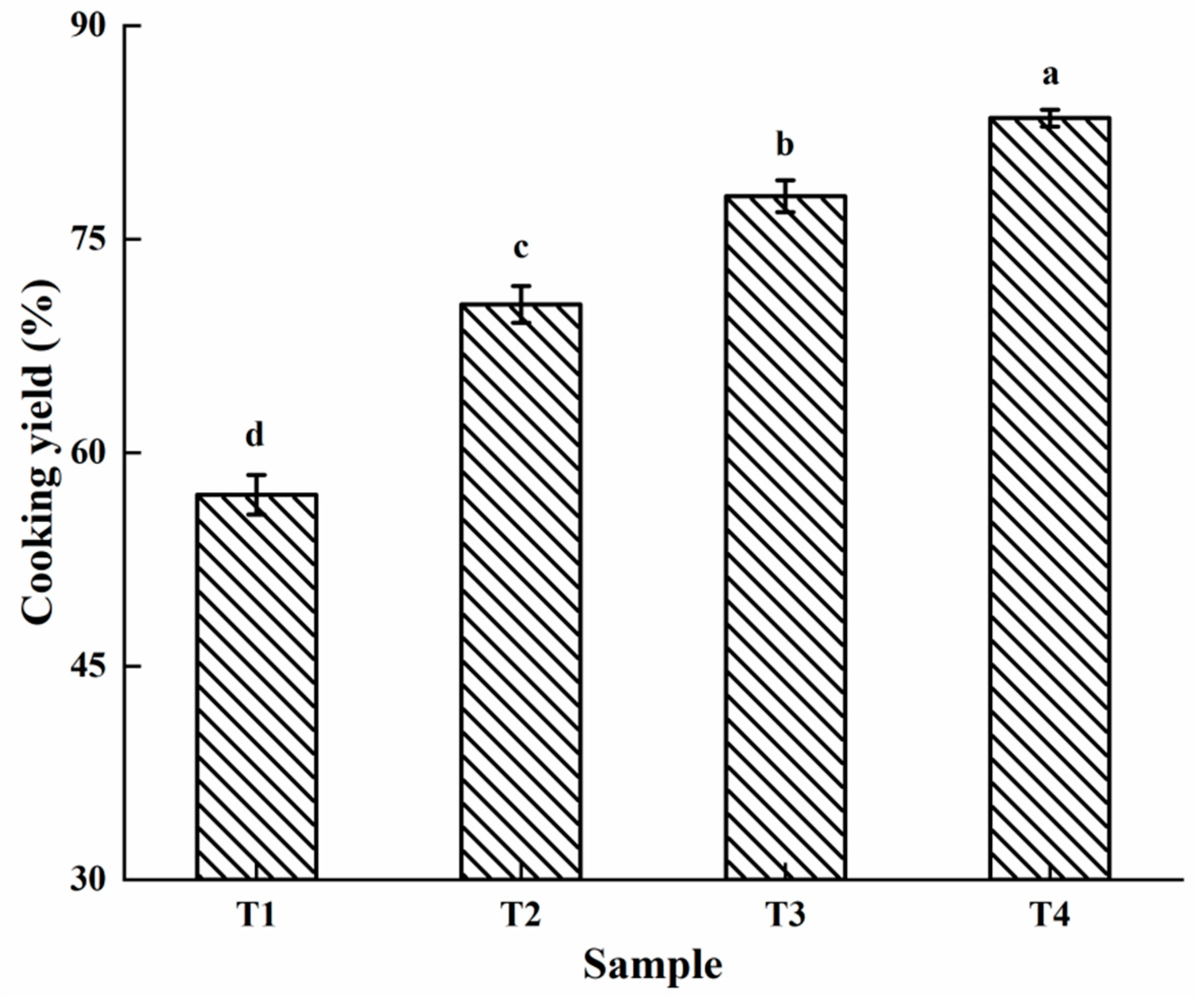
2.1. Cooking Yield
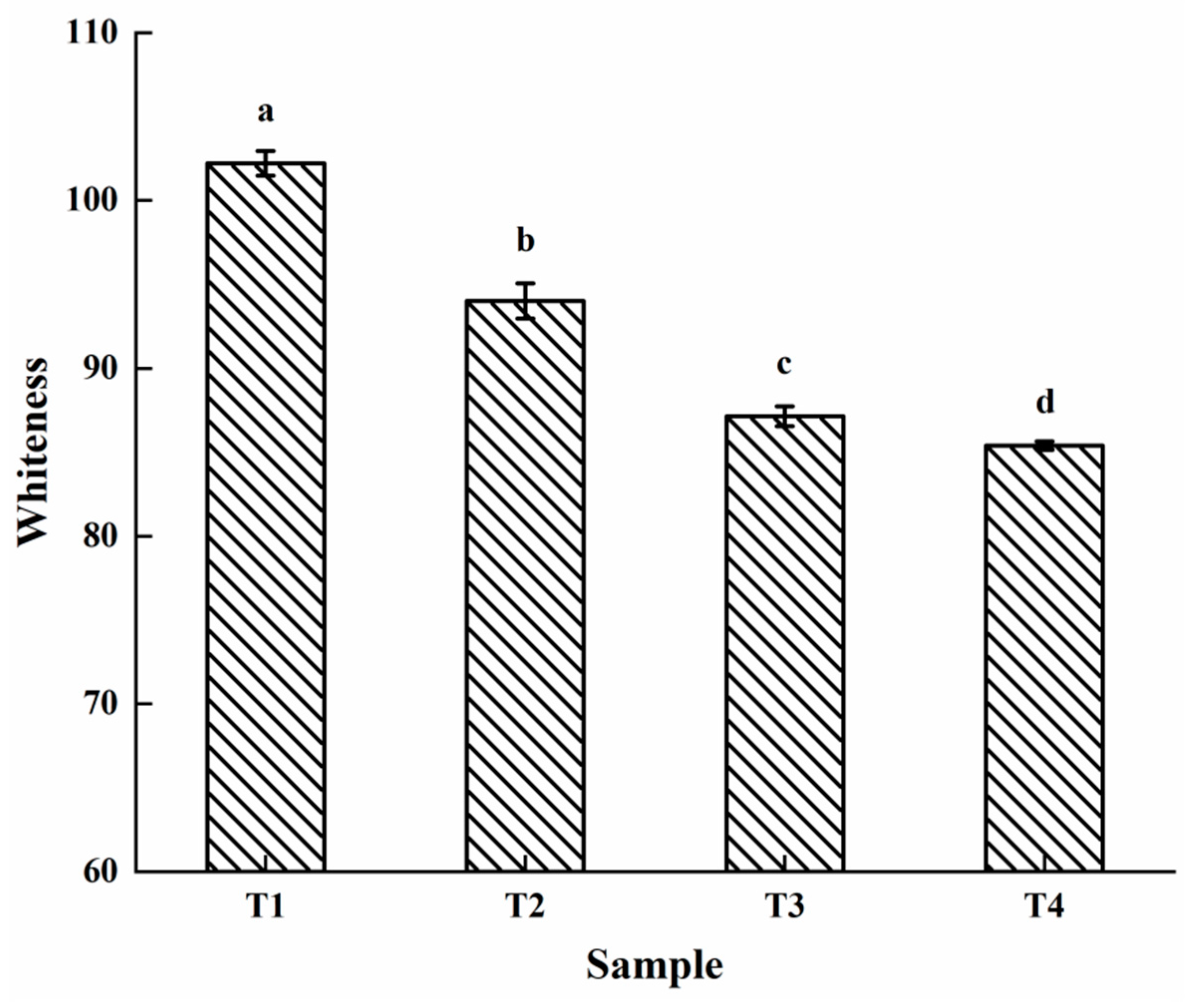
2.2. Whiteness
2.3. Textural Properties
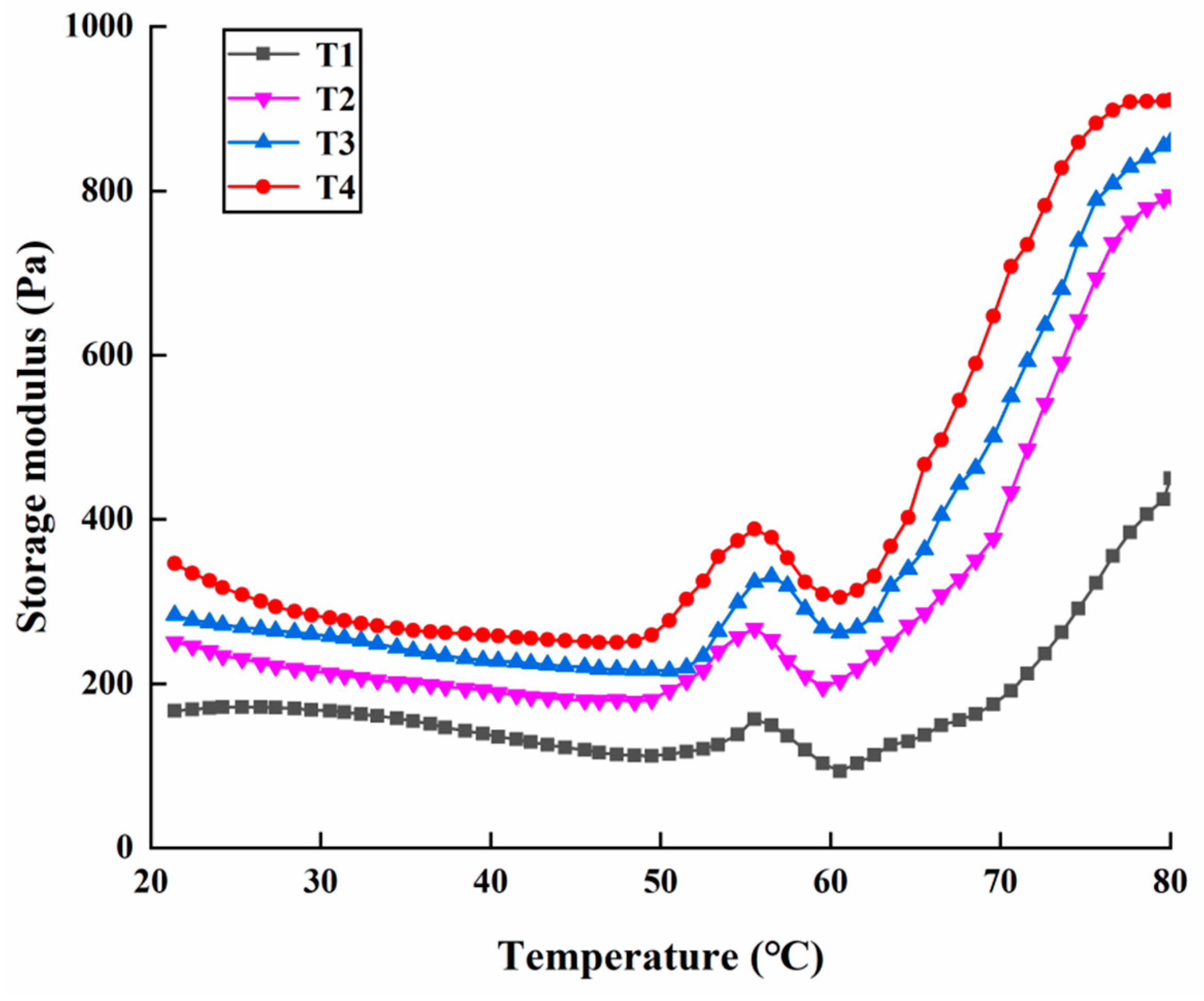
2.4. Rheological Properties
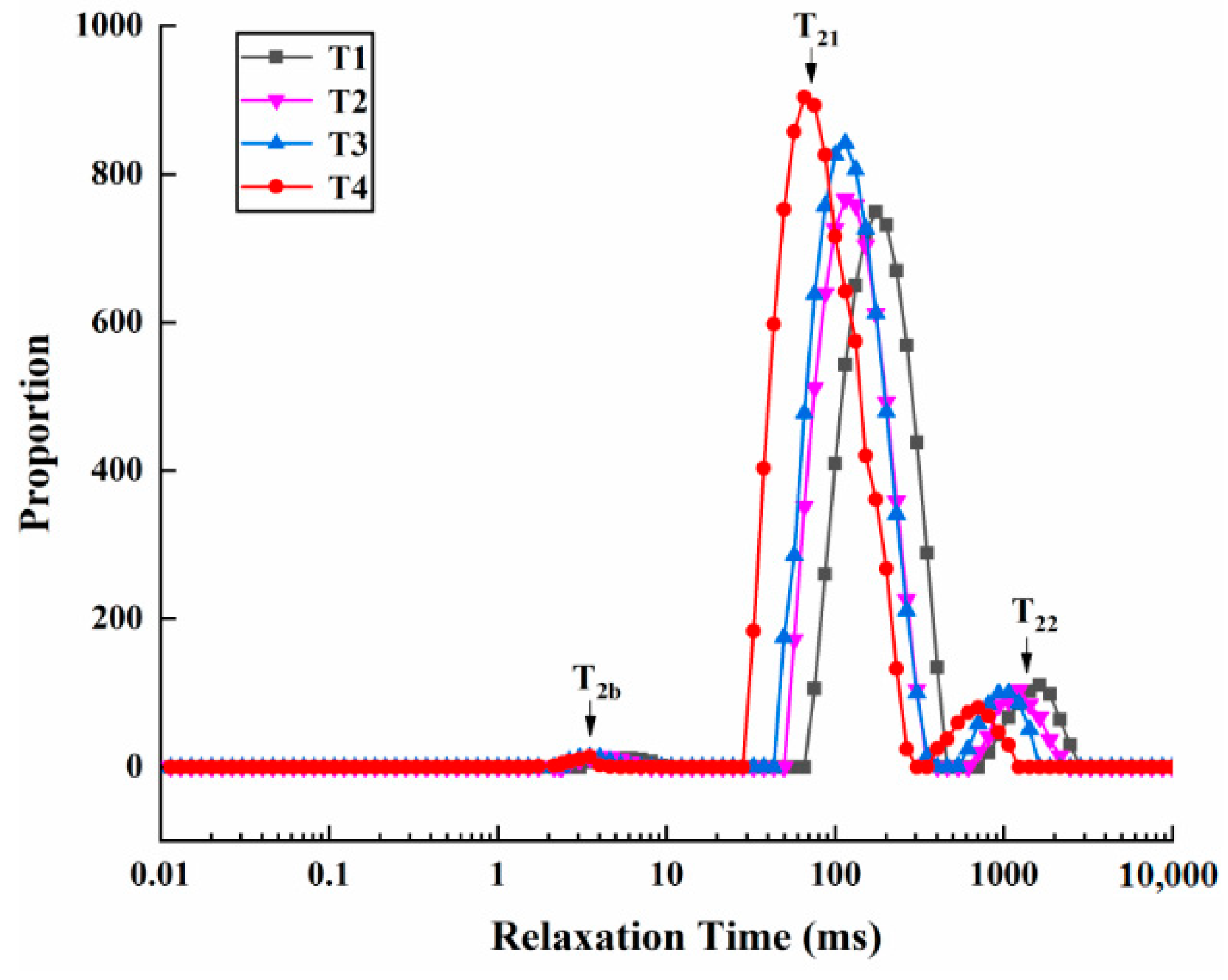
2.5. LF-NMR Measurement
3. Materials and methods
3.1. Experimental Materials
3.2. Extraction of PSE Meat Myofibrillar Proteins
3.3. Preparation of the Solution and Gel of PSE Meat Myofibrillar Proteins
3.4. Cooking Yield
3.5. Whiteness
3.6. Textural Properties
3.7. Dynamic Rheological Properties
3.8. Low-Field Nuclear Magnetic Resonance (LF-NMR)
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paula, M.M.D.; Haddad, G.D.S.; Rodrigues, L.M.; Júnior, A.A.; Romos, A.D.S.; Ramos, E.M. Effects of PSE meat and salt concentration on the technological and sensory characteristics of restructured cooked hams. Meat Sci. 2019, 152, 96–103. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Shao, Y.G.; Liu, G.J.; Xing, S.J.; Zhang, L.; Zhu, M.R.; Xu, Y.L.; Wang, Z.R. Proteomics analysis as an approach to understand the formation of pale, soft, and exudative (PSE) pork. Meat Sci. 2021, 177, 108353. [Google Scholar]
- Liu, R.; Wu, G.Y.; Li, K.Y.; Ge, Q.F.; Wu, M.G.; Yu, H.; Wu, S.L.; Bao, W.B. Comparative Study on Pale, Soft and Exudative (PSE) and Red, Firm and Non-Exudative (RFN) Pork: Protein Changes during Aging and the Differential Protein Expression of the Myofibrillar Fraction at 1 h Postmortem. Foods 2021, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Kang, Z.L.; Zhao, Y.Y.; Xu, X.L.; Zhou, G.H. Use of High-Intensity Ultrasound to Functional Properties of Batter Suspensions Prepared from PSE-like Chicken Breast Meat. Food Bioprocess Technol. 2014, 7, 346–3477. [Google Scholar] [CrossRef]
