Synthesis, Characterization and Anticancer Efficacy Studies of Iridium (III) Polypyridyl Complexes against Colon Cancer HCT116 Cells
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Method
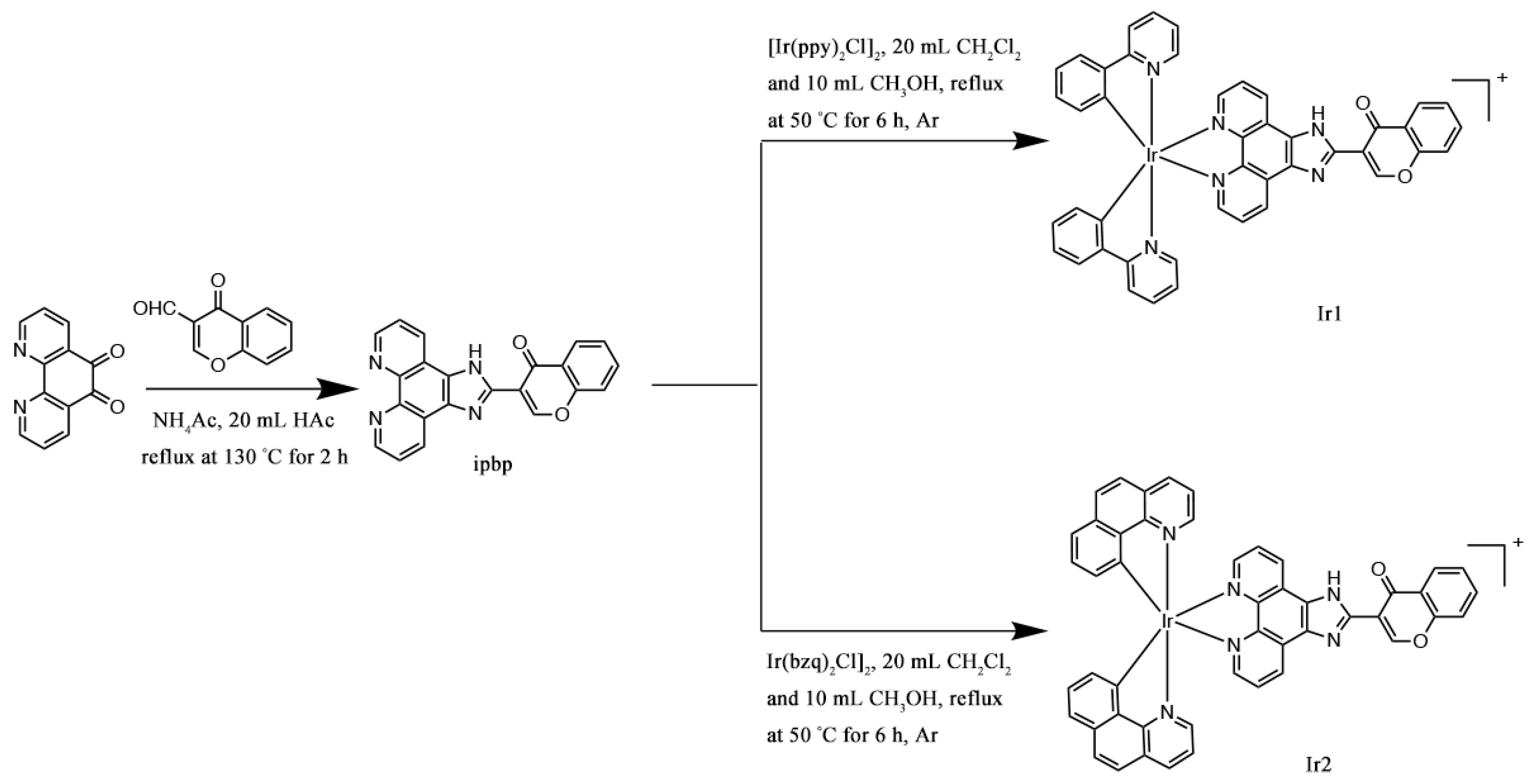
2.2. Synthesis of Complexes
2.2.1. Synthesis of [Ir(ppy)2(ipbp)](PF6) (Ir1)
2.2.2. Synthesis of [Ir(bzq)2(ipbp)](PF6) (Ir2)
2.3. Cell Culture
2.4. Cell Viability Determination
2.5. Cellular Uptake Studies
2.6. Studies of Inhibitory Efficacy in 3D Model
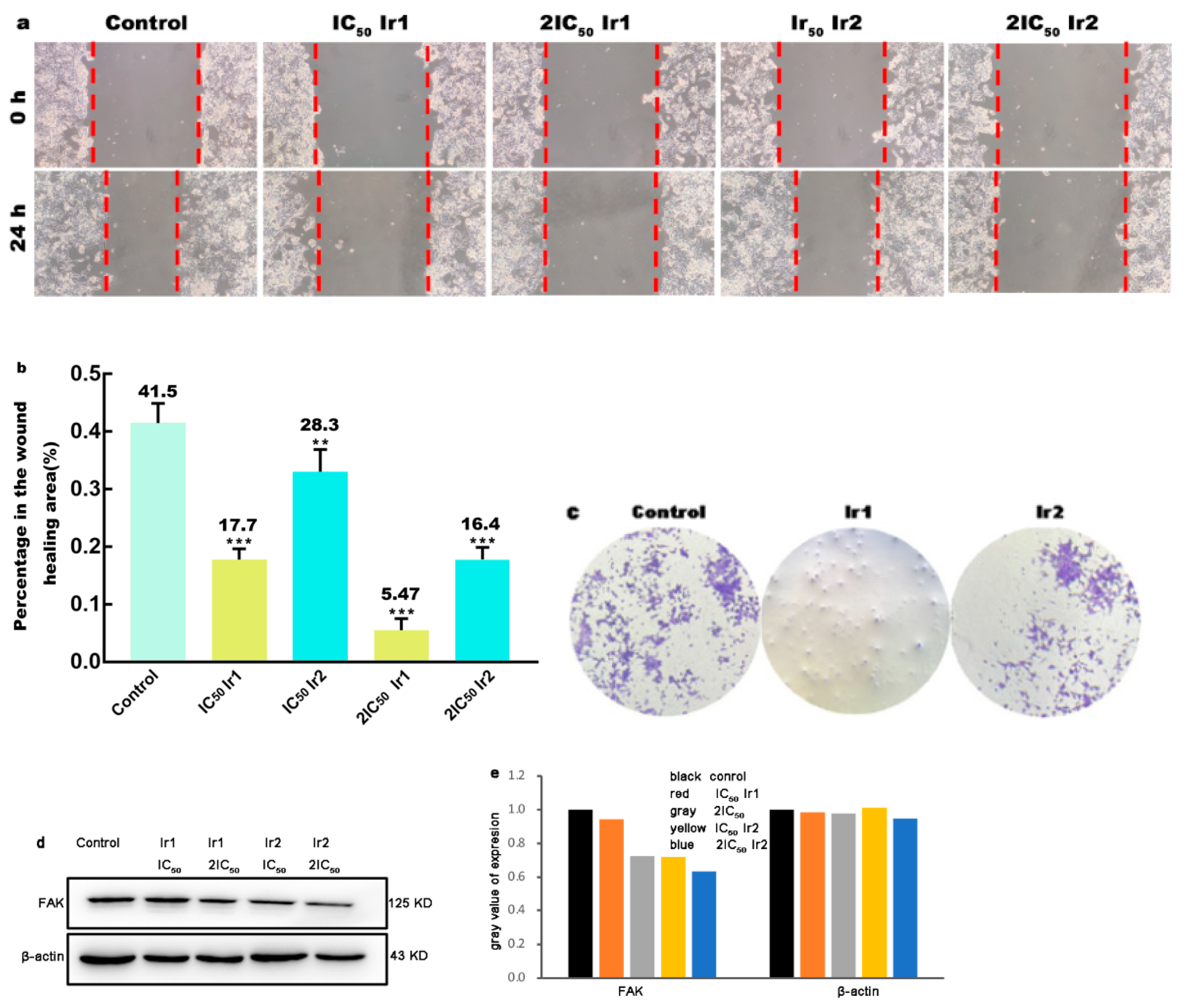
2.7. Colony-Forming Assay
2.8. Wound Scratch Studies
2.9. Cell Cycle Arrest Studies
2.10. Detection of Intracellular Reactive Oxygen Species
2.11. Glutathione (GSH) Measurement
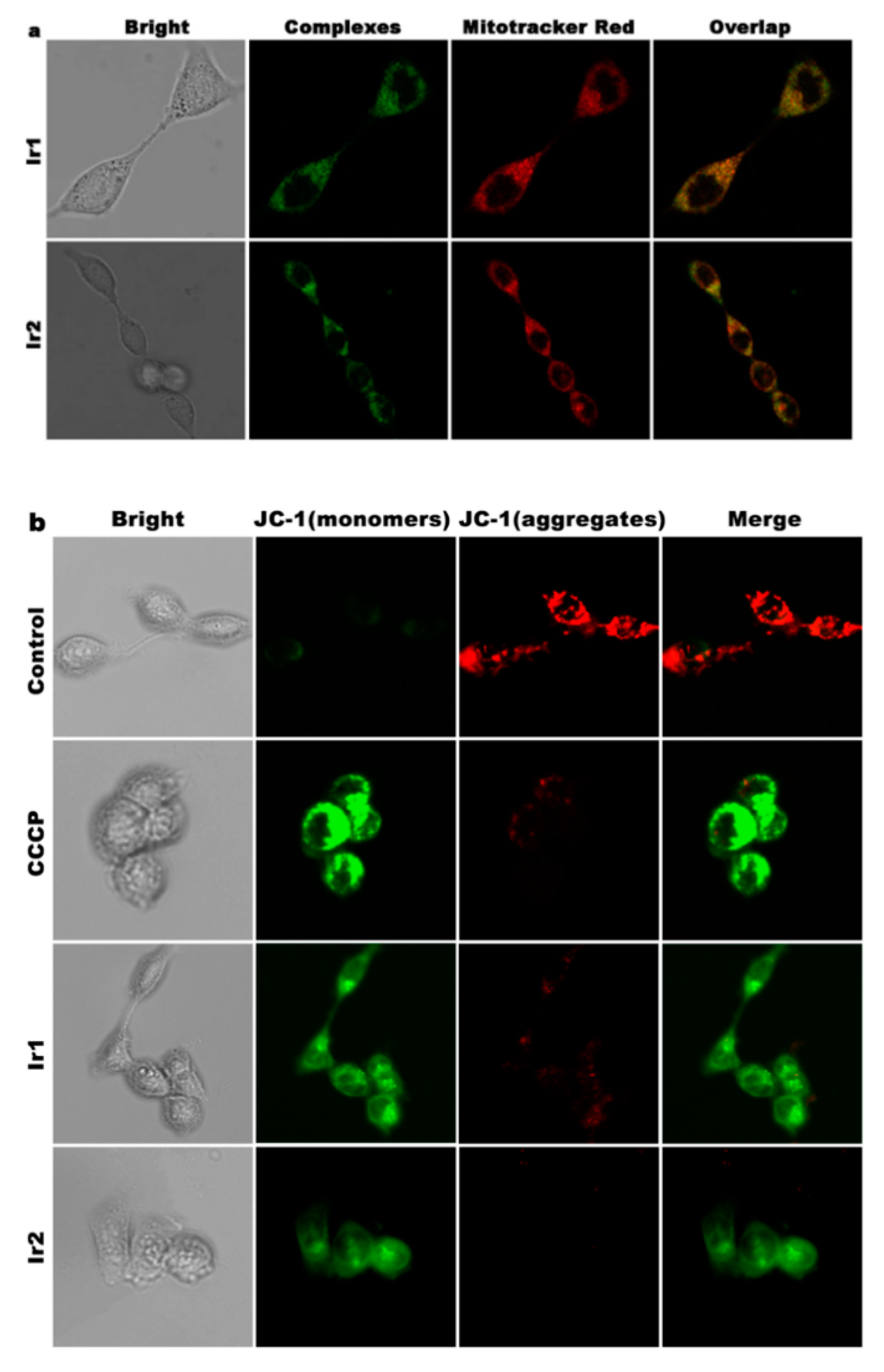
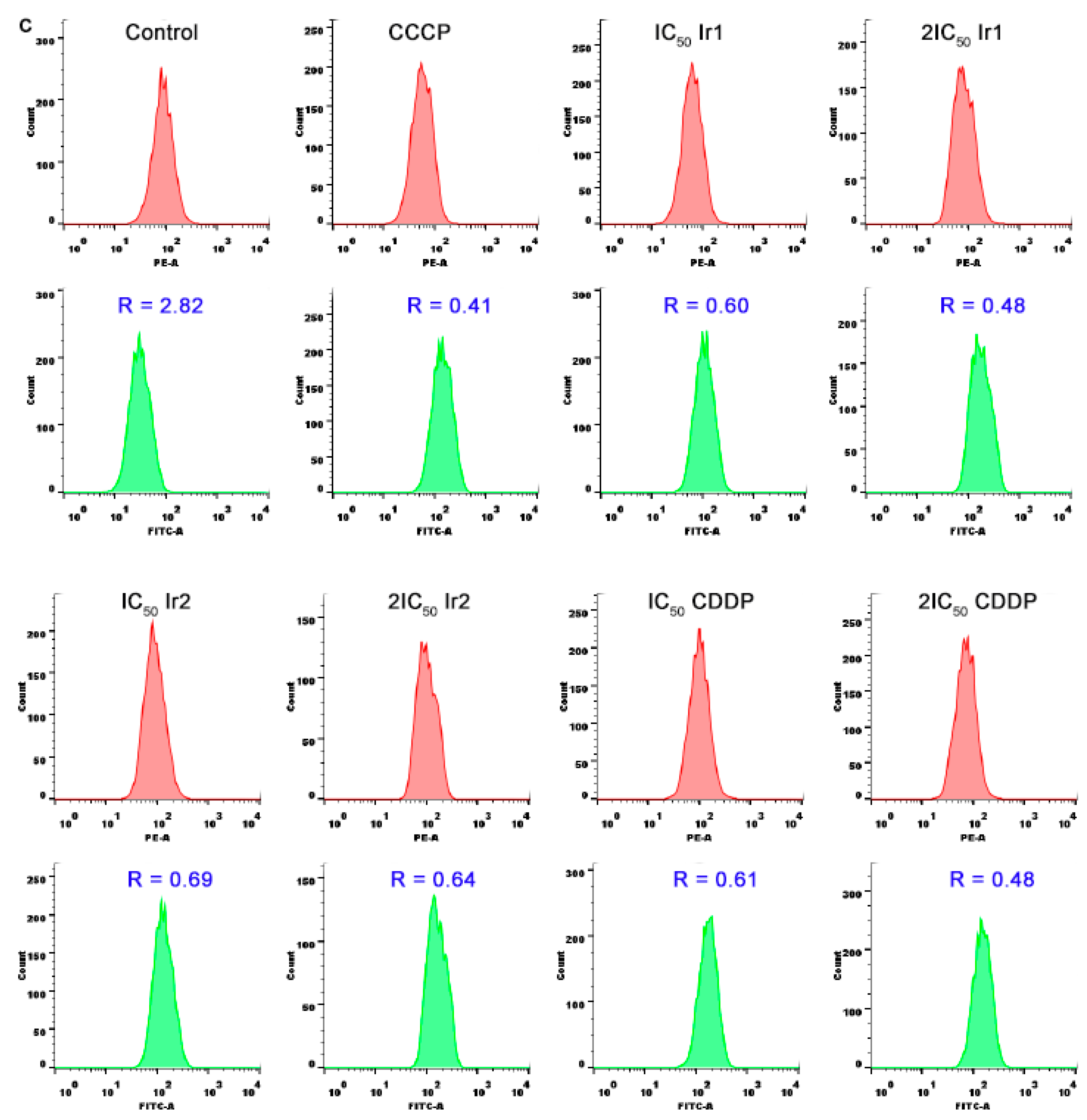
2.12. Mitochondrial Membrane Potential Detection
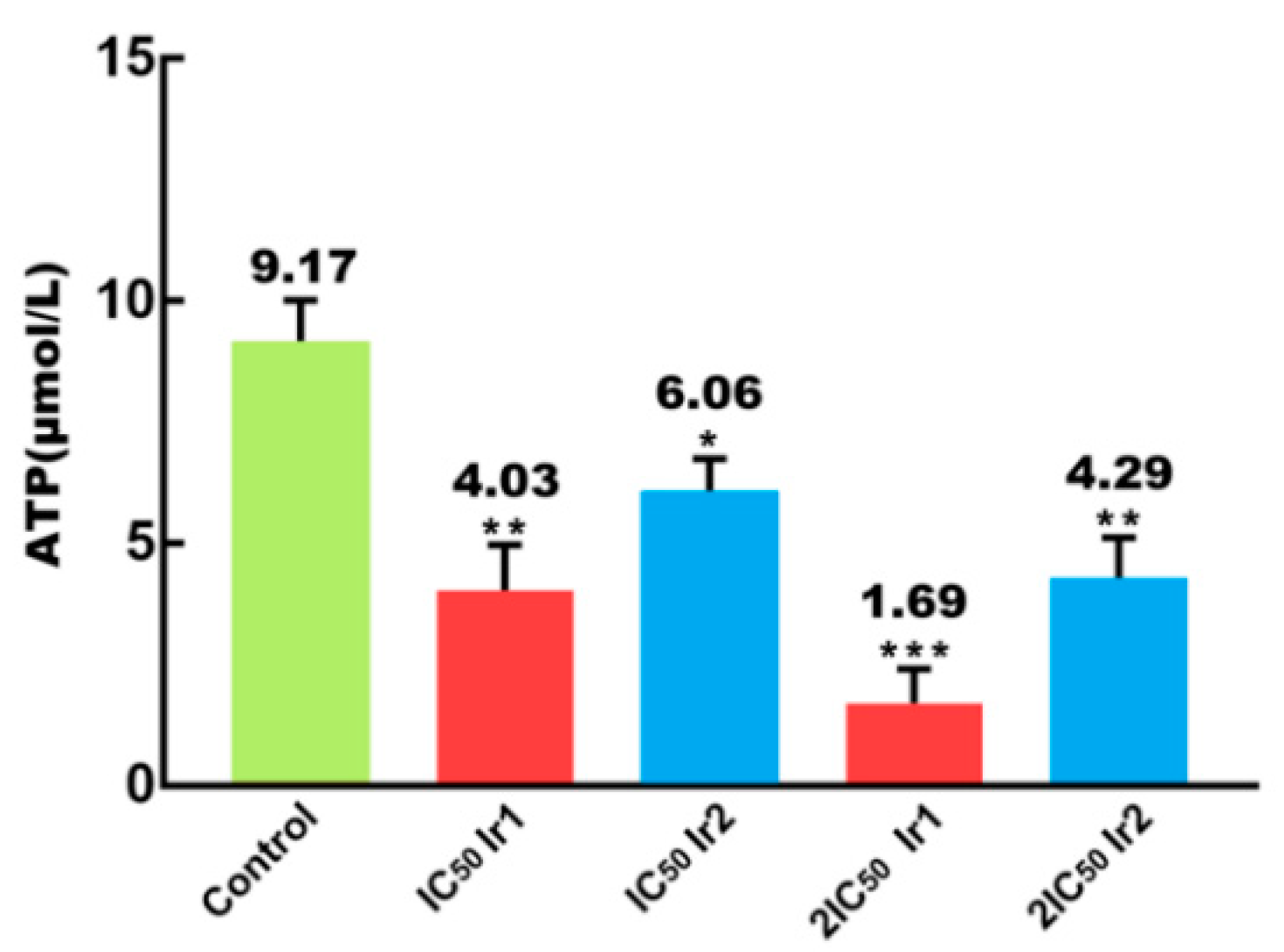
2.13. Assay of ATP Content
2.14. Cell Apoptosis Studies
2.15. Expression of Bcl-2 Family Proteins
2.16. Immunostaining Analysis
2.17. Data Analysis
3. Results and Discussion
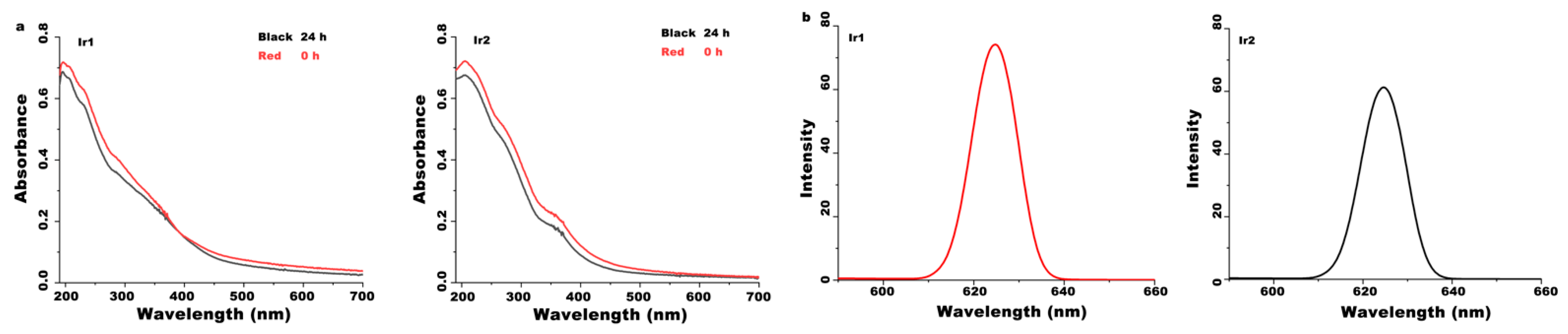
3.1. Synthesis and Characterization
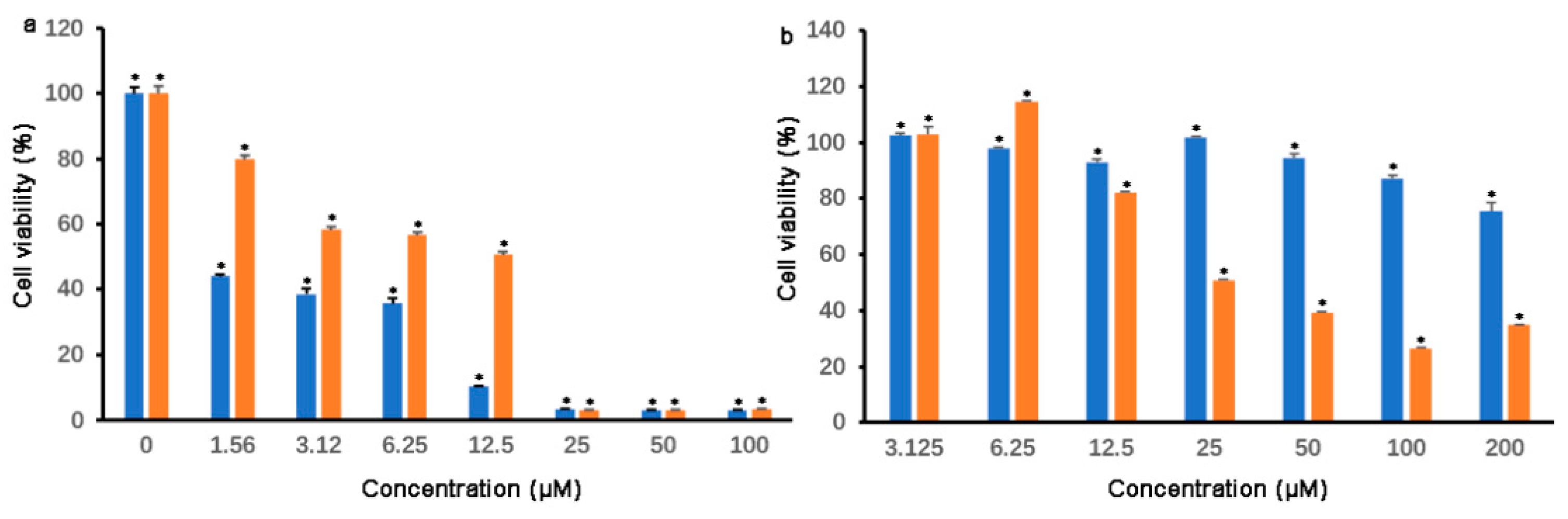
3.2. Cell Viability and IC50 Determination
3.3. Cell Uptake Studies
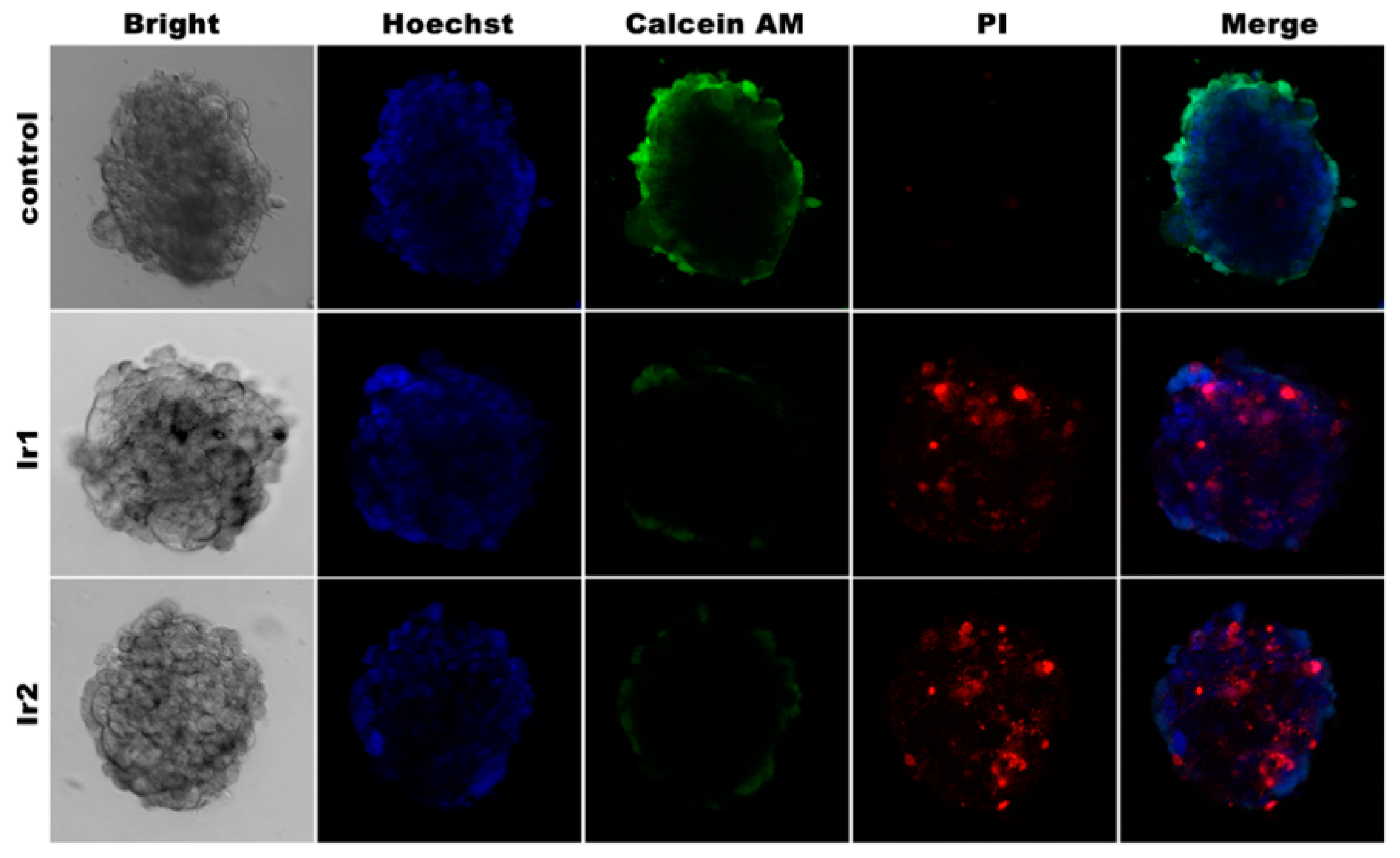
3.4. Cell 3D Model Studies
3.5. Wound Healing Studies
3.6. Location and the Change of Mitochondrial Membrane Potential
3.7. Intracellular ATP Content Determination
3.8. Intracellular ROS Levels Assay
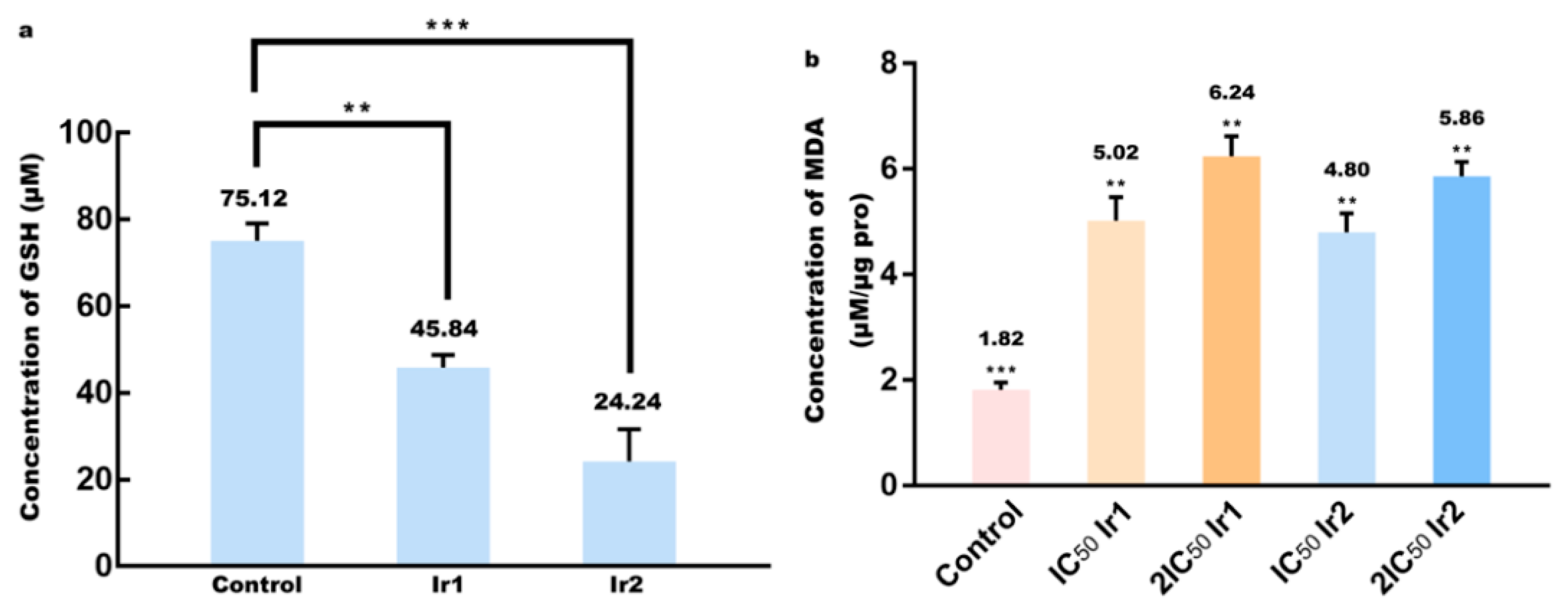
3.9. Determination of Glutathione and Lipid Peroxidation
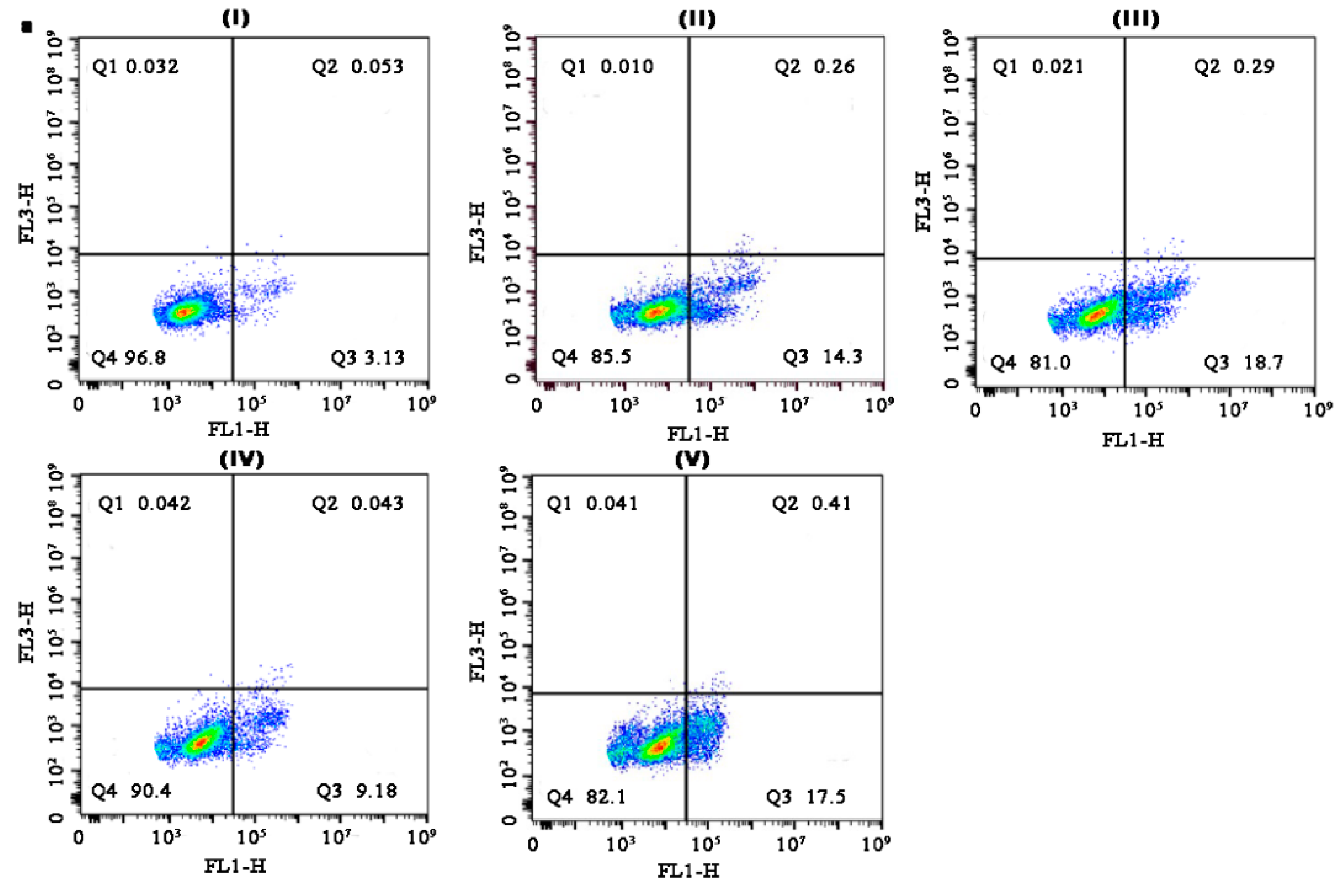
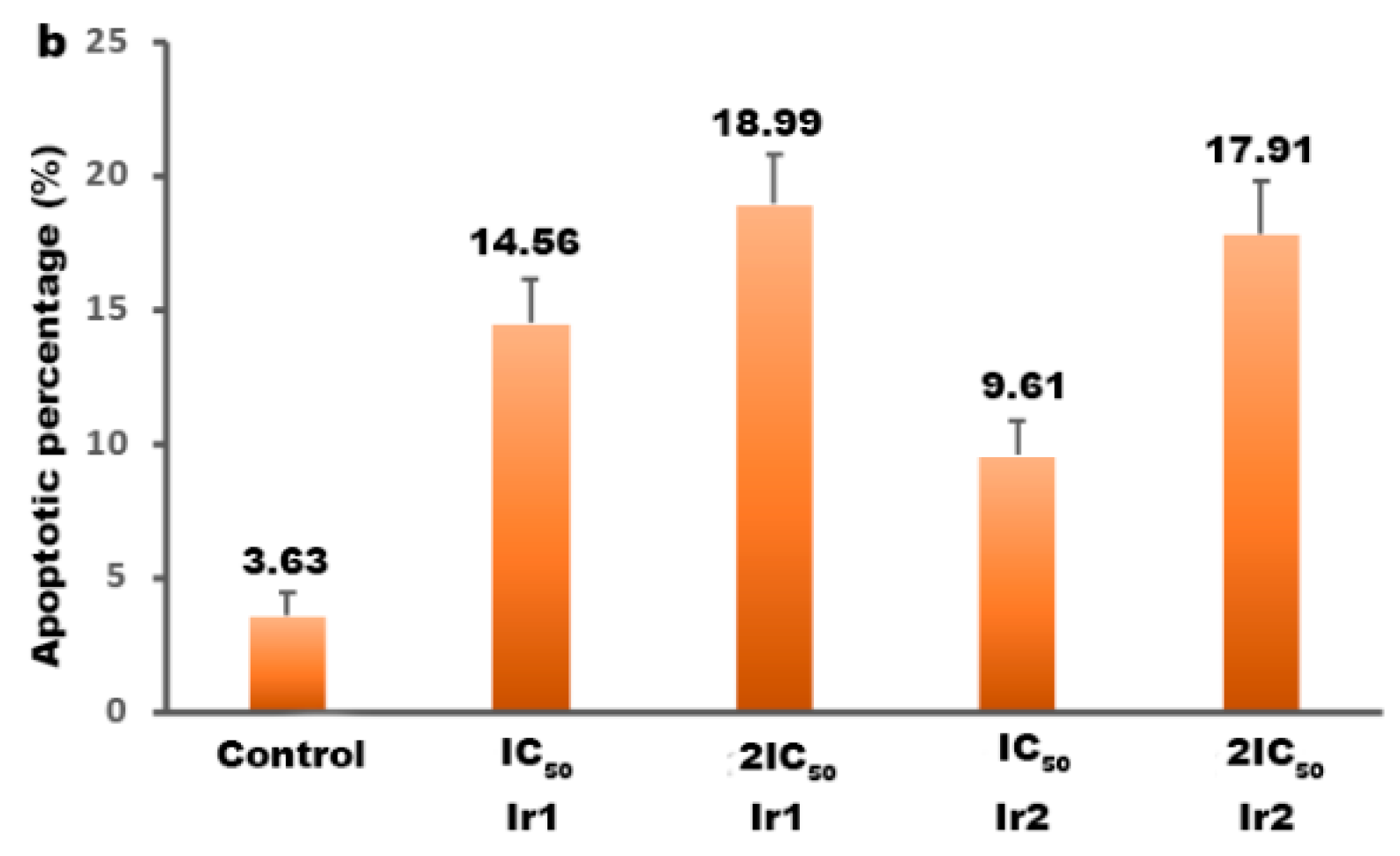
3.10. Apoptotic Efficacy Studies
3.11. Cell Cycle Distribution Studies
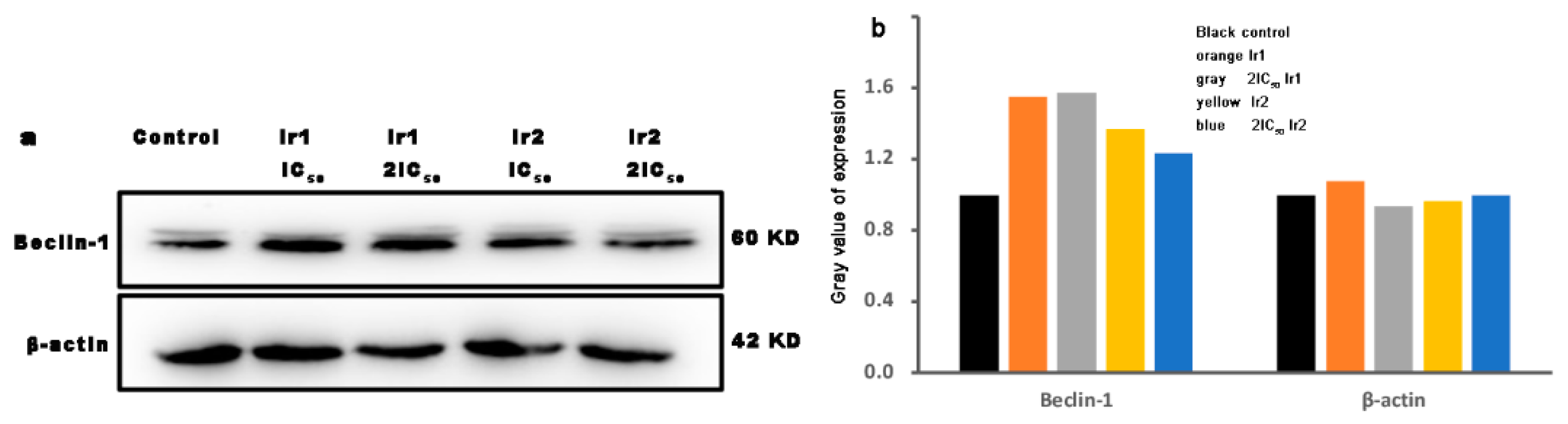
3.12. Autophagy Assay
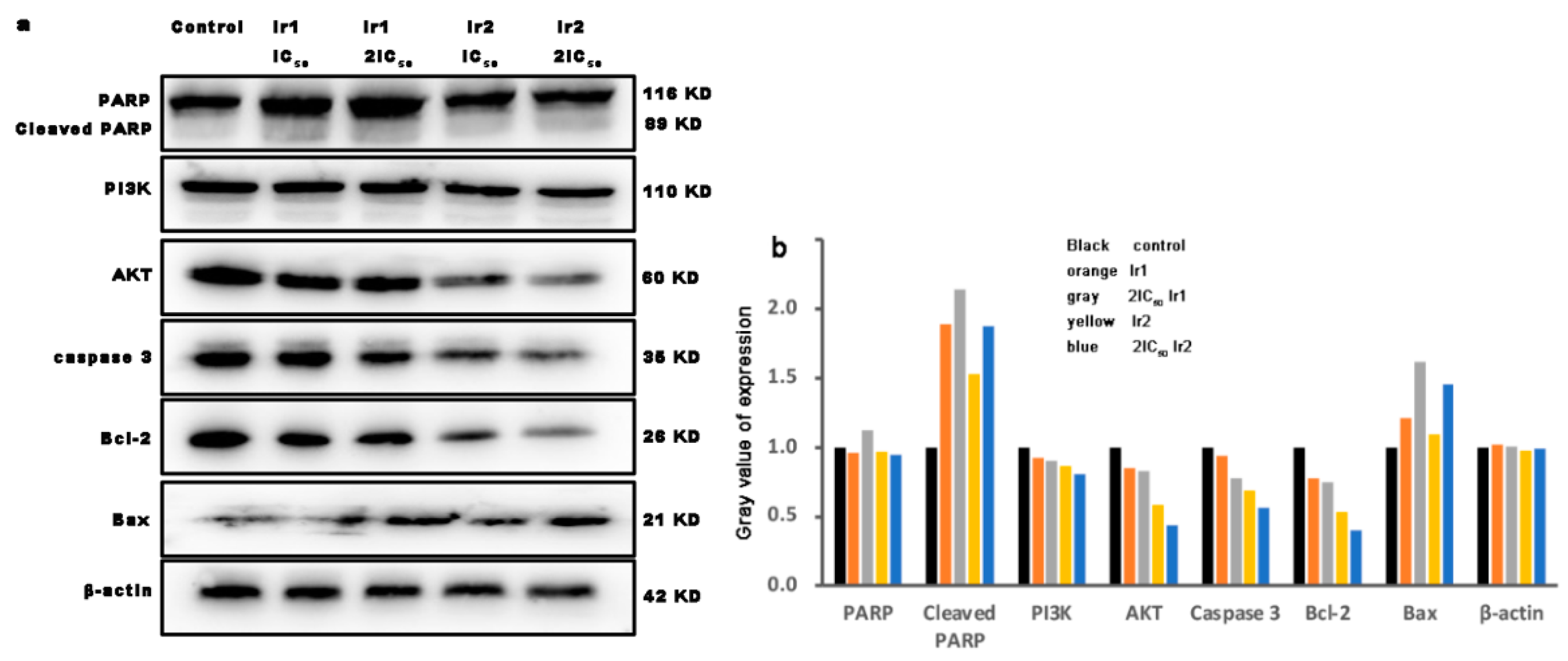
3.13. The Expression of Bcl-2 Family Proteins
3.14. Immunogenic Cell Death
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ATP | adenine nucleoside triphosphate |
| Bax | Bcl-2-associated x protein |
| Bcl-2 | B-cell lymphoma-2 |
| Bzq | benzo[h]quinolone |
| Calcein-AM. | calcein-acetoxymethylester |
| CCCP | carbonylcyanide-m-chlorophenylhydrazone |
| CDK4 | cyclin-dependent kinase |
