Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential
Abstract
1. Introduction
2. Results and Discussion
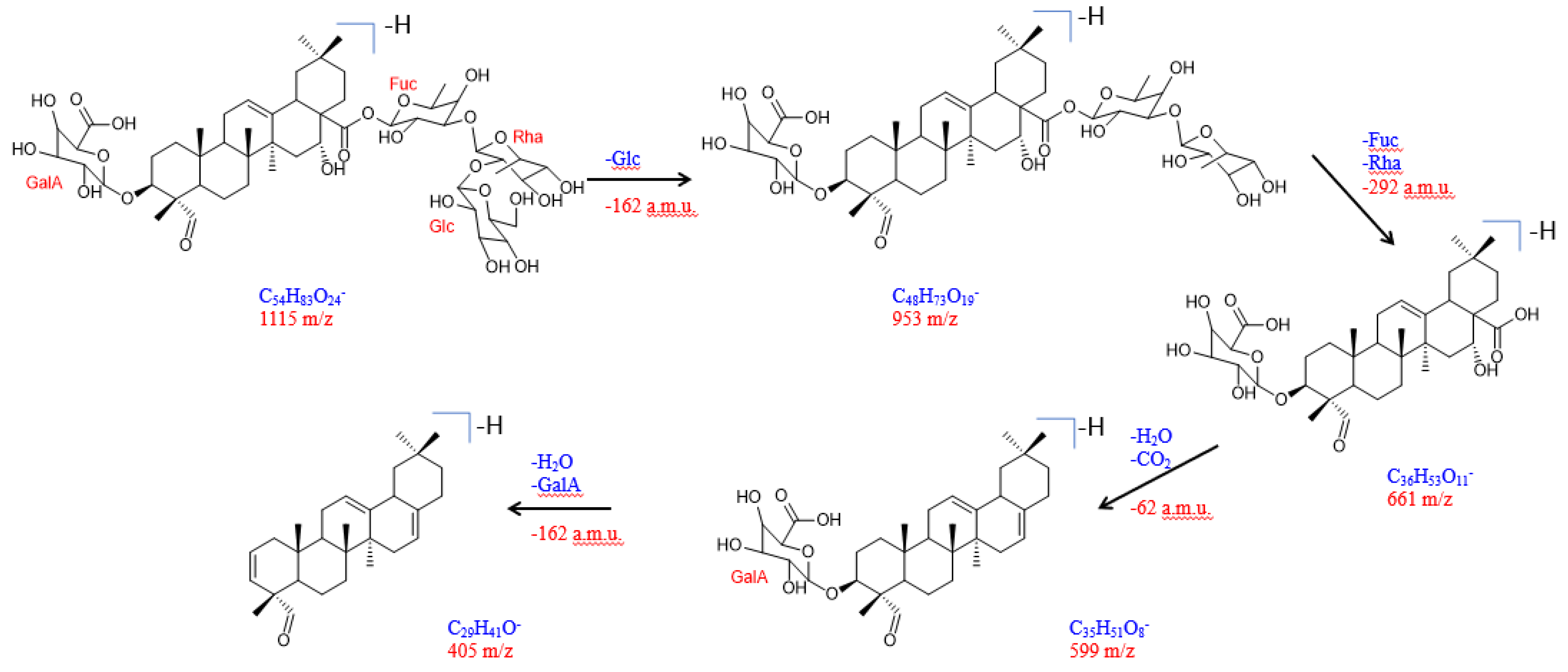
2.1. Isolation and Structural Elucidation of Compound 1
2.2. Biological Evaluation of Compound 1
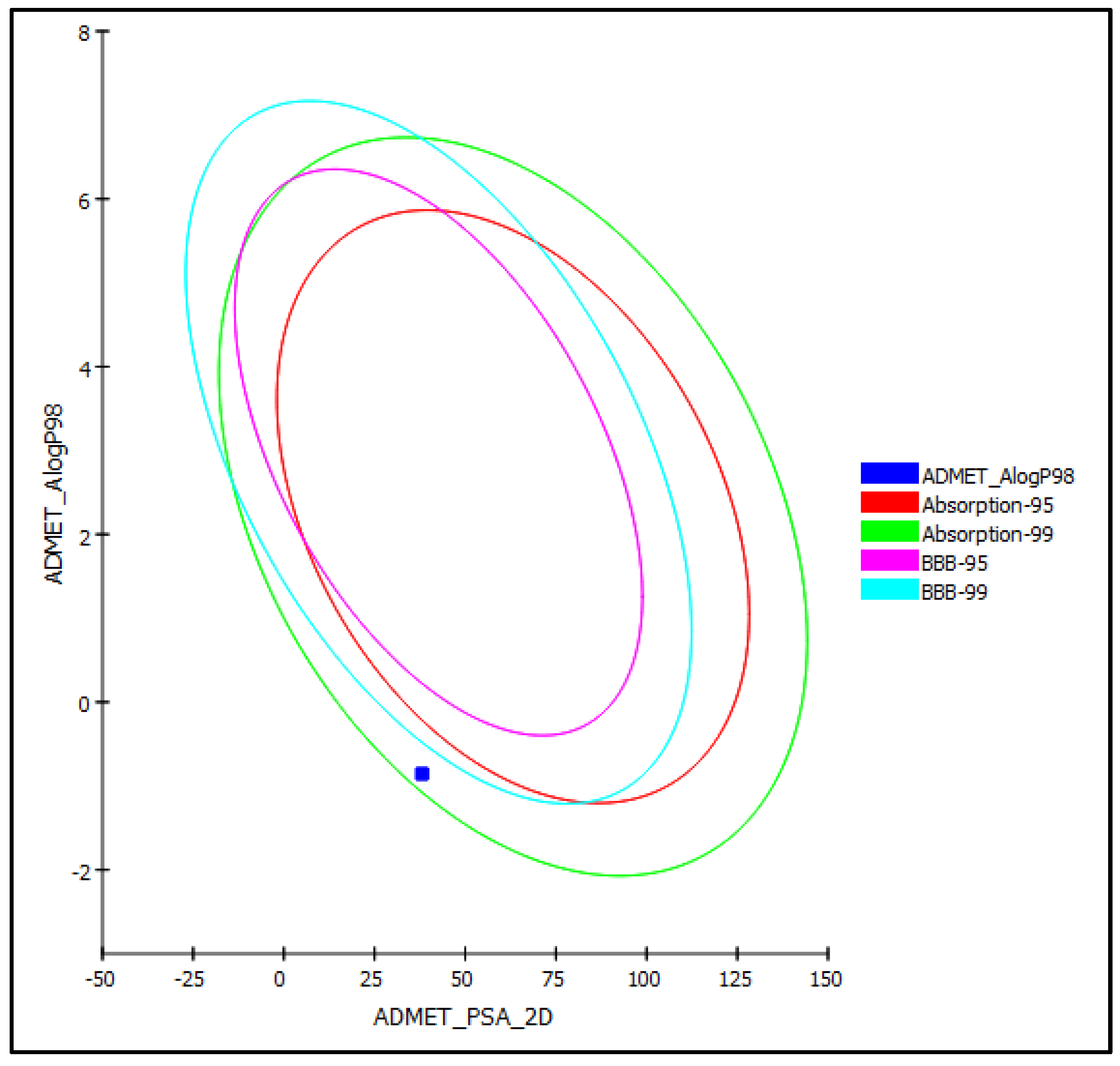
2.3. In Silico Evaluation of the Pharmacodynamic, Pharmacokinetic, and Toxicity Properties of Silviridoside
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Antioxidant Assays
3.6. Enzyme Inhibition Assays
3.7. In Silico Evaluation of Silviridoside
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress. Sport Med. 2006, 36, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.; Rajauria, G. Optimisation of ultrasound frequency, extraction time and solvent for the recovery of polyphenols, phlorotannins and associated antioxidant activity from brown seaweeds. Mar. Drug. 2020, 18, 250. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ru, Y.; Wang, Z.; He, X.; Kong, K.-W.; Zheng, T.; Zhang, X. Phytochemical composition, antioxidant activity, and enzyme inhibitory activities (α-glucosidase, xanthine oxidase, and acetylcholinesterase) of Musella lasiocarpa. Molecules 2021, 26, 4472. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P. Anti-inflammatory activity of a polyphenolic extract from Arabidopsis thaliana in vitro and in vivo models of Alzheimer’s disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Sakna, S.T.; El-Fiky, N.M.; Shabana, M.M.; Wessjohann, L.A. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC–PDA–qTOF-MS and chemometrics. Phytochemistry 2015, 119, 41–50. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, J.; Wang, Y.; Chen, J.; Xi, J. Nanozymes as enzyme inhibitors. Int. J. Nanomed. 2021, 16, 1143–1155. [Google Scholar] [CrossRef]
- Jabir, N.R.; Khan, F.R.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Birks, J.S. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Adhikari, A.; Devkota, H.; Takano, A.; Masuda, K.; Nakane, T.; Basnet, P.; Skalko-Basnet, N. Screening of Nepalese crude drugs traditionally used to treat hyperpigmentation: In vitro tyrosinase inhibition. Int.J. Cosm. Sci. 2008, 30, 353–360. [Google Scholar] [CrossRef]
