Phytochemical Profiles, Antioxidant, Cytotoxic, and Anti-Inflammatory Activities of Traditional Medicinal Plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
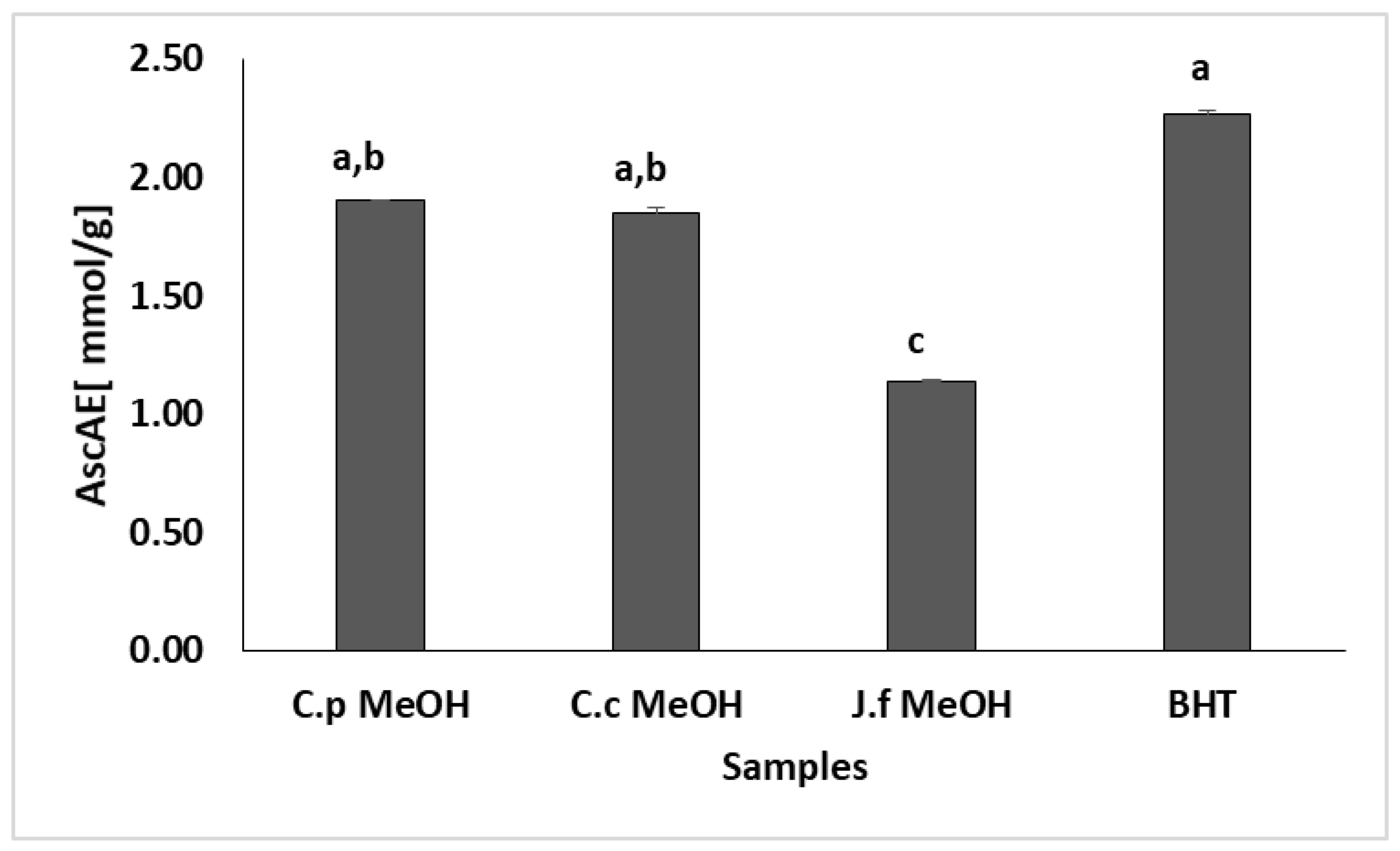
2.2. Antioxidant Activity
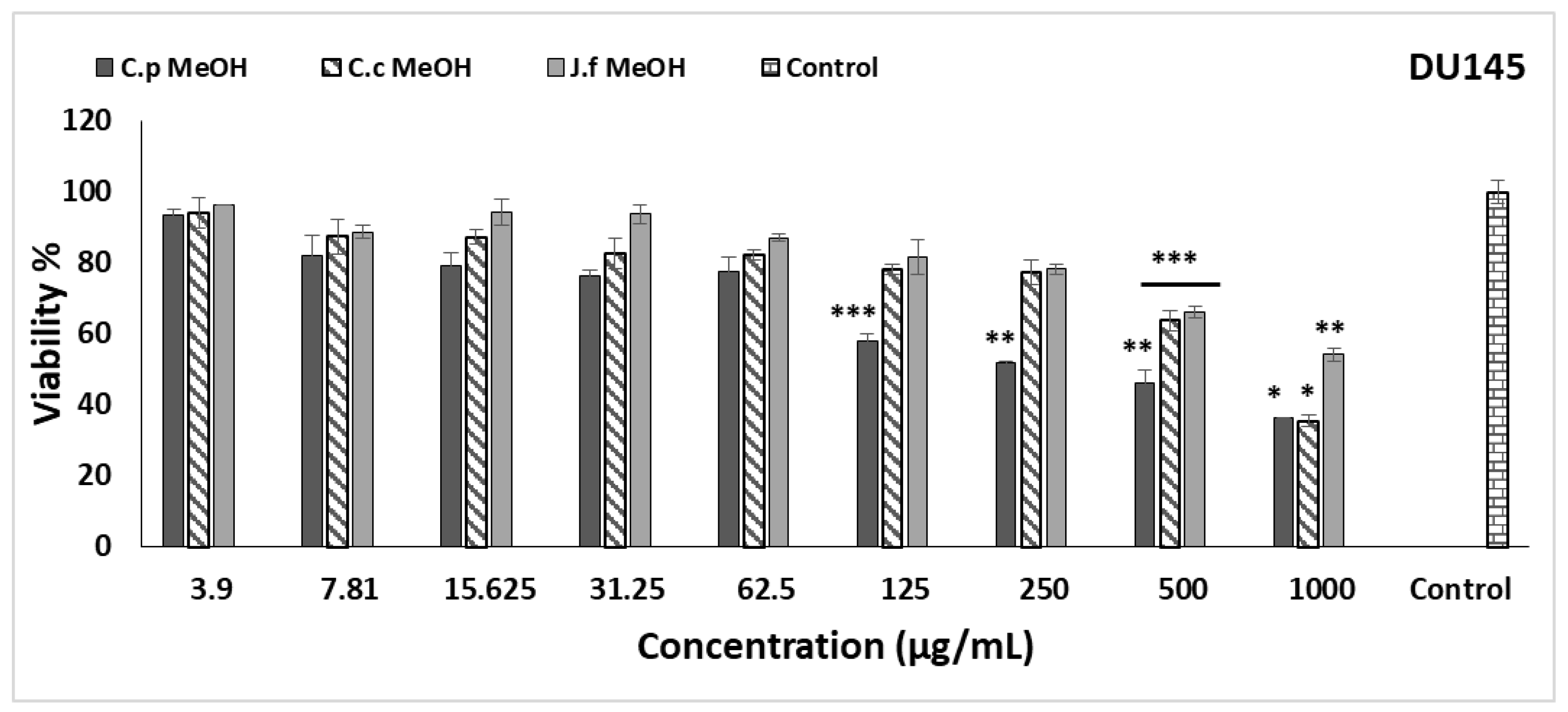
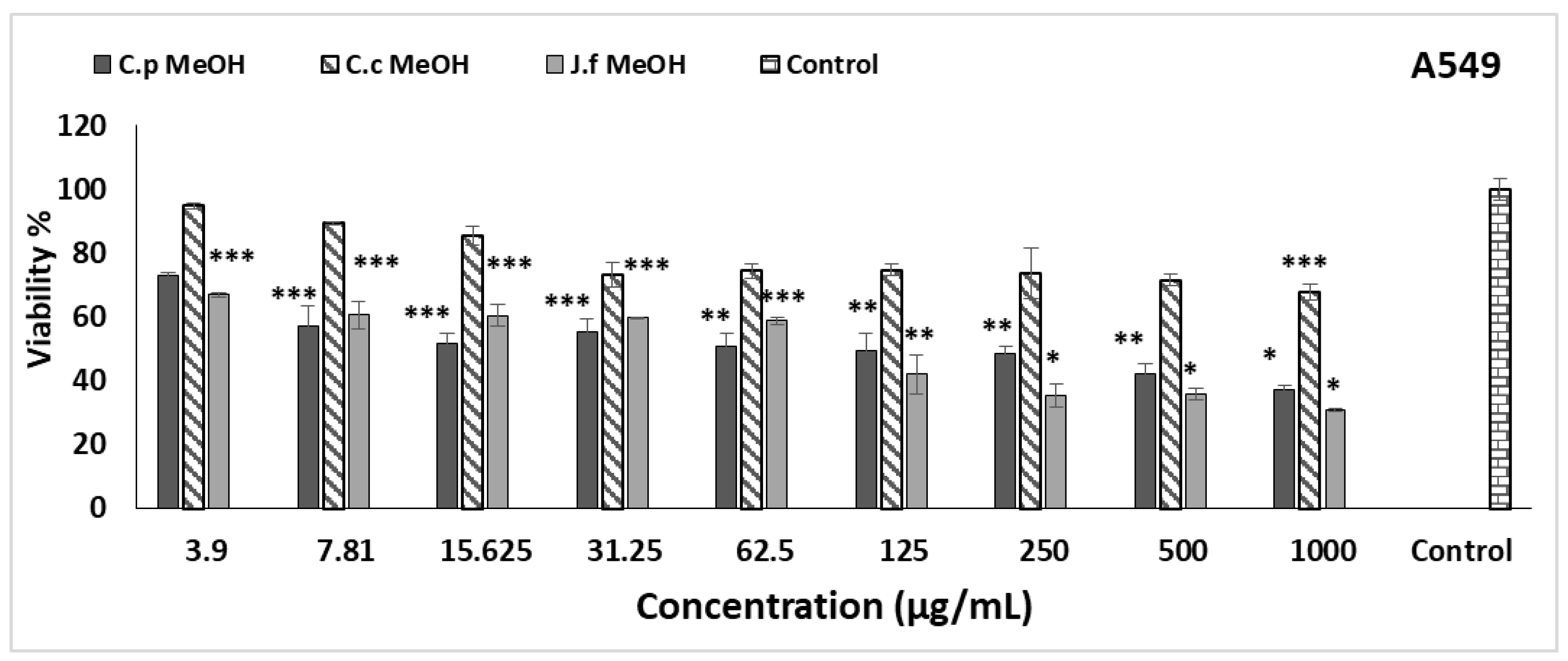
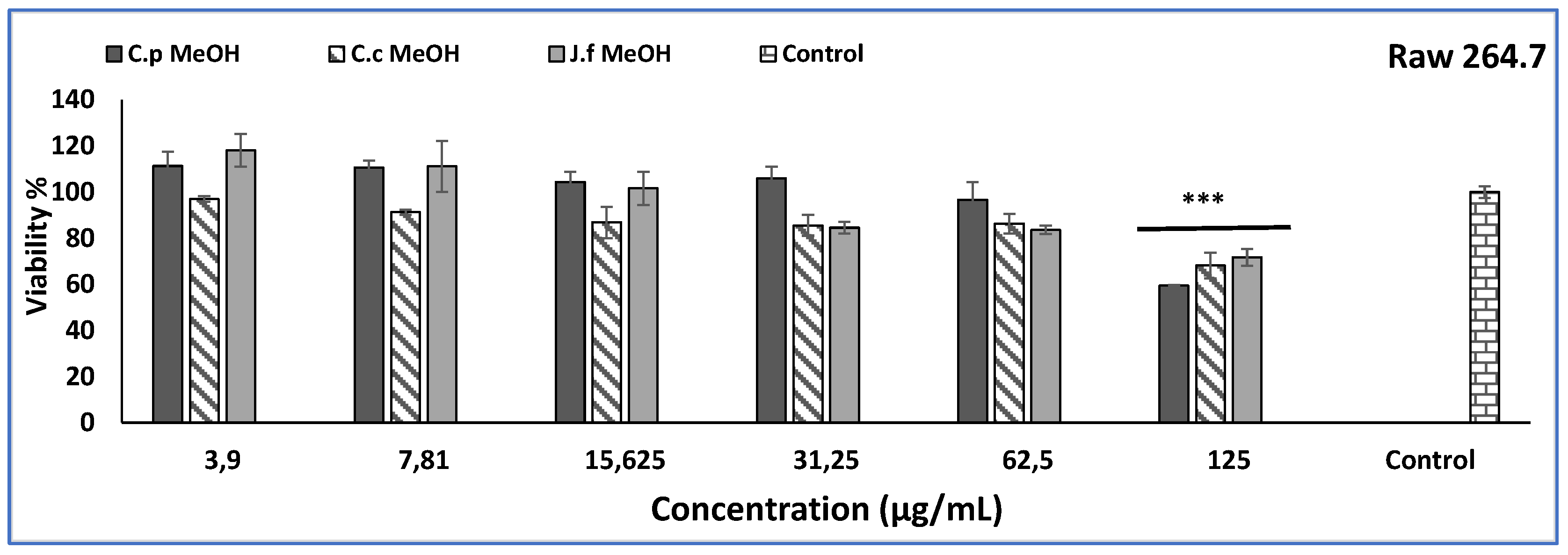
2.3. Cytotoxic Activity
2.4. Anti-Inflammatory Activity
3. Materials and Method
3.1. Plant Material
3.2. Sample Preparation
3.3. Chemical Analysis
3.3.1. Determination of Total Phenolic Content
3.3.2. Determination of Total Flavonoid Content
3.3.3. High Performance Liquid Chromatography (HPLC) Analysis
3.4. Antioxidant Activity
3.4.1. DPPH● Radical Scavenging Activity
3.4.2. ABTS●+ Radical Scavenging Activity
3.4.3. Iron(III) to iron(II) reduction activity (FRAP)
3.5. Cytotoxic Activity
3.5.1. Cell Lines and Cell Culture Methods
3.5.2. Determination of Cell Viability Assay
3.6. Anti-Inflammatory Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Campo, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Kültür, Ş.; Bona, M.; Nath, E.Ö. A new species of Centaurea (Asteraceae) from East Anatolia, Turkey. Phytotaxa 2016, 247, 085–091. [Google Scholar] [CrossRef]
- Baytop, T. Therapy with Medicinal Plants in Turkey-Past and Present, 2nd ed.; Nobel Tıp Basımevi: Istanbul, Turkey, 1999. [Google Scholar]
- Dalar, A.; Konczak, I. Botanicals from Eastern Anatolia Region of Turkey: Antioxidant capacity and phenolic constituents of endemic herbal medicines. J. Nat. Med. 2012, 2, 126–135. [Google Scholar] [CrossRef]
- Servi, H.; Çelik, S.; Göktürk, R.S. Essential oil composition of two endemic Centaurea species from Turkey. Nat. Volatiles Essent. Oils. 2019, 6, 18–24. [Google Scholar]
- Güner, A.; Aslan, S.; Ekim, T.; Vural, M.; Babaç, M.T. Türkiye Bitkileri Listesi (Damarlı Bitkiler); Flora Dizisi, I., Ed.; Nezahat Gökyigit Botanik Bahçesi Yayınları: Istanbul, Turkey, 2012. [Google Scholar]
- Taştan, P.; Fafal, T.; Tüzün, B.S.; Gönenç, T.; Demirci, B.; Kivcak, B. Composition of essential oil and fatty acids of Centaurea pichleri ssp. pichleri. Int. J. Second. Metab. 2017, 4, 37–42. [Google Scholar] [CrossRef]