| CTR | cell surface calreticulin |
| DAPI | 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride |
| DCHF | 2′,7′-dichloro-3,6-fluorandiol |
| DCHF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| DCF | dichlorofluorescein |
| DEME | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethylsulfoxide |
| FAK | focal adhesion kinase |
| FBS | fetal bovine serum |
| FITC | fluorescein isothiocyanate |
| GSH | glutathione |
| HCT116 | human colon cancer cells |
| Hppy | 2-phenylpyridine |
| HSP70 | heat-shock protein 70 |
| ICD | immunogenic cell death |
| Ipbp | 3-(1H-imidazo[4,5-f][1,10]phenanthrolin-2yl)-4H-chromen-4-one |
| JC-1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide |
| MMP | mitochondrial membrane potential |
| MTT | 3-(4,5-dimethylthiazole)-2,5-diphenyltetraazolium bromide |
| NPIP | 2-(2-nitrophenyl)-1H-imidazo[4,5-f][1,10]phenanthroline |
| PARP | poly ADP-ribose polymerase |
| PI | propidium iodide |
| Piq | 1-phenylisoquinoline |
| PBS | phosphate buffer saline |
| Ppy | deprotonated phenylpyridine |
| PVDF | polyvinylidene fluoride |
| RIPA | 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate |
| ROS | reactive oxygen species |
| TBST | 150 mM NaCl, 20 mM Tris-HCl, 0.05% Tween 20 |
| TMS | tetramethylsilane |
| Triton X-100 | polyethylene glycol octylphenyl ether |
| Tween 70 | polyoxyethylene monolaurate sorbaitan |
References
- Stewart, B.W.; Wild, C.P. World Cancer Report 2014; IARC Nonserial Publication; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Fardi, M.; Soladi, S.; Farshdousti, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes. Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Vegerhof, A.; Barnoy, E.A.; Motiei, M.; Malka, D.; Danan, Y.; Zalevsky, Z.; Popovtzer, R. Targeted Magnetic Nanoparticles for Mechanical Lysis of Tumor Cells by Low-Amplitude Alternating Magnetic Field. Materials 2016, 9, 943. [Google Scholar] [CrossRef] [PubMed]
- Vegerhof, A.; Motei, M.; Rudinzky, A.; Malka, D.; Popovtzer, R.; Zalevsky, Z. Thermal therapy with magnetic nanoparticles for cell destruction. Biomed. Opt. Express 2016, 7, 4581–4594. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Speer, R.J. Organo-platinum complexes as antitumor agents (review). Anticancer Res. 1982, 2, 173–186. [Google Scholar]
