Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compounds 1–15
2.2. Antioxidant, Tyrosinase and Acetylcholinesterase Inhibitory Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction, Partition and Purification
3.4. Computational Section
3.5. ABTS Radical Scavenging Activity
3.6. DPPH Radical Scavenging Activity
3.7. PTIO Radical Scavenging Activity
3.8. Tyrosinase Inhibitory Activity
3.9. Acetylcholinesterase Inhibitory Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar]
- Xian, D.; Guo, M.; Xu, J.; Yang, Y.; Zhao, Y.; Zhong, J. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021, 26, 134–146. [Google Scholar]
- Ağagündüz, D. Determination of the total antioxidant and oxidant status of some galactagogue and herbal teas. Food Sci. Hum. Wellness 2020, 9, 377–382. [Google Scholar]
- Jiang, H.; Wu, F.; Jiang, X.; Pu, Y.F.; Shen, L.R.; Wu, C.Y.; Bai, H.J. Antioxidative, cytoprotective and whitening activities of fragrant pear fruits at different growth stages. Front. Nutr. 2022, 9, 1020855. [Google Scholar]
- Bayazid, A.B.; Jang, Y.A.; Jeong, S.A.; Lim, B.O. Cypress tree (Chamaecyparis obtusa) Bark extract inhibits melanogenesis through repressing CREB and MITF signalling pathways in α-MSH-stimulated B16F10 cells. Food Agric. Immunol. 2022, 33, 498–510. [Google Scholar]
- Li, C.; Fu, Y.; Dai, H.; Wang, Q.; Gao, R.; Zhang, Y. Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism. Food Sci. Hum. Wellness 2022, 11, 218–229. [Google Scholar]
- Zhang, F.; Li, S.; Liu, C.; Fang, K.; Jiang, Y.; Zhang, J.; Lan, J.; Zhu, L.; Pang, H.; Wang, G. Rapid screening for acetylcholinesterase inhibitors in Selaginella doederleinii Hieron by using functionalized magnetic Fe3O4 nanoparticles. Talanta 2022, 243, 123284. [Google Scholar]
- Sandupama, P.; Munasinghe, D.; Jayasinghe, M. Coconut oil as a therapeutic treatment for Alzheimer’s disease: A review. J. Future Foods 2022, 2, 41–52. [Google Scholar]
- Tuzimski, T.; Petruczynik, A. Determination of anti-Alzheimer’s disease activity of selected plant ingredients. Molecules 2022, 27, 3222. [Google Scholar]
- Ye, H.; Tao, X.; Zhang, W.; Chen, Y.; Yu, Q.; Xie, J. Food-derived bioactive peptides: Production, biological activities, opportunities and challenges. J. Future Foods 2022, 2, 294–306. [Google Scholar]