- Veres, K.; Csupor-Löffler, B.; Lázár, A.; Hohmann, J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Sci. World J. 2012, 2012, 489646. [Google Scholar] [CrossRef]
- Weaver, S.E. The biology of Canadian weeds. 115. Conyza canadensis. Can. J. Plant Sci. 2001, 81, 867–875. [Google Scholar] [CrossRef]
- Polat, R.; Satıl, F. An ethnobotanical survey of medicinal plants in Edremit Gulf (Balıkesir–Turkey). J. Ethnopharmacol. 2012, 139, 626–641. [Google Scholar] [CrossRef]
- Ayaz, F.; Sarimahmut, M.; Kucukboyaci, N.; Ulukaya, E. Cytotoxic effect of Conyza canadensis (L.) cronquist on human lung cancer cell lines. Turk. J. Pharm. Sci. 2016, 13, 342–346. [Google Scholar] [CrossRef]
- Yohanan, R.; Jeyarani, N.J.; Devipriya, V.; Rather, S.A.; Kasana, S.; Thakur, J.; Dwivedi, D.; Pandey, A.K. Evaluating genetic diversity within genus Jasminum L. (Oleaceae) using intersimple sequence repeats (ISSR) marker. Proceedings of the National Academy of Sciences. Proc. Natl. Acad. Sci. India. Sect. B Biol. Sci. 2020, 90, 531–540. [Google Scholar] [CrossRef]
- Akkol, E.K.; Kozan, E.; Bardakci, H.; Barak, T.H.; Khalilpour, S. Potential anthelmintic and antioxidant activities of Jasminum fruticans L. and its phytochemical analysis. Pharm. Sci. 2021, 28, 481–491. [Google Scholar] [CrossRef]
- Honda, G.; Yeşilada, E.; Tabata, M.; Sezik, E.; Fujita, T.; Takeda, Y.; Takaishi, Y.; Tanaka, T. Traditional medicine in Turkey VI. Folk medicine in West Anatolia: Afyon, Kütahya, Denizli, Muğla, Aydin provinces. J. Ethnopharmacol. 1996, 53, 75–87. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights, and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Kozan, E.; Küpeli, E.; Yesilada, E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J. Ethnopharmacol. 2006, 108, 211–216. [Google Scholar] [CrossRef]