- Rosenberg, B. Noble Metal Complexes in Cancer Chemotherapy. Adv. Exp. Med. Biol. 1978, 91, 129–150. [Google Scholar] [CrossRef]
- Wang, D.; Lippard, S.J. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discov. 2005, 4, 307–320. [Google Scholar] [CrossRef]
- Klein, A.V.; Hambley, T.W. Platinum Drug Distribution in Cancer Cells and Tumors. Chem. Rev. 2009, 109, 4911–4920. [Google Scholar] [CrossRef]
- Gao, E.-J.; Wang, K.-H.; Gu, X.-F.; Yu, Y.; Sun, Y.-G.; Zhang, W.-Z.; Yin, H.-X.; Wu, Q.; Zhu, M.-C.; Yan, X.-M. A novel binuclear palladium complex with benzothiazole-2-thiolate: Synthesis, crystal structure and interaction with DNA. J. Inorg. Biochem. 2007, 101, 1404–1409. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef]
- YLiu, Z.; Vinje, J.; Pacifico, C.; Natile, G.; Sletten, E. Formation of adenine-N3/guanine-N7 cross-link in the reaction of trans-oriented platinum substrates with dinucleotides. J. Am. Chem. Soc. 2002, 124, 12854–12862. [Google Scholar]
- Gust, R.; Beck, W.; Jaouen, G.; Schönenberger, H. Optimization of cisplatin for the treatment of hormone dependent tumoral diseases. Coord. Chem. Rev. 2009, 253, 2742–2759. [Google Scholar] [CrossRef]
- He, M.; Du, F.; Zhang, W.-Y.; Yi, Q.-Y.; Wang, Y.-J.; Yin, H.; Bai, L.; Gu, Y.-Y.; Liu, Y.-J. Photoinduced anticancer effect evaluation of ruthenium(II) polypyridyl complexes toward human lung cancer A549 cells. Polyhedron 2019, 165, 97–110. [Google Scholar] [CrossRef]
- Yi, Q.-Y.; Wang, Y.-J.; He, M.; Zhang, W.-Y.; Du, F.; Gu, Y.-Y.; Bai, L.; Liu, Y.-J. Induction of apoptosis in SGC-7901 cells by ruthenium(II) complexes through ROS-mediated lysosome–mitochondria dysfunction and inhibition of PI3K/AKT/mTOR pathways. Transit. Met. Chem. 2018, 44, 187–205. [Google Scholar] [CrossRef]
- Brindell, M.; Gurgul, I.; Janczy-Cempa, E.; Gajda-Morszewski, P.; Mazuryk, O. Moving Ru polypyridyl complexes beyond cytotoxic activity towards metastasis inhibition. J. Inorg. Biochem. 2021, 226, 111652. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Haribabu, J.; Chien, C.M.; Sabapathi, G.; Chou, C.; Karvembu, R.; Venuvanalingam, P.; Ching, W.M.; Tsai, M.L.; Hsu, S.C.N. Half-sandwich Ru(η6-p-cymene) complexes featuring pyrazole appended ligands: Synthesis, DNA binding and in vitro cytotoxicity. J. Inorg. Biochem. 