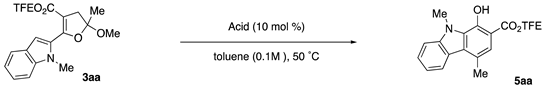
Abstract
The development of a Lewis acid-catalyzed, intramolecular ring-opening benzannulation of 5-(indolyl)2,3-dihydrofuran acetals is described. The resulting 1-hydroxycarbazole-2-carboxylates are formed in up to 90% yield in 1 h. The dihydrofuran acetals are readily accessed from the reactions of enol ethers and α-diazo-β-indolyl-β-ketoesters. To highlight the method’s synthetic utility, a formal total synthesis of murrayafoline A, a bioactive carbazole-containing natural product, was undertaken.
1. Introduction
The carbazole scaffold and its derivatives represent a privileged class of nitrogen-containing heteroaromatic structures often found in bioactive natural products and pharmaceutical drugs (Figure 1) [1,2,3,4,5,6,7]. For instance, Celiptium® is a marketed drug utilized for metastatic breast cancer [8], while carvedilol is used treat high blood pressure and heart failure [9]. In another example, carprofen is an anti-flammatory drug prescribed in veterinary medicine [10]. Carbazoles are also frequently employed in the development of advanced materials due to their conjugated tricyclic structure, which provides a tunable π-extended system [11]. This feature has been exploited for optical and thermoelectronic applications such as fluorescent dyes for bioimaging and conductive polymers for transistors, light emitting diodes, biosensors, and photovoltaic devices [12,13,14].
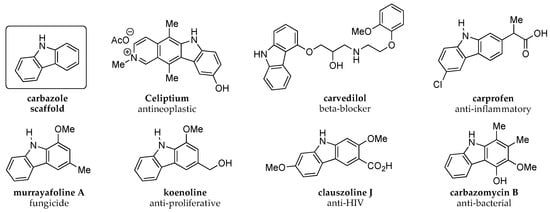
Figure 1.
The Carbazole Scaffold and Representative Bioactive Derivatives.
The synthesis of carbazoles generally follows two distinct approaches (Figure 2): (a) an annulation reaction to generate the central pyrrole ring or (b) a benzannulation reaction in which a benzene ring is appended on the five-member ring of an indole [15,16,17,18,19]. While several syntheses have been reported [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44], the abundant presence of the carbazole core in bioactive molecules and photoelectronic materials makes them attractive targets for the development of new synthetic methodologies, often targeting new substitution patterns, modularity and mild reaction conditions.
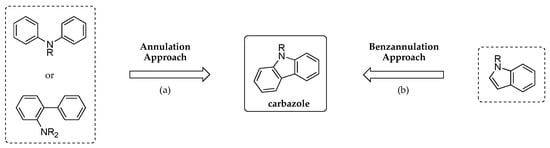
Figure 2.
Standard approaches to the carbazole framework.
Our lab previously established a Lewis acid-catalyzed approach to intramolecular benzannulation using (hetero)aryl-substituted 2,3-dihydrofuran acetals as precursors (Figure 3) [45,46]. Vicinal hydroxybenzoates are thus produced following dihydrofuran ring opening (via acetal hydrolysis), enolate isomerization, intramolecular π-attack (on the resulting oxonium II), and subsequent alcohol elimination. We demonstrated the versatility of this benzannulation approach using dihydrofurans substituted with arenes and oxygen- and sulfur-containing-heteroaromatics, resulting in the formation of naphthalenes, benzofurans, dibenzo[b,d]furans, phenanthrenes, and benzothiophenes in good to high yields (Figure 3a) [45]. More recently, we demonstrated that a (2-pyrrolyl)-substituted 2,3-dihydrofuran acetal was readily converted to the corresponding 7-hydroxyindole-6-carboxylate in 77% yield (Figure 3b)[46].
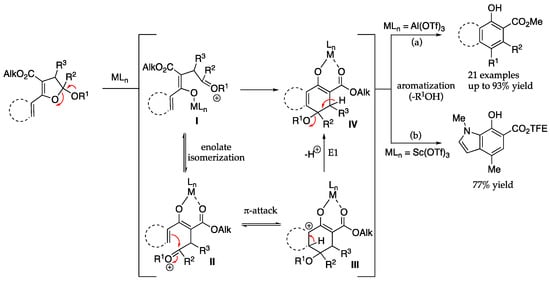
Figure 3.
Dihydrofuran acetals as building blocks for benzannulation to generate (a) arenes and oxygen-/sulfur-containg heteroaromatics and (b) indoles [45,46].
Substituted 1-hydroxycarbazoles are an important class of bioactive carbazoles that have been the targets of synthetic efforts over the last 15 years [47,48]. Many of these efforts begin with commercial 1-hydroxycarbazole, requiring numerous steps for regioselective substituent installation [49]. Direct regioselective methods to 1-hydroxycarbazoles are relatively scarce in the literature. Thus, a need still exists for the efficient syntheses of these structural motifs. Encouraged by the successful example of indole formation from a dihydrofuran acetal [46], we herein discuss our efforts to develop efficient Lewis acid-catalyzed intramolecular ring-opening benzannulations of the corresponding indolyl-substituted dihydrofuran acetals to form 1-hydroxy-9H-carbazole-2-carboxylates (Scheme 1).

Scheme 1.
Proposed intramolecular ring-opening benzannulation approach to 1-hydroxycarbazoles.
2. Results and Discussion
2.1. Synthesis of Dihydrofuran Acetals 3
Synthesis of dihydrofuran acetals 3 were accomplished through the Cu(hfacac)2-catalyzed decomposition of N-indolyl α-diazo-β-ketoesters 1 in the presence of enol ethers 2 (Scheme 2) [46]. In most cases, the dihydrofuran-forming reaction proceeded as expected with yields up to 76%. The outliers included the reactions with 1-aryl-substituted enol ethers 2f–2i, dihydropyran 2k, and 5-methyl-2,3-dihydrofuran 2l.
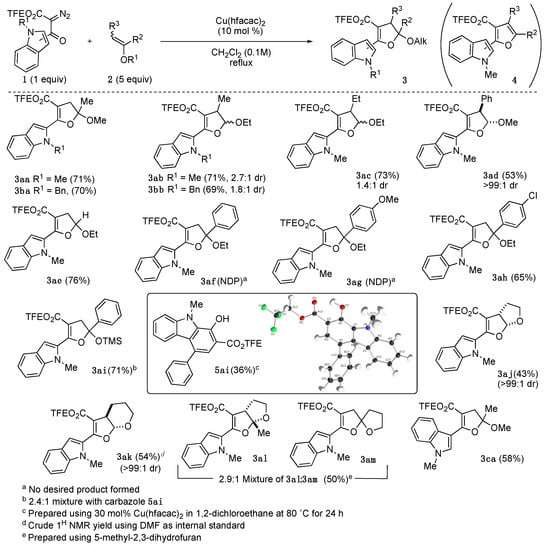
Scheme 2.
Synthesis of Dihydrofuran Acetals 3.
Under the reaction conditions, no dihydrofuran products were detected with the 1-aryl-susbtituted ethyl enol ethers 2f and 2g. Instead, the corresponding furans (4af and 4ag) were obtained. It is likely that the dihydrofuran serves as a short-lived intermediate which readily undergoes Cu-promoted EtOH elimination to provide the conjugated furan. In contrast, when a p-chloro group is placed on the phenyl ring, dihydrofuran 3ah is readily formed in 65% yield. Employing the corresponding 1-aryl silyl enol ether 2i in the reaction conditions, unexpectedly provided a 2.4:1 inseparable mixture of dihydrofuran 3ai (50%) along with carbazole 5ai (21%). After some minor attempts to design direct one-pot or tandem transformations, we determined that carbazole 5ai could only be isolated in up to 36% yield when diazo 1a and enol ether 2i were treated with 20 mol% Cu(hfacac)2 in 1,2-dichloroethane at 70 °C for 24 h.
For tetrahydropyran 2k, the desired dihydrofuran 3ak was obtained as an inseparable mixture with unidentifiable material in a 56% 1H NMR yield. The crude mixture was carried forward. 5-Methyl-2,3-dihydrofuran 2l underwent partial in situ alkene isomerization to form 2-methylene tetrahydrofuran 2m. This isomerization resulted in the formation of a 2.9:1 mixture of fused-bicyclic dihydrofuran 3al and spirocyclic dihydrofuran 3am in 50% total yield.
2.2. Acid Screening for Ring-Opening Benzannulation
Dihydrofuran 3aa was selected as the initial system to begin optimizing the ring-opening benzannulation (Table 1). Based on our previous work, we began by screening Lewis and Brønsted acids at 10 mol% catalyst loading in toluene at 70 °C. We confirmed that no reaction occurs in the absence of an acid catalyst (entry 1). Al(OTf)3, the best performing Lewis acid in our previous study, provided carbazole 5aa in 79% yield (entry 2). Both Sc(OTf)3 and Ga(OTf)3 gave the carbazole with yields of 71% and 66%, respectively (entries 3 and 4). Divalent metals, Zn(OTf)2 and Mg(OTf)2, offered little or no product (entries 5 and 6). In contrast, Yb(OTf)3 generated carbazole 5aa in 90% yield (entry 7). In(OTf)3 afforded 82% yield of carbazole (entry 8), while tetravalent Hf(OTf)4 gave the product in 79% yield (entry 9). To test the influence of any potential TfOH formed in the reaction, we ran a series of control reactions. First, YbCl3 and AlCl3 were employed in the reaction. YbCl3 gave low conversion with only 24% yield of 5aa (entry 10). AlCl3 provided 5aa in 80% yield (entry 11). With TfOH, 5aa was formed in 66% yield (entry 12). Given that TfOH provided good reactivity as well, pTsOH•H2O was used as another comparative Brønsted acid. Carbazole 5aa was formed in 55% yield along with 19% yield of furan 4aa (entry 13). These control reactions demonstrate that while Lewis acid catalysis is definitely in play, we must acknowledge the presence of cooperative catalysis by TfOH. We decided to move forward with Yb(OTf)3 as the Lewis acid catalyst of choice given the high product yield. Further changes to either the temperature, solvent, or concentration failed to provide better product outcomes.

Table 1.
Acid Screening 1.
2.3. Benzannulation Substrate Scope
With dihydrofuran 3aa readily converting to carbazole 5aa with high yield, we next sought to explore the benzannulation reaction with the other synthesized substrates. We first examined 3-substituted dihydrofuran acetals 3ab–3ad (Scheme 3). Under the reactions, 2-ethoxy-3-methyl-substituted dihydrofuran 3ab (as a 3.4:1 trans:cis diastereomeric mixture) gave partial conversion to the corresponding carbazole-2-carboxylate 5ab in 58% yield (Scheme 3a). Interestingly, the recovered dihydrofuran (15%) was the cis-diastereomer. This outcome equates to a ~3.8:1 carbazole:cis-dihydrofuran ratio which closely parallels the initial dihydrofuran trans:cis ratio. Similar results were obtained with 3-ethyl substituted dihydrofuran 3ac (as 1.4:1 trans:cis mixture). Carbazole 5ac was formed in 42% yield along with 31% of recovered cis-dihydrofuran, a matching ~1.4:1 ratio (Scheme 3b). Seemingly contradictory, the trans-3-phenyl-substituted substrate 3ad afforded only trace (~5%) benzannulation product (Scheme 3c). Instead, epimerization of the acetal center occurred to generate cis-3ad in 64% yield, which is fairly unreactive under the reaction conditions. It is plausible that the phenyl substituent participates in the mechanism through anchimeric assistance resulting in stabilization of the dihydrofuran and reversible ring-opening. Thus, we can infer that the trans-dihydrofuran isomers react faster than the corresponding cis-isomers under the reaction conditions.
