A Novel Method to Construct 2-Aminobenzofurans via [4 + 1] Cycloaddition Reaction of In Situ Generated Ortho-Quinone Methides with Isocyanides
Abstract
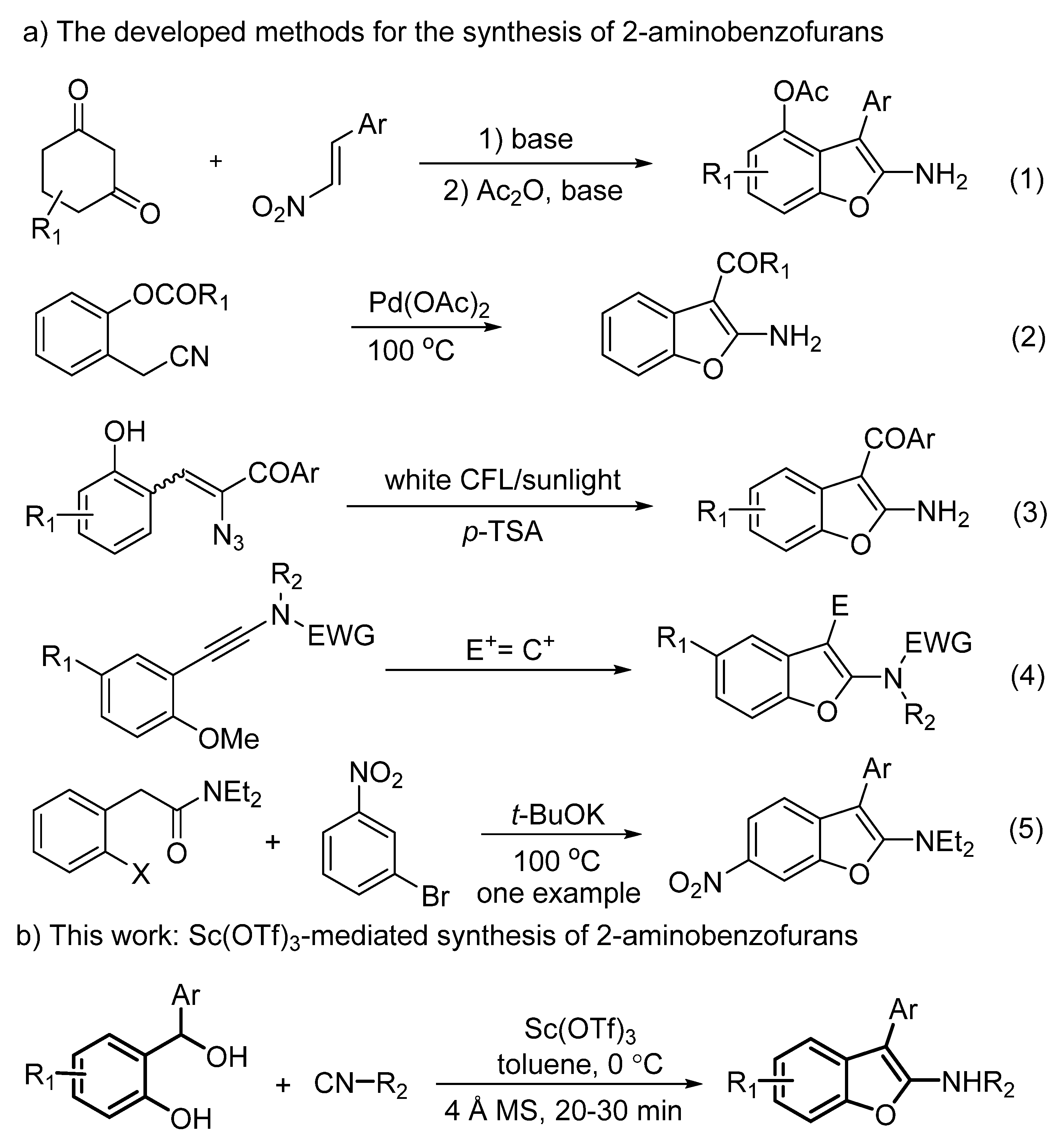
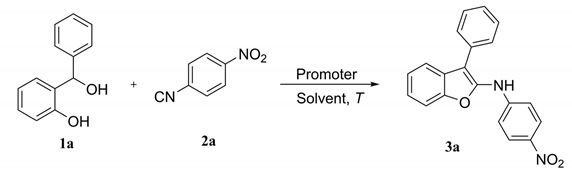
1. Introduction
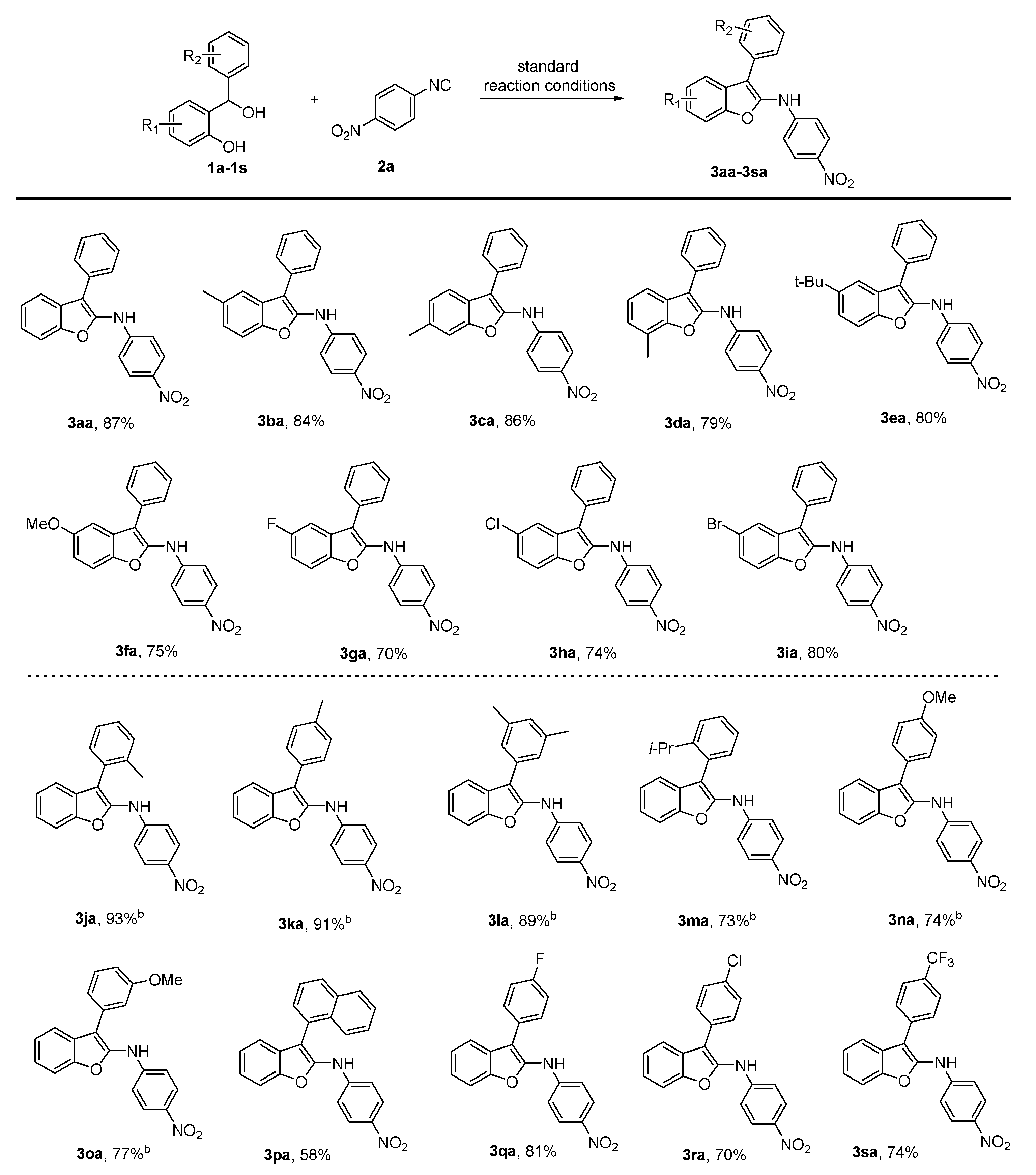
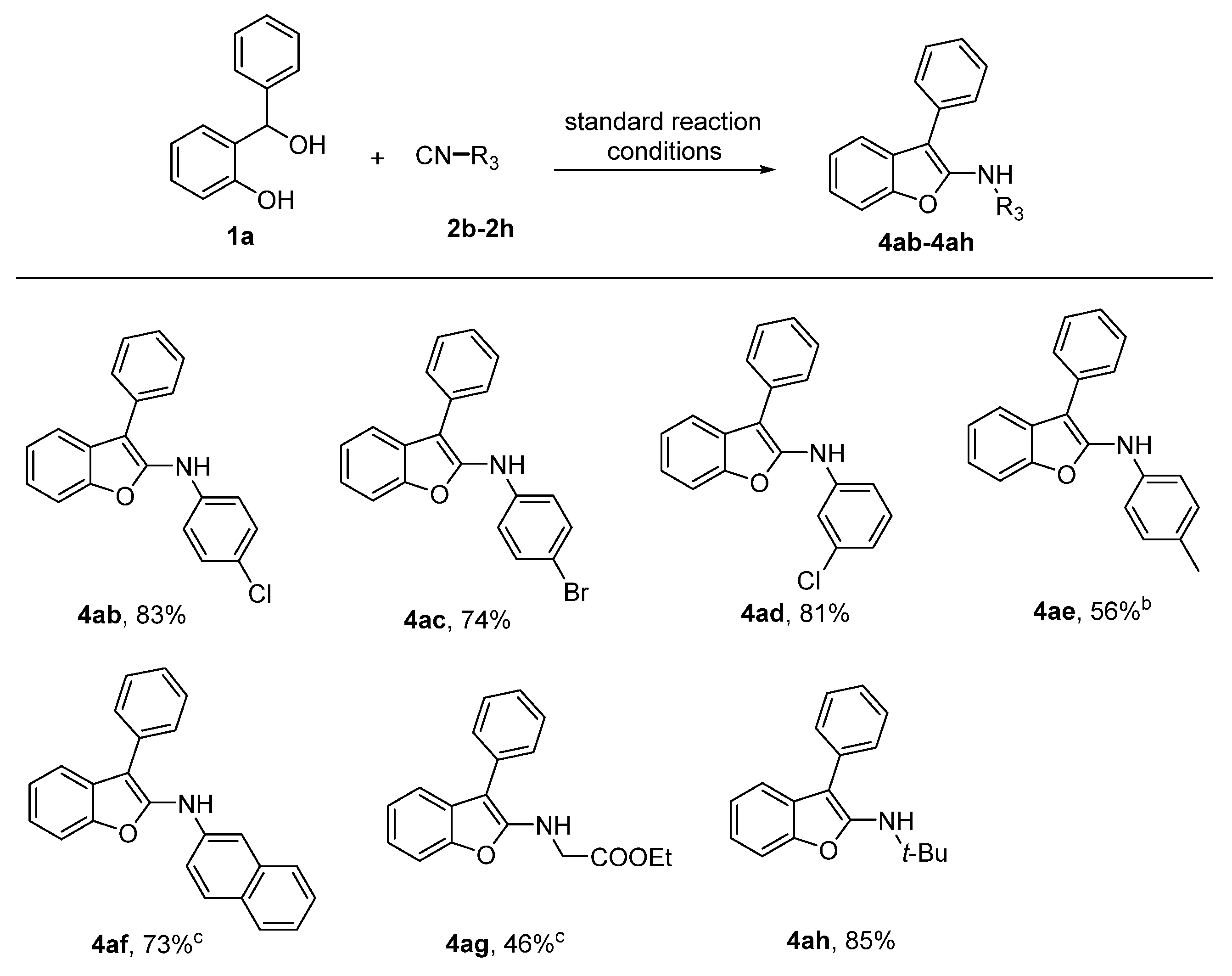
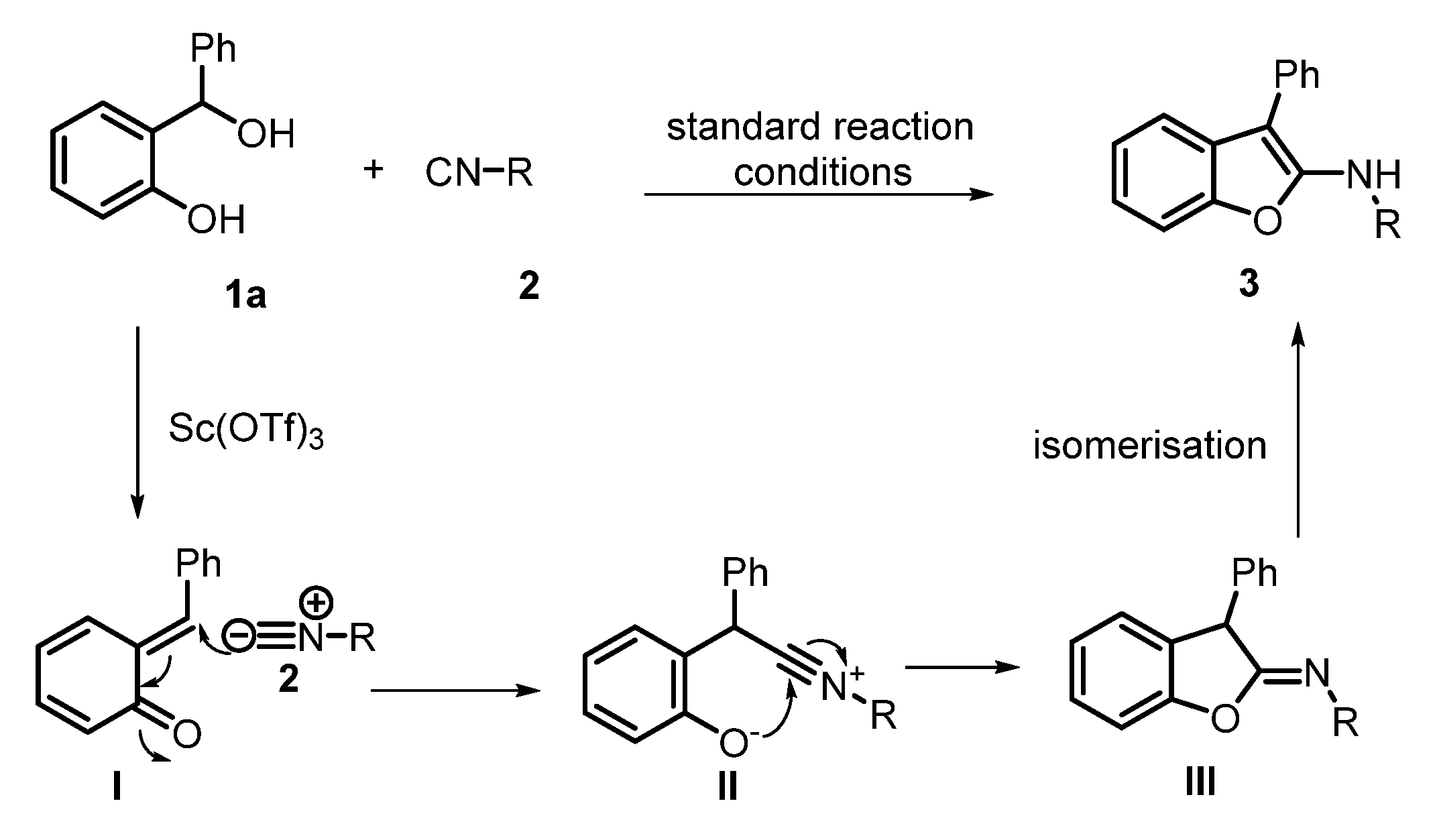
2. Results
3. Experimental
3.1. General Procedures