2019, 194, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Fandzloch, M.; Dobrzańska, L.; Jędrzejewski, T.; Jezierska, J.; Wiśniewska, J.; Łakomska, I. Synthesis, structure and biological evaluation of ruthenium(III) complexes of triazolopyrimidines with anticancer properties. JBIC J. Biol. Inorg. Chem. 2019, 25, 109–124. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Deng, Y.; Li, B.; Li, C.; Lin, Y.; Yang, D.; Zhang, H.; Chen, L.; Wang, T. Novel cyclometalated Ru(II) complexes containing isoquinoline ligands: Synthesis, characterization, cellular uptake and in vitro cytotoxicity. Eur. J. Med. Chem. 2020, 203, 112562. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, X.; Liu, H.; Shi, C.; Yuan, Y.; Bai, L.; Liao, X.; Zhang, Y.; Liu, Y. Enhanced in vitro cytotoxicity and antitumor activity in vivo of iridium(III) complexes liposomes targeting endoplasmic reticulum and mitochondria. J. Inorg. Biochem. 2022, 233. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Ge, X.; Zhang, Y.; Xie, Y.; Hao, Y.; Wu, D.; Zhao, J.; Yuan, X.-A.; Tian, L.; et al. Dual functions of iridium(III) 2-phenylpyridine complexes: Metastasis inhibition and lysosomal damage. J. Inorg. Biochem. 2020, 205, 110983. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, C.; Wu, X.; Li, W.; Huang, C.; Liang, L.; Chen, J.; Wang, Y.; Liu, Y. Synthesis and anticancer activity in vitro and in vivo evaluation of iridium(III) complexes on mouse melanoma B16 cells. J. Inorg. Biochem. 2022, 232. [Google Scholar] [CrossRef]
- Gu, Y.; Wen, H.; Zhang, Y.; Bai, L.; Zhou, Y.; Zhang, H.; Tian, L.; Hao, J.; Liu, Y. Studies of anticancer activity in vivo and in vitro behaviors of liposomes encapsulated iridium(III) complex. JBIC J. Biol. Inorg. Chem. 2021, 26, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hao, H.; Ge, X.; He, X.; Liu, Y.; Wang, Y.; Wang, H.; Shao, M.; Jing, Z.; Tian, L.; et al. Triphenylamine-appended cyclometallated iridium(III) complexes: Preparation, photophysical properties and application in biology/luminescence imaging. J. Inorg. Biochem. 2019, 199, 110757. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, Y.; Zhang, H.; Zhou, Y.; Li, W.; Yuan, Y.; Hao, J.; Yang, L.; Liu, Y. Synthesis and evaluation of iridium(III) complexes on antineoplastic activity against human gastric carcinoma SGC-7901 cells. JBIC J. Biol. Inorg. Chem. 2021, 26, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, B.; Xu, C.-X.; He, L.; Wan, Y.-C.; Ji, L.-N.; Mao, Z.-W. Mitochondria-targeted phosphorescent cyclometalated iridium(III) complexes: Synthesis, characterization, and anticancer properties. JBIC J. Biol. Inorg. Chem. 2020, 25, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tian, L.; Xiao, R.; Zhou, Y.; Zhang, Y.; Hao, J.; Liu, Y.; Wang, J. Anticancer effect evaluation in vitro and in vivo of iridium(III) polypyridyl complexes targeting DNA and mitochondria. Bioorganic Chem. 2021, 115, 105290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, W.; Zhang, H.; Zhou, Y.; Tian, L.; Hao, J.; Yuan, Y.; Li, W.; Liu, Y. Increasing anticancer effect in vitro and vivo of liposome-encapsulated iridium(III) complexes on BEL-7402 cells. J. Inorg. Biochem. 2021, 225, 111622. [Google Scholar] [CrossRef]
- Gilewska, A.; Barszcz, B.; Masternak, J.; Kazimierczuk, K.; Sitkowski, J.; Wietrzyk, J.; Turlej, E. Similarities and differences in d6 low-spin ruthenium, rhodium and iridium half-sandwich complexes: Synthesis, structure, cytotoxicity and interaction with biological targets. JBIC J. Biol. Inorg. Chem. 2019, 24, 591–606. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Ge, X.; Zhang, S.; Xu, Z.; Gao, W. Design, synthesis, and evaluation of phosphorescent Ir(III) complexes with anticancer activity. J. Inorg. Biochem. 2019, 197, 110703. [Google Scholar] [CrossRef]
- Nohara, A.; Umetani, T.; Sanno, Y. Studies on antianaphylactic agents-I: A facile synthesis of 4-oxo-4H-1-benzopyran-3-carboxaldehydes by vilsmeier reagents. Tetrahedron 1974, 30, 3553–3561. [Google Scholar] [CrossRef]
- Recanatini, M.; Bisi, A.; Cavall, A.; Belluti, F.; Gobbi, S.; Rampa, A.; Palzer, P.; Palusczak, A.; Hartmann, R.W. A new class of nonsteroidal aromatase inhibitors: Design and synthesis of chromone and xanthone derivatives and inhibition of the P450 enzymes aromatase and 17r-Hydroxylase/C17,20-Lyase. J. Med. Chem. 2001, 44, 672–680. [Google Scholar] [CrossRef]