- Lesiow, T.; Rentfrow, G.K.; Xiong, Y.L. Polyphosphate and myofibrillar protein extract promote transglutaminase-mediated enhancements of rheological and textural properties of PSE pork meat batters. Meat Sci. 2017, 128, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xue, S.; Xu, Y.; Xu, X.; Han, M. Improved gelation functionalities of myofibrillar protein from pale, soft and exudative chicken breast meat by nonenzymatic glycation with glucosamine. Int. J. Food Sci. Technol. 2018, 53, 2006–2014. [Google Scholar] [CrossRef]
- dos Santos, V.M.O.; Caldara, F.R.; de Oliveira Seno, L.; Feijó, G.L.D.; Garcia, R.G.; Altemio, Â.D.C. Marinade with alkaline solutions for the improvement of pork quality. Pesqui. Agropecuária Bras. 2012, 47, 1655–1662. [Google Scholar] [CrossRef][Green Version]
- Murthy, L.N.; Phadke, G.G.; Jeyakumari, A.; Ravishankar, C.N. Effect of added calcium and heat setting on gel forming and functional properties of Sardinella fimbriata surimi. J. Food Sci. Technol. 2020, 5, 101007. [Google Scholar] [CrossRef]
- Lu, F.; Kang, Z.L.; Wei, L.P.; Li, Y.P. Effect of sodium bicarbonate on gel properties and protein conformation of phosphorus-free chicken meat batters. Arab. J. Chem. 2021, 14, 102969. [Google Scholar] [CrossRef]