- Wei, J.; Wang, B.; Chen, Y.; Wang, Q.; Ahmed, A.F.; Cui, L.; Xi, X.; Kang, W. Effects of two triterpenoids from Nigella sativa seeds on insulin resistance of 3T3-L1 adipocytes. Front. Nutr. 2022, 9, 995550. [Google Scholar]
- Li, J.; Zhao, S.; Wang, M.; Wang, Y.; Ren, C. Germplasm resources and research progress of Cercis L. J. Northwest For. Univ. 2021, 36, 145–152. [Google Scholar]
- Zhang, J.J.; Zhou, L.; Cui, L.L.; Liu, Z.H.; Wei, J.F.; Kang, W.Y. Antioxidant and alpha-glucosidase inhibitiory activity of Cercis chinensis flowers. Food Sci. Hum. Wellness 2020, 9, 313–319. [Google Scholar]
- Wei, Q.; Gui, Q.; Qiu, Z.; Xu, F.; Ji, X. Microwave-assisted extraction and antioxidant activities in vitro of polysaccharides from Cercis chinensis Bunge flowers. Food Sci. 2015, 36, 39–44. [Google Scholar]
- Shi, M.; He, N.; Li, W.; Li, C.; Kang, W. Simultaneous determination of myricetrin, quercitrin and afzelin in leaves of Cercis chinensis by a fast and effective method of ionic liquid microextraction coupled with HPLC. Chem. Cent. J. 2018, 12, 23. [Google Scholar]
- Zhang, J.J.; Zhang, Y.; Kang, W.Y. Advances in research of chemical constituents and pharmacological activites of Cercis Linn. Chin. Pharm. J. 2014, 49, 1782–1784. [Google Scholar]
- He, N.; Wang, P.; Niu, Y.; Chen, J.; Li, C.; Kang, W. Evaluation antithrombotic activity and action mechanism of myricitrin. Ind. Crops Prod. 2019, 129, 536–541. [Google Scholar]
- Yin, Z.; Zhang, J.; Guo, Q.; Sun, K.; Chen, L.; Zhang, W.; Yang, B.; Kang, W. Two novel heteroglycan with coagulant activity from flowers of Cercis chinensis Bunge. J. Mol. Struct. 2021, 1243, 130756. [Google Scholar]
- Shu, P.; Li, Y.; Luo, Y.; Yu, M.; Fei, Y.; Liu, W.; Yang, Y.; Wei, X.; Zhang, Y.; Tu, T.; et al. Isolation, characterization and antioxidant, tyrosinase inhibitory activities of constituents from the flowers of Cercis glabra ‘Spring-1’. Rec. Nat. Prod. 2021, 15, 254–260. [Google Scholar]
- Shu, P.; Yang, Y.; Zhang, H.; Li, N.; Liu, G.; Zhang, J.; Zhao, Q.; Wei, X.; Yi, W.; Sun, N.; et al. Isolation and characterization of antioxidative monoterpenes from Cynanchum atratum roots. Biosci. Biotechnol. Biochem. 2022, 86, 585–589. [Google Scholar]
