Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis
Abstract
1. Introduction
2. Results and Discussion
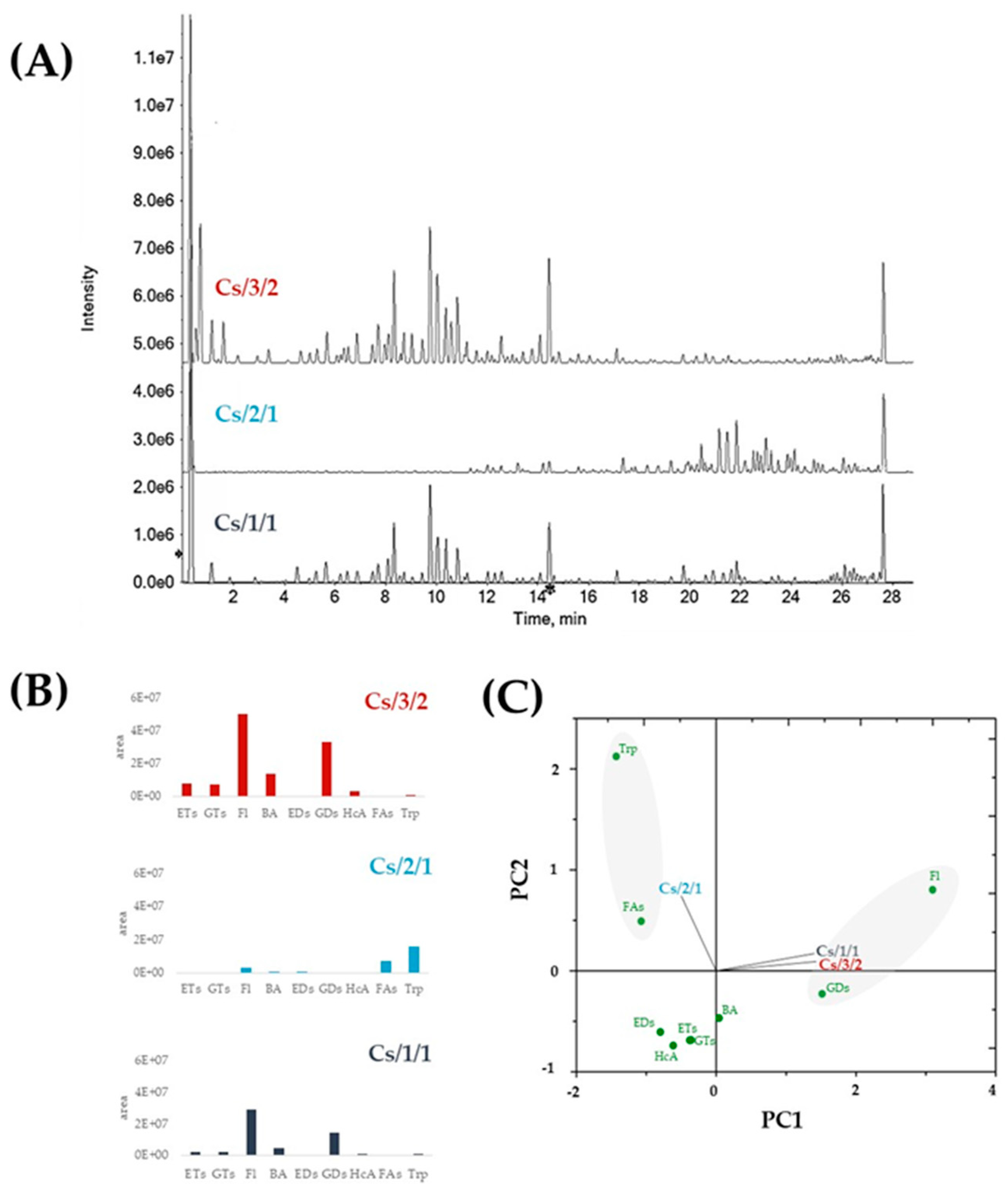
2.1. Chemical Investigation of Alcoholic Leaf Extract and Cs/2/1 and Cs/3/2 Fractions
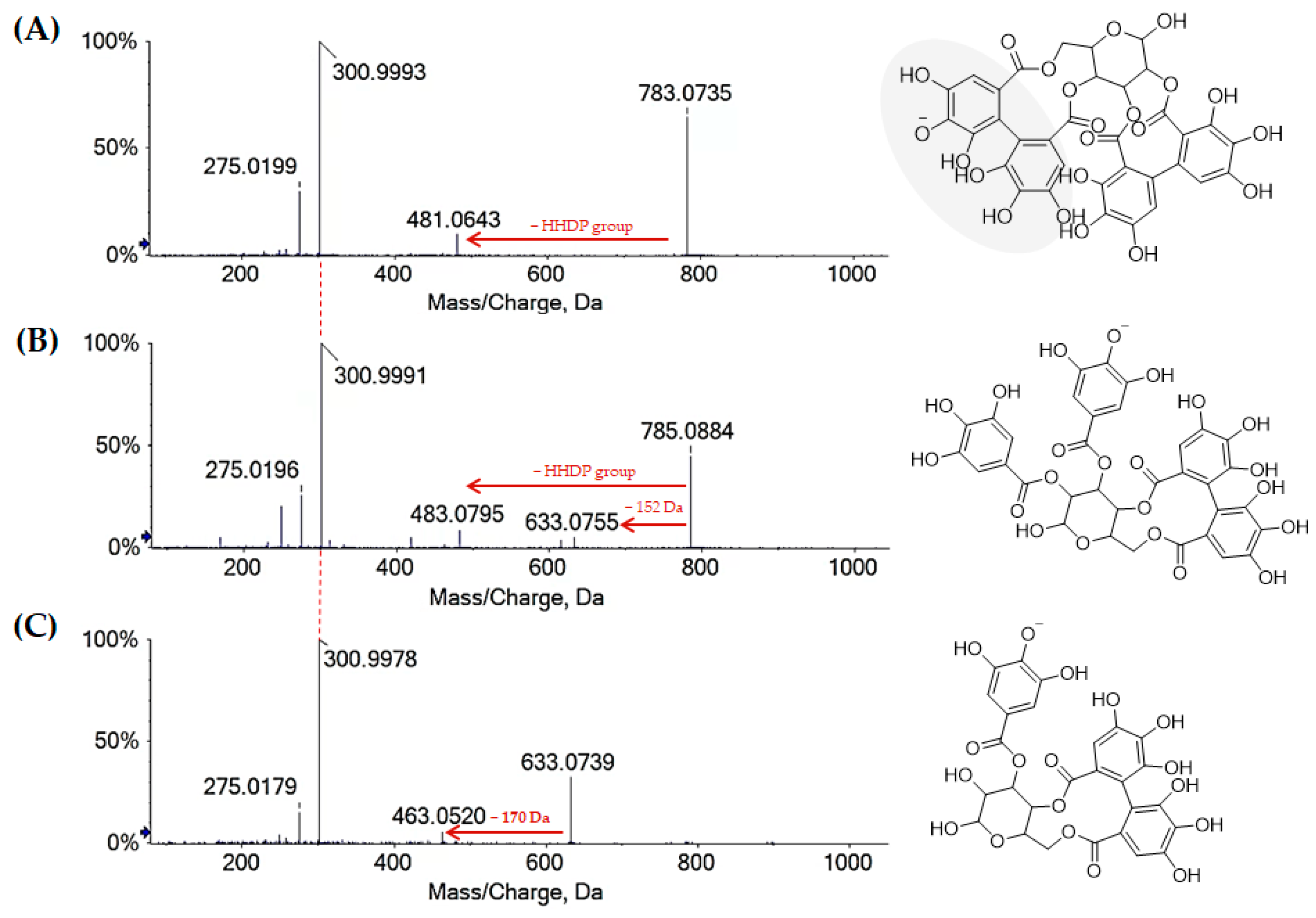
2.1.1. Chestnut Leaf Tannins and Beyond
2.1.2. Chestnut Leaf Flavonoids
2.1.3. Chestnut Leaf Lipids
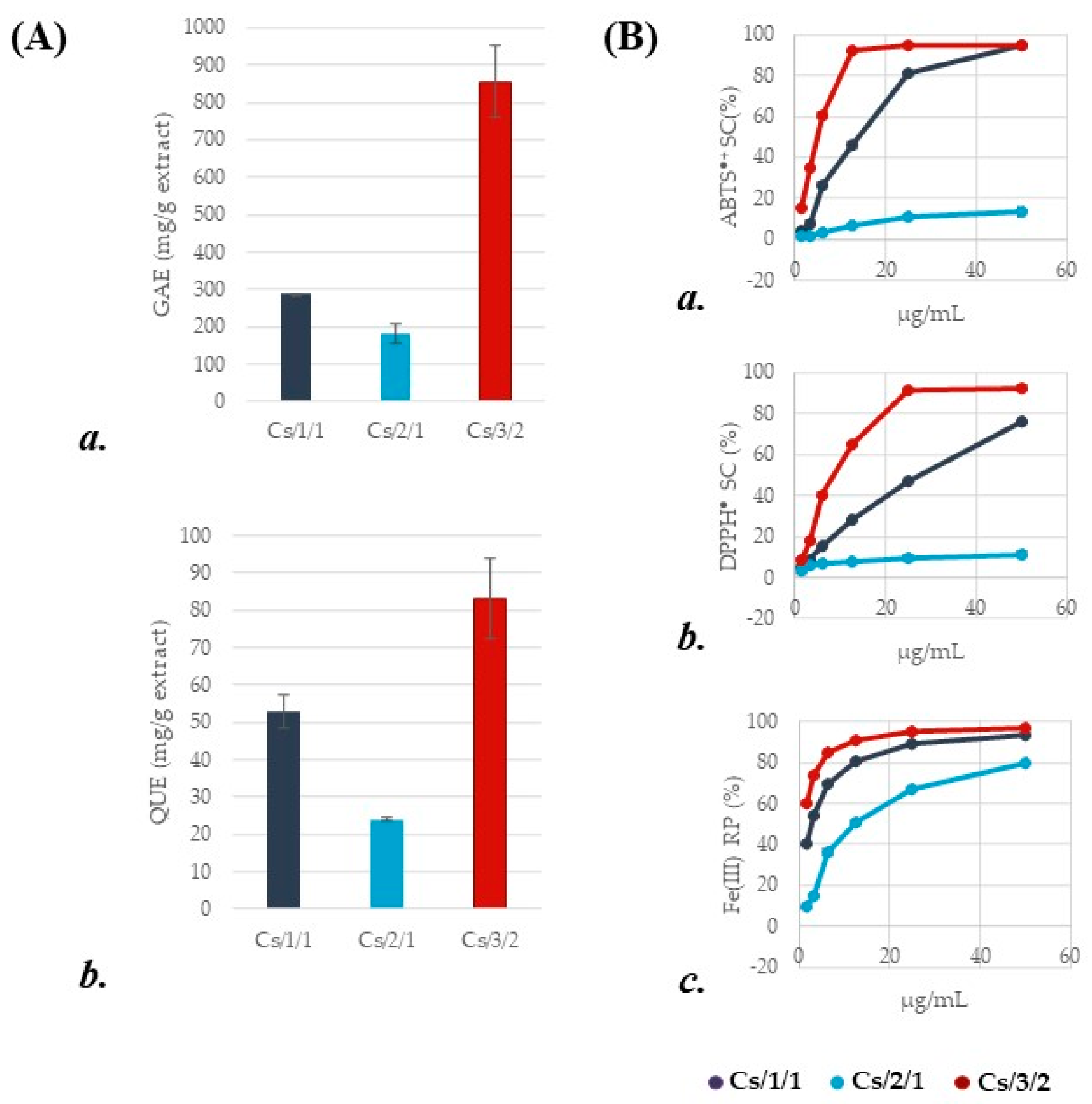
2.2. Effects of Chestnut Leaf Alcoholic Extract and Its Fractions on In Vitro Rumen Fermentative Activity
3. Material and Methods
3.1. Plant Collection, Fractionation, and Evaluation of Leaf Chemical Composition
3.2. UHPLC-HRMS and MS/MS Parameters and UV-Vis Analyses
3.3. Antioxidant Assessment
3.3.1. Determination of DPPH (2,2′-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Capacity
3.3.2. Determination of ABTS [2,2′-Azinobis-(3-Ethylbenzothiazolin-6-Sulfonic Acid)] Radical Cation Scavenging Capacity
3.3.3. Determination of Potassium Ferricyanide Reducing Power (PFRAP)
3.4. Determination of Total Phenolic Content
3.5. Determination of Total Flavonoid Content
3.6. In Vitro Fermentation
3.7. Fermentation End-Product Assessment
3.8. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 4 September 2022).
- Demirkesen, I.; Mert, B.; Sumnu, G.; Sahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010, 101, 329–336. [Google Scholar] [CrossRef]
- Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2014, 29, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. J.Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; de la Luz Cádiz-Gurrea, M.; de Freitas, V.; Costa, P.C.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: Is cosmetic industry a prominent opportunity to valorize this agro-waste? Biol. Life Sci. Forum 2021, 6, 24. [Google Scholar]
- Almeida, I.F.; Valentão, P.; Andrade, P.B.; Seabra, R.M.; Pereira, T.M.; Amaral, M.H.; Paulo, C.C.; Bahia, M.F. In vivo skin irritation potential of a Castanea sativa (Chestnut) leaf extract, a putative natural antioxidant for topical application. Basic Clin. Pharmacol. Toxicol. 2008, 103, 461–467. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-Assisted Extraction as a Green Technology Approach to Recover Polyphenols from Castanea sativa Shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal treatment of chestnut shells (Castanea sativa) to produce oligosaccharides and antioxidant compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef]
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P.P. Promising new applications of Castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin E profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2017, 32, 1022–1032. [Google Scholar] [CrossRef]
- Śliwiński, B.J.; Soliva, C.R.; Machmüller, A.; Kreuzer, M. Efficacy of plant extracts rich in secondary constituents to modify rumen fermentation. Anim. Feed Scie. Tech. 2002, 101, 101–114. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, S.; Li, Y.; Zhang, S.; Qi, X.; Guo, K.; Guo, Y.; Fortina, R. Pilot Study of the effects of polyphenols from chestnut involucre on methane production, volatile fatty acids, and ammonia concentration during in vitro rumen fermentation. Animals 2021, 11, 108. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Dietary phytochemicals as rumen modifiers: A review of the effects on microbial populations. Antonie Van Leeuwenhoek 2009, 96, 363–375. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Pacifico, S. UHPLC-ESI-QqTOF analysis and in vitro rumen fermentation for exploiting Fagus sylvatica leaf in ruminant diet. Molecules 2022, 27, 2217. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Buccioni, A.; Cappucci, A.; Mele, M. Methane Emission from enteric fermentation: Methanogenesis and fermentation. In Climate Change Impact on Livestock: Adaptation and Mitigation; Sejian, V., Gaughan, J., Baumgard, L., Prasad, C., Eds.; Springer: New Delhi, India, 2015; pp. 171–186. [Google Scholar]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.N.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol Ecol. 2018, 94, 61. [Google Scholar]
- Mannelli, F.; Daghio, M.; Alves, S.P.; Bessa, R.J.B.; Minieri, S.; Giovannetti, L.; Conte, G.; Mele, M.; Messini, A.; Rapaccini, A.; et al. Effects of chestnut tannin extract, vescalagin and gallic acid on the dimethyl acetals profile and microbial community composition in rumen liquor: An in vitro study. Microorganisms 2019, 7, 202. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Fitri, A.; Suwignyo, B.; Elfahmi; Hidayatik, N.; Kumalasari, N.R.; Irawan, A.; Jayanegara, A. The utilisation of tannin extract as a dietary additive in ruminant nutrition: A meta-analysis. Animals 2021, 11, 3317. [Google Scholar] [CrossRef]
- Sariyildiz, T.; Anderson, J.M. Variation in the chemical composition of green leaves and leaf litters from three deciduous tree species growing on different soil types. For. Ecol. Manag. 2005, 210, 303–319. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and in vitro preliminary anti-inflammatory activity of Castanea sativa (Italian Cultivar “Marrone di Roccadaspide” PGI) burs, leaves, and chestnuts extracts and their metabolite profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable tannins from sweet chestnut fractions obtained by a sustainable and eco-friendly industrial process. Nat. Prod. Commun. 2016, 11, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Galvão, M.A.M.; de Arruda, A.O.; Bezerra, I.C.F.; Ferreira, M.R.A.; Soares, L.A.L. Evaluation of the Folin-Ciocalteu Method and Quantification of Total Tannins in Stem Barks and Pods from Libidibia ferrea (Mart. ex Tul) L. P. Queiroz. Braz. Arch. Biol. Technol. 2018, 61, 586. [Google Scholar] [CrossRef]
- Woo, Y.; Oh, J.; Kim, J.-S. Suppression of Nrf2 Activity by Chestnut Leaf Extract Increases Chemosensitivity of Breast Cancer Stem Cells to Paclitaxel. Nutrients 2017, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Schink, A.; Neumann, J.; Leifke, A.L.; Ziegler, K.; Fröhlich-Nowoisky, J.; Cremer, C.; Eckhard, T.; Weber, B.; Poshl, U.; Schuppan, D.; et al. Screening of herbal extracts for TLR2- and TLR4-dependent anti-inflammatory effects. PLoS ONE 2018, 13, e0203907. [Google Scholar] [CrossRef] [PubMed]
- Barreira, J.; Ferreira, I.; Oliveira, M.; Pereira, J. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant Potential of Chestnut (Castanea sativa L.) and Almond (Prunus dulcis L.) By-products. Int. J. Food Sci. 2010, 16, 209–221. [Google Scholar] [CrossRef]
- Almeida, I.F.; Fernandes, E.; Lima, J.L.F.C.; Costa, P.C.; Bahia, M.F. Protective effect of Castanea sativa and Quercus robur leaf extracts against oxygen and nitrogen reactive species. J. Photochem. Photobiol. 2008, 91, 87–95. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Mari, A.; Balato, A.; Filosa, R.; Lembo, S.; Napolitano, A.; Piacente, S. Phenolics from Castanea sativa leaves and their effects on UVB-induced damage. Nat. Prod. Res. 2018, 32, 1170–1175. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Comandini, P.; Hrelia, S. Sweet Chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell. Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.; Dobrowolska, A.; Kicel, A.; Owczarek, A.; Bazylko, A.; Granica, S.; Piwowarski, J.P.; Olszewska, M.A. Polyphenolic profile, antioxidant and anti-inflammatory activity of eastern teaberry (Gaultheria procumbens L.) leaf extracts. Molecules 2014, 19, 20498–20520. [Google Scholar] [CrossRef] [PubMed]