- Kang, Z.; Shang, X.; Li, Y.; Ma, H. Effect of Ultrasound-Assisted Sodium Bicarbonate Treatment on Aggregation and Conformation of Reduced-Salt Pork Myofibrillar Protein. Molecules 2022, 27, 7493. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Kang, Z.L.; Ma, H.J.; Xu, X.L.; Zhou, G.H. Effect of sodium chloride or sodium bicarbonate in the chicken batters: A physico-chemical and Raman spectroscopy study. Food Hydrocoll. 2018, 83, 222–228. [Google Scholar] [CrossRef]
- Li, Y.P.; Kang, Z.L.; Sukmanov, V.; Ma, H.J. Effects of soy protein isolate on gel properties and water holding capacity of low-salt pork myofibrillar protein under high pressure processing. Meat Sci. 2021, 176, 108471. [Google Scholar] [CrossRef]
- Kang, Z.L.; Zhang, X.H.; Li, K.; Li, Y.P.; Lu, F.; Ma, H.J.; Song, Z.J.; Zhao, S.M.; Zhu, M.M. Effects of sodium bicarbonate on the gel properties, water distribution and mobility of low-salt pork batters. LWT-Food Sci. Technol. 2021, 139, 110567. [Google Scholar] [CrossRef]
- Zou, X.; Kang, Z.L.; Li, Y.P.; Ma, H.J. Effect of sodium bicarbonate on solubility, conformation and emulsion properties of pale, soft and exudative meat myofibrillar proteins. LWT-Food Sci. Technol. 2022, 157, 113097. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Zhao, H.; Sun, W.; Cui, C. Effects of oxidative modification on gel properties of isolated porcine myofibrillar protein by peroxyl radicals. Meat Sci. 2014, 96, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Salvador, P.; Toldrà, M.; Saguer, E.; Carretero, C.; Parés, D. Microstructure-function relationships of heat-induced gels of porcine haemoglobin. Food Hydrocoll. 2009, 23, 1654–1659. [Google Scholar] [CrossRef]