- Shu, P.; Fei, Y.; Li, Y.; Xu, T.; Lou, Y.; Yang, Y.; Zhang, H.; Li, N.; Wei, X.; Xiao, F.; et al. Isolation and characterization of bioactive phenolic compounds from Cinnamomum camphora barks. Holzforschung 2022, 76, 391–396. [Google Scholar]
- Liu, D.Y.; Shi, X.F.; Wang, D.D.; He, F.J.; Ma, Q.H.; Fan, B. Two new myricetin glycosides from pine needles of Cedrus deodara. Chem. Nat. Comp. 2011, 47, 704–707. [Google Scholar]
- Xu, J.; Li, X.; Zhang, P.; Li, Z.L.; Wang, Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch. Pharm. Res. 2005, 28, 395–399. [Google Scholar]
- Abdullah, N.H.; Salim, F.; Ahmad, R. Chemical constituents of malaysian U. cordata var. ferruginea and their in vitro α-glucosidase inhibitory activities. Molecules 2016, 21, 525. [Google Scholar]
- Shen, G.; Oh, S.R.; Min, B.S.; Lee, J.; Ahn, K.S.; Kim, Y.H.; Lee, H.K. Phytochemical investigation of Tiarella polyphylla. Arch. Pharm. Res. 2008, 31, 10–16. [Google Scholar]
- Abdullaeva, R.K.; Bobakulov, K.M.; Nishanbaev, S.Z.; Beshko, N.Y.; Sham’yanov, I.D.; Abdullaev, N.D. Secondary metabolites from the aerial part of Mausolea eriocarpa. Chem. Nat. Compd. 2016, 52, 913–914. [Google Scholar]
- Li, M.; Xia, Z.; Li, B.; Tian, Y.; Zhang, G.; Xu, C.; Dong, J. Chemical constituents from Ginkgo biloba L. male flowers and their biological activities. Med. Chem. Res. 2019, 28, 1557–1566. [Google Scholar]
- Gedara, S.R.; Galala, A.A. New cytotoxic spirostane saponin and biflavonoid glycoside from the leaves of Acacia saligna (Labill.) H.L. Wendl. Nat. Prod. Res. 2014, 28, 324–329. [Google Scholar]
- Allaoua, Z.; Benkhaled, M.; Dibi, A.; Long, C.; Aberkane, M.C.; Bouzidi, S.; Kassah-Laouar, A.; Haba, H. Chemical composition, antioxidant and antibacterial properties of Pteranthus dichotomus from Algerian Sahara. Nat. Prod. Res. 2016, 30, 700–704. [Google Scholar]
- Gao, L.; Xu, X.; Yang, J. Chemical constituents of the roots of Rheum officinale. Chem. Nat. Compd. 2013, 49, 603–605. [Google Scholar]
- Kumboonma, P.; Senawong, T.; Saenglee, S.; Yenjai, C.; Phaosiri, C. New histone deacetylase inhibitors from the twigs of Melanorrhoea usitata. Med. Chem. Res. 2018, 27, 2004–2015. [Google Scholar]