- Baydoun, S.; Chalak, L.; Dalleh, H.; Arnold, N. Ethnopharmacological survey of medicinal plants used in traditional medicine by the communities of Mount Hermon, Lebanon. J. Ethnopharmacol. 2015, 173, 139–156. [Google Scholar] [CrossRef]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Karamenderes, C.; Konyalioglu, S.; Khan, S.; Khan, I.A. Total phenolic contents, free radical scavenging activities and inhibitory effects on the activation of NF-kappa B of eight Centaurea L. species. Phytother. Res. 2007, 21, 488–491. [Google Scholar] [CrossRef]
- Erol-Dayi, Ö.; Pekmez, M.; Bona, M.; Aras-Perk, A.; Arda, N. Total phenolic contents, antioxidant activities cytotoxicity of three Centaurea species: C. calcitrapa subsp. calcitrapa, C. ptosimopappa C. spicata. Free. Radic. Antioxid. 2011, 1, 31–36. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Carradori, S.; Mocan, A.M.; Aktumsek, A. Total phenolics, flavonoids, condensed tannins content of eight Centaurea species and their broad inhibitory activities against cholinesterase, tyrosinase, α-amylase and α-glucosidase. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 195–200. [Google Scholar] [CrossRef]
- El Guiche, R.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimie, A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the South of Morocco. Int. J. New Technol. Res. 2015, 1, 263695. [Google Scholar]
- Hidalgo, G.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Ayla, S.; Okur, M.E.; Günal, M.Y.; Özdemir, E.M.; Çiçek Polat, D.; Yoltaş, A.; Biçeroğlu, Ö.; Karahüseyinoğlu, S. Wound healing effects of methanol extract of Laurocerasus officinalis roem. Biotech. Histochem. 2019, 94, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Okur, M.E.; Ayla, Ş.; Karadağ, A.E.; Polat, D.Ç.; Demirci, S.; Seçkin, İ. Opuntia ficus indica fruits ameliorate cisplatin-induced nephrotoxicity in mice. Biol. Pharm. Bull. 2020, 43, 831–838. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food. Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A role of gallic acid in oxidative damage diseases: A comprehensive review. Nat. Prod. Commun. 2019, 14, 1–9. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Acet, T. Determining the phenolic components by using HPLC and biological activity of Centaurea triumfetti. Plant Biosyst. 2021, 155, 159–164. [Google Scholar] [CrossRef]