- Elraey, M.; Elgamal, A. Quercetin -3-O-β-D-glucuronide butyl ester from Vitis vinifera leaves of potent anti-helicobacter pylori activity and impact of its combination with clarithromycin. Egypt. J. Chem. 2022, 65, 2–4. [Google Scholar]
- Liebisch, G.; Fahy, E.; Aoki, J.; Dennis, E.A.; Durand, T.; Ejsing, C.S.; Fedorova, M.; Feussner, I.; Griffiths, W.J.; Köfeler, H.; et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J. Lipid Res. 2020, 61, 1539–1555. [Google Scholar] [CrossRef]
- Hou, W.; Zhou, H.; Khalil, M.B.; Seebun, D.; Bennett, S.A.L.; Figeys, D. Lyso-form fragment ions facilitate the determination of stereospecificity of diacyl glycerophospholipids. Rapid Commun. Mass Spectrom. 2010, 25, 205–217. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.; Nhiem, N.X.; Song, J.-H.; Ko, H.-J.; Kim, S.H. Cycloartane-type triterpenoid derivatives and a flavonoid glycoside from the burs of Castanea crenata. Phytochemistry 2019, 158, 135–141. [Google Scholar] [CrossRef]
- Quave, C.L.; Lyles, J.T.; Kavanaugh, J.S.; Nelson, K.; Parlet, C.P.; Crosby, H.A.; Heilmann, P.K.; Horswill, A.R. Castanea sativa (European Chestnut) leaf extracts rich in ursene and oleanene derivatives block Staphylococcus aureus virulence and pathogenesis without detectable resistance. PLoS ONE 2015, 10, e0136486. [Google Scholar] [CrossRef]
- Pérez, A.J.; Pecio, Ł.; Kowalczyk, M.; Kontek, R.; Gajek, G.; Stopinsek, L.; Mirt, I.; Stochmal, A.; Oleszek, W. Cytotoxic triterpenoids isolated from sweet chestnut heartwood (Castanea sativa) and their health benefits implication. Food Chem. Toxicol. 2017, 109, 863–870. [Google Scholar] [CrossRef]
- Jayanegara, A.; Marquardt, S.; Kreuzer, M.; Leiber, F. Nutrient and energy content, in vitro ruminal fermentation characteristics and methanogenic potential of alpine forage plant species during early summer. J. Sci Food Agric. 2011, 91, 1863–1870. [Google Scholar] [CrossRef]
- Hassanat, F.; Benchaar, C. Assessment of the effect of condensed (acacia and quebracho) and hydrolysable (chestnut and valonea) tannins on rumen fermentation and methane production in vitro. J. Sci. Food Agric. 2012, 93, 332–339. [Google Scholar] [CrossRef]
- Witzig, M.; Zeder, M.; Rodehutscord, M. Effect of the ionophore monensin and tannin extracts supplemented to grass silage on populations of ruminal cellulolytics and methanogens in vitro. Anaerobe 2018, 50, 44–54. [Google Scholar] [CrossRef]
- Sarnataro, C.; Spanghero, M. In vitro rumen fermentation of feed substrates added with chestnut tannins or an extract from Stevia rebaudiana Bertoni. Anim Nutr. 2020, 6, 54–60. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2017, 4, 137–150. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2010, 91, 24–37. [Google Scholar] [CrossRef]
- Castro-Montoya, J.M.; Makkar, H.P.S.; Becker, K. Chemical composition of rumen microbial fraction and fermentation parameters as affected by tannins and saponins using an in vitro rumen fermentation system. Can. J. Anim. Sci. 2011, 91, 433–448. [Google Scholar] [CrossRef]
- Martínez, T.F.; McAllister, T.A.; Wang, Y.; Reuter, T. Effects of tannic acid and quebracho tannins on in vitro ruminal fermentation of wheat and corn grain. J. Sci. Food Agric. 2006, 86, 1244–1256. [Google Scholar] [CrossRef]