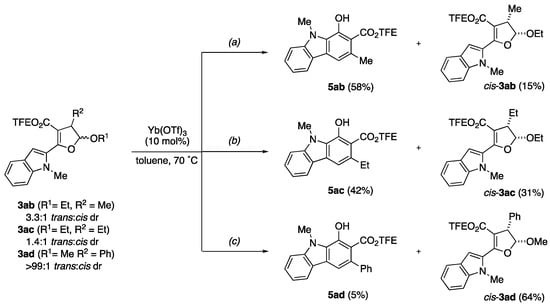
Scheme 3.
Unexpected Conversion Issues for 4-Substituted Dihydrofuran Acetals (a) 3ab, (b) 3ac, and (c) 3ad.
To overcome this reactivity issue, we first turned to revisiting some of the other Lewis acids that effectively formed the carbazole. Disappointingly, similar outcomes were obtained whether In(OTf)3, Sc(OTf)3, or Al(OTf)3 was used in place of Yb(OTf)3. At this point, we went back to our previous work for inspiration and decided to try Al(OTf)3 with a drop of H2O [45]. These conditions previously prevented furan formation from the corresponding dihydrofuran acetals. Satisfyingly, subjecting dihydrofurans 3ab–3ad to Al(OTf)3 and 1 drop of H2O, provided the desired carbazoles 5ab, 5ac, and 5ad in 67%, 62%, and 65% yields, respectively (Scheme 4). Our rationale for the shift in reactivity with the added water is the likely formation of TfOH (in line with the catalyst screen) which facilitates generation of dihydrofuran hemiacetal intermediate V that undergoes ring-opening. To confirm our hypothesis, we treated 3ad with TfOH (10 mol%) in toluene at 70 °C and obtained carbazole 5ad in 54% yield.
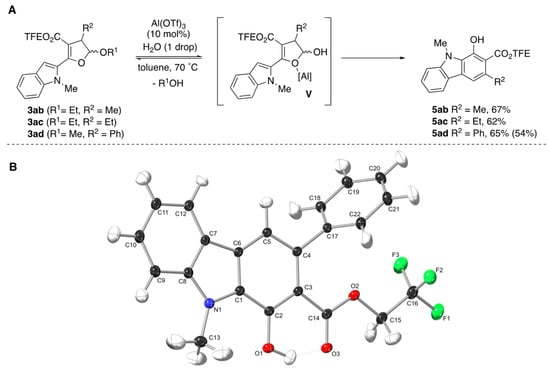
Scheme 4.
(A) Effect of Al(OTf)3 and H2O (1 drop) on Benzannulations of Dihydrofurans 3ab–3ad and (B) the Crystal Structure of 5ad (drawn at 50% probability level). Yield in parentheses represents product yield using TfOH (10 mol%).
With two sets of reaction conditions, we proceeded forward with the exploration of the substrate scope. Figure 4 summarizes the outcomes of the study, including those systems previously discussed (entries 1–4). The trisubstituted dihydrofuran 3ab (derived from ethyl vinyl ether) smoothly converted using Yb(OTf)3 to its 1-hydroxy carbazole-2-carboxylate 5ab in 81% yield (entry 5). For 2-(4-chlorophenyl)-2-methoxy dihydrofuran 3ah, elimination to furan 4ah was the predominant outcome observed for both Yb(OTf)3 and Al(OTf)3/H2O. The formed carbazole 5ah (5% for Yb and 19% for Al) was inseparable from the furan product in both cases (entry 6). Next, we examined the fused bicyclic dihydrofurans 3aj and 3ak (entries 7 and 8). Dihydrofuran 3aj was synthesized as the cis-diastereomer. Under the Al(OTf)3/H2O conditions, only furan 4aj was obtained. After some minor optimization, we found that Al(OTf)3 without added water produced a 78% yield of lactono-carbazole 6aj which resulted from the intramolecular transesterification of 5aj (entry 7). Under the same conditions (Al(OTf)3 with no added water), tetrahydropyran-fused dihydrofuran acetal 3ak readily provided carbazole 5ak in 22% (entry 8). No lactonization was observed which is consistent with entropic and enthalpic considerations for seven-membered ring formation [50]. Based on the reactions with the bicyclic dihydrofurans, we rationalized two things relative to the tetrasubstituted monocyclic dihydrofurans 3ab–3ad: (1) Diastereoselectivity does not seem to be a factor in the reactivity; (2) It is likely that hemiacetal formation is unfavorable with the added water due to the stability of the bicyclic acetal framework. When the mixture of fused-bicyclic dihydrofuran 3al and spirocyclic dihydrofuran 3am was subjected to the same conditions (Al(OTf)3, no water), lactono-carbazole 6al was obtained in 61% yield along with 19% yield of carbazole 5am (entry 9).
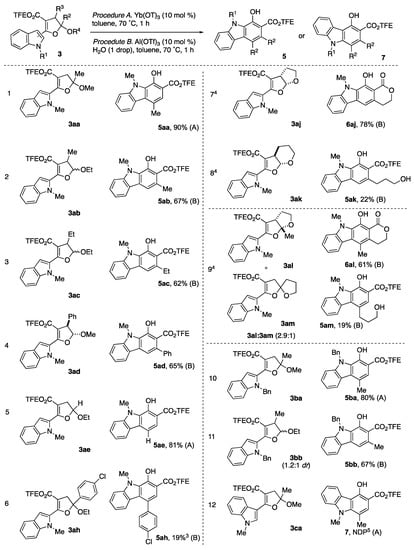
Figure 4.
Substrate Scope of the Ring Opening Benzannulation of Dihydrofuran Acetals 3. 1 Procedure A: Reaction performed with dihydrofuran acetal 3 and Yb(OTf)3 (10 mol%) in toluene (0.1 M) at 70 °C for 1 h. 2 Procedure B: Reaction performed with dihydrofuran acetal 3, Al(OTf)3 (10 mol%), H2O (one drop) in toluene (0.1 M) at 70 °C for 1 h. 3 Inseparable mixture with furan 4ah. 4 No water was added. 5 No desired product formed using either procedure. Obtained furan 4ca instead.
For the future purposes of synthesis, the effects of changing the N-methyl substituent to a N-benzyl group was explored. The corresponding carbazole 5ba was obtained in 80% yield using Yb(OTf)3 for dihydrofuran 3ba (entry 10), whereas Al(OTf)3/H2O was used to generate 67% yield of carbazole 5bb from dihydrofuran 3bb (entry 11).
Lastly, we attempted the benzannulation of the 3-indolyl dihydrofuran 3ca (entry 12). In all cases, regardless of what procedure or Lewis acid employed, only furan 4ca was obtained.
2.4. Formal Synthesis of Murrayafoline A
Using carbazole 5bb as a precursor, we undertook the formal synthesis of murrayafoline A (8, Scheme 5). Murrayafoline A is a monomeric carbazolic alkaloid that has been isolated from kilograms of the dried powdered roots of several species of the genus Murraya, Glycosmis, and Clausena in up to 3% yield [51,52,53]. Murrayafoline A was shown to exhibit a number of interesting biological activities including strong fungicidal activity (12.5 µg dose against C. cucumerinum) [52], cancer growth inhibitory activity (HT-1080 cells) [53], and Wnt/β-catenin signaling antagonist activity [54]. It has been the target of numerous several syntheses due to its role as a precursor to access a variety of congeners and non-natural derivatives. From carbazole 5bb, we could readily access N-benzyl murrayafoline A 9 in 66% yield over two steps following Krapcho decarbalkoxylation and O-methylation. Carbazole 9 has been shown by Mal and coworkers [47] to readily convert to the target molecule in in one step.
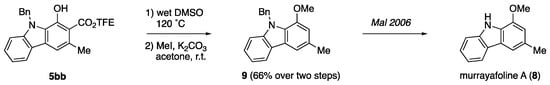
Scheme 5.
Formal Synthesis of Murrayafoline A (8) [30].
3. Experimental
A. General. All reactions were performed under protection of N2 in flame-dried glassware unless water was applied as solvent. Chromatographic purification was performed as flash chromatography with Silicycle SiliaFlash P60 silica gel (40–63 µm) or preparative thin-layer chromatography (prep-TLC) using silica gel F254 (1000 µm) plates and solvents indicated as eluent with 0.1–0.5 bar pressure. For quantitative flash chromatography, technical grades solvents were utilized. Analytical thin-layer chromatography (TLC) was performed on Silicycle SiliaPlate TLC silica gel F254 (250 µm) TLC glass plates. Visualization was accomplished with UV light. Infrared (IR) spectra were obtained via thin film IR on a salt plate using a Nicolet 6700 Fourier-transform infrared spectrophotometer. The IR bands are characterized as broad (br), weak (w), medium (m), and strong (s). Proton and carbon nuclear magnetic resonance spectra (Supplementary Materials: 1H NMR, 13C NMR and 19F NMR) were recorded on a Bruker 400 MHz spectrometer or on a Bruker 500 MHz spectrometer or on a Bruker 700 MHz spectrometer with solvent resonances as the internal standard (1H NMR: CDCl3 at 7.26 ppm; 13C NMR: CDCl3 at 77.0 ppm). 1H NMR data are reported as follows: chemical shift (ppm), multiplicity (s = singlet, d = doublet, dd = doublet of doublets, dt = doublet of triplets, ddd = doublet of doublet of doublets, t = triplet, q = quartet, p = pentet, m = multiplet, br = broad), coupling constants (Hz), and integration. Mass spectra were obtained through EI on a Micromass AutoSpec machine or through ESI on a Thermo Orbitrap XL. The accurate mass analyses run in EI mode were at a mass resolution of 10,000 and were calibrated using PFK (perfluorokerosene) as an internal standard. The accurate mass analyses run in EI mode were at a mass resolution of 30,000 using the calibration mixture supplied by Thermo. Crystal structures for carbazoles 5ad (Deposition Number 2213553) and 5ai (Deposition Number 2213554) were deposited in the Cambridge Structural Database.
B. Synthesis of Indolyl-α-diazo-β-ketoesters 1. General Procedure: Using the following modified literature procedure [55]: To a dry flask charged with a stir bar and the corresponding indole carboxylic acid (1.0 equiv.), dry CH2Cl2 was added to make a 0.5 M solution, followed by addition of catalytic DMF (a few drops). The solution was then cooled to 0 °C and oxalyl chloride (1.2 equiv.) was slowly added over 1 min. After 15 min, the reaction was allowed to warm to room temperature with continued stirring. After 3 h at room temperature, the reaction was concentrated under reduced pressure and the acid chloride residue was dissolved in dry THF to make a 1 M solution (keeping it under inert atmosphere). The solution was added slowly to the prepared enolate at −78 °C. The enolate was prepared by first adding LHMDS (1 M in THF, 3.0 equiv.) to a dry flask charged with a stir bar under nitrogen and cooling to −78 °C. The corresponding acetate (1.05 equiv.) was added to the solution of LHMDS in one shot and stirred for 45 min at −78 °C. After 30 min from the addition of the acid chloride to the enolate solution, the reaction was quenched with 0.5 M HCl at −78 °C, extracted with EtOAc three times, dried using Na2SO4, and filtered through celite. The combined organic layers were concentrated under reduced pressure and purified by flash chromatography on silica gel using 15–30% EtOAc/Hexanes as the mobile phase to produce pure product II. To an ice-cold solution of II (1.0 equiv.) and 4-acetamidobenzenesulfonyl azide (p-ABSA, 1.05 equiv.) in MeCN (0.2 M), NEt3 (1.1 equiv.) was slowly added. The reaction was left to warm gradually to room temperature overnight. Upon completion, the reaction mixture was vacuum-filtered through a celite plug to remove the formed solids. The filtrate was concentrated under vacuum and the obtained residue was purified on silica gel (15–30% EtOAC/Hexanes) to afford pure diazo compound 1.