- Bertram, H.C.; Kristensen, M.; Andersen, H.J. Functionality of myofibrillar proteins as affected by pH, ionic strength and heat treatment-a low-field NMR study. Meat Sci. 2004, 68, 249–256. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xiong, S.B.; Xie, B.J.; Qin, L.H. Role of secondary structures in the gelation of porcine myosin at different pH values. Meat Sci. 2008, 80, 632–639. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, H.; Xu, P.; Jiang, D.; Zhang, X.; Xu, W.; Wang, D. Combined effect of ultrasound and sodium bicarbonate marination on chicken breast tenderness and its molecular mechanism. Ult. Sonochem. 2019, 59, 104735. [Google Scholar] [CrossRef]
- Petracci, M.; Laghi, L.; Rimini, S.; Rocculi, P.; Capozzi, F.; Cavani, C. Chicken Breast Meat Marinated with Increasing Levels of Sodium Bicarbonate. J. Poult. Sci. 2014, 51, 206–212. [Google Scholar] [CrossRef]
- Wachirasiri, K.; Wanlapa, S.; Uttapap, D.; Puttanlek, C.; Rungsardthong, V. Changing in processing yield and physical properties of frozen white shrimp (Penaeus vannamei) treated with lysine and sodium bicarbonate. Int. J. Food Sci. Technol. 2016, 52, 763–771. [Google Scholar] [CrossRef]
- Alvarado, C.; Sams, A. Injection marination strategies for remediation of pale, exudative broiler breast meat. Poult. Sci. 2003, 82, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.R.; Naveena, B.M.; Muthukumar, M.; Babji, Y.; Murthy, T.R.K. Effect of chilling, polyphosphate and bicarbonate on quality characteristics of broiler breast meat. Br. Poult. Sci. 2003, 46, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Wangtueai, S.; Noomhorm, A.; Regenstein, J.M. Effect of Microbial Transglutaminase on Gel Properties and Film Characteristics of Gelatin from Lizardfish (Saurida spp.) Scales. J. Food Sci. 2010, 75, 731–739. [Google Scholar] [CrossRef]