- Makkar, H.P. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Díaz Carrasco, J.M.; Cabral, C.; Redondo, L.M.; Pin Viso, N.D.; Colombatto, D.; Farber, M.D.; Fernández Miyakawa, M.E. Impact of Chestnut and Quebracho Tannins on Rumen Microbiota of Bovines. Biomed Res. Int. 2017, 2017, 9610810. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Trabalza, M.M.; Lupi, P.; Conte, G.; et al. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef]
- Moss, A.R.; Jouany, J.P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high forage diet 1,2. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef] [PubMed]
- Avila, A.S.; Zambom, M.A.; Faccenda, A.; Werle, C.H.; Almeida, A.R.E.; Schneider, C.R.; Grunevald, D.G.; Faciola, A.P. Black Wattle (Acacia mearnsii) condensed tannins as feed additives to lactating dairy cows. Animals 2020, 10, 662. [Google Scholar] [CrossRef] [PubMed]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. Int. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, L.R.; Henry, D.D.; Ciriaco, F.M.; Quintela, J.C.; DiLorenzo, N.; Ipharraguerre, I.R. Triterpenes from Olea europaea modulate in vitro ruminal fermentation. Transl. Anim. Sci. 2022, 6, txac056. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, Ruminal Fermentation, Ciliate Protozoal Populations, and Milk Production from Dairy Cows Fed Cinnamaldehyde, Quebracho Condensed Tannin, or Yucca schidigera Saponin Extracts. Int. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef]
- Jäger, E.J. Rothmaler-Exkursionsflora Von Deutschland; Springer: Berlin/Heidelberg, Germany, 2017; p. 930. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005; p. 24. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and no starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Pacifico, S.; Gallicchio, M.; Lorenz, P.; Duckstein, S.M.; Potenza, N.; Galasso, S.; Marciano, S.; Fiorentino, A.; Stintzing, F.C.; Monaco, P. Neuroprotective potential of Laurus nobilis antioxidant polyphenol-enriched leaf extracts. Chem. Res. Toxicol. 2014, 27, 611–626. [Google Scholar] [CrossRef]
- Formato, M.; Piccolella, S.; Zidorn, C.; Pacifico, S. UHPLC-HRMS Analysis of Fagus sylvatica (Fagaceae) Leaves: A Renewable Source of Antioxidant Polyphenols. Antioxidants 2021, 10, 1140. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; and France, J.A. Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I.; Raso, G.; Todaro, M. Silage of prickly pears (Opuntia spp.) juice by-products. Animals 2020, 10, 1716. [Google Scholar] [CrossRef]
- EC Council. Regulation 882/2004 on Official controls performed to ensure verification of compliance with feed and food law, animal health and animal welfare rules. Off. J. Eur. Union 2004, L191/1, 1–52. [Google Scholar]
- Pelagalli, A.; Musco, N.; Trotta, N.; Cutrignelli, M.I.; Di Francia, A.; Infascelli, F.; Tudisco, R.; Lombardi, P.; Vastolo, A.; Calabrò, S. Chemical characterisation and in vitro gas production kinetics of eight faba bean varieties. Animals 2020, 10, 398. [Google Scholar] [CrossRef]
- Calabrò, S.; Musco, N.; Roberti, F.; Vastolo, A.; Coppola, M.; Esposito, L.; Cutrignelli, M.I. Fermentability characteristics of different Saccharomyces cerevisiae cell wall using cat faeces as inoculum. Ital. J. Anim. Sci. 2020, 19, 186–193. [Google Scholar] [CrossRef]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. Anim. Sci. J. 2001, 73, 313–322. [Google Scholar] [CrossRef]
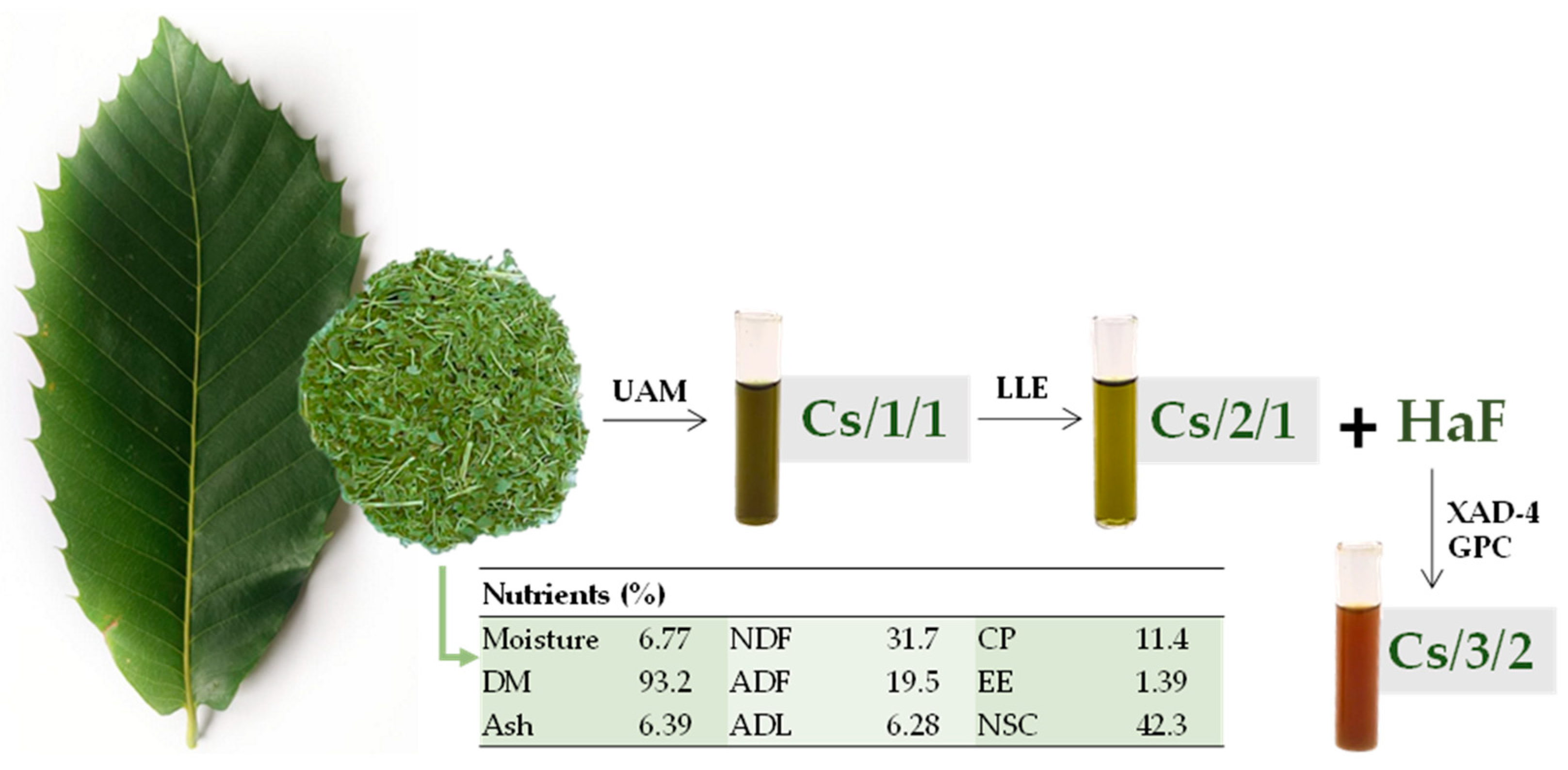