3.2. Typical Procedure for Synthesis of 3aa
- N-(4-Nitrophenyl)-3-phenylbenzofuran-2-amine (3aa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.5, yield: 87%, red solid, mp 123 °C.IR: 3309, 1639, 1589, 1494, 1393, 1311, 1242, 1190, 1114. 1H NMR (400 MHz, Chloroform-d) δ 8.16 (d, J = 8.8 Hz, 2H), 7.71 (d, J = 7.0 Hz, 1H), 7.58 (d, J = 7.3 Hz, 2H), 7.50 (q, J = 7.4 Hz, 3H), 7.37 (dt, J = 13.6, 6.9 Hz, 3H), 7.02 (d, J = 8.8 Hz, 2H), 6.66 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 151.2, 148.3, 145.3, 141.0, 131.2, 129.6, 128.2, 128.1, 127.6, 126.0, 124.3, 123.6, 119.5, 114.6, 111.1, 108.1. ESI-HRMS: m/z calcd for C20H15N2O3 [M + H]+: 331.1077, found: 331.1075.
- 5-Methyl-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ba): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 84%, red solid, mp 108 °C. IR: 3368, 2922, 1586, 1492, 1323, 1239, 1192, 1110. 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 9.1 Hz, 2H), 7.58–7.51 (m, 2H), 7.51–7.42 (m, 3H), 7.41–7.33 (m, 2H), 7.18–7.12 (dd, J = 8.4 Hz, 0.8Hz, 1H), 7.03–6.95 (m, 2H), 6.54 (s, 1H), 2.47 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 149.6, 148.3, 145.3, 141.1, 133.1, 131.4, 129.2, 128.2, 128.0, 127.6, 126.0, 125.4, 119.4, 114.5, 110.6, 108.0, 21.5. ESI-HRMS: m/z calcd for C21H17N2O3 [M + H]+: 345.1234, found: 345.1239.