- Matsuura, H.; Yoshihara, T.; Ichihara, A.; Kikuta, Y.; Koda, Y. Tuber-forming substances in jerusalem artichoke (Helianthus tuberosus L.). Biosci. Biotechnol. Biochem. 1993, 57, 1253–1256. [Google Scholar]
- Shrestha, S.; Natarajan, S.; Park, J.H.; Lee, D.Y.; Cho, J.G.; Kim, G.S.; Jeon, Y.J.; Yeon, S.W.; Yang, D.C.; Baek, N.I. Potential neuroprotective flavonoid-based inhibitors of CDK5/p25 from Rhus parviflora. Bioorg. Med. Chem. Lett 2013, 23, 5150–5154. [Google Scholar]
- Thu, N.T.; The Hung, N.; Thuy An, N.T.; Vinh, L.B.; Binh, B.T.; Thu, N.T.B.; Khoi, N.M.; Ha, D.T. Four new phenolic compounds from the fruit of Cornus officinalis (Cornaceae) and their anti-inflammatory activity in RAW 264.7 cells. Nat. Prod. Res. 2021, 36, 3806–3812. [Google Scholar]
- Xie, Y.; Li, X.; Xu, J.; Jiang, Q.; Xie, H.; He, J.; Chen, D. Two phenolic antioxidants in Suoyang enhance viability of •OH-damaged mesenchymal stem cells: Comparison and mechanistic chemistry. Chem. Cent. J. 2017, 11, 84. [Google Scholar]
- Elloumi, W.; Maalej, A.; Ortiz, S.; Michel, S.; Chamkha, M.; Boutefnouchet, S.; Sayadi, S. Pistacia lentiscus L. distilled leaves as a potential cosmeceutical ingredient: Phytochemical characterization, transdermal diffusion, and anti-elastase and anti-tyrosinase activities. Molecules 2022, 27, 855. [Google Scholar]
- Tang, H.C.; Chen, Y.C. Identification of tyrosinase inhibitors from traditional Chinese medicines for the management of hyperpigmentation. Springerplus 2015, 4, 184. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar]
- Vo, T.S.; Le, T.T.; Kim, S.Y.; Ngo, D.H. The role of myricetin from Rhodomyrtus tomentosa (Aiton) Hassk fruits on downregulation of FcɛRI-mediated mast cell activation. J. Food Biochem. 2020, 44, e13143. [Google Scholar]
- Wu, M.; Liu, M.; Wang, F.; Cai, J.; Luo, Q.; Li, S.; Zhu, J.; Tang, Z.; Fang, Z.; Wang, C.; et al. The inhibition mechanism of polyphenols from Phyllanthus emblica Linn. fruit on acetylcholinesterase: A interaction, kinetic, spectroscopic, and molecular simulation study. Food Res. Int. 2022, 158, 111497. [Google Scholar]
- Zhu, P.F.; Cheng, G.G.; Zhao, L.Q.; Khan, A.; Yang, X.W.; Zhang, B.Y.; Li, M.C.; Liu, Y.P.; Luo, X.D. Antioxidant and cytoprotective effects of new diarylheptanoids from Rhynchanthus beesianus. J. Agric. Food Chem. 2021, 69, 6229–6239. [Google Scholar]