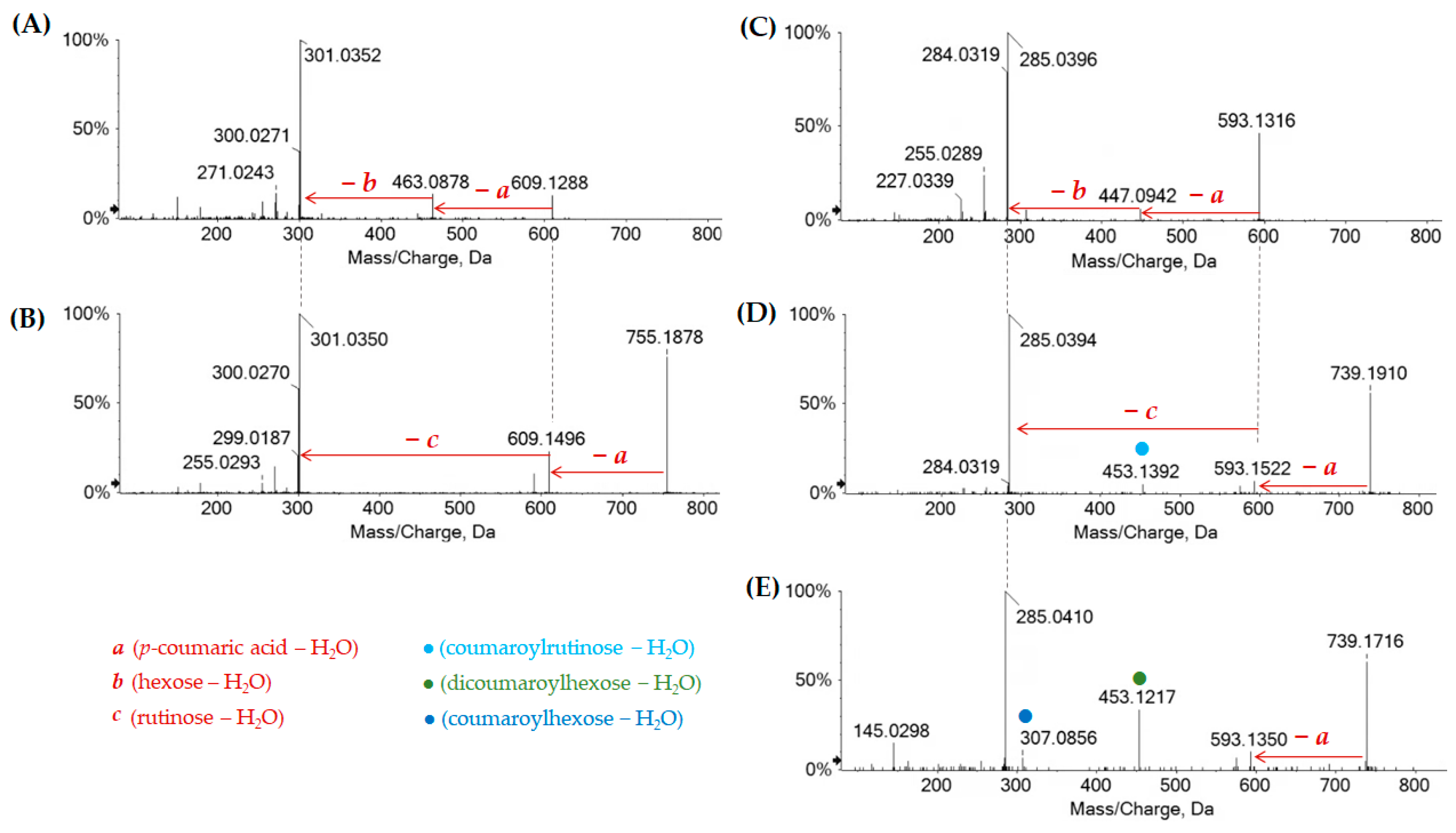
| Peak | Rt | Tentative Assignment | Formula | [M-H]− Found (m/z) | Error (ppm) | RDB | Cs/1/1 | Cs/2/1 | Cs/3/2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.288 | Quinic acid | C7H12O6 | 191.0562 | 0.5 | 2 | ● | ● | |
| 10 | 1.260 | Dihydroxybenzoic acid hexoside | C13H16O9 | 315.0724 | 0.8 | 6 | ● | ● | |
| 20 | 3.929 | p-Coumaric acid hexoside I | C15H18O8 | 325.0926 | −0.9 | 7 | ● | ||
| 22 | 4.609 | p-Coumaric acid hexoside II | C15H18O8 | 325.0926 | −0.9 | 7 | ● | ● | |
| 24 | 4.988 | Caffeic acid hexoside | C15H18O9 | 341.0871 | −1.2 | 7 | ● | ||
| 26 | 5.628 | 5-O-Caffeoyl quinic acid | C16H18O9 | 353.0884 | 1.7 | 8 | ● | ● | |
| 35 | 7.080 | 3-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0925 | −1.2 | 8 | ● | ● | |
| 36 | 7.218 | 3-O-p-Coumaroyl quinic acid | C16H18O8 | 337.0933 | 1.2 | 8 | ● | ● | |
| 56 | 10.379 | Benzyl-dihydroxybenzoate-O-pentosyl hexoside I | C25H30O13 | 537.1624 | 1.9 | 11 | ● | ● | |
| 59 | 10.635 | Benzyl-dihydroxybenzoate-O-hexoside | C20H22O9 | 405.1192 | 0.2 | 10 | ● | ● | ● |
| 74 | 13.160 | Benzyl-dihydroxybenzoate-O-pentosyl hexoside II | C25H30O13 | 537.1635 | 4.0 | 11 | ● | ● | ● |
| Peak | Rt | Tentative Assignment | Formula | [M-H]− Found (m/z) | Error (ppm) | Cs/1/1 | Cs/2/1 | Cs/3/2 |
|---|---|---|---|---|---|---|---|---|
| 2 | 0.602 | Galloyl hexose I | C13H16O10 | 331.0670 | −0.2 | ● | ● | |
| 3 | 0.680 | Pyrogallol gallic acid | C13H10O8 | 293.0302 | 0.1 | ● | ● | |
| 4 | 0.680 | Hexahydroxydiphenic acid | C14H10O10 | 337.0204 | 0.8 | ● | ● | |
| 5 | 0.721 | Gallic acid | C7H6O5 | 169.0151 | 5.0 | ● | ||
| 6 | 0.776 | Galloyl hexose II | C13H16O10 | 331.0669 | −0.5 | ● | ||
| 7 | 1.008 | Digalloyl hexose I | C20H20O14 | 483.0785 | 1.0 | ● | ||
| 8 | 1.124 | 3,4,5-trihydroxybenzyl hexoside I (e.g., crenatin) | C13H18O9 | 317.0879 | 0.3 | ● | ● | |
| 9 | 1.163 | NHTP-HHDP-hexose I | C41H26O26 | 933.0640; 466.0328 [M-2H]2− | 1.8 | ● | ● | |
| 11 | 1.417 | 3,4,5-trihydroxybenzyl hexoside II | C13H18O9 | 317.0877 | −0.3 | ● | ||
| 12 | 1.535 | Galloyl hexose III | C13H16O10 | 331.0674 | 1.0 | ● | ||
| 13 | 1.632 | Bis-HHDP-hexose I | C34H24O22 | 783.0700 | 1.7 | ● | ● | |
| 14 | 1.768 | Galloyl shikimic acid I | C14H14O9 | 325.0554 | −3.4 | ● | ||
| 15 | 1.827 | NHTP-HHDP-hexose II | C41H26O26 | 933.0641; 466.0326 [M-2H]2− | 0.2 | ● | ● | |
| 16 | 2.087 | Galloyl shikimic acid II | C14H14O9 | 325.0563 | −0.6 | ● | ||
| 17 | 2.187 | Galloyl dihydroxybenzoic acid | C14H10O8 | 305.0305 | 0.7 | ● | ||
| 18 | 2.385 | Galloyl shikimic acid III | C14H14O9 | 325.0566 | −0.1 | ● | ||
| 19 | 3.314 | Bis-HHDP-hexose II | C34H24O22 | 783.0696 | 1.2 | ● | ||
| 21 | 4.589 | Galloyl-chebuloyl-HHDP-hexose I | C41H30O27 | 953.0901 | −0.1 | ● | ||
| 23 | 4.966 | HHDP-valoneoyl-hexose | C41H28O27 | 951.0761 | 1.7 | ● | ● | |
| 25 | 5.268 | Digalloyl-HHDP-hexose I | C34H26O22 | 785.0857 | 1.8 | ● | ● | |
| 27 | 6.004 | Galloyl-HHDP-hexose | C27H22O18 | 633.0742 | 1.8 | ● | ● | |
| 28 | 6.004 | Galloyl-bis-HHDP-hexose I | C41H28O26 | 935.0818 | 2.1 | ● | ||
| 29 | 6.244 | Methylvaloneoyl–NHTP–hexose | C49H32O31 | 557.0405 [M-2H]2− | 1.8 | ● | ● | |
| 30 | 6.165 | Digalloyl hexose II | C20H20O14 | 483.0788 | 1.6 | ● | ● | |
| 31 | 6.560 | Galloyl-bis-HHDP-hexose II (e.g., stachyurin/casuarinin) | C41H28O26 | 935.0801 | 0.5 | ● | ● | |
| 32 | 6.500 | Galloyl-chebuloyl-HHDP-hexose II (e.g., chebulagic acid) | C41H30O27 | 953.0909 | 0.8 | ● | ● | |
| 33 | 6.546 | Trigalloyl hexose I | C27H24O18 | 635.0892 | 0.3 | ● | ● | |