- Liu, Y.J.; Cao, H.; Yao, J.H.; Li, H.; Yuan, Y.X.; Ji, L.N. Synthesis, characterization, and DNA interaction studies of the ruthenium(II) complexes [Ru(bpy)2(ipbp)]2+ and [Ru(ipbp)(phen)2]2+ (ipbp = 3-(1H-Imidazo[4,5-f][1,10]phenanthrolin-2-yl)-4H-1-benzopyran-2-one; bpy = 2,2′-bipyridine; phen = 1,10-phenanthroline). Helv. Chim. Acta 2004, 87, 3119–3130. [Google Scholar]
- Sprouse, S.; King, K.A.; Spellane, P.J.; Watts, R.J. Photophysical effects of metal-carbon.sigma. bonds in ortho-metalated complexes of iridium (III) and rhodium (III). J. Am. Chem. Soc. 1984, 106, 6647–6653. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Diphenyltetrazolium bromide ( MTT ) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Z.; Ye, B.H.; Wang, L.; Ji, L.N.; Zhou, J.Y.; Li, R.H. Bis(2,2′-bipyridine)ruthenium(II) complexes with imidazo[4,5-f][1,10]-phenanthroline or 2-phenylimidazo[4,5-f][1,10]phenanthroline. J. Chem. Soc. Dalton Trans. 1997, 8, 1395–1402. [Google Scholar] [CrossRef]
- Kordestani, N.; Rudbari, H.A.; Fernandes, A.R.; Raposo, L.R.; Baptista, P.V.; Ferreira, D.; Bruno, G.; Bella, G.; Scopelliti, R.; Braun, J.D.; et al. Antiproliferative Activities of Diimine-Based Mixed Ligand Copper(II) Complexes. ACS Comb. Sci. 2020, 22, 89–99. [Google Scholar] [CrossRef]
- Dandash, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Nasri, S.; Brégier, F.; Granet, R.; Karam, W.; Diab-Assaf, M.; Sol, V.; Liagre, B. In vitro anticancer activity of new gold(III) porphyrin complexes in colon cancer cells. J. Inorg. Biochem. 2017, 177, 27–38. [Google Scholar] [CrossRef]
- Bautista, H.R.; Díaz, R.O.S.; Shen, L.Z.Q.; Orvain, C.; Gaiddon, C.; Lagadec, R.L.; Ryabov, A.D. Impact of cyclometalated ruthe-nium(II) complexes on lactate dehydrogenase activity and cytotoxicity in gastric and colon cancer cells. J. Inorg. Biochem. 2016, 163, 28–38. [Google Scholar] [CrossRef]
- Gu, Y.; Wen, H.; Bai, L.; Zhou, Y.; Zhang, H.; Tian, L.; Zhang, Y.; Hao, J.; Liu, Y. Exploring anticancer efficiency of mitochondria-targeted cyclometalated iridium(III) complexes. J. Inorg. Biochem. 2020, 212, 111215. [Google Scholar] [CrossRef]