- Yırtıcı, Ü.; Ergene, A.; Atalar, M.N.; Adem, Ş. Phytochemical composition, antioxidant, enzyme inhibition, antimicrobial effects, and molecular docking studies of Centaurea sivasica. S. Afr. J. Bot. 2022, 144, 58–71. [Google Scholar] [CrossRef]
- Abood, M.A.; Kadhim, E.J. Phytochemical Investigation of Some Active Components in Iraqi Conyza canadensis (Syn. Erigeron canadensis). IJDDT 2021, 11, 669–675. [Google Scholar]
- Van Vuuren, S.F.; Viljoen, A.M.; Őzek, T.; Demirci, B.; Başer, K.H.C. Seasonal and geographical variation of Heteropyxis natalensis essential oil and the effect thereof on the antimicrobial activity. S. Afr. J. Bot. 2007, 73, 441–448. [Google Scholar] [CrossRef]
- Polat, D.Ç.; Coskun, M. Quantitative determination by HPLC-DAD of icariin, epimedin A, epimedin B, and epimedin C in Epimedium (Berberidaceae) species growing in Turkey. Nat. Prod. Commun. 2016, 11, 1665–1666. [Google Scholar] [CrossRef]
- Polat, D.Ç.; Eryılmaz, M.; Akalın, K.; Coşkun, M. Antimicrobial activity of grapefruit seed. Hacet. Univ. Eczacı. Fak. Derg. 2018, 38, 1–3. [Google Scholar]
- İlgün, S.; Karatoprak, G.Ş.; Polat, D.Ç.; Şafak, E.K.; Yıldız, G.; Küpeli Akkol, E.; Sobarzo-Sánchez, E. Phytochemical Composition and Biological Activities of Arctium minus (Hill) Bernh.: A Potential Candidate as Antioxidant, Enzyme Inhibitor, and Cytotoxic Agent. Antioxidants 2022, 11, 1852. [Google Scholar] [CrossRef]
- Takatsuka, M.; Goto, S.; Kobayashi, K.; Otsuka, Y.; Shimada, Y. Evaluation of pure antioxidative capacity of antioxidants: ESR spectroscopy of stable radicals by DPPH and ABTS assays with singular value decomposition. Food Biosci. 2022, 48, 101714. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Assessment of the antioxidant potential and fatty acid composition of four Centaurea L. taxa from Turkey. Food Chem. 2013, 141, 91–97. [Google Scholar] [CrossRef]
- Hayet, E.; Maha, M.; Samia, A.; Ali, M.M.; Souhir, B.; Abderaouf, K.; Mighri, Z.; Mahjoub, A. Antibacterial, antioxidant and cytotoxic activities of extracts of Conyza canadensis (L.) Cronquist growing in Tunisia. Med. Chem. Res. 2009, 18, 447–454. [Google Scholar] [CrossRef]