- Liu, M.Y.; Zeng, F.; Shen, Y.; Wang, Y.Y.; Zhang, N.; Geng, F. Bioguided isolation and structure identification of acetylcholinesterase enzyme inhibitors from Drynariae rhizome. J. Anal. Methods Chem. 2020, 2020, 2971841. [Google Scholar]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar]
- Ja’afar Muhammad, K.; Jamil, S.; Basar, N.; Mohd Arriffin, N.; Tijjani Idris, M.; Jibril, S.; Temilola Akanji, F. Antioxidant, antimicrobial and antityrosinase activities of phytochemicals from the leaves of Globimetula braunii (Engler) Van Tiegh (Loranthaceae). Bull. Chem. Soc. Ethiop. 2022, 36, 387–397. [Google Scholar]
- Nguyen, T.T.H.; Nguyen, V.T.; Van Cuong, P.; Thanh, T.N.; Le Thi, T.A.; Huong, D.T.M.; Truong, B.N.; Litaudon, M.; The, S.N. A new flavonoid from the leaves of Garcinia mckeaniana Craib and α-glucosidase and acetylcholinesterase inhibitory activities. Nat. Prod. Res. 2022, 36, 5074–5080. [Google Scholar]
- Naksuriya, O.; Okonogi, S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discoveries Ther. 2015, 9, 136–141. [Google Scholar]
- Kim, Y.J. Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull. 2007, 30, 1052–1055. [Google Scholar]
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020, 327, 126984. [Google Scholar]
- Durmaz, G. Freeze-dried ABTS•+ method: A ready-to-use radical powder to assess antioxidant capacity of vegetable oils. Food Chem. 2012, 133, 1658–1663. [Google Scholar]
- Li, X. 2-Phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) radical scavenging: A new and simple antioxidant assay in vitro. J. Agric. Food Chem. 2017, 65, 6288–6297. [Google Scholar]
- Razak, D.L.A.; Fadzil, N.H.M.; Jamaluddin, A.; Rashid, N.Y.A.; Sani, N.A.; Manan, M.A. Effects of different extracting conditions on anti-tyrosinase and antioxidant activities of Schizophyllum commune fruit bodies. Biocatal. Agric. Biotechnol. 2019, 19, 101116. [Google Scholar]
- Kim, J.M.; Chang, S.M.; Kim, I.H.; Kim, Y.E.; Hwang, J.H.; Kim, K.S.; Kim, W.S. Design of optimal solvent for extraction of bio-active ingredients from mulberry leaves. Biochem. Eng. J. 2007, 37, 271–278. [Google Scholar]
- Wiemann, J.; Loesche, A.; Csuk, R. Novel dehydroabietylamine derivatives as potent inhibitors of acetylcholinesterase. Bioorg. Chem. 2017, 74, 145–157. [Google Scholar]