| 34 | 6.819 | Chesnatin | C27H26O18 | 637.1064 | 2.8 | ● | ● | |
| 37 | 7.297 | Digalloyl-HHDP-hexose II | C34H26O22 | 785.0856 | 1.7 | ● | ● | |
| 38 | 7.417 | Digalloyl deoxyhexose | C20H20O13 | 467.0843 | 2.5 | ● | ● | |
| 39 | 8.110 | Isochesnatin | C27H26O18 | 637.1063 | 2.6 | ● | ● | |
| 40 | 8.327 | Galloyl phenol hexoside I (e.g., cretanin) | C20H22O13 | 469.0996 | 1.8 | ● | ● | |
| 41 | 8.581 | Galloyl-bis-HHDP-hexose III | C41H28O26 | 935.0797; 467.0375 [M-2H]2− | 0.1 | ● | ● | |
| 42 | 8.721 | Galloyl phenol hexoside II | C20H22O13 | 469.0997 | 2.0 | ● | ● | |
| 43 | 8.919 | Trigalloyl hexose II | C27H24O18 | 635.0905 | 2.4 | ● | ● | |
| 44 | 9.000 | Trigalloyl-valoneoyl-HHDP-hexose | C48H34O31 | 552.0490 [M-2H]2− | 1.1 | ● | ● | |
| 45 | 9.455 | Trigalloyl-HHDP-hexose | C41H30O26 | 937.0947; 468.0454 [M-2H]2− | −0.6 | ● | ● | |
| 47 | 9.704 | Tetragalloyl hexose | C34H28O22 | 787.1007 | 1.0 | ● | ● | |
| 48 | 9.704 | Ellagic acid pentoside | C19H14O12 | 433.0415 | 0.6 | ● | ● | |
| 49 | 9.754 | Chestanin | C40H42O26 | 937.1894 | 0.3 | ● | ● | |
| 58 | 10.592 | Isochestanin | C40H42O26 | 937.1904 | 1.3 | ● | ● | |
| 69 | 11.980 | Trimethylellagic acid hexose | C23H24O13 | 551.057 [M+FA]− | n.c. | ● | ● | ● |
| 81 | 14.174 | Trimethylellagic acid deoxyhexose | C23H22O12 | 535.1106 [M+FA]− | n.c. | ● | ● | ● |
| 82 | 14.174 | Trimethylellagic acid I | C17H12O8 | 343.0455 | −1.3 | ● | ● | |
| 87 | 15.139 | Trimethylellagic acid II | C17H12O8 | 343.0459 | −0.1 | ● | ● |
| Peak | Rt | Tentative Assignment | Formula | [M-H]− Found (m/z) | Error (ppm) | Cs/1/1 | Cs/2/1 | Cs/3/2 |
|---|---|---|---|---|---|---|---|---|
| 46 | 9.595 | Quercetin pentosyl-hexoside | C26H28O16 | 595.1318 | 2.3 | ● | ● | |
| 50 | 9.969 | Quercetin hexuronide I | C21H18O13 | 477.0686 | 2.6 | ● | ● | |
| 51 | 9.911 | Quercetin 3-O-hexoside I | C12H20O12 | 463.0893 | 2.4 | ● | ● | |
| 52 | 10.009 | Rutin | C27H30O16 | 609.1479 | 2.9 | ● | ● | |
| 53 | 10.067 | Quercetin 3-O-hexoside II | C12H20O12 | 463.0892 | 2.2 | ● | ● | |
| 54 | 10.315 | Quercetin hexuronide II | C21H18O13 | 477.0680 | 1.1 | ● | ● | |
| 55 | 10.315 | Quercetin 3-O-hexoside III | C12H20O12 | 463.0890 | 1.7 | ● | ● | |
| 57 | 10.515 | Quercetin 3-O-pentoside | C20H18O11 | 433.0786 | 1.5 | ● | ● | |
| 60 | 10.810 | Kaempferol deoxyhexosyl-hexoside I | C27H30O15 | 593.1533 | 3.5 | ● | ● | |
| 61 | 10.887 | Isorhamnetin deoxyhexoside I | C21H20O11 | 447.0941 | 1.8 | ● | ● | |
| 62 | 11.099 | Isorhamnetin deoxyhexosyl-hexoside I | C28H32O16 | 623.1632 | 2.2 | ● | ● | |
| 63 | 11.176 | Kaempferol deoxyhexosyl-hexoside II | C27H30O15 | 593.1526 | 2.4 | ● | ● | |
| 64 | 11.196 | Isorhamnetin 3-O-hexoside I | C22H22O12 | 477.1046 | 1.6 | ● | ● | |
| 65 | 11.293 | Isorhamnetin deoxyhexoside II | C21H20O11 | 447.0935 | 0.5 | ● | ● | |
| 66 | 11.293 | Isorhamnetin hexuronide | C22H20O13 | 491.0841 | 2.0 | ● | ● | |
| 67 | 11.510 | Isorhamnetin deoxyhexosyl-hexoside II | C28H32O16 | 623.1633 | 2.3 | ● | ● | |
| 68 | 11.570 | Isorhamnetin 3-O-hexoside II | C22H22O12 | 477.1051 | 2.6 | ● | ● | |
| 70 | 12.181 | Kaempferol acetylhexoside I | C23H22O12 | 489.1058 | 4.0 | ● | ● | ● |
| 71 | 12.402 | Quercetin | C15H10O7 | 301.0354 | −0.3 | ● | ● | ● |
| 72 | 12.737 | Luteolin | C15H10O6 | 285.0398 | −2.3 | ● | ● | ● |
| 73 | 12.819 | Kaempferol acetylhexoside II | C23H22O12 | 489.1051 | 2.6 | ● | ● | |
| 75 | 13.729 | Quercetin p-coumaroyl-hexoside | C30H26O14 | 609.1258 | 1.3 | ● | ● | |
| 76 | 13.749 | Quercetin p-coumaroyl- deoxyhexosyl-hexoside I | C36H36O18 | 755.1854 | 3.3 | ● | ● | |