2,2,2-Trifluoroethyl 2-diazo-3-(1-methyl-1H-indol-2-yl)-3-oxopropanoate (1a): Prepared by general diazo synthesis route, and started with N-methylindole-2-carboxylic acid (5.00 g, 28.5 mmol). It afforded 7.76 g (23.9 mmol, 84% over 3 steps) final product 1a as a yellow solid. 1H NMR (700 MHz, CDCl3) δ 7.69 (dt, J = 8.1, 1.0 Hz, 1H), 7.42–7.36 (m, 2H), 7.20 (s, 1H), 7.17 (ddd, J = 7.9, 5.0, 2.7 Hz, 1H), 4.65 (q, J = 8.2 Hz, 2H), 3.97 (s, 3H). 13C NMR (176 MHz, CDCl3) δ 175.5, 159.6, 140.1, 132.3, 126.1, 125.6, 123.1, 122.6 (q, J = 277.7 Hz), 120.9, 111.9, 110.2, 60.5 (q, J = 37.4 Hz), 31.8. IR 2950.79 (w), 2142.71 (s), 1741.70 (s), 1728.65 (s), 1613.26 (m), 1315.51 (s), 1274.18 (s), 1170. 92 (s), 1108.74 (m). HMRS (ESI) m/z: [M + H]+ calc. for. C14H10F3N3O3, 326.0747; Found 326.0748.
2,2,2-Trifluoroethyl 3-(1-benzyl-1H-indol-2-yl)-2-diazo-3-oxopropanoate (1b): Prepared by general diazo synthesis route, and started with N-benzylindole-2-carboxylic acid (2.00 g, 7.96 mmol) [55]. It afforded 2.46 g (6.14 mmol, 77% over 3 steps) final product 1b as a yellow gel. 1H NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 3.4 Hz, 2H), 7.30 (s, 1H), 7.28–7.17 (m, 4H), 7.06–7.02 (m, 2H), 5.71 (s, 2H), 4.63 (q, J = 8.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 175.6, 159.5, 139.8, 137.9, 132.2, 128.6, 127.2, 126.3, 126.2, 125.8, 123.1, 122.6 (q, J = 277.5 Hz), 121.2, 112.7, 110.8, 75.3, 60.5 (q, J = 37.1 Hz), 48.0. IR 3031.90 (m), 2139.42 (s), 1764.60 (s), 1717.14 (m), 1665.47 (s), 1283.34 (m), 1167.42 (s), 1141.86 (m). HMRS (ESI) m/z: [M + H]+ calc. C20H14F3N3O3, 402.1060; Found 402.1060.
2,2,2-Trifluoroethyl 2-diazo-3-(1-methyl-1H-indol-3-yl)-3-oxopropanoate (1c): Prepared by general diazo synthesis route, and started with N-methylindole-3-carboxylic acid (5.00 g, 28.5 mmol). It afforded 7.32 g (22.5 mmol, 79.0% over 3 steps) final product 1c as a yellow solid. 1H NMR (700 MHz, CDCl3) δ 8.38–8.34 (m, 1H), 8.19 (s, 1H), 7.39–7.28 (m, 3H), 4.63 (q, J = 8.3 Hz, 2H), 3.85 (s, 3H). 13C NMR (176 MHz, CDCl3) δ 176.2, 160.3, 137.6, 136.8, 127.6, 123.5, 122.9, 122.8 (q, J = 277.7 Hz), 122.5, 113.1, 109.6, 60.3 (q, J = 37.1 Hz), 33.7. IR 3148. 66 (m), 3012.10 (w), 2916.12 (w), 2148.29 (s), 1740.71 (s), 1731.42 (s), 1583.12 (m), 1307.18 (s), 1279.46 (s), 1167.31 (s), 1120.22 (m). HMRS (ESI) m/z: [M + H]+ calc. for. C14H10F3N3O3, 326.0747; Found 326.0731.
C. Synthesis of 2,3-dihydrofuran acetals 3.General Procedure: To a dry flask charged with a stir bar and a solution of Cu(hfacac)2 (29 mg, 61.5 μmol) and 2 (3.07 mmol) in anhydrous CH2Cl2 (6 mL) was added diazo 1 (615 μmol). The reaction was stirred vigorously under reflux condition for overnight or monitored by TLC for completion. After consuming all starting diazo, the mixture was diluted with Et2O (30 mL) and washed with saturated thiourea (20 mL). The organic layer was dried over Na2SO4, concentrated under reduced pressure, and purified by column chromatography. Since the 19F signals of all DHF acetal products have similar chemical shifts (−73.3 ppm to −73.8 ppm) and coupling constant (t, J = 8.5 Hz), only the 19F NMR of 3aa is reported and attached.
2,2,2-Trifluoroethyl 5-methoxy-5-methyl-2-(1-methyl-1H-indol-2-yl)-4,5-dihydrofuran-3-carboxylate (3aa): Prepared following general procedure using 2-methoxypropene 2a (221 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.39 in 20% EtOAC/Hexanes) afforded 3aa as pale-yellow solid (181 mg, 79%). 1H NMR (700 MHz, CDCl3) δ 7.65 (dt, J = 8.0, 1.0 Hz, 1H), 7.34 (dq, J = 8.4, 1.0 Hz, 1H), 7.30 (ddd, J = 8.3, 6.9, 1.2 Hz, 1H), 7.17 (d, J = 1.0 Hz, 1H), 7.13 (ddd, J = 8.0, 6.9, 1.1 Hz, 1H), 4.47 (qq, J = 8.5, 4.2 Hz, 2H), 3.81 (s, 3H), 3.43 (s, 3H), 3.21 (d, J = 16.6 Hz, 1H), 3.09 (d, J = 16.6 Hz, 1H), 1.73 (s, 3H). 13C NMR (176 MHz, CDCl3) δ 162.3, 158.3, 138.7, 127.7, 126.8, 123.9, 123.15 (q, J = 277.0 Hz), 121.9, 120.1, 111.6, 109.7, 108.9, 102.7, 59.82 (q, J = 36.4 Hz), 50.4, 40.5, 31.8, 24.5. 19F NMR (471 MHz, CDCl3) δ −73.43 (t, J = 8.5 Hz, 3F). IR 3050.91(m), 2945.23, 2836.57(m), 1720.57(s), 1711.31(s), 1168.64(s), 1106.59(s), 1047.59(s). HMRS (ESI) m/z: [M + H]+ calc. for C18H18F3NO4, 370.1260; Found 370.1262.
2,2,2-Trifluoroethyl 2-(1-benzyl-1H-indol-2-yl)-5-methoxy-5-methyl-4,5-dihydrofuran-3-carboxylate (3ba): Prepared following general procedure using 2-methoxypropene 2a (221 mg, 3.07 mmol) and diazo 1b (247 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.52 in 20% EtOAC/Hexanes) afforded 3ba as pale-yellow solid (192 mg, 70%). 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.9 Hz, 1H), 7.29 (s, 1H), 7.28–7.16 (m, 5H), 7.14 (ddd, J = 8.0, 6.3, 1.6 Hz, 1H), 7.00 (d, J = 6.9 Hz, 2H), 5.49 (s, 2H), 4.47 (qd, J = 8.5, 2.1 Hz, 2H), 3.17 (s, 3H), 3.13 (d, J = 16.7 Hz, 1H), 2.99 (d, J = 16.6 Hz, 1H), 1.57 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.3, 158.0, 138.3, 138.0, 128.5, 127.6, 127.1, 127.1, 126.0, 124.0, 123.1 (d, J = 277.4 Hz), 121.9, 120.4, 111.6, 110.3, 109.7, 102.9, 59.8 (q, J = 36.3 Hz), 50.2, 48.5, 40.6, 24.1. IR 3061.65(m), 3032.41(m), 2930.32(m), 2851.80(m), 1720.26(s), 1708.31(s), 1167.72(s), 1160.70(s), 1048.76(s). HMRS (ESI) m/z: [M + H]+ calc. for C24H22F3NO4, 446.1573; Found 446.1576.
2,2,2-Trifluoroethyl 5-ethoxy-4-methyl-2-(1-methyl-1H-indol-2-yl)-4,5-dihydrofuran-3-carboxylate (3ab): Prepared following general procedure using 1-ethoxypropene (mixture of cis:trans = 2.7:1) 2b (265 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.45 in 20% EtOAC/Hexanes) afforded 3ab (167 mg, 70%) as a mixture of diastereomers (dr = 3.3:1). 1H NMR (500 MHz, CDCl3) for trans isomer: δ 7.64 (d, J = 7.7 Hz, 1H), 7.35–7.27 (m, 2H), 7.13 (d, J = 6.6 Hz, 1H), 7.03 (s, 1H), 5.74 (d, J = 7.5 Hz, 1H), 4.57–4.48 (m, 1H), 4.44–4.35 (m, 1H), 3.78 (s, 3H), 3.74–3.66 (m, 1H), 3.51 (p, J = 7.1 Hz, 1H), 1.34 (d, J = 7.0 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H); For cis isomer: δ 7.64 (d, J = 7.7 Hz, 1H), 7.35–7.27 (m, 2H), 7.16–7.09 (m, 2H), 5.27 (d, J = 1.8 Hz, 1H), 4.57–4.48 (m, 1H), 4.44–4.35 (m, 1H), 3.80 (s, 3H), 3.74–3.66 (m, 1H), 3.27 (qd, J = 7.1, 1.9 Hz, 1H),1.34 (d, J = 7.0 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H); 13C NMR (126 MHz, CDCl3) for trans isomer (major isomer): δ 162.6, 158.1, 138.5, 128.2, 126.8, 123.51, 121.7, 120.0, 111.5, 109.6, 107.9, 107.8, 65.8, 59.6 (q, J = 36.3 Hz), 40.8, 31.5, 15.0, 11.8. The 13C signal that is directly coupled by fluorine is not reported due to the low intensity. IR 3058.43 (m), 2978.89 (s), 2933.93 (s), 1720.49 (s), 1711.45 (s), 1626.02 (m), 1281.91 (m), 1167.26 (s), 1084.81(s). HMRS (ESI) m/z: [M + H]+ calc. for C19H20F3NO4, 384.1417; Found 384.1417.