- Şeker Karatoprak, G.; Yücel Aşık, Ç.; Çakır, A.; Köngül Şafak, E. In vitro pharmacological screening of antioxidant, cytotoxic and enzyme inhibitory activities of Citrus aurantifolia Linn. Dried fruit extract. Int. J. Environ. Health Res. 2021, 31, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Egawa, S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018, 25, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2018, 7, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Molnár, J.; Forgo, P.; Vasas, A.; Kele, Z.; Hohmann, J. Antiproliferative constituents of the roots of Conyza canadensis. Planta Med. 2011, 77, 1183–1188. [Google Scholar] [CrossRef]
- Choi, D.H.; Hwang, H.S. Anti-inflammation activity of brazilin in TNF-α induced human psoriasis dermatitis skin model. Appl. Biol. Chem. 2019, 62, 1–9. [Google Scholar] [CrossRef]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef]
- Todorović-Raković, N. The role of cytokines in the evolution of cancer: IFN-γ paradigm. Cytokine 2022, 151, 155442. [Google Scholar] [CrossRef]
- Christodoulou, C.; Choy, E.H. Joint inflammation and cytokine inhibition in rheumatoid arthritis. Clin. Exp. Med. 2006, 6, 13–19. [Google Scholar] [CrossRef]
- Caramori, G.; Adcock, I.M.; Di Stefano, A.; Chung, K.F. Cytokine inhibition in the treatment of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 397. [Google Scholar] [CrossRef]
- Acıkara, Ö.B.; Ilhan, M.; Kurtul, E.; Šmejkal, K.; Akkol, E.K. Inhibitory activity of Podospermum canum and its active components on collagenase, elastase, and hyaluronidase enzymes. Bioorg. Chem. 2019, 93, 103330. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free-radical scavenging action of medicinal herbs from Ghana, Thonningia sanguinea on experimentally ınduced liver ınjuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Koşar, M.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and phenolic composition of Salvia virgata Jacq. From Turkey. J. Agric. Food Chem. 2008, 56, 2369–2374. [Google Scholar] [CrossRef]