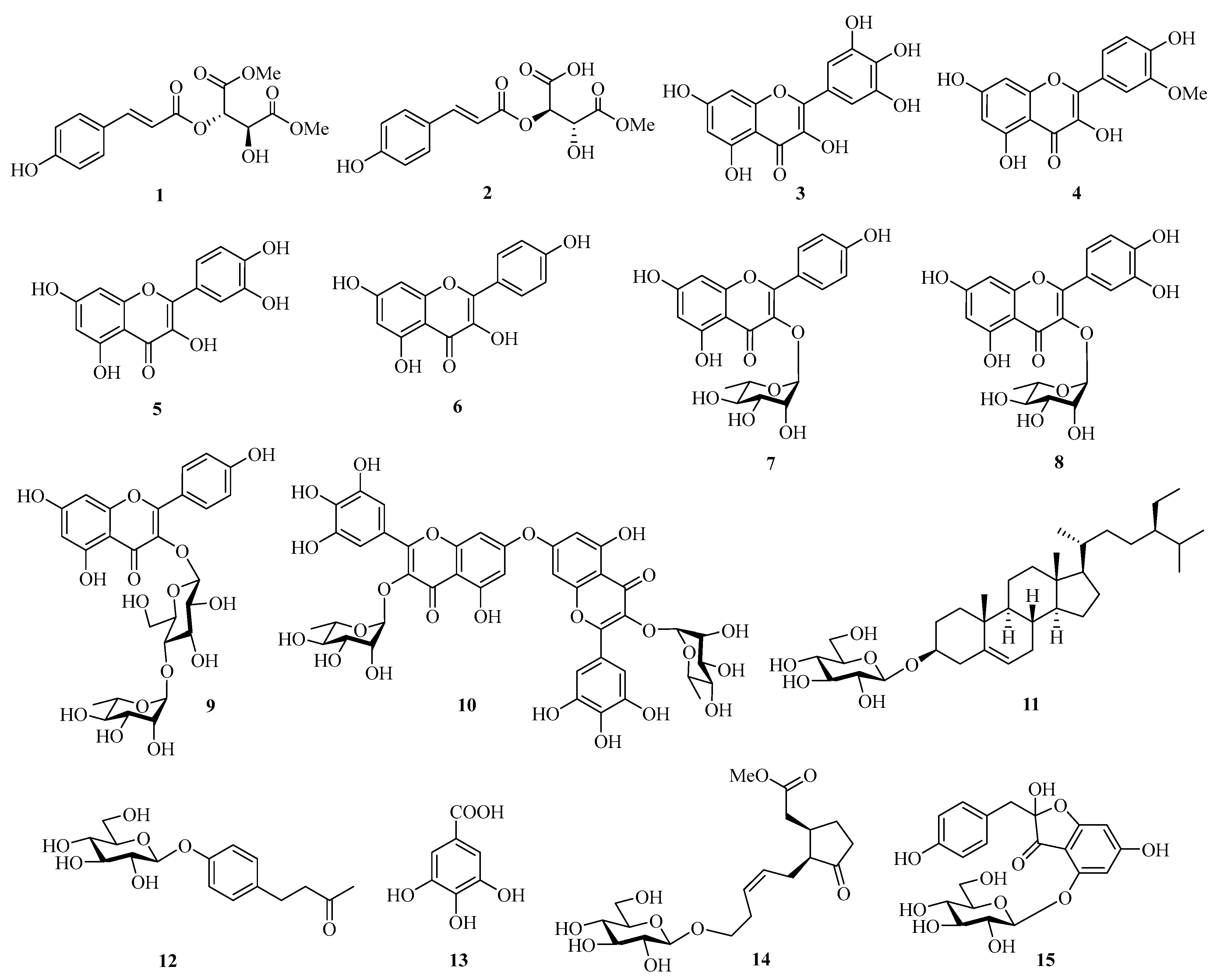


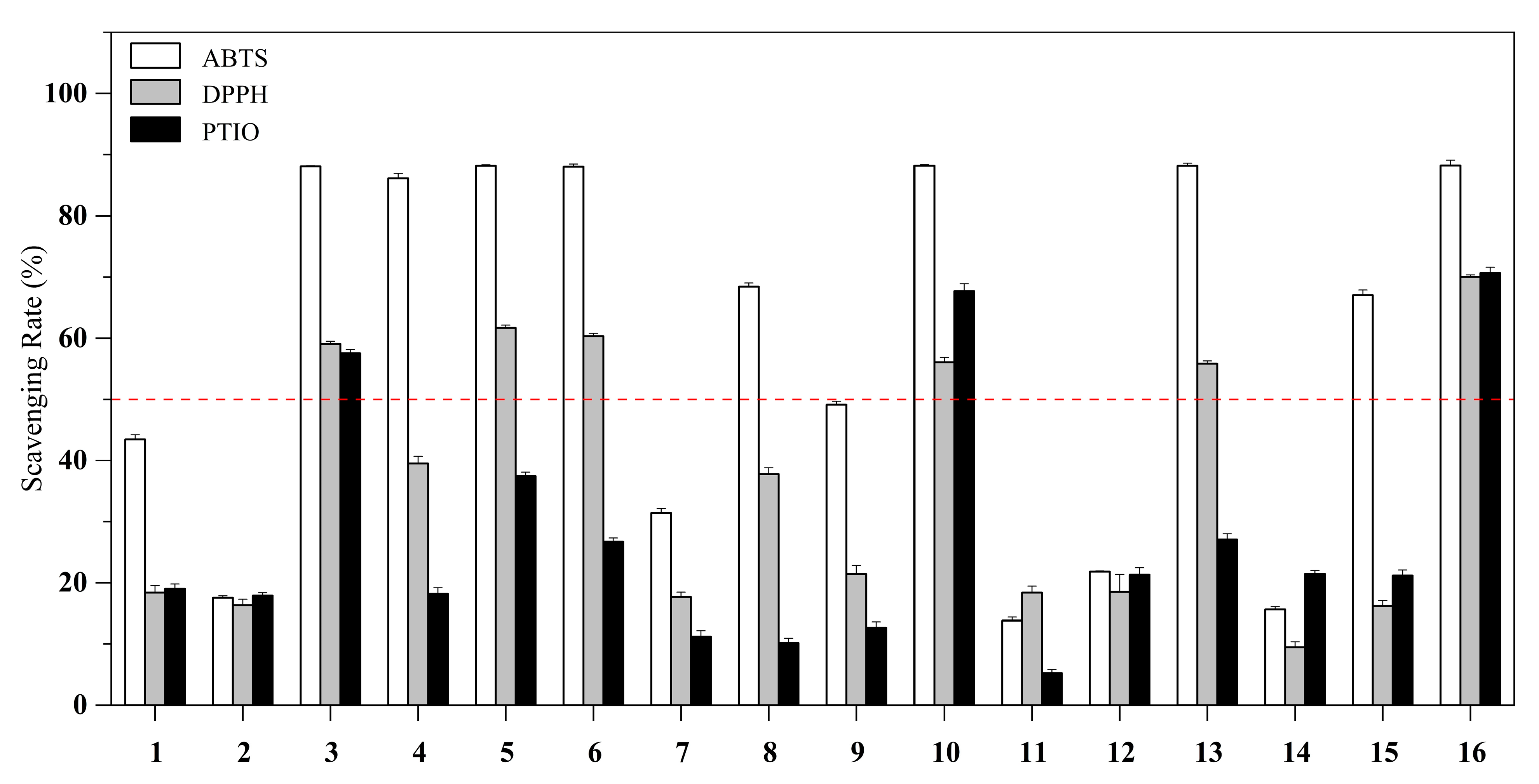

| Compound | ABTS Radical Scavenging Activity (%) [a] | IC50 (μM) | DPPH Radical Scavenging Activity (%) [a] | IC50 (mM) | PTIO Radical Scavenging Activity (%) [a] | IC50 (mM) |
|---|---|---|---|---|---|---|
| 1 | 43.44 ± 0.75 | >500 | 18.38 ± 1.16 | >100 | 19.01 ± 0.78 | >100 |
| 2 | 17.57 ± 0.32 | >500 | 16.35 ± 0.99 | >100 | 17.93 ± 0.44 | >100 |
| 3 | 88.10 ± 0.10 | 94 | 59.08 ± 0.43 | 0.87 | 57.55 ± 0.62 | 2.56 |
| 4 | 86.14 ± 0.81 | >500 | 39.46 ± 1.25 | 6.25 | 18.18 ± 0.99 | 44.27 |
| 5 | 88.19 ± 0.16 | 97 | 61.67 ± 0.46 | 0.59 | 37.43 ± 0.65 | 6.36 |
| 6 | 88.04 ± 0.43 | 108 | 60.32 ± 0.47 | 0.59 | 26.71 ± 0.61 | 14.74 |
| 7 | 31.43 ± 0.73 | >500 | 17.70 ± 0.77 | >100 | 11.17 ± 0.97 | >100 |
| 8 | 68.42 ± 0.60 | >500 | 37.77 ± 1.03 | 6.56 | 10.13 ± 0.77 | >100 |
| 9 | 49.15 ± 0.56 | >500 | 21.42 ± 1.43 | >100 | 12.63 ± 1.03 | 27.64 |
| 10 | 88.20 ± 0.16 | 45 | 56.03 ± 0.79 | 0.56 | 67.73 ± 1.17 | 0.53 |
| 11 | 13.86 ± 0.57 | >500 | 18.38 ± 1.06 | >100 | 5.27 ± 0.56 | >100 |
| 12 | 21.82 ± 0.10 | >500 | 18.49 ± 2.86 | 34.87 | 21.31 ± 1.19 | >100 |
| 13 | 88.19 ± 0.43 | 179 | 55.80 ± 0.44 | 0.77 | 27.07 ± 0.93 | >100 |
| 14 | 15.67 ± 0.44 | >500 | 9.47 ± 0.86 | >100 | 21.44 ± 0.55 | >100 |
| 15 | 67.06 ± 0.85 | >500 | 16.23 ± 0.89 | >100 | 21.17 ± 0.92 | >100 |
| l-ascorbic acid | 88.24 ± 0.83 | 156 | 70.01 ± 0.34 | 0.44 | 70.65 ± 0.94 | 1.61 |
| Compound | Tyrosinase Inhibition (%) [a] | IC50 (mM) | Compound | Acetylcholinesterase Inhibition (%) [a] | IC50 (μM) |
|---|---|---|---|---|---|
| 1 | 53.13 ± 0.65 | 2.63 | 1 | 38.43 ± 0.20 | >1000 |
| 2 | 53.54 ± 0.90 | 2.59 | 2 | 39.46 ± 0.25 | >1000 |
| 3 | 60.00 ± 1.49 | 1.39 | 3 | 85.27 ± 0.06 | 276 |
| 4 | 44.17 ± 0.85 | 4.69 | 4 | 68.13 ± 0.24 | 664 |
| 5 | 77.29 ± 1.52 | 0.64 | 5 | 83.65 ± 0.48 | 345 |
| 6 | 57.50 ± 0.82 | 1.69 | 6 | 82.21 ± 0.09 | 377 |
| 7 | 32.71 ± 1.51 | 9.24 | 7 | 41.69 ± 0.48 | >1000 |
| 8 | 39.17 ± 0.86 | 4.96 | 8 | 40.11 ± 0.31 | >1000 |
| 9 | 23.54 ± 1.04 | NA | 9 | 39.90 ± 0.33 | >1000 |
| 10 | 66.04 ± 0.62 | 0.65 | 10 | 61.30 ± 0.38 | 374 |
| 11 | 29.79 ± 0.88 | 8.84 | 11 | 40.83 ± 0.41 | >1000 |