2,2,2-Trifluoroethyl 2-(1-benzyl-1H-indol-2-yl)-5-ethoxy-4-methyl-4,5-dihydrofuran-3-carboxylate (3bb): Prepared following general procedure but set up as 1 g (2.5 mmol) scale: using 1-ethoxypropene (mixture of cis:trans = 1.2:1) 2b (1.07 g, 12.46 mmol), diazo 1b (1.00 g, 2.49 mmol) and Cu(hfacac)2 (119 mg, 0.249 mmol) in anhydrous CH2Cl2 (24 mL). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.48 in 20% EtOAC/Hexanes) afforded 3bb as the mixture of diastereomers (dr = 1.81: 1) (800 mg, 70%). 1H NMR (500 MHz, CDCl3) for trans isomer: δ 7.74–7.67 (m, 1H), 7.30–7.19 (m, 5H), 7.18–7.11 (m, 2H), 7.07 (d, J = 6.8 Hz, 2H), 5.60 (d, J = 7.5 Hz, 1H), 5.46 (d, J = 6.5 Hz, 1H), 5.44 (d, J = 6.5 Hz, 1H), 4.59–4.47 (m, 1H), 4.48–4.36 (m, 1H), 3.68–3.55 (m, 1H), 3.52–3.46 (m, 1H), 3.42 (p, J = 7.1 Hz, 1H), 1.28 (d, J = 7.1 Hz, 3H), 1.16 (d, J = 7.1 Hz, 3H); For cis isomer: δ 7.74–7.67 (m, 1H), 7.30–7.19 (m, 5H), 7.18–7.11 (m, 2H), 7.07 (d, J = 6.8 Hz, 2H), 5.49 (d, J = 6.5 Hz, 1H), 5.46 (d, J = 4.7 Hz, 1H), 5.17 (d, J = 1.9 Hz, 1H), 4.59–4.47 (m, 1H), 4.48–4.36 (m, 1H), 3.68–3.55 (m, 1H), 3.52–3.46 (m, 1H), 3.23 (qd, J = 7.1, 2.0 Hz, 1H), 1.28 (d, J = 7.1 Hz, 3H), 1.15 (d, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) for both diastereomers: δ 162.4, 162.2, 157.80, 157.78, 138.2, 138.1, 138.0, 137.9, 128.42, 128.38, 127.2, 127.1, 126.2, 126.0, 123.9, 123.7, 121.9, 121.8, 120.3, 120.2, 111.5, 110.3, 110.2, 109.5, 109.4, 109.1, 109.0, 107.7, 65.5, 64.6, 59.7 (q, J = 36.3 Hz) 59.6 (q, J = 36.3 Hz), 48.49, 48.46, 44.0, 40.7, 17.4, 14.9, 14.8, 11.6. IR 3063.84 (w), 2977.64 (m), 2930.38 (m), 1711.27 (s), 1708.59 (s), 1281.37 (s), 1167.60 (s). HMRS (ESI) m/z: [M + H]+ calc. for C25H24F3NO4, 460.1730; Found 460.1731.
2,2,2-Trifluoroethyl 5-ethoxy-4-ethyl-2-(1-methyl-1H-indol-2-yl)-4,5-dihydrofuran-3-carboxylate (3ac): Prepared following general procedure using 1-ethoxybutene (mixture of cis:trans = 1.5:1) 2c (308 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.48 in 20% EtOAC/Hexanes) afforded 3ac as the mixture of diastereomers (dr = 1.4:1) (179 mg, 73%). 1H NMR (400 MHz, CDCl3) for trans isomer: δ 7.65 (d, J = 7.0 Hz, 1H), 7.39–7.25 (m, 2H), 7.19–7.09 (m,1H), 7.02 (d, J = 0.9 Hz, 1H), 5.78 (d, J = 7.4 Hz, 1H), 4.58–4.33 (m, 2H), 3.98 (dqd, J = 9.6, 7.1, 3.9 Hz, 1H), 3.78 (s, 3H), 3.78–3.66 (m, 1H), 3.38 (ddd, J = 9.3, 7.3, 3.5 Hz, 1H), 1.96–1.79 (m, 2H), 1.30 (t, J = 6.9 Hz, 3H), 1.06 (t, J = 7.4 Hz, 3H); For cis isomer: δ 7.66 (d, J = 7.0 Hz, 1H), 7.39–7.25 (m, 2H), 7.19–7.09 (m, 2H), 5.37 (d, J = 1.9 Hz, 1H), 4.58–4.33 (m, 2H), 3.98 (dqd, J = 9.6, 7.1, 3.9 Hz, 1H), 3.78 (s, 3H), 3.78–3.66 (m, 1H), 3.21 (ddd, J = 8.6, 3.9, 1.9 Hz, 1H), 1.73–1.55 (m, 2H),1.32 (t, J = 6.9 Hz, 3H), 1.02 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) for both diastereomers: δ 162.6, 162.3, 158.3, 138.6, 138.4, 128.4, 128.1, 126.9, 126.8, 123.7, 123.5, 121.8, 121.6, 120.03, 119.95, 109.7, 109.6, 108.6, 107.8, 107.68, 107.65, 107.2, 65.9, 64.7, 59.7 (q, J = 36.3 Hz), 59.6 (q, J = 36.3 Hz), 50.4, 47.2, 31.5, 31.4, 24.3, 19.6, 15.1, 15.0, 12.3, 10.4. The 13C signal that is directly coupled by fluorine is not reported due to the low intensity. IR 2968.85 (m), 2936.33 (w), 2877.54 (w), 1720.23 (s), 1711.33 (m), 1277.33 (m), 1168.55 (s), 1084.69 (s). HMRS (ESI) m/z: [M + H]+ calc. for C20H22F3NO4, 398.1574; Found 398.1576.
2,2,2-Trifluoroethyl 5-methoxy-2-(1-methyl-1H-indol-2-yl)-4-phenyl-4,5-dihydrofuran-3-carboxylate (3ad): Prepared following general procedure using beta-methoxystyrene (pure cis isomer) 2d (413 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.33 in 20% EtOAC/Hexanes) afforded 3ad as yellow gel (140 mg, 53%). 1H NMR (500 MHz, CDCl3) δ 7.69 (dt, J = 7.9, 1.0 Hz, 1H), 7.40–7.29 (m, 7H), 7.25 (d, J = 0.9 Hz, 1H), 7.16 (ddd, J = 7.9, 6.8, 1.1 Hz, 1H), 5.86 (d, J = 7.8 Hz, 1H), 4.66 (d, J = 7.8 Hz, 1H), 3.89 (s, 3H), 3.50 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 162.0, 159.0, 138.8, 135.7, 129.2, 128.0, 127.6, 127.2, 126.8, 123.9, 122.8 (q, J = 277.5 Hz), 121.9, 120.2, 109.7, 108.9, 108.6, 107.9, 59.6 (q, J = 36.5 Hz), 57.7, 52.5, 31.8. IR 3061.65 (w), 3030.38 (w), 2937.40 (m), 2845.72 (w), 1720.41 (s), 1711.54 (m), 1277.50 (m), 1168.60 (s), 1092.46 (s). HMRS (ESI) m/z: [M + H]+ calc. for C23H20F3NO4, 432.1417; Found 432.1418.
2,2,2-Trifluoroethyl 5-ethoxy-2-(1-methyl-1H-indol-2-yl)-4,5-dihydrofuran-3-carboxylate (3ae): Prepared following general procedure using ethoxyethene 2e (413 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.39 in 20% EtOAC/Hexanes) afforded 3ae as yellow solid (171 mg, 75%). 1H NMR (500 MHz, CDCl3) δ 7.66 (dt, J = 8.0, 1.0 Hz, 1H), 7.37–7.28 (m, 2H), 7.16 (d, J = 0.8 Hz, 1H), 7.14 (ddd, J = 7.9, 6.8, 1.1 Hz, 1H), 5.75 (dd, J = 7.4, 2.8 Hz, 1H), 4.55–4.41 (m, 2H), 3.97 (dq, J = 9.5, 7.1 Hz, 1H), 3.81 (s, 3H), 3.71 (dq, J = 9.5, 7.1 Hz, 1H), 3.34 (dd, J = 16.6, 7.4 Hz, 1H), 3.05 (dd, J = 16.6, 2.8 Hz, 1H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.3, 158.1, 138.5, 127.8, 126.8, 123.7, 123.1 (q, J = 277.3 Hz), 121.8, 120.0, 109.7, 108.7, 105.2, 102.8, 64.8, 59.7 (q, J = 36.4 Hz), 37.2, 31.7, 15.1. IR 2977.71 (m), 2938.65 (w), 1720.30 (s), 1711.16 (m), 1629.92 (m), 1283.07 (s), 1247.21 (s), 1169.49 (s), 1080.27 (s). HMRS (ESI) m/z: [M + H]+ calc. for C18H18F3NO4, 370.1261; Found 370.1262.
2,2,2-Trifluoroethyl 5-(4-chlorophenyl)-5-ethoxy-2-(1-methyl-1H-indol-2-yl)-4,5-dihydrofuran-3-carboxylate (3ah): Prepared following general procedure using 1-chloro-4-(1-ethoxyvinyl)benzene 2h (562 mg, 3.07 mmol, which was prepared according to previous reported literature [46]) and diazo 1a (200 mg, 615 μmol). Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.52 in 20% EtOAC/Hexanes) afforded 3ah as yellow solid (192 mg, 65%). 1H NMR (500 MHz, CDCl3) δ 7.71 (dt, J = 8.0, 1.0 Hz, 1H), 7.50–7.47 (m, 2H), 7.44–7.39 (m, 3H), 7.35 (ddd, J = 8.3, 6.9, 1.2 Hz, 1H), 7.28 (d, J = 0.8 Hz, 1H), 7.18 (ddd, J = 8.0, 6.8, 1.1 Hz, 1H), 4.50 (qd, J = 8.5, 2.6 Hz, 2H), 3.90 (s, 3H), 3.71 (dq, J = 9.5, 7.1 Hz, 1H), 3.57 (d, J = 16.6 Hz, 1H), 3.41 (dq, J = 9.4, 7.1 Hz, 1H), 3.31 (d, J = 16.6 Hz, 1H), 1.24 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 162.1, 157.7, 138.7, 138.6, 134.6, 128.9, 127.7, 127.1, 126.8, 123.9, 123.1 (q, J = 277.5 Hz), 121.8, 120.2, 111.4, 109.7, 108.9, 103.5, 59.9 (d, J = 36.4 Hz), 59.7, 44.8, 31.9, 15.3. IR 3058.41 (w), 2978.18 (m), 2936.26 (w), 1724.57 (s), 1711.38 (m), 1283.63 (m), 1234.06 (m), 1171.08 (s), 1093.74 (s). HMRS (ESI) m/z: [M + H]+ calc. for C24H21ClF3NO4, 480.1184; Found 480.1186.
Reaction of Diazo1aand Trimethyl((1-phenylvinyl)oxy)silane2i. Following the general procedure using trimethyl((1-phenylvinyl)oxy)silane 2i (591 mg, 3.07 mmol) and diazo 1a (200 mg, 615 μmol), an inseparable mixture of dihydrofuran 3ai, carbazole 5ai, and acetophenone (hydrolysis product of enol silane 2i) was isolated following column chromatography (1–5% EtOAC/Hexanes). According to quantitative 1H-NMR (with DMF as the internal standard), the yields of 3ai and 5ai were 50% and 21%, respectively.