- Şeker Karatoprak, G.; Yücel, Ç.; Kaytan, H.Ç.; İlgün, S.; Köngül Şafak, E.; Koşar, M. Antioxidant and Cytotoxic Activities of Aerial and Underground Parts of Hypericum scabrum L. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 2107–2113. [Google Scholar] [CrossRef]
- Şeker Karatoprak, G.; İlgün, S.; Koşar, M. Phenolic Composition, Anti-Inflammatory, Antioxidant, and Antimicrobial Activities of Alchemilla mollis (Buser) Rothm. Chem. Biodivers. 2017, 14, e1700150. [Google Scholar] [CrossRef]

| Methanol Extract of Centaurea pichleri subsp. pichleri | Methanol Extract of Conyza canadensis | Methanol Extract of Jasminum fruticans | |
|---|---|---|---|
| Total Phenol (mgGAE/gextract) | 98.19 ± 1.64 | 71.34 ± 0.53 | 97.41 ± 0.92 |
| Total Flavonoid (mgCA/gextract) | 21.85 ± 0.64 | 18.91 ± 1.46 | 19.45 ± 0.84 |
| Chlorogenic acid (% ± SD **) | 2.202 ± 0.014 | 1.110 ± 0.011 | ND * |
| p-Coumaric acid (% ± SD **) | ND * | ND * | 0.061 ± 0.007 |
| Ferulic acid (% ± SD **) | ND * | ND * | 0.077 ± 0.005 |
| Gallic acid (% ± SD **) | 0.031 ± 0.002 | 0.249 ± 0.101 | 0.271 ± 0.054 |
| Hyperoside (% ± SD **) | ND * | ND * | ND * |
| Rutin (% ± SD **) | 0.049 ± 0.002 | ND * | 0.949 ± 0.008 |
| Calibration Range (μg/mL) | Linear Equation | Correlation Factor (r2 ± SD *) | LOD (μg/mL) | LOQ (μg/mL) | |
|---|---|---|---|---|---|
| Chlorogenic acid | 25–250 | y = 7.2394 x − 167.78 | 0.993 ± 0.002 | 0.006 | 0.020 |
| p-Coumaric acid | 50–500 | y = 21.376 x + 206.94 | 0.999 ± 0.0005 | 0.0008 | 0.002 |
| Ferulic acid | 50–500 | y = 18.588 x + 5.3289 | 0.992 ± 0.001 | 0.0003 | 0.001 |
| Gallic acid | 50–500 | y = 44.783 x + 109.09 | 0.998 ± 0.001 | 0.00003 | 0.0001 |
| Hyperoside | 50–500 | y = 34.63 x + 636.51 | 0.992 ± 0.002 | 0.00005 | 0.0001 |
| Rutin | 50–500 | y = 58.596 x − 61.545 | 0.9952 ± 0.002 | 0.00002 | 0.00007 |
| Amount (μg/mL) | Intra-Day Precision (RSD * %) | Inter-Day Precision (RSD * %) | |
|---|---|---|---|
| Chlorogenic acid | 50 200 500 | 0.704 0.616 0.046 | 0.295 0.006 0.651 |
| p-Coumaric acid | 50 200 500 | 1.367 0.069 0.142 | 0.311 1.203 0.651 |
| Ferulic acid | 50 200 500 | 0.570 0.046 0.094 | 0.132 0.532 0.321 |