- Janibekov, A.A.; Youssef, F.S.; Ashour, M.L.; Mamadalieva, N.Z. New flavonoid glycosides from two Astragalus species (Fabaceae) and validation of their antihyperglycaemic activity using molecular modelling and in vitro studies. Ind. Crops Prod. 2018, 118, 142–148. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Ebada, S.S.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2017, 69, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Paşayeva, L.; Özalp, B.; Fatullayev, H. Potential enzyme inhibitory properties of extracts and fractions from fruit latex of Ficus carica-based on inhibition of α-amylase and α-glucosidase. J. Food Meas. Character. 2020, 14, 2819–2827. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Hussain, H.; Zengin, G.; Mollica, A.; Al Musayeib, N.M.; Ashour, M.L.; Westermann, B.; Wessjohann, L.A. Validation of the antioxidant and enzyme inhibitory potential of selected triterpenes using in vitro and in silico studies, and the evaluation of their ADMET properties. Molecules. 2021, 26, 6331. [Google Scholar] [CrossRef] [PubMed]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Altyar, A.E.; Al-Azizi, M.M.; Ashour, M.L. A Comprehensive insight on the health benefits and phytoconstituents of Camellia sinensis and recent approaches for its quality control. Antioxidants 2019, 8, 455. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Lafont, R.; Wink, M. Diversity of secondary metabolites in the genus Silene L. (Caryophyllaceae)—Structures, distribution, and biological properties. Diversity 2014, 6, 415–499. [Google Scholar] [CrossRef]
- Yildiz, K.; Cirpici, A.H. Taxonomic revision of Silene (Caryophyllaceae) sections Siphonomorpha, Lasiostemones, Sclerocalycinae, Chloranthae, Tataricae, and Otites in Turkey. Turk. J. Bot. 2013, 37, 191–218. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Janibekov, A.A.; Girault, J.-P.; Lafont, R. Two minor phytoecdysteroids of the plant Silene viridiflora. Nat. Prod. Commun. 2010, 5, 1934578X1000501013. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Ul’chenko, N.; Yuldasheva, N.; Egamberdieva, D.; Zhanibekov, A.; Dzhukharova, M.K.; Glushenkova, A. Fatty-acid composition and antibacterial activity of CHCl3 extracts of three plants of the genus Silene. Chem. Nat. Compound. 2010, 46, 95–96. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Böhmdorfer, S.; Zengin, G.; Bacher, M.; Potthast, A.; Akramov, D.K.; Janibekov, A.; Rosenau, T. Phytochemical and biological activities of Silene viridiflora extractives. Development and validation of a HPTLC method for quantification of 20-hydroxyecdysone. Ind. Crops Prod. 2019, 129, 542–548. [Google Scholar] [CrossRef]