| 77 | 13.769 | Kaempferol p-coumaroyl-hexoside I | C30H26O13 | 593.1326 | 2.4 | ● | ● | ● |
| 78 | 13.968 | Quercetin hexuronide butyl-ester | C25H26O14 | 533.1316 | 2.9 | ● | ||
| 79 | 13.968 | Quercetin p-coumaroyl- deoxyhexosil-hexoside II | C36H36O18 | 755.1850 | 3.3 | ● | ● | |
| 80 | 13.984 | Kaempferol | C15H10O6 | 285.0403 | −0.6 | ● | ● | ● |
| 83 | 14.366 | Kaempferol p-coumaroyl deoxyhexosyl hexoside I | C36H36O17 | 739.1894 | 1.9 | ● | ● | |
| 85 | 14.561 | Kaempferol p-coumaroyl-hexoside II | C30H26O13 | 593.1311 | 1.7 | ● | ● | ● |
| 86 | 14.643 | Kaempferol p-coumaroyl deoxyhexosyl hexoside II | C36H36O17 | 739.1899 | 2.9 | ● | ● | |
| 88 | 15.563 | Kaempferol acetyl p-coumaroyl-hexoside | C32H28O14 | 635.1432 | 4.0 | ● | ● | ● |
| 89 | 15.799 | Methylkaempferol | C16H12O6 | 299.0559 | −0.7 | ● | ● | ● |
| 91 | 17.088 | Kaempferol di-p-coumaroyl hexoside I | C39H32O15 | 739.1695 | 3.6 | ● | ● | ● |
| 92 | 17.284 | Kaempferol di-p-coumaroyl hexoside II | C39H32O15 | 739.1694 | 3.3 | ● | ● | ● |
| 93 | 17.313 | Acacetin | C16H12O5 | 283.0611 | −0.3 | ● | ● | |
| 96 | 18.337 | Kaempferol acetyl di-p-coumaroyl hexoside I | C41H34O16 | 781.1792 | 2.3 | ● | ● | ● |
| 97 | 18.594 | Kaempferol acetyl di-p-coumaroyl hexoside II | C41H34O16 | 781.1797 | 2.9 | ● | ● | ● |
| 100 | 19.954 | Kaempferol di-acetyl di-p-coumaroyl hexoside I | C43H36O17 | 823.1879 | −0.1 | ● | ● | |
| 102 | 20.144 | Kaempferol di-acetyl di-p-coumaroyl hexoside II | C43H36O17 | 823.1885 | 0.6 | ● | ● |
| Peak | Rt | Tentative Assignment | Formula | [M-H]− Found (m/z) | Error (ppm) | Cs/1/1 | Cs/2/1 | Cs/3/2 |
|---|---|---|---|---|---|---|---|---|
| 84 | 14.436 | Traumatic acid | C12H20O4 | 227.1291 | 1.0 | ● | ● | ● |
| 90 | 16.019 | 3,7,24-trihydroxy-cycloartene-28-oic acid hexoside | C36H58O10 | 695.4037 [M+FA]− | n.c. | ● | ● | ● |
| 94 | 17.343 | 3,7-dihydroxynorcycloartane 24,28-dioic acid (e.g., castaartancrenoic acid A) | C27H42O6 | 461.2921 | 2.7 | ● | ● | ● |
| 95 | 17.856 | Cycloartane-type triterpene | C30H50O6 | 505.3544 | 1.9 | ● | ● | ● |
| 98 | 18.752 | Cycloartane-type triterpene | C27H42O5 | 445.2956 | −0.8 | ● | ● | ● |
| 99 | 19.845 | Cycloartane-type triterpene | C30H48O5 | 487.3435 | 1.2 | ● | ||
| 101 | 20.044 | Cycloartane-type triterpene | C30H48O5 | 487.3437 | 1.6 | ● | ||
| 103 | 20.256 | Cycloartane-type triterpene | C30H48O5 | 487.3438 | 1.8 | ● | ||
| 104 | 20.425 | Pentacyclic triterpene | C30H48O6 | 503.3383 | 1.0 | ● | ||
| 105 | 20.606 | DGMG (18:3) | C33H56O14 | 675.3601 | 0.5 | ● | ● | ● |
| 106 | 20.721 | 9-oxooctadeca-10,12-dienoic acid | C18H32O3 | 293.2123 | 0.3 | ● | ||
| 107 | 20.721 | Pentacyclic triterpene | C30H48O6 | 503.3385 | 1.4 | ● | ||
| 108 | 21.100 | 9-oxooctadeca-10,12,15 trienoic acid | C18H28O3 | 291.1965 | −0.2 | ● | ||
| 109 | 21.318 | l-PA (18:3) | C21H37O7P | 431.2212 | 1.8 | ● | ● | ● |
| 110 | 21.846 | Pentacyclic triterpene | C30H48O4 | 471.3501 517.3558 [M+FA]− | 4.5 | ● | ● | |
| 111 | 22.313 | l-PA (16:0) | C19H39O7P | 409.2358 | −0.6 | ● | ● | ● |
| 112 | 22.513 | Pentacyclic triterpene | C30H48O4 | 471.3487 | 1.5 | ● | ||
| 113 | 22.654 | Pentacyclic triterpene | C30H48O5 | 487.3440 | 2.9 | ● | ||
| 114 | 22.695 | Pentacyclic triterpene | C30H48O4 | 471.3480 | 0.1 | ● | ||
| 115 | 22.784 | Pentacyclic triterpene | C30H48O5 | 487.3433 | 0.8 | ● | ||
| 116 | 22.975 | Linolenic acid | C18H30O2 | 277.2172 | −0.4 | ● | ||
| 117 | 23.115 | Pentacyclic triterpene | C30H48O4 | 471.3483 | 0.6 | ● | ||