- Bertram, H.C.; Meyer, R.L.; Wu, Z.; Zhou, X.; Andersen, H.J. Water Distribution and Microstructure in Enhanced Pork. J. Agric. Food Chem. 2008, 56, 7201–7207. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Zhou, P.; Zhang, X.; Wang, J. Effects of high pressure modification on conformation and gelation properties of myofibrillar protein. Food Chem. 2017, 217, 678–686. [Google Scholar] [CrossRef]
- Tobin, B.D.; O’Sullivan, M.G.; Hamill, R.M.; Kerry, J.P. Effect of Varying Salt and Fat Levels on the Sensory and Physiochemical Quality of Frankfurters. Meat Sci. 2012, 92, 659–666. [Google Scholar] [CrossRef]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhang, W.; Liu, R.; Liu, Y.; Xing, L.; Han, M.; Kang, Z.L.; Xu, X.L.; Zhou, G.H. Improved gel functionality of myofibrillar proteins incorporation with sugarcane dietary fiber. Food Res. Int. 2017, 100, 586–594. [Google Scholar] [CrossRef]
- Kang, Z.L.; Zhu, D.; Li, B.; Ma, H.; Song, Z. Effect of pre-emulsified sesame oil on physical-chemical and rheological properties of pork batters. Food Sci. Technol. Int. 2017, 37, 620–626. [Google Scholar] [CrossRef][Green Version]
- Han, M.; Zhang, Y.; Fei, Y.; Xu, X.; Zhou, G. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2009, 228, 665–670. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.; Yang, K.; Yin, X.; Ma, J.; Li, Z.; Sun, W.; Han, M. Effect of low-frequency magnetic field on the gel properties of pork myofibrillar proteins. Food Chem. 2019, 274, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.T.Y.; Omana, D.A.; Betti, M. Application of high pressure processing to improve the functional properties of pale, soft, and exudative (PSE)-like turkey meat. Innov. Food Sci. Emerg. Technol. 2011, 12, 216–225. [Google Scholar] [CrossRef]
- Mohan, A.; Jaico, T.; Kerr, W.; Singh, R. Functional properties of bicarbonates on physicochemical attributes of ground beef. LWT-Food Sci. Technol. 2016, 70, 333–341. [Google Scholar] [CrossRef]
- Kang, Z.L.; Chen, F.S.; Ma, H.J. Effect of pre-emulsified soy oil with soy protein isolate in frankfurters: A physical-chemical and Raman spectroscopy study. LWT-Food Sci. Technol. 2016, 74, 465–471. [Google Scholar] [CrossRef]
- Kang, Z.L.; Bai, R.; Lu, F.; Zhang, T.; Gao, Z.; Zhao, S.M.; Zhu, M.M.; Ma, H.J. Effects of high pressure homogenization on the solubility, foaming, and gel properties of soy 11S globulin. Food Hydrocoll. 2022, 124, 107261. [Google Scholar] [CrossRef]
| Sample | Hardness (g) | Springiness | Cohesiveness | Chewiness (g.mm) |
|---|---|---|---|---|
| T1 | 110.52 ± 7.76 d | 0.57 ± 0.06 d | 0.33 ± 0.02 d | 21.08 ± 2.95 d |
| T2 | 141.33 ± 7.49 c | 0.72 ± 0.06 c | 0.44 ± 0.04 c | 48.88 ± 4.55 c |
| T3 | 157.27 ± 1.52 b | 0.83 ± 0.03 b | 0.52 ± 0.02 b | 75.99 ± 3.01 b |
| T4 | 248.43 ± 16.15 a | 0.91 ± 0.02 a | 0.57 ± 0.05 a | 115.36 ± 2.64 a |
| Scheme | Initial Relaxation Time (ms) | Peak Ration (%) | ||||
|---|---|---|---|---|---|---|
| T2b | T21 | T22 | P2b | P21 | P22 | |
| T1 | 3.20 ± 0.27 a | 69.08 ± 5.69 a | 740.69 ± 61.00 a | 0.78 ± 0.04 a | 84.03 ± 2.35 c | 13.25 ± 2.74 a |