- Eshmirzaeva, N.; Khidyrova, N.; Khodzhaeva, M.; Mezhlumyan, L.; Shakhidoyatov, K.M. Chemical Composition of Silene viridiflora. Chem. Nat. Compound. 2005, 41, 451–453. [Google Scholar] [CrossRef]
- Dzakhangirova, M.; Syrov, V. Experimental evaluation of effect of stimulation of sum of ecdysteroids from Silene brachuica and S. viridiflora for erythropoiesis in laboratory animals. Pathology 2005, 2, 7–9. [Google Scholar]
- Shakhmurova, G.; Mamadalieva, N.; Zhanibekov, A.; Khushbaktova, Z.; Syrov, V. Effect of total ecdysteroid preparation from Silene viridiflora on the immune state of experimental animals under normal and secondary immunodeficiency conditions. Pharm. Chem. J. 2012, 46, 222–224. [Google Scholar] [CrossRef]
- Bechkri, S.; Magid, A.A.; Sayagh, C.; Berrehal, D.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, Z.; Kabouche, A. Triterpene saponins from Silene gallica collected in North-Eastern Algeria. Phytochemistry 2020, 172, 112274. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. The antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-d-glucopyranosyl)-hederagenin. Phytother. Res. 2006, 20, 130–134. [Google Scholar] [CrossRef]
- Nguyen, N.-H.; Ha, T.K.Q.; Yang, J.-L.; Pham, H.T.T.; Oh, W.K. Triterpenoids from the genus Gynostemma: Chemistry and pharmacological activities. J. Ethnopharmacol. 2021, 268, 113574. [Google Scholar] [CrossRef]
- Zandoná, G.P.; Bagatini, L.; Woloszyn, N.; de Souza Cardoso, J.; Hoffmann, J.F.; Moroni, L.S.; Stefanello, F.M.; Junges, A.; Rombaldi, C.V. Extraction and characterization of phytochemical compounds from araçazeiro (Psidium cattleianum) leaf: Putative antioxidant and antimicrobial properties. Food Res. Int. 2020, 137, 109573. [Google Scholar] [CrossRef]
- Rauf, A.; Jehan, N. Natural products as a potential enzyme inhibitors from medicinal plants. Enzym. Inhib. Act. 2017, 165, 177. [Google Scholar]
- Yue, J.; Xu, J.; Cao, J.; Zhang, X.; Zhao, Y. Cucurbitane triterpenoids from Momordica charantia L. and their inhibitory activity against α-glucosidase, α-amylase and protein tyrosine phosphatase 1B (PTP1B). J. Funct. Food. 2017, 37, 624–631. [Google Scholar] [CrossRef]
- El Sayed, A.M.; AbdElSattar, E.; Khalil, M.N. New calogenin pregnane glycoside derivative from Huernia saudi-arabica and its lipase and α-glucosidase inhibitory activities. Biomed. Pharmacother. 2020, 127, 110143. [Google Scholar] [CrossRef]