2,2,2-Trifluoroethyl 2-(1-methyl-1H-indol-2-yl)-3a,4,5,6a-tetrahydrofuro[2,3-b]furan-3-carboxylate (3aj): Prepared following general procedure using dihydrofuran 2j (215 mg, 3.07 mmol), diazo 1a (200 mg, 615 μmol) and 20 mol% Cu(hfacac)2 (59 mg, 123 μmol). This reaction was kept at reflux for 2 d. Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.3 in 20% EtOAC/Hexanes) afforded 3aj as yellow gel (112 mg, 50%). 1H NMR (500 MHz, CDCl3) δ 7.68 (dt, J = 7.9, 1.0 Hz, 1H), 7.37–7.30 (m, 2H), 7.25 (s, 1H), 7.15 (ddd, J = 7.9, 6.6, 1.4 Hz, 1H), 6.34 (d, J = 6.3 Hz, 1H), 4.63 (dq, J = 12.7, 8.5 Hz, 1H), 4.45 (dq, J = 12.7, 8.5 Hz, 1H), 4.19 (ddd, J = 8.7, 5.6, 2.4 Hz, 1H), 4.07–4.02 (m, 1H), 3.90–3.84 (m, 1H), 3.82 (s, 3H), 2.25 (dt, J = 8.4, 4.0 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 162.0, 160.6, 138.7, 127.0, 126.6, 124.0, 123.1 (q, J = 277.5 Hz), 121.8, 120.1, 109.9, 109.7, 109.3, 104.1, 67.1, 59.7 (q, J = 36.3 Hz), 47.6, 31.9, 31.8. IR 2958.56 (m), 2881.14 (m), 1721.91 (s), 1711.23 (m), 1619.47 (m), 1280.55 (s), 1233.10 (s), 1168.90 (s), 1077.28 (s). HMRS (ESI) m/z: [M + H]+ calc. for C18H16F3NO4, 368.1104; Found 368.1104.
2,2,2-Trifluoroethyl 2-(1-methyl-1H-indol-2-yl)-3a,5,6,7a-tetrahydro-4H-furo[2,3-b]pyran-3-carboxylate (3ak): Prepared following general procedure using 2,3-dihydropyran 2k (259 mg, 3.07 mmol), diazo 1a (200 mg, 615 μmol) and 20 mol% Cu(hfacac)2 (59 mg, 123 μmol). The reaction was kept at reflux for 3 d for complete consumption of starting material. Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.36 in 20% EtOAC/Hexanes) afforded 3ak as yellow gel. DMF was applied as internal NMR standard to quantify the yield (54%), since there were some undefined impurities. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 8.0 Hz, 1H), 7.36–7.28 (m, 2H), 7.21 (s, 1H), 7.12 (ddd, J = 7.9, 6.8, 1.2 Hz, 1H), 6.03 (d, J = 7.3 Hz, 1H), 4.55 (dq, J = 12.8, 8.5 Hz, 1H), 4.45 (dq, J = 12.7, 8.5 Hz, 1H), 3.94–3.86 (m, 2H), 3.82 (s, 3H), 3.21 (td, J = 7.3, 6.2 Hz, 1H), 2.23–2.13 (m, 1H), 1.79–1.70 (m, 3H). 13C NMR (126 MHz, CDCl3) δ 162.5, 159.3, 138.9, 127.6, 126.8, 124.0, 123.2 (q, J = 277.7 Hz), 121.9, 120.2, 109.8, 109.4, 108.1, 104.8, 61.7, 59.7 (q, J = 36.4 Hz), 38.2, 31.9, 23.5, 20.3. IR 3057.59 (w), 2949.15 (m), 1720.38 (s), 1711.18 (m), 1618.58 (m), 1613.47 (m), 1279.06 (m), 1161.26 (s), 1093.65 (s). HMRS (ESI) m/z: [M + H]+ calc. for C19H18F3NO4, 382.1261; Found 382.1261.
Reaction of Diazo1aand 5-methyl 2,3-dihydrofuran (2l). Following the general procedure with commercial 5-methyl 2,3-dihydropyran 2l (259 mg, 3.07 mmol), diazo 1a (200 mg, 615 μmol) and 20 mol% Cu(hfacac)2 (59 mg, 123 μmol), a 2.85:1 mixture (50% total yield according to 1H-NMR) of dihydrofurans 3al and 3am was isolated due to the in situ isomerization of 2l to 2-methylene tetrahydrofuran (2m) in the reaction pot. Interestingly, if synthesized 5-methyl 2,3-dihydropyran 2l [56] was used (see Supporting Information for 1H-NMR) instead of the commercial material, only 3am was isolated as the major product when the reaction was run at 65 °C. Carbazole 5ai could be prepared directly in 36% yield by the reaction of diazo 1a and 2i using Cu(hfacac)2 (30 mol%) with 1,2-dichloroethane as the solvent at 80 °C for 24 h.
2,2,2-Trifluoroethyl 2-(1-methyl-1H-indol-2-yl)-1,6-dioxaspiro[4.4]non-2-ene-3-carboxylate (3am): Prepared according to what is described previously. Purification via silica gel column chromatography (5% EtOAc/Hexanes, Rf = 0.3 in 20% EtOAc/Hexanes) afforded 3am as yellow gel (132 mg, 56%). 1H NMR (500 MHz, CDCl3) δ 7.63 (dd, J = 7.9, 1.0 Hz, 1H), 7.34–7.26 (m, 2H), 7.14–7.09 (m, 2H), 4.53–4.40 (m, 2H), 4.19–4.09 (m, 2H), 3.78 (s, 3H), 3.31 (d, J = 16.6 Hz, 1H), 3.27 (d, J = 16.6 Hz, 1H), 2.47–2.42 (m, 1H), 2.26–2.17 (m, 1H), 2.16–2.07 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 162.3, 157.8, 138.6, 127.9, 126.8, 123.7, 123.2 (q, J = 277.3 Hz), 121.8, 120.0, 117.7, 109.6, 108.6, 102.6, 69.1, 59.7 (q, J = 36.4 Hz), 38.5, 36.7, 31.6, 23.9. IR 3058.34 (w), 2922.83 (s), 2853.16 (m), 1720.57 (s), 1614.31 (s), 1463.21 (m), 1284.80 (s), 1171.14 (s). HMRS (ESI) m/z: [M + H]+ calc. for 382.1261; Found 382.1261.
2,2,2-Trifluoroethyl 5-methoxy-5-methyl-2-(1-methyl-1H-indol-3-yl)-4,5-dihydrofuran-3-carboxylate (3ca): Prepared following general procedure using 2-methoxypropene 2a (221 mg, 3.07 mmol) and diazo 1c (200 mg, 615 μmol). The reaction reached completion within 1 h under reflux. Purification via silica gel column chromatography (5% EtOAC/Hexanes, Rf = 0.5 in 20% EtOAC/Hexanes) afforded 3ca as yellow solid (132 mg, 58%). 1H NMR (500 MHz, CDCl3) δ 8.88 (s, 1H), 8.19 (d, J = 8.0 Hz, 1H), 7.38 (d, J = 8.2 Hz, 1H), 7.32 (t, 1H), 7.27 (d, J = 1.1 Hz, 1H), 4.63–4.50 (m, 2H), 3.89 (s, 3H), 3.44 (s, 3H), 3.24–3.04 (m, 2H), 1.78 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 164.2, 163.5, 136.8, 136.6, 126.9, 123.5 (d, J = 277.6 Hz), 122.7, 122.7, 121.5, 110.5, 109.7, 104.4, 94.5, 59.5 (q, J = 35.8 Hz), 50.2, 39.7, 33.5, 25.1. IR 3113.53 (w), 2938.52 (w), 1763.42 (s), 1759.62 (s), 1653.54 (s), 1530.37 (m), 1282.47 (m), 1169.54 (s), 1087.58 (m). HMRS (ESI) m/z: [M + H]+ calc. for C18H18F3NO4, 370.1260; Found 370.1262.
D. Ring-opening benzannulation of DHF toward 1-hydroxycarbazoles 5 and 6.
General procedure A: To a vial or pressured tube charged with a stir bar and a solution of DHF 3 in toluene (0.1 M) was added Yb(OTf)3 (10 mol%). Then, the suspension was set under sonication for 1 min. The reaction was stirred under 70 °C for 1 h or monitored by TLC for completion. After consuming all DHF, the mixture was concentrated under reduced pressure, and purified by column chromatography.
General procedure B: To a vial or pressured tube charged with a stir bar and a solution of DHF 3 in toluene (0.1 M) was added Al(OTf)3 (10 mol%). Then, the suspension was set under sonication for 1 min, and 1 small drop of water (~4 mg) was added to the mixture. After the mixture was sonicated for another 5 min, the reaction was stirred vigorously under 70 °C for 1 h. After consuming all DHF, the mixture was concentrated under reduced pressure, and purified by column chromatography.
Note: Since the 19F signals of all carbazole products have similar chemical shifts (−73.3 ppm to −73.8 ppm) with same coupling constant (t, J = 8.5 Hz), only the 19F NMR of 5aa is reported and attached.
2,2,2-Trifluoroethyl 1-hydroxy-4,9-dimethyl-9H-carbazole-2-carboxylate (5aa): Prepared following general procedure A using DHF 3aa (89 mg, 0.24 mmol) and Yb(OTf)3 (15 mg, 24 μmol) in toluene (2.4 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5aa as yellow crystal (73 mg, 90%). 1H NMR (700 MHz, CDCl3) δ 10.99 (s, 1H), 8.21 (dt, J = 8.0, 1.0 Hz, 1H), 7.56 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.40 (s, 1H), 7.28 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 4.75 (q, J = 8.4 Hz, 2H), 4.25 (s, 3H), 2.80 (s, 3H). 13C NMR (176 MHz, CDCl3) δ 169.7, 149.6, 142.5, 128.4, 128.1, 126.8, 124.1, 123.3, 123.0 (q, J = 277.7 Hz), 122.8, 119.6, 119.5, 109.1, 105.7, 60.6 (q, J = 36.7 Hz), 32.1, 20.4. 19F NMR (471 MHz, CDCl3) δ −73.44 (t, J = 8.5 Hz, 3F). IR 3057.01 (w), 2963.78 (w), 1670.40 (s), 1278.20 (s), 1234.57 (s), 1157.37 (s), 1127.22 (s). HMRS (ESI) m/z: [M + H]+ calc. for C17H14F3NO3, 338.0999; Found 338.0998.
2,2,2-Trifluoroethyl 1-hydroxy-3,9-dimethyl-9H-carbazole-2-carboxylate (5ab): Prepared following general procedure B using DHF 3ab (110 mg, 0.287 mmol) and Al(OTf)3 (14 mg, 29 μmol) in toluene (2.8 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5ab as yellow crystal (65 mg, 67%). 1H NMR (400 MHz, CDCl3) δ 11.83 (s, 1H), 7.52 (ddd, J = 8.3, 7.0, 1.2 Hz, 1H), 7.41–7.37 (m, 2H), 7.23 (ddd, J = 7.9, 7.1, 1.0 Hz, 1H), 4.72 (q, J = 8.4 Hz, 2H), 4.19 (s, 3H), 2.67 (d, J = 0.9 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 171.3, 152.7, 142.8, 130.4, 128.0, 127.3, 123.1 (q, J = 277.8 Hz) 121.7, 121.0, 119.2, 114.3, 109.2, 106.3, 60.9 (q, J = 36.8 Hz), 32.0, 24.5. IR 3030.13 (w), 2961.61 (m), 2937.58 (m), 1649.38 (s), 1637.42 (s), 1316.84 (s), 1272.05 (s), 1158.44 (s), 1036.63 (s). HMRS (ESI) m/z: [M + H]+ calc. for C17H14F3NO3, 338.0999; Found 338.0998.