| T2 | 2.38 ± 0.11 b | 54.74 ± 4.30 b | 586.95 ± 46.14 b | 0.76 ± 0.25 a | 87.90 ± 1.87 b | 9.53 ± 0.94 b |
| T3 | 2.30 ± 0.10 b | 41.41 ± 3.26 c | 444.00 ± 34.90 c | 0.74 ± 0.01 a | 91.24 ± 0.16 a | 5.78 ± 0.33 c |
| T4 | 2.19 ± 0.03 b | 29.90 ± 2.46 d | 335.87 ± 26.40 d | 0.66 ± 0.04 a | 93.65 ± 0.20 a | 3.04 ± 0.53 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-W.; Zou, X.-L.; Yao, P.-L.; Kang, Z.-L.; Ma, H.-J. Changes in Gel Characteristics, Rheological Properties, and Water Migration of PSE Meat Myofibrillar Proteins with Different Amounts of Sodium Bicarbonate. Molecules 2022, 27, 8853. https://doi.org/10.3390/molecules27248853
Wu Z-W, Zou X-L, Yao P-L, Kang Z-L, Ma H-J. Changes in Gel Characteristics, Rheological Properties, and Water Migration of PSE Meat Myofibrillar Proteins with Different Amounts of Sodium Bicarbonate. Molecules. 2022; 27(24):8853. https://doi.org/10.3390/molecules27248853
Chicago/Turabian StyleWu, Zhong-Wei, Xiao-Li Zou, Peng-Lei Yao, Zhuang-Li Kang, and Han-Jun Ma. 2022. "Changes in Gel Characteristics, Rheological Properties, and Water Migration of PSE Meat Myofibrillar Proteins with Different Amounts of Sodium Bicarbonate" Molecules 27, no. 24: 8853. https://doi.org/10.3390/molecules27248853
APA StyleWu, Z.-W., Zou, X.-L., Yao, P.-L., Kang, Z.-L., & Ma, H.-J. (2022). Changes in Gel Characteristics, Rheological Properties, and Water Migration of PSE Meat Myofibrillar Proteins with Different Amounts of Sodium Bicarbonate. Molecules, 27(24), 8853. https://doi.org/10.3390/molecules27248853