- Apak, R.A.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Ovidi, E.; Musayeib, N.M.A.; Ashour, M.L. Morphology, anatomy and secondary metabolites investigations of Premna odorata Blanco and evaluation of its anti-tuberculosis activity using in vitro and in silico studies. Plants 2021, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; Youssef, F.S.; Alahdal, A.M.; Almasri, D.M.; Ashour, M.L. Anti-Hyperglycaemic evaluation of Buddleia indica leaves using in vitro, in vivo and in silico studies and its correlation with the major phytoconstituents. Plants 2021, 10, 2351. [Google Scholar] [CrossRef] [PubMed]

| No. | δC | δH (J/Hz) | Selected NOESY Cross-Peaks | No. | δC | δH (J/Hz) | Selected NOESY Cross-Peaks |
|---|---|---|---|---|---|---|---|
| 1 | 37.8 | 1.01, m; 1.58, m | 16 | 72.4 | 4.34, br,s | 26 | |
| 2 | 24.2 | 1.62, m; 1.96, m | 17 | 47.9 | 11, 12 | ||
| 3 | 80.2 | 3.82, m | 5, 1′ | 18 | 40.5 | 2.86, m | 15a, 19a, 22a, 30 |
| 4 | 54.5 | 19 | 46.5 | 0.99, m; 2.25, m | |||
| 5 | 46.7 | 1.25, m | 3 | 20 | 30.2 | ||
| 6 | 19.8 | 0.77, m; 1.34, m | 21 | 35.0 | 1.08, m; 1.92, m | ||
| 7 | 31.8 | 1.26, m; 1.37, m | 22 | 30.7 | 1.63, m; 1.79, m | ||
| 8 | 41.1 | 23 | 207.0 | 9.35, s | 2b, 3, 5, 6b, 24, 1′ | ||
| 9 | 46.1 | 1.62, m | 24 | 9.8 | 0.98, s | ||
| 10 | 35.5 | 25 | 15.5 | 0.91, s | |||
| 11 | 22.9 | 1.82, m | 26 | 16.7 | 0.65, s | 12, 16 | |
| 12 | 121.2 | 5.22, m | 27 | 26.4 | 1.32, s | ||
| 13 | 143.2 | 28 | 175.0 | ||||
| 14 | 39.4 | 29 | 32.9 | 0.84, s | |||
| 15 | 34.8 | 1.64, m; 1.26, m | 30 | 24.3 | 0.91, s |
| No. | δC | δH (J/Hz) | Selected HMBC Cross-Peaks | Selected NOESY Cross-Peaks |
|---|---|---|---|---|
| GalA | ||||
| 1′ | 102.90 | 3.98, d (7.6) | 3 | 3 |
| 2′ | 73.71 | 2.82, m | ||
| 3′ | 72.16 | 3.02, m | ||
| 4′ | 76.89 | 3.03, m | ||
| 5′ | 73.81 | 3.11, m | 6´ | |
| 6′ | 172.61 | |||
| Rhamnose | ||||
| 1″ | 99.39 | 5.42, br,s | 2″, 3″, 5″, 2″″ | 3″″ |
| 2″ | 81.50 | 3.82, m | 1′′′ | |
| 3″ | 70.23 | 3.41, m | ||
| 4″ | 72.47 | 3.12, m | ||
| 5″ | 68.59 | 3.48, m | ||
| 6″ | 18.16 | 1.10, d (6.1) | 4″, 5″ | |
| Glucose | ||||
| 1′′′ | 105.90 | 4.24, d (7.7) | 2″ | 2″ |
| 2′′′ | 74.04 | 3.01, m | ||
| 3′′′ | 76.50 | 3.12, m | ||
| 4′′′ | 70.17 | 3.03, m | ||
| 5′′′ | 76.85 | 3.09, m | ||
| 6′′′ | 61.32 | 3.70, dd (10.5, 5.8) | ||
| 3.43, m | ||||
| Fucose | ||||
| 1″″ | 93.03 | 5.22 d (8.0) | 28 | |
| 2″″ | 74.66 | 3.55, m | 4″″ | |
| 3″″ | 74.38 | 3.53, m | 1″″, 2″″ | 1″ |
| 4″″ | 71.32 | 3.38, m | ||
| 5″″ | 70.69 | 3.58, m | 1″″ | |
| 6″″ | 16.30 | 1.05, d (6.3) | 4″″, 5″″ | |
| OH Groups | ||||