- Yugandhar, D.; Nayak, V.L.; Archana, S.; Shekar, K.C.; Srivastava, A.K. Design, synthesis and anticancer properties of novel oxa/azaspiro[4,5]trienones as potent apoptosis inducers through mitochondrial disruption. Eur. J. Med. Chem. 2015, 101, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.; Brockhoff, G.; Goepferich, A. Targeting drugs to mitochondria. Eur. J. Pharm. Biopharm. 2012, 82, 1–18. [Google Scholar] [CrossRef]
- Abu-Gosh, S.E.; Kolvazon, N.; Tirosh, B.; Ringel, I.; Yavin, E. Multiple Triphenylphosphonium Cations Shuttle a Hydrophilic Peptide into Mitochondria. Mol. Pharm. 2009, 6, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Malugin, A.; Kopeèková, P.; Kopeèek, J. HPMA Copolymer-bound doxorubicin induces apoptosis in ovarian carcinoma cells by the disruption of mitochondrial function. Mol. Pharm. 2006, 3, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Hajare, S.N.; Subramanian, M.; Gautam, S.; Sharma, A. Induction of apoptosis in human cancer cells by a Bacillus lipopeptide bacillomycin D. Biochimie 2013, 95, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, D.; Cheltsov, A.; Sergienko, E.; Vasile, S.; Golubkov, V.; Aleshin, A.E.; Levin, T.; Traer, E.; Hann, B.; Freimuth, J.; et al. TRAIL-Based High Throughput Screening Reveals a Link between TRAIL-Mediated Apoptosis and Glutathione Reductase, a Key Component of Oxidative Stress Response. PLoS ONE 2015, 10, e0129566. [Google Scholar] [CrossRef]
- Wan, D.; Lai, S.-H.; Zeng, C.-C.; Zhang, C.; Tang, B.; Liu, Y.-J. Ruthenium(II) polypyridyl complexes: Synthesis, characterization and anticancer activity studies on BEL-7402 cells. J. Inorg. Biochem. 2017, 173, 1–11. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Harper, J. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular Uptake, Intracellular Trafficking, and Cytotoxicity of Nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Tang, B.; Shen, F.; Wan, D.; Guo, B.-H.; Wang, Y.-J.; Yi, Q.-Y.; Liu, Y.-J. DNA-binding, molecular docking studies and biological activity studies of ruthenium(ii) polypyridyl complexes. RSC Adv. 2017, 7, 34945–34958. [Google Scholar] [CrossRef]