2,2,2-Trifluoroethyl 3-ethyl-1-hydroxy-9-methyl-9H-carbazole-2-carboxylate (5ac): Prepared following general procedure B using DHF 3ac (108 mg, 0.272 mmol) and Al(OTf)3 (13 mg, 27 μmol) in toluene (2.7 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5ac as yellow crystal (59 mg, 62%). 1H NMR (500 MHz, CDCl3) δ 11.80 (s, 1H), 8.03 (dt, J = 7.8, 1.0 Hz, 1H), 7.53 (ddd, J = 8.3, 7.1, 1.2 Hz, 1H), 7.44 (s, 1H), 7.40 (dt, J = 8.4, 0.9 Hz, 1H), 7.24 (ddd, J = 7.9, 7.1, 0.9 Hz, 1H), 4.74 (q, J = 8.4 Hz, 2H), 4.19 (s, 3H), 3.07 (q, J = 7.4 Hz, 2H), 1.29 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 171.1, 152.6, 142.8, 136.9, 128.1, 127.3 (d, J = 2.3 Hz), 123.0 (q, J = 277.2 Hz), 121.8, 121.0, 119.2, 113.1, 109.2, 105.7, 61.0 (q, J = 36.8 Hz), 32.0, 30.1, 16.8. IR 3026.24 (w), 2954.17 (m), 2870.75 (w), 1649.94 (s), 1315.64 (s), 1259.43 (s), 1158.68 (s), 1123.55 (s), 1036.82 (m). HMRS (ESI) m/z: [M + H]+ calc. for C18H16F3NO3, 352.1155; Found 352.1153.
2,2,2-Trifluoroethyl 1-hydroxy-9-methyl-3-phenyl-9H-carbazole-2-carboxylate (5ad): Prepared following general procedure B using DHF 3ad (100 mg, 0.232 mmol) and Al(OTf)3 (11 mg, 23 μmol) in toluene (2.3 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5ad as yellow crystal (60 mg, 65%). 1H NMR (500 MHz, CDCl3) δ 11.38 (s, 1H), 8.02 (dt, J = 7.9, 1.0 Hz, 1H), 7.56 (ddd, J = 8.3, 7.0, 1.2 Hz, 1H), 7.51 (s, 1H), 7.46–7.33 (m, 6H), 7.26 (ddd, J = 7.9, 6.6, 0.9 Hz, 1H), 4.38 (q, J = 8.4 Hz, 2H), 4.26 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 170.7, 151.2, 143.5, 142.8, 135.0, 128.4, 127.7, 127.4, 126.4, 122.2 (q, J = 277.5 Hz), 122.0, 121.1, 119.6, 114.9, 109.3, 106.1, 60.6 (q, J = 37.2 Hz), 32.1. IR 3022.72 (w), 2916.53 (w), 1655.22 (s), 1321.40 (s), 1281.60 (m), 1159.52 (s), 1030.67 (m). HMRS (ESI) m/z: [M + H]+ calc. for C22H16F3NO3, 400.1155; Found 400.1154.
2,2,2-Trifluoroethyl 1-hydroxy-9-methyl-9H-carbazole-2-carboxylate (5ae): Prepared following general procedure A using DHF 3ae (150 mg, 0.406 mmol) and Yb(OTf)3 (25 mg, 41 μmol) in toluene (4 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5ae as yellow crystal (106 mg, 81%). 1H NMR (500 MHz, CDCl3) δ 11.15 (s, 1H), 8.07 (dt, J = 7.8, 1.0 Hz, 1H), 7.65 (d, J = 8.4 Hz, 1H), 7.60–7.51 (m, 2H), 7.43 (dt, J = 8.4, 0.9 Hz, 1H), 7.26 (ddd, J = 7.9, 7.1, 0.9 Hz, 1H), 4.75 (q, J = 8.3 Hz, 2H), 4.23 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 151.2, 142.4, 129.4, 128.3, 127.4, 123.0 (q, J = 277.3 Hz), 122.0, 121.1, 119.5, 119.5, 111.5, 109.2, 106.2, 60.6 (q, J = 36.9 Hz), 32.1. IR 3061.58 (w), 2964.60 (m), 2933.49 (w), 1668.50 (s), 1630.09 (m), 1464.87 (s), 1250.61 (s), 1144.95 (s), 1040.08 (m). HMRS (ESI) m/z: [M + H]+ calc. for C16H12F3NO3, 324.0842; Found 324.0840.
Reaction of dihydrofuran3ahwith Al(OTf)3: Following the general procedure B with dihydrofuran 3ah, Al(OTf)3, and no added, an inseparable mixture of carbazole 5ah and furan 4ah was formed. Since product 5ah and 4ah run at similar Rf value, q-NMR was applied to quantify the yield of each one. According to quantitative 1H-NMR (with DMF as internal standard), the respective yield of 5ah and 4ah in the mixture were 19% and 68%.
2,2,2-Trifluoroethyl 1-hydroxy-9-methyl-4-phenyl-9H-carbazole-2-carboxylate (5ai): To a dry flask charged with a stir bar and the mixture of 3ai and 5ai prepared in previous section, dry 1,2-DCE (6 mL) was added, followed by addition of Cu(hfacac)2 (59 mg, 123 μmol, 20 mol%.). The solution was then heated to 80 °C for 24 h, diluted with Et2O (30 mL) and washed with saturated thiourea (20 mL). The organic layer was dried over Na2SO4, concentrated under reduced pressure, and purified by column chromatography (silica gel, 0–5% EtOAC/Hexanes), which afforded 5ai as pale-yellow crystal (88 mg, 36% over two steps). 1H NMR (500 MHz, CDCl3) δ 11.18 (s, 1H), 7.57–7.46 (m, 7H), 7.43 (dt, J = 8.4, 0.9 Hz, 1H), 7.35 (dt, J = 8.1, 1.0 Hz, 1H), 6.99 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H), 4.75 (q, J = 8.4 Hz, 2H), 4.30 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.7, 150.5, 142.7, 140.3, 129.4, 129.1, 128.6, 128.5, 127.6, 127.1, 127.0, 123.1, 122.9 (q, J = 277.4 Hz), 121.8, 120.2, 119.2, 109.1, 105.9, 60.6 (q, J = 37.2 Hz), 32.2. IR 3056.30 (w), 2946.60 (w), 1658.71 (s), 1465.88 (m), 1236.68 (s), 1234.71 (s) 1155.21 (s), 1128.94 (m). HMRS (ESI) m/z: [M-H]− calc. for C22H16F3NO3, 398.1010; Found 398.1004.
11-Hydroxy-10-methyl-4,10-dihydropyrano[3,4-b]carbazol-1(3H)-one (6aj): Prepared following general procedure B using DHF 3aj (88 mg, 0.24 mmol) and Al(OTf)3 (11 mg, 24 μmol) in toluene (2.4 mL) without addition of water. Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.38 in 20% EtOAC/Hexanes) afforded 5ae as yellow crystal (50 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 12.01 (s, 1H), 8.02 (dt, J = 7.8, 1.0 Hz, 1H), 7.54 (ddd, J = 8.3, 7.1, 1.2 Hz, 1H), 7.42 (dt, J = 8.4, 0.9 Hz, 1H), 7.36 (t, J = 1.1 Hz, 1H), 7.24 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H), 4.62 (dd, J = 6.4, 5.6 Hz, 2H), 4.21 (s, 3H), 3.19 (ddd, J = 6.6, 5.5, 1.1 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 171.3, 151.5, 142.6, 128.7, 128.4, 127.5, 121.8, 121.0, 119.4, 109.3, 109.0, 104.0, 68.8, 31.9, 28.1. IR 3056.02 (w), 2914.82 (m), 1648.69 (s), 1468.19 (m), 1302.74 (s), 1253.14 (s), 1124.21 (s). HMRS (ESI) m/z: [M + H]+ calc. for C16H13NO3, 268.0968; Found 268.0968.
2,2,2-Trifluoroethyl 1-hydroxy-3-(3-hydroxypropyl)-9-methyl-9H-carbazole-2-carboxylate (5ak): Prepared following general procedure B using DHF 3ak (75 mg, 0.24 mmol) and Al(OTf)3 (9 mg, 20 μmol) in toluene (2 mL) without addition of water. Purification via silica gel column chromatography (10–30% EtOAC/Hexanes, Rf = 0.14 in 20% EtOAC/Hexanes) afforded 5ak as yellow crystal (25 mg, 33%). 1H NMR (500 MHz, CDCl3) δ 11.81 (s, 1H), 8.03 (dt, J = 7.8, 1.0 Hz, 1H), 7.53 (ddd, J = 8.3, 7.0, 1.2 Hz, 1H), 7.46 (s, 1H), 7.41 (dt, J = 8.4, 0.9 Hz, 1H), 7.24 (ddd, J = 7.9, 7.0, 0.9 Hz, 1H), 4.78 (q, J = 8.4 Hz, 2H), 4.21 (s, 3H), 3.72 (t, J = 6.4 Hz, 2H), 3.16–3.12 (m, 2H), 1.95–1.88 (m, 2H), 1.49 (s, br, 1H). 13C NMR (126 MHz, CDCl3) δ 171.0, 152.9, 142.8, 134.1, 128.1, 127.5, 127.4, 123.1 (q, J = 277.3 Hz), 121.7, 121.1, 119.3, 114.1, 109.2, 105.7, 62.4, 61.0 (q, J = 36.8 Hz), 35.2, 33.3, 32.1. IR 3324.04 (w, br), 2958.85 (m), 2925.22 (m), 1647.07 (s), 1434.70 (m), 1313.12 (s), 1256.98 (s), 1157.55 (s), 1035.10 (s). HMRS (ESI) m/z: [M + H]+ calc. for C19H18F3NO4, 382.1261; Found 382.1259.
11-Hydroxy-5,10-dimethyl-4,10-dihydropyrano[3,4-b]carbazol-1(3H)-one (6al) and 2,2,2-Trifluoroethyl 1-hydroxy-4-(3-hydroxypropyl)-9-methyl-9H-carbazole-2-carboxylate (5am): To a vial charged with a stir bar and a 2.85:1 mixture of 3al and 3am (100 mg, 0.262 mmol) in toluene (2.5 mL) was added Al(OTf)3 (12 mg, 26.2 μmol). The suspension was set under sonication for 1 min, followed by stirred and heated under 70 °C for 1 h. Then, another portion of Al(OTf)3 (12 mg, 26.2 μmol) was added to the reaction followed by increasing temperature to 85 °C for additional 1 h. After consuming all DHF, the mixture was concentrated under reduced pressure, and purified by column chromatography.
6al: Purification on a silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.38 in 20% EtOAC/Hexanes) afforded 5al as yellow crystal (45 mg, 61%). 1H NMR (400 MHz, CDCl3) δ 12.06 (s, 1H), 8.24 (d, J = 8.1 Hz, 1H), 7.55 (ddd, J = 8.3, 7.0, 1.1 Hz, 2H), 7.44 (dt, J = 8.3, 0.9 Hz, 2H), 7.25 (ddd, J = 8.1, 7.1, 1.1 Hz, 1H), 4.60 (dd, J = 6.5, 5.6 Hz, 3H), 4.22 (s, 5H), 3.15 (t, J = 6.1 Hz, 3H), 2.69 (s, 5H). 13C NMR (101 MHz, CDCl3) δ 171.8, 150.0, 142.7, 127.9, 127.4, 126.8, 125.4, 123.5, 122.7, 119.6, 119.3, 109.1, 103.9, 68.3, 32.0, 24.9, 15.6. IR 2994.64 (w), 2922.00 (m), 2851.13 (w), 1654.70 (s), 1626.95 (m), 1317.00 (s), 1224. 95 (s), 1158. 46 (m), 1134.11 (m). HMRS (ESI) m/z: [M + H]+ calc. for C17H15NO3, 282.1125; Found 282.1118.