| 12 | 35.63 ± 1.01 | 7.47 | 12 | 39.84 ± 0.53 | >1000 |
| 13 | 80.00 ± 0.78 | 0.59 | 13 | 71.74 ± 0.40 | >1000 |
| 14 | 37.71 ± 0.56 | NA | 14 | 47.18 ± 0.16 | >1000 |
| 15 | 42.08 ± 0.54 | 4.04 | 15 | 39.91 ± 0.36 | NA |
| kojic acid | 84.38 ± 0.31 | 0.63 | donepezil | 91.17 ± 0.23 | 3.3 |
| Position | 1 | 2 | Ceroffester D [34] | |||
|---|---|---|---|---|---|---|
| δC | δH (mult, J in Hz) | δC | δH (mult, J in Hz) | δC | δH (mult, J in Hz) | |
| 1 | 127.1 | 127.0 | 127.0 | |||
| 2 | 131.6 | 7.48 (d, 8.0) | 131.4 | 7.46 (d, 8.4) | 131.6 | 7.49 (d, 8.7) |
| 3 | 117.0 | 6.82 (d, 8.0) | 116.9 | 6.82 (d, 8.4) | 117.0 | 6.85 (d, 8.7) |
| 4 | 161.7 | 161.4 | 161.5 | |||
| 5 | 117.0 | 6.82 (d, 8.0) | 116.9 | 6.82 (d, 8.4) | 117.0 | 6.85 (d, 8.7) |
| 6 | 131.6 | 7.48 (d, 8.0) | 131.4 | 7.46 (d, 8.4) | 131.6 | 7.49 (d, 8.7) |
| 7 | 148.3 | 7.73 (d, 16.0) | 148.0 | 7.72 (d, 16.0) | 148.2 | 7.73 (d, 15.9) |
| 8 | 113.8 | 6.36 (d, 16.0) | 113.9 | 6.36 (d, 16.0) | 113.9 | 6.42 (d, 15.9) |
| 9 | 167.8 | 167.9 | 167.9 | |||
| 1′ | 169.3 | 170.4 | 169.1 | |||
| 2′ | 74.8 | 5.57 (d, 2.4) | 74.6 | 5.57 (d, 2.4) | 75.3 | 5.62 (d, 2.9) |
| 3′ | 72.0 | 4.81 (d, 2.4) | 71.9 | 4.86 (d, 2.4) | 72.1 | 4.77 (d, 2.9) |
| 4′ | 172.4 | 172.6 | 172.2 | |||
| 1′-OCH3 | 53.3 | 3.79 (s) | 53.2 | 3.77 (s) | ||
| 4′-OCH3 | 53.2 | 3.74 (s) | 53.2 | 3.74(s) | 53.2 | 3.82 (s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lou, Y.; Xu, T.; Cao, H.; Zhao, Q.; Zhang, P.; Shu, P. Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves. Molecules 2022, 27, 8667. https://doi.org/10.3390/molecules27248667
Lou Y, Xu T, Cao H, Zhao Q, Zhang P, Shu P. Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves. Molecules. 2022; 27(24):8667. https://doi.org/10.3390/molecules27248667
Chicago/Turabian StyleLou, Yueyue, Ting Xu, Huaqiang Cao, Qiuyue Zhao, Pengpai Zhang, and Penghua Shu. 2022. "Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves" Molecules 27, no. 24: 8667. https://doi.org/10.3390/molecules27248667
APA StyleLou, Y., Xu, T., Cao, H., Zhao, Q., Zhang, P., & Shu, P. (2022). Natural Antioxidants, Tyrosinase and Acetylcholinesterase Inhibitors from Cercis glabra Leaves. Molecules, 27(24), 8667. https://doi.org/10.3390/molecules27248667