- Xu, J.; Bai, L.; Gu, Y.-Y.; Du, F.; Zhang, W.-Y.; He, M.; Liu, Y.-J. Synthesis, characterization, apoptosis, ROS, autophagy and western blotting studies of cyclometalated iridium(III) complexes. Inorg. Chem. Commun. 2019, 111, 107594. [Google Scholar] [CrossRef]
- Cai, F.; Chen, M.; Zha, D.; Zhang, P.; Zhang, X.; Cao, N.; Wang, J.; He, Y.; Fan, X.; Zhang, W.; et al. Curcumol potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer. Oncotarget 2017, 8, 115526–115545. [Google Scholar] [CrossRef]
- Wu, S.-W.; Su, C.-H.; Ho, Y.-C.; Huang-Liu, R.; Tseng, C.-C.; Chiang, Y.-W.; Yeh, K.-L.; Lee, S.-S.; Chen, W.-Y.; Chen, C.-J.; et al. Genotoxic effects of 1-nitropyrene in macrophages are mediated through a p53-dependent pathway involving cytochrome c release, caspase activation, and PARP-1 cleavage. Ecotoxicol. Environ. Saf. 2021, 213, 112062. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, R.; Xie, L.; Liao, X.; Xiong, K.; Ree, T.W.; Chen, Y.; Ji, L.; Chao, H. An ER-targeting iridium(III) complex which induces immunogenic cell death in non-small cell lung cancer. Angew. Chem. Int. Ed. Engl. 2021, 60, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Guido, K.; Lorenzo, G.; Oliver, K.; Laurence, Z. Immunogenic cell death in cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, B.; Wang, Y.; Wang, D.; Xue, X.; Nie, Y. Synthesis, Characterization and Anticancer Efficacy Studies of Iridium (III) Polypyridyl Complexes against Colon Cancer HCT116 Cells. Molecules 2022, 27, 5434. https://doi.org/10.3390/molecules27175434
Xie B, Wang Y, Wang D, Xue X, Nie Y. Synthesis, Characterization and Anticancer Efficacy Studies of Iridium (III) Polypyridyl Complexes against Colon Cancer HCT116 Cells. Molecules. 2022; 27(17):5434. https://doi.org/10.3390/molecules27175434
Chicago/Turabian StyleXie, Biao, Yi Wang, Di Wang, Xingkui Xue, and Yuqiang Nie. 2022. "Synthesis, Characterization and Anticancer Efficacy Studies of Iridium (III) Polypyridyl Complexes against Colon Cancer HCT116 Cells" Molecules 27, no. 17: 5434. https://doi.org/10.3390/molecules27175434
APA StyleXie, B., Wang, Y., Wang, D., Xue, X., & Nie, Y. (2022). Synthesis, Characterization and Anticancer Efficacy Studies of Iridium (III) Polypyridyl Complexes against Colon Cancer HCT116 Cells. Molecules, 27(17), 5434. https://doi.org/10.3390/molecules27175434