5am: Purification via silica gel column chromatography (10–30% EtOAC/Hexanes, Rf = 0.14 in 20% EtOAC/Hexanes) afforded 5am as pale-yellow crystal (19 mg, 19%). 1H NMR (500 MHz, CDCl3) δ 11.03 (s, 1H), 8.20 (dt, J = 8.1, 0.9 Hz, 1H), 7.56 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.47 (dt, J = 8.3, 0.9 Hz, 1H), 7.43 (s, 1H), 7.28 (ddd, J = 8.1, 7.1, 1.1 Hz, 1H), 4.76 (q, J = 8.3 Hz, 2H), 4.26 (s, 3H), 3.84 (t, J = 6.3 Hz, 2H), 3.30–3.21 (m, 2H), 2.13–2.05 (m, 2H). 1.41 (s, br, 1H). 13C NMR (126 MHz, CDCl3) δ 169.6, 149.8, 142.6, 128.8, 128.1, 127.4, 126.8, 123.3, 123.0 (q, J = 277.5 Hz), 121.9, 119.7, 118.8, 109.2, 105.8, 62.6, 60.6 (q, J = 37.0 Hz), 32.5, 32.1, 30.0. IR 3325.97 (s, br), 2931.32 (m), 2891.86 (w), 1662.28 (s), 1332.21 (s), 1278.97 (s), 1239.36 (s), 1153.52(s), 1040.43 (s). HMRS (ESI) m/z: [M + H]+ calc. for C19H18F3NO4, 382.1261; Found 382.1258.
2,2,2-Trifluoroethyl 9-benzyl-1-hydroxy-4-methyl-9H-carbazole-2-carboxylate (5ba): Prepared following general procedure A using DHF 3ba (175 mg, 0.393 mmol) and Yb(OTf)3 (24 mg, 39 μmol) in toluene (3.9 mL). Purification via silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5ba as yellow crystal (133 mg, 82%). 1H NMR (500 MHz, CDCl3) δ 11.36 (s, 1H), 8.58 (dt, J = 8.0, 0.9 Hz, 1H), 7.84 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 7.80–7.74 (m, 2H), 7.67–7.52 (m, 4H), 7.53–7.47 (m, 2H), 6.30 (s, 2H), 5.07 (q, J = 8.3 Hz, 2H), 3.16 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 169.5, 149.20, 142.0, 138.6, 128.5, 128.3, 127.9, 127.1, 126.9, 126.3, 124.0, 123.3, 123.04, 122.97 (q, J = 277.1 Hz), 119.9, 119.8, 109.8, 105.9, 60.6 (q, J = 36.8 Hz), 48.5, 20.4. IR 2969.19 (w), 2918.79 (w), 1664.19 (s), 1459.67 (s), 1302.85 (m), 1156.55 (s), 1122.03 (s). HMRS (ESI) m/z: [M + H]+ calc. for C23H18F3NO3, 414.1311; Found 414.1313.
2,2,2-Trifluoroethyl 9-benzyl-1-hydroxy-3-methyl-9H-carbazole-2-carboxylate (5bb): Prepared following general procedure B using DHF 3bb (624 mg, 1.36 mmol) and Al(OTf)3 (64 mg, 136 μmol) in toluene (14 mL) with couple drops of water. Purification on a silica gel column chromatography (1–10% EtOAC/Hexanes, Rf = 0.59 in 20% EtOAC/Hexanes) afforded 5bb as yellow crystal (376 mg, 67%). 1H NMR (500 MHz, CDCl3) δ 11.88 (s, 1H), 8.06 (d, J = 7.8 Hz, 1H), 7.49–7.45 (m, 2H), 7.38 (d, J = 8.3 Hz, 1H), 7.26–7.18 (m, 4H), 7.17–7.13 (m, 2H), 5.93 (s, 2H), 4.74 (q, J = 8.3 Hz, 2H), 2.71 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 171.3, 152.5, 142.4, 138.8, 130.9, 128.5, 128.4, 127.5, 127.1, 126.9, 126.4, 123.0 (q, J = 277.4 Hz), 122.0, 121.1, 119.6, 114.4, 109.9, 106.7, 60.9 (q, J = 37.0 Hz), 48.5, 24.6. IR 3030.93 (w), 2974.09 (w), 2936.23 (m), 1645.01 (s), 1435.07 (s), 1314.23 (s), 1251.83 (s), 1150.59 (s), 1038.06 (s). HMRS (ESI) m/z: [M + H]+ calc. for C23H18F3NO3, 414.1311; Found 414.1313.
E. Synthesis of benzyl-protected Murrayafoline A (9) from Carbazole 5bb: The conversion from 5bb to murrayafoline A was inspired by a reported literature procedure [57]. Wet DMSO (10 mL) was added to a vial charged with a stir bar and 5bb (310 mg, 0.75 mmol). The mixture was heating at 120 °C for overnight (~18 h), poured into 40 mL brine, extracted with 50 mL EtOAc. The organic layer was washed with brine (2 × 40 mL) and the combined aqueous layer was extracted with another 50 mL EtOAc. The combined organic layer was dried over Na2SO4, concentrated under reduced pressure, and purified by column chromatography to afford the decarboxylated carbazole product as a yellow solid (151 mg, 70%, with 18% recovery of 5bb). Next, anhydrous acetone (20 mL) was added to a flask charged with a stir bar, decarboxylated product (151 mg, 0.525 mmol), and K2CO3 (363 mg, 2.63 mmol, 5 eq.). The mixture was stirred at 0 °C for 20 min, then MeI (373 mg, 2.63 mmol, 5 eq.) was slowly added to cold suspension. The reaction mixture was removed from the ice-bath stirred at room temperature overnight (~18 h). The reaction was concentrated under reduced pressure, re-dissolved in Et2O (60 mL), and washed with brine (30 mL). The organic layer was dried over Na2SO4, concentrated under reduced pressure, and purified by column chromatography to afford N-benzyl murrayafoline A (9) as a yellow gel (150 mg, 94%). Characterizations were consistent with previously reported literature [48] and 1H-NMR spectrum is attached.
4. Conclusions
In summary, we have reported a Lewis acid-catalyzed synthesis of substituted 1-hydroxycarbazole-2-carboxylates in yields up to 90%. The approach employs 2,3-dihydrofuran acetals as substrates for an intramolecular ring-opening benzannulation. The dihydrofurans are readily accessed from the Cu(II)-catalyzed reaction of enol ethers and α-diazo-β-indolyl-β-ketoesters. The substituent pattern of the enol ether is ultimately reflected in the carbazole products as 1,1-disubstituted enol ethers afford 4-substituted-1-hydroxycarbazole-2-carboxylates, 1,2-disubstituted enol ethers provide the corresponding 3-substituted-1-hydroxycarbazole derivatives, and a 1,1,2-trisubstituted enol ether gives the 3,4-disubstituted-1-hydroxycarbazole-2-carboxylates. Thus, a modular approach to dihydrofurans allows for strategic synthetic design to access carbazole structural diversity. As an example, a formal synthesis of murrayafoline A, a bioactive natural product, was outlined.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238344/s1, The charts for 1H, 13C, and representative 19F NMRs are available online.
Author Contributions
Conceptualization, S.F., G.G.F. and S.Y.; methodology, S.Y.; formal analysis, S.F. and S.Y.; investigation, S.F. and S.Y.; resources, S.F.; data curation, S.Y., H.E.A. and N.E.P.; writing—original draft preparation, S.F., S.Y. and G.G.F.; writing—review and editing, S.Y., G.G.F. and H.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation (CHE-2102472) and by Georgia Tech through the Leddy Family Fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article or the Supplementary Materials here. Samples of diazo compounds, dihydrofurans, and carbazoles are available from the authors.
Acknowledgments
The authors would like to thank Caria Evans for her analytical support toward IR data collection. Single-crystal diffraction experiments were performed at the Georgia Tech SCXRD facility directed by John Bacsa.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Knölker, H.-J.; Reddy, K.R. Isolation and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2002, 102, 4303–4428. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.W.; Reddy, K.R.; Knölker, H.-J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193–3328. [Google Scholar] [CrossRef] [PubMed]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole Scaffolds in Cancer Therapy: A Review from 2012 to 2018. J. Enzyme Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef] [PubMed]
- Knölker, H.-J.; Reddy, K.R. Chapter 4—Biological and Pharmacological Activities of Carbazole Alkaloids. In The Alkaloids; Cordell, G.A., Ed.; Academic Press: London, UK, 2008; Volume 65, pp. 181–193. [Google Scholar]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent Developments and Biological Activities of N-Substituted Carbazole Derivatives: A Review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef]
- Głuszyńska, A. Biological Potential of Carbazole Derivatives. Eur. J. Med. Chem. 2015, 94, 405–426. [Google Scholar] [CrossRef]
- Caruso, A.; Ceramella, J.; Iacopetta, D.; Saturnino, C.; Mauro, M.V.; Bruno, R.; Aquaro, S.; Sinicropi, M.S. Carbazole Derivatives as Antiviral Agents: An Overview. Molecules 2019, 24, 1912. [Google Scholar] [CrossRef]
- Juret, P.; Heron, J.F.; Couette, J.E.; Delozier, T.; Le Talaer, J.Y. Hydroxy-9-methyl-2-ellipticinium for osseous metastases from breast cancer: A 5 year experience. Cancer Treat. Rep. 1982, 66, 1909–1916. [Google Scholar]
- Wisler, J.W.; DeWire, S.M.; Whalen, E.J.; Violin, J.D.; Drake, M.T.; Ahn, S.; Shenoy, S.K.; Lefkowitz, R.J. A unique mechanism of -blocker action: Carvedilol stimulates-arrestin signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 16657–16662. [Google Scholar] [CrossRef]
- Fox, S.M.; Johnston, S.A. Use of carprofen for the treatment of pain and inflammation in dogs. J. Am. Vet. Med. Assoc. 1997, 210, 1493–1498. [Google Scholar]
- Grazulevicius, J.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Li, J.; Grimsdale, A.C. Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 2010, 39, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, J.; Zhang, J. A Review on Synthesis of Carbazole-based Chromophores as Organic Light-emitting Materials. Curr. Org. Chem. 2012, 16, 2014–2025. [Google Scholar] [CrossRef]
- Yin, J.; Ma, Y.; Li, G.; Peng, M.; Lin, W. A versatile small-molecule fluorescence scaffold: Carbazole derivatives for bioimaging. Coord. Chem. Rev. 2020, 412, 213257. [Google Scholar] [CrossRef]
- Aggarwal, T.; Verma, A.K. Recent Advances in the Synthesis of Carbazoles from Indoles. Org. Biomol. Chem. 2019, 17, 8330–8342. [Google Scholar] [CrossRef]
- Banerjee, A.; Kundu, S.; Bhattacharyya, A.; Sahu, S.; Maji, M.S. Benzannulation Strategies for the Synthesis of Carbazoles, Indolocarbazoles, Benzocarbazoles, and Carbolines. Org. Chem. Front. 2021, 8, 2710–2771. [Google Scholar] [CrossRef]
- Georgiades, S.N.; Nicolaou, P.G. Chapter One—Recent Advances in Carbazole Syntheses. Adv. Heterocycl. Chem. 2019, 129, 1–88. [Google Scholar] [CrossRef]
- Roy, J.; Jana, A.K.; Mal, D. Recent Trends in the Synthesis of Carbazoles: An Update. Tetrahedron 2012, 68, 6099–6121. [Google Scholar] [CrossRef]
- Yaqub, G.; Hussain, E.; Rehman, M.A.; Mateen, B. Review on advancements in synthesis of carbazoles. Asian J. Chem. 2009, 21, 2485–2520. [Google Scholar]
- For Pertinent Examples of Carbazole Synthesis (since 2017), See Refs 20–44. SciFinder; Chemical Abstracts Service: Columbus, OH; Research Topic: “Carbazole Synthesis”. Available online: https://scifinder.cas.org (accessed on 22 November 2022).