| 118 | 23.215 | Pentacyclic triterpene (e.g., ursolic or oleanolic acid) | C30H48O3 | 455.3537 | 1.4 | ● | ||
| 119 | 23.496 | Linoleic acid | C18H32O2 | 279.2324 | −2.0 | ● |
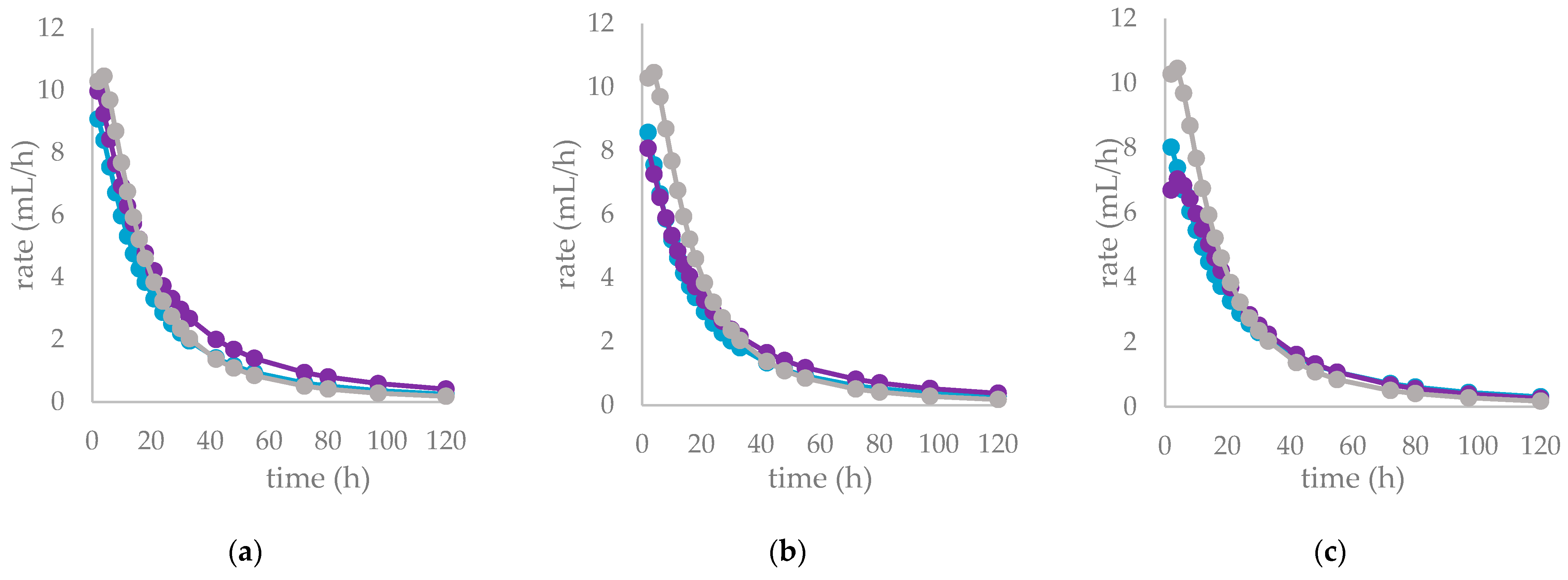
| (A) | (B) | |||
|---|---|---|---|---|
| control diet | Cs/1/1 |  | ||
| Parameter | 50 mg | 200 mg | ||
| OMD (%) | 74.9 | 65.0 ** | 68.9 ** | |
| OMCV (mL/g) | 244 | 208 *** | 234 NS | |
| Tmax (h) | 3.11 | 1.49 ** | 1.24 ** | |
| Rmax (mL/h) | 10.6 | 9.14 NS | 10.1 NS | |
| control diet | Cs/2/1 | 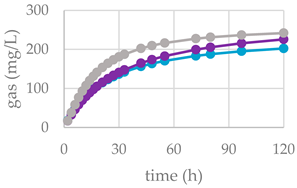 | ||
| 50 mg | 200 mg | |||
| OMD (%) | 74.9 | 64.9 ** | 65.9 *** | |
| OMCV (mL/g) | 244 | 194 *** | 184 *** | |
| Tmax (h) | 3.11 | 0.46 *** | 0.33 *** | |
| Rmax (mL/h) | 10.6 | 9.25 NS | 8.69 * | |
| control diet | Cs/3/2 | 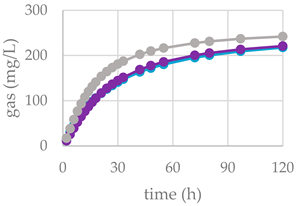 | ||
| 50 mg | 200 mg | |||
| OMD (%) | 74.9 | 67.7 ** | 67.5 ** | |
| OMCV (mL/g) | 244 | 215 ** | 219 ** | |
| Tmax (h) | 3.11 | 1.07 ** | 3.91 NS | |
| Rmax (mL/h) | 10.6 | 8.17 * | 7.04 ** | |
| Control Diet | Cs/1/1 | Cs/2/1 | Cs/3/2 | MSE | ||||
|---|---|---|---|---|---|---|---|---|
| 50 mg | 200 mg | 50 mg | 200 mg | 50 mg | 200 mg | |||
| OMD | 44.7 | 44.8 NS | 56.3 *** | 47.3 NS | 54.6 *** | 47.0 NS | 56.3 *** | 0.61 |
| CH4 (mL/g iOM) | 10.3 | 2.41 *** | 2.14 *** | 2.56 *** | 2.43 *** | 6.96 ** | 6.63 *** | 0.15 |
| CH4(mL/d OM) | 23.1 | 5.38 *** | 3.79 *** | 5.41 *** | 4.45 *** | 14.8 ** | 11.8 *** | 0.71 |
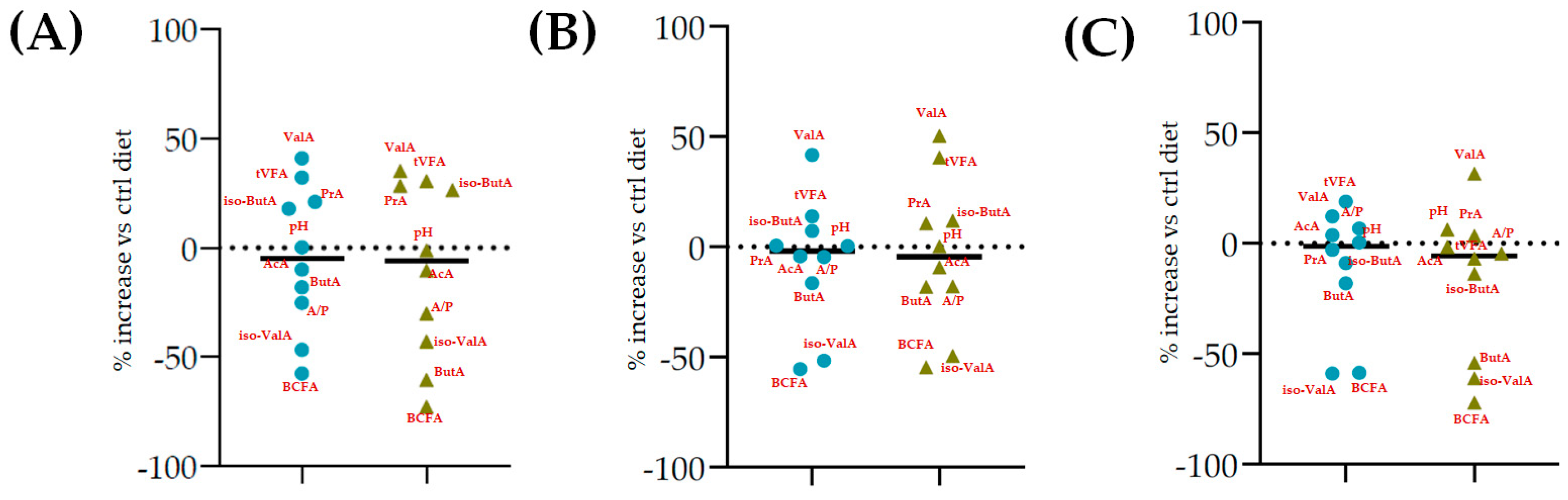
| Control Diet | Cs/1/1 | Cs/2/1 | Cs/3/2 | MSE | ||||
|---|---|---|---|---|---|---|---|---|
| 50 mg | 200 mg | 50 mg | 200 mg | 50 mg | 200 mg | |||
| pH | 6.20 | 6.46 *** | 6.43 *** | 6.49 *** | 6.35 ** | 6.34 ** | 6.31 * | 0.0006 |
| Total VFA (mmol/L) | 57.2 | 75.0 *** | 76.9 *** | 68.7 ** | 88.2 *** | 80.4 *** | 73.5 *** | 1.33 |
| AcA (% VFA) | 64.7 | 56.8 ** | 54.8 ** | 55.5 ** | 55.9 ** | 55.8 ** | 60.3 * | 1.59 |
| PrA (% VFA) | 15.1 | 21.5 ** | 24.9 *** | 22.3 *** | 22.3 *** | 23.5 ** | 19.7 *** | 0.98 |
| ButA (% VFA) | 11.9 | 16.5 * | 15.4 * | 16.5 * | 16.6 * | 16.1 * | 16.0 * | 1.03 |
| ValA (% VFA) | 3.98 | 2.21 *** | 2.25 *** | 3.05 ** | 2.28 *** | 2.53 ** | 1.73 *** | 0.020 |
| iso-ButA (% VFA) | 0.99 | 0.80 * | 0.69 * | 0.72 * | 0.93 NS | 0.77 * | 0.62 ** | 0.002 |
| iso-ValA (% VFA) | 3.25 | 2.17 *** | 2.24 *** | 1.89 *** | 1.96 *** | 1.29 *** | 1.65 *** | 0.003 |
| BCFA (% VFA) | 7.42 | 3.95 *** | 3.80 *** | 3.08 *** | 3.28 *** | 2.56 *** | 3.09 *** | 0.014 |
| A/P | 4.27 | 2.64 *** | 2.20 *** | 2.50 *** | 2.50 *** | 2.38 *** | 3.06 *** | 0.03 |
| AcA PrA ButA ValA iso-ButA iso-ValA | 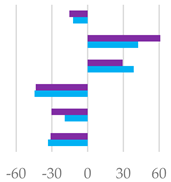 |  | 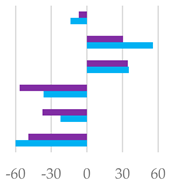 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules 2022, 27, 8662. https://doi.org/10.3390/molecules27248662
Formato M, Vastolo A, Piccolella S, Calabrò S, Cutrignelli MI, Zidorn C, Pacifico S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules. 2022; 27(24):8662. https://doi.org/10.3390/molecules27248662
Chicago/Turabian StyleFormato, Marialuisa, Alessandro Vastolo, Simona Piccolella, Serena Calabrò, Monica Isabella Cutrignelli, Christian Zidorn, and Severina Pacifico. 2022. "Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis" Molecules 27, no. 24: 8662. https://doi.org/10.3390/molecules27248662
APA StyleFormato, M., Vastolo, A., Piccolella, S., Calabrò, S., Cutrignelli, M. I., Zidorn, C., & Pacifico, S. (2022). Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules, 27(24), 8662. https://doi.org/10.3390/molecules27248662