| Gallic acid | 25 100 250 | 0.733 2.710 2.149 | 2.007 3.189 2.493 |
| Hyperoside | 50 200 500 | 1.559 0.807 0.208 | 3.137 1.068 0.452 |
| Rutin | 50 200 500 | 0.076 0.622 0.011 | 0.352 0.878 0.453 |
| Standards | Concentration in Sample (mg/mL) | Amount Spiked (mg/mL) | Mean Amount Found in the Mixture (mg/mL) | Mean Recovery (% ± SD *) | RSD ** (%) |
|---|---|---|---|---|---|
| Chlorogenic acid | 0.08 | 0.04 0.08 0.16 | 0.06 0.08 0.12 | 103.771 ± 0.719 103.224 ± 1.055 98.243 ± 2.726 | 1.753 1.159 2.774 |
| p-Coumaric acid | 0.002 | 0.001 0.002 0.004 | 0.0015 0.002 0.003 | 104.073 ± 2.039 101.827 ± 2.219 101.437 ± 2.657 | 1.959 2.179 2.619 |
| Ferulic acid | 0.003 | 0.0015 0.003 0.006 | 0.00375 0.003 0.0045 | 102.858 ± 2.357 102.396 ± 1.797 102.446 ± 2.448 | 2.292 1.755 2.389 |
| Gallic acid | 0.01 | 0.005 0.01 0.02 | 0.0075 0.01 0.015 | 97.996 ± 2.301 96.539 ± 1.280 98.900 ± 2.254 | 2.348 1.326 2.279 |
| Rutin | 0.03 | 0.015 0.03 0.06 | 0.0225 0.03 0.045 | 103.019 ± 1.806 102.549 ± 1.189 102.946 ± 1.906 | 1.753 1.159 1.852 |
| Centaurea pichleri subsp. pichleri | Conyza canadensis | Jasminum fruticans | BHT | |||||
|---|---|---|---|---|---|---|---|---|
| Conc. (mg/mL) | DPPH Inhibition (%) | ABTS TEAC mmol/L/Trolox | DPPH Inhibition (%) | ABTS TEAC mmol/L/Trolox | DPPH Inhibition (%) | ABTS TEAC mmol/L/Trolox | DPPH Inhibition (%) | ABTS TEAC mmol/L/Trolox |
| 4 | 77.11 ± 0.82 a | 2.56 ± 0.04 * | 77.44 ± 0.24 a | 2.56 ± 0.005 * | 73.18 ± 0.15 a,b | 2.57 ± 0.03 * | 84.19 ± 1.27 a | 2.58 ± 0.01 * |
| 2 | 73.42 ± 0.54 a,b | 2.55 ± 0.0 *5 | 76.43 ± 3.92 a,b | 2.56 ± 0.03 * | 53.67 ± 0.99 b | 2.56 ± 0.003 * | 84.01 ± 0.8 a | 2.57 ± 0.03 * |
| 1 | 58.37 ± 2.90 b | 2.49 ± 0.0 *3 | 43.12 ± 1.87 c | 2.26 ± 0.13 ** | 26.40 ± 2.18 d | 2.35 ± 0.15 ** | 83.92 ± 2.14 a | 2.56 ± 0.12 * |
| 0.5 | 29.10 ± 3.33 d | 1.66 ± 0.06 *** | 20.36 ± 0.29 d,e | 1.44 ± 0.07 *** | 11.72 ± 0.45 e | 1.59 ± 0.11 *** | 82.17 ± 4.13 a | 2.56 ± 0.08 * |
| TNF-α (pg/mL) | PGE2 (pg/mL) | IFNƔ (pg/mL) | NO (µM) | |||||
|---|---|---|---|---|---|---|---|---|