- Munoz-Torres, M.A.; Martinez-Lara, F.; Solas, M.; Suarez-Pantiga, S.; Sanz, R. “Back-to-Front” Indole and Carbazole Synthesis from N,N-Bis-(2-bromoallyl)amines by Carbolithiation Reactions with Gold-Catalysis. Adv. Synth. Catal. 2022, 364, 3716–3724. [Google Scholar] [CrossRef]
- Barrera, E.; Hernandez-Benitez, R.I.; Gonzalez-Gonzalez, C.A.; Escalante, C.H.; Fuentes-Benites, A.; Gonzalez-Romero, C.; Becerra-Martinez, E.; Delgado, F.; Tamariz, J. Synthesis of Diarylamines and Methylcarbazoles and Formal Total Synthesis of Alkaloids Ellipticine and Olivacine. Eur. J. Org. Chem. 2022, 2022, e202200364. [Google Scholar] [CrossRef]
- Campbell, E.; Taladriz-Sender, A.; Paisley, O.I.; Kennedy, A.R.; Bush, J.T.; Burley, G.A. A Chemo- and regioselective tandem [3 + 2]heteroannulation strategy for carbazole synthesis: Combining two mechanistically distinct bond-forming processes. ChemRxiv 2021, 87, 4603–4616. [Google Scholar] [CrossRef] [PubMed]
- Reshma, H.P.; Emmanuel, B.D.; Beevi, J.; Dharan, S.S. Insilico design, synthesis and biological evaluation of novel carbazole derivatives. J. Pharm. Sci. Res. 2021, 13, 8–18. [Google Scholar]
- Merkushev, A.A.; Makarov, A.S.; Shpuntov, P.M.; Abaev, V.T.; Trushkov, I.V.; Uchuskin, M.G. Oxidative Rearrangement of 2-(2-Aminobenzyl)furans: Synthesis of Functionalized Indoles and Carbazoles. Eur. J. Org. Chem. 2021, 2021, 1274–1285. [Google Scholar] [CrossRef]
- Alavi, S.; Lin, J.-B.; Grover, H.K. Copper-Catalyzed Annulation of Indolyl α-Diazocarbonyl Compounds Leads to Structurally Rearranged Carbazoles. Org. Lett. 2021, 23, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Points, G.L., III; Beaudry, C.M. Regioselective Synthesis of Substituted Carbazoles, Bicarbazoles, and Clausine C. Org. Lett. 2021, 23, 6882–6885. [Google Scholar] [CrossRef]
- Faltracco, M.; Damian, M.; Ruijter, E. Synthesis of Carbazoles and Dihydrocarbazoles by a Divergent Cascade Reaction of Donor–Acceptor Cyclopropanes. Org. Lett. 2021, 23, 7592–7596. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, D.; Zeng, J.; Liu, Y.; Bu, X.; Yang, X. Benzocarbazole Synthesis via Visible-Light-Accelerated Rh(III)-Catalyzed C–H Annulation of Aromatic Amines with Bicyclic Alkenes. Org. Lett. 2021, 23, 7740–7745. [Google Scholar] [CrossRef]
- Faltracco, M.; Ortega-Rosales, S.; Janssen, E.; Cioc, R.C.; Vande Velde, C.M.L.; Ruijter, E. Synthesis of Carbazoles by a Diverted Bischler–Napieralski Cascade Reaction. Org. Lett. 2021, 23, 3100–3104. [Google Scholar] [CrossRef]
- Hao, T.; Huang, L.; Wei, Y.; Shi, M. Copper-Catalyzed Synthesis of Indolyl Benzo[b]Carbazoles and Their Photoluminescence Property. Org. Lett. 2021, 23, 5133–5137. [Google Scholar] [CrossRef]
- Wu, C.-J.; Cao, W.-X.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Tandem [2+2] Cycloaddition/Rearrangement toward Carbazoles by Visible-Light Photocatalysis. Org. Lett. 2021, 23, 2135–2139. [Google Scholar] [CrossRef]
- Reddy, C.R.; Srinivasu, E.; Sathish, P.; Subbarao, M.; Donthiri, R.R. One-Pot Arylative Benzannulation of 2-Carbonyl-3-Propargyl Indoles with Boronic Acids Leading to Arylated Carbazoles. J. Org. Chem. 2021, 86, 1118–1132. [Google Scholar] [CrossRef]
- Guo, T.; Han, L.; Wang, T.; Lei, L.; Zhang, J.; Xu, D. Copper-Catalyzed Three-Component Formal [3 + 1 + 2] Benzannulation for Carbazole and Indole Synthesis. J. Org. Chem. 2020, 85, 9117–9128. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Song, L.; Hashmi, A.S.K. Synthesis of Carbazoles and Related Heterocycles from Sulfilimines by Intramolecular C−H Aminations. Angew. Chem. Int. Ed. 2020, 59, 12342. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Samineni, R.; Pabbaraja, S.; Mehta, G. A General Carbazole Synthesis via Stitching of Indole-Ynones with Nitromethanes: Application to Total Synthesis of Carbazomycin A, Calothrixin B, and Staurosporinone. Org. Lett. 2019, 21, 3372–3376. [Google Scholar] [CrossRef]
- Bal, A.; Maiti, S.; Mal, P. Iodine(III)-Enabled Distal C-H Functionalization of Biarylsulfonanilides. J. Org. Chem. 2018, 83, 11278–11287. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Bose, A.; Mal, P. Oxidative N-Arylation for Carbazole Synthesis by C-C Bond Activation. J. Org. Chem. 2018, 83, 8127–8138. [Google Scholar] [CrossRef]
- Tharra, P.; Baire, B. Regioselective Cyclization of (Indol-3-yl)pentyn-3-ols as an Approach to (Tetrahydro)carbazoles. Org. Lett. 2018, 20, 1118–1121. [Google Scholar] [CrossRef]
- Shao, C.; Zhou, B.; Wu, Z.; Ji, X.; Zhang, Y. Synthesis of Carbazoles from 2-Iodobiphenyls by Palladium-Catalyzed C−H Activation and Amination with Diaziridinone. Adv. Synth. Catal. 2018, 360, 887–892. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Rocchi, D.; Tejero, T.; Merino, P.; Menendez, J.C. One-pot synthesis of functionalized carbazoles via a CAN-catalyzed multicomponent process comprising a C-H activation step. J. Org. Chem. 2017, 82, 7492–7502. [Google Scholar] [CrossRef]
- Alimi, I.; Remy, R.; Bochet, C.G. Photochemical C-H Activation: Generation of Indole and Carbazole Libraries, and First Total Synthesis of Clausenawalline D. Eur. J. Org. Chem. 2017, 2017, 3197–3210. [Google Scholar] [CrossRef]
- Maiti, S.; Mal, P. Dehydrogenative Aromatic Ring Fusion for Carbazole Synthesis via C-C/C-N Bond Formation and Alkyl Migration. Org. Lett. 2017, 19, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gin, E.; Wasinska-Kalwa, M.; Banwell, M.G.; Carr, P.D. A Palladium-Catalyzed Ullmann Cross-Coupling/Reductive Cyclization Route to the Carbazole Natural Products 3-Methyl-9H-carbazole, Glycoborine, Glycozoline, Clauszoline K, Mukonine, and Karapinchamine A. J. Org. Chem. 2017, 82, 4148–4159. [Google Scholar] [CrossRef] [PubMed]
- Aponte-Guzmán, J.; Phun, L.H.; Cavitt, M.A.; Taylor, J.E., Jr.; Davy, J.C.; France, S. Catalytic, Cascade Ring-Opening Benzannulations of 2,3-Dihydrofuran O,O- and N,O-Acetals. Chem. Eur. J. 2016, 22, 10405–10409. [Google Scholar] [CrossRef] [PubMed]
- Guerra Faura, G.; Nguyen, T.; France, S. Catalyst-Controlled Chemodivergent Reactions of 2-Pyrrolyl-α-diazo-β-ketoesters and Enol Ethers: Synthesis of 1,2-Dihydrofuran Acetals and Highly Substituted Indoles. J. Org. Chem. 2021, 86, 10088–10104. [Google Scholar] [CrossRef]
- Mal, D.; Senapati, B.; Pahari, P. Regioselective synthesis of 1-hydroxycarbazoles via anionic [4 + 2] cycloaddition of furoindolones: A short synthesis of murrayafoline-A. Tetrahedron Lett. 2006, 47, 1071–1075. [Google Scholar] [CrossRef]
- Youn, S.W.; Kim, Y.H.; Jo, Y.H. Palladium-Catalyzed Regioselective Synthesis of 1-Hydroxycarbazoles Under Aerobic Conditions. Adv. Synth. Catal. 2019, 361, 462–468. [Google Scholar] [CrossRef]
- Padmaja, P.; Reddy, N. Padmaja Pannala and Reddy Narayana, Hydroxycarbazoles as Starting Materials in Organic Syntheses. Curr. Org. Synth. 2015, 12, 3–19. [Google Scholar] [CrossRef]
- Galli, C.; Mandolini, L. The Role of Ring Strain on the Ease of Ring Closure of Bifunctional Chain Molecules. Eur. J. Org. Chem. 2000, 2000, 3117–3125. [Google Scholar] [CrossRef]
- Itoigawa, M.; Kashiwada, Y.; Ito, C.; Furukawa, H.; Tachibana, Y.; Bastow, K.F.; Lee, K.-H. Antitumor Agents. 203. Carbazole Alkaloid Murrayaquinone A and Related Synthetic Carbazolequinones as Cytotoxic Agents. J. Nat. Prod. 2000, 63, 893–897. [Google Scholar] [CrossRef]
- Cuong, N.M.; Wilhelm, H.; Porzel, A.; Arnold, N.; Wessjohann, L. 1-O-Substituted derivatives of murrayafoline A and their antifungal properties. Nat. Prod. Res. 2008, 22, 950–954. [Google Scholar] [CrossRef]
- Cui, C.-B.; Yan, S.-Y.; Cai, B.; Yao, X.-S. Carbazole alkaloids as new cell cycle inhibitor and apopTosis inducers from Clausena dunniana. J. Asian Nat. Prod. Res. 2002, 4, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Gwak, J.; Cho, M.; Ryu, M.-J.; Lee, J.-H.; Kim, S.K.; Kim, Y.H.; Lee, G.W.; Yun, M.-Y.; Cuong, N.M.; et al. Murrayafoline A attenuates the Wnt/β-catenin pathway by promoting the degradation of intracellular β-catenin proteins. Biochem. Biophys. Res. Commun. 2010, 391, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.N.; Martin, M.C.; Shenje, R.; France, S. Calcium-Catalyzed Formal [5 + 2] Cycloadditions of Alkylidene β-Ketoesters with Olefins: Chemodivergent Synthesis of Highly Functionalized Cyclohepta[b]Indole Derivatives. Org. Lett. 2019, 21, 7268–7273. [Google Scholar] [CrossRef] [PubMed]
- Cuzzupe, A.N.; Hutton, C.A.; Lilly, M.J.; Mann, R.K.; McRae, K.J.; Zammit, S.C.; Rizzacasa, M.A. Total Synthesis of the Epidermal Growth Factor Inhibitor (-)-Reveromycin B. J. Org. Chem. 2001, 66, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Scheidt, K.A.; Downey, C.W. Synthesis of (-)-Epibatidine. Org. Lett. 2001, 3, 3009–3012. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).