| 3″-OH | 4.39, d (9.3) | |||
| 2′-OH | 4.46, d (4.8) | |||
| 6″″-OH | 4.61, t (5.6) | |||
| 4″-OH | 4.76, d (4.8) | |||
| 4’’’-OH | 4.75, m | |||
| 16-OH | 4.81, d (4.8) | |||
| 4″″-OH | 4.93, d (5.8) | |||
| 3″″-OH | 4.96, d (5.0) | |||
| 2’’’’-OH | 5.20, m | |||
| 3′and 4′-OH | 4.75 | |||
| Antioxidant Activity a | Silviridoside | Enzyme Inhibitory Activity b | Silviridoside |
|---|---|---|---|
| DPPH (mg TE/g) | 2.32 ± 0.48 | AChE (mg GALAE/g) | 2.52 ± 0.48 |
| ABTS (mg TE/g) | 1.24 ± 0.29 | BChE (mg GALAE/g) | 7.16 ± 0.04 |
| FRAP (mg TE/g) | 5.13 ± 0.31 | Tyrosinase (mg KAE/g) | 38.83 ± 0.45 |
| CUPRAC (mg TE/g) | 9.59 ± 0.52 | Amylase (mmol ACAE/g) | 0.19 ± 0.01 |
| PHD (mmol TE/g) | 0.28 ± 0.01 | Glucosidase (mmol ACAE/g) | 1.21 ± 0.01 |
| MCA (mg EDTAE/g) | 6.62 ± 0.35 |
| Compounds | Silviridoside |
|---|---|
| ADMET Descriptors | |
| Absorption Level a | 1 |
| Solubility Level b | 5 |
| BBB Level c | 4 |
| PPB Level d | False |
| CPY2D6 | NI |
| Hepatotoxicity | Toxic |
| PSA-2D e | 38.12 |
| Alog p98 f | −0.86 |
| TOPKAT Descriptors | |
| Ames Prediction | Non-mutagenic |
| Rat Oral LD50 (g/kg.bw) | 1.116 |
| Rat Chronic LOAEL (g/kg.bw) | 0.075 |
| Rat Female NTP | Non-carcinogenic |
| Rat Male NTP | Carcinogenic |
| Skin Irritancy | Mild |
| Eye Irritancy | Severe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhmudova, M.M.; Bacher, M.; Zengin, G.; Rosenau, T.; Youssef, F.S.; Almasri, D.M.; Elhady, S.S.; Mamadalieva, N.Z. Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential. Molecules 2022, 27, 8781. https://doi.org/10.3390/molecules27248781
Makhmudova MM, Bacher M, Zengin G, Rosenau T, Youssef FS, Almasri DM, Elhady SS, Mamadalieva NZ. Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential. Molecules. 2022; 27(24):8781. https://doi.org/10.3390/molecules27248781
Chicago/Turabian StyleMakhmudova, Markhabo M., Markus Bacher, Gokhan Zengin, Thomas Rosenau, Fadia S. Youssef, Diena M. Almasri, Sameh S. Elhady, and Nilufar Z. Mamadalieva. 2022. "Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential" Molecules 27, no. 24: 8781. https://doi.org/10.3390/molecules27248781
APA StyleMakhmudova, M. M., Bacher, M., Zengin, G., Rosenau, T., Youssef, F. S., Almasri, D. M., Elhady, S. S., & Mamadalieva, N. Z. (2022). Silviridoside: A New Triterpene Glycoside from Silene viridiflora with Promising Antioxidant and Enzyme Inhibitory Potential. Molecules, 27(24), 8781. https://doi.org/10.3390/molecules27248781