| 31.25 µg/mL | 62.5 µg/mL | 31.25 µg/mL | 62.5 µg/mL | 31.25 µg/mL | 62.5 µg/mL | 31.25 µg/mL | 62.5 µg/mL | |
| Centaurea pichleri subsp. pichleri | 2009.12 ± 18.96 *** | 1953.57 ± 21.48 ** | 1922.73 ± 6.48 ** | 1854.17 ± 9.47 ** | 112.41 ± 9.14 *** | 99.1 ± 3.65 ** | 47.81 ± 7.15 ** | 38.48 ± 3.62 ** |
| Conyza canadensis | 2451.29 ± 18.19 | 2312.42 ± 25.53 | 2315.54 ± 21.47 | 2259.89 ± 17.82 | 151.24 ± 3.75 | 132.14 ± 5.58 | 75.47 ± 8.14 | 67.64 ± 5.12 |
| Jasminum fruticans | 2217.191 ± 11.58 *** | 2109.82 ± 9.56 *** | 2157.87 ± 25.89 *** | 2121.62 ± 22.49 *** | 126.45 ± 7.59 | 115.63 ± 8.74 *** | 61.91 ± 4.18 *** | 55.72 ± 2.41 *** |
| Control | 986.4 ± 8.94 * | 1017.16 ± 18.79 * | 48.51 ± 4.72 * | 9.11 ± 2.74 * | ||||
| LPS group | 2517.73 ± 6.55 | 2416.72 ± 9.57 | 157.78 ± 9.63 | 83.15 ± 4.17 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polat, D.Ç.; İlgün, S.; Karatoprak, G.Ş.; Akkol, E.K.; Capasso, R. Phytochemical Profiles, Antioxidant, Cytotoxic, and Anti-Inflammatory Activities of Traditional Medicinal Plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans. Molecules 2022, 27, 8249. https://doi.org/10.3390/molecules27238249
Polat DÇ, İlgün S, Karatoprak GŞ, Akkol EK, Capasso R. Phytochemical Profiles, Antioxidant, Cytotoxic, and Anti-Inflammatory Activities of Traditional Medicinal Plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans. Molecules. 2022; 27(23):8249. https://doi.org/10.3390/molecules27238249
Chicago/Turabian StylePolat, Derya Çiçek, Selen İlgün, Gökçe Şeker Karatoprak, Esra Küpeli Akkol, and Raffaele Capasso. 2022. "Phytochemical Profiles, Antioxidant, Cytotoxic, and Anti-Inflammatory Activities of Traditional Medicinal Plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans" Molecules 27, no. 23: 8249. https://doi.org/10.3390/molecules27238249
APA StylePolat, D. Ç., İlgün, S., Karatoprak, G. Ş., Akkol, E. K., & Capasso, R. (2022). Phytochemical Profiles, Antioxidant, Cytotoxic, and Anti-Inflammatory Activities of Traditional Medicinal Plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans. Molecules, 27(23), 8249. https://doi.org/10.3390/molecules27238249