- 6-Methyl-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ca): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 86%, red solid, mp 109 °C. IR: 3360, 2920, 1596, 1496, 1322, 1304, 1248, 1188, 1109. 1H NMR (400 MHz, CDCl3) δ 8.13 (d, J = 9.1 Hz, 2H), 7.56 (dd, J = 11.8, 7.7 Hz, 3H), 7.46 (t, J = 7.6 Hz, 2H), 7.35 (t, J = 7.4 Hz, 1H), 7.31 (s, 1H), 7.14 (d, J = 7.9 Hz, 1H), 6.99–6.91 (m, 2H), 6.53 (s, 1H), 2.51 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 151.7, 148.7, 144.4, 141.0, 134.8, 131.4, 129.2, 128.1, 127.6, 126.0, 125.3, 124.8, 119.2, 114.4, 111.4, 109.0, 21.7. ESI-HRMS: m/z calcd for C21H17N2O3 [M + H]+: 345.1234, found: 345.1234.
- 7-Methyl-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3da): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 79%, red solid, mp 141 °C. IR: 3364, 2923, 1591, 1501, 1385, 1325, 1248, 1183, 1110. 1H NMR (400 MHz, CDCl3) δ 8.19–8.11 (m, 2H), 7.59–7.51 (m, 3H), 7.47 (t, J = 7.6 Hz, 2H), 7.36 (t, J = 7.4 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 7.3 Hz, 1H), 7.03–6.96 (m, 2H), 6.60 (s, 1H), 2.56 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.2, 148.4, 144.9, 141.0, 131.4, 129.2, 128.2, 127.6, 127.5, 126.0, 125.4, 123.6, 121.4, 117.1, 114.5, 108.6, 15.0. ESI-HRMS: m/z calcd for C21H17N2O3 [M + H]+: 345.1234, found: 345.1255.
- 5-(tert-butyl)-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ea): Petroleum ether: ethyl acetate = 15:1, Rf = 0.7, yield: 80%, red solid, mp 97 °C. IR: 3356, 2960, 1591, 1503, 1340, 1285, 1186, 1111. 1H NMR (400 MHz, Chloroform-d) δ 8.13 (d, J = 9.0 Hz, 2H), 7.68 (s, 1H), 7.56 (d, J = 7.2 Hz, 2H), 7.49 (t, J = 7.5 Hz, 2H), 7.43 (s, 2H), 7.38 (d, J = 7.2 Hz, 1H), 6.98 (d, J = 9.0 Hz, 2H), 6.62 (s, 1H), 1.41 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 149.5, 148.5, 146.8, 145.3, 141.0, 131.4, 129.3, 128.3, 127.6, 126.0, 122.2, 115.7, 114.5, 110.5, 108.7, 34.9, 31.9. ESI-HRMS: m/z calcd for C24H23N2O3 [M + H]+: 387.1703, found: 387.1704.
- 5-Methoxy-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3fa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.4, yield: 75%, red solid, mp 165 °C. IR: 3371, 2931, 1586, 1479, 1322, 1296, 1225, 1191, 1152, 1110. 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 9.1 Hz, 2H), 7.56–7.51 (m, 2H), 7.48 (t, J = 7.6 Hz, 2H), 7.42–7.34 (m, 2H), 7.12 (d, J = 2.5 Hz, 1H), 7.05–6.97 (m, 2H), 6.92 (dd, J = 8.9, 2.6 Hz, 1H), 6.62 (s, 1H), 3.85 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 156.6, 148.1, 146.0, 146.0, 141.1, 131.3, 129.3, 128.7, 128.2, 127.6, 126.0, 114.6, 112.3, 111.6, 107.9, 102.5, 56.0. ESI-HRMS: m/z calcd for C21H17N2O4 [M + H]+: 361.1183, found: 361.1187.
- 5-Fluoro-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ga): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 70%, red solid, mp 141 °C. IR: 3343, 1586, 1502, 1476, 1325, 1311, 1242, 1195, 1140, 1110. 1H NMR (400 MHz, DMSO-d6) δ 10.05 (s, 1H), 8.10 (d, J = 9.0 Hz, 2H), 7.62 (dd, J = 10.3, 5.9 Hz, 3H), 7.53–7.41 (m, 3H), 7.36 (t, J = 7.3 Hz, 1H), 7.17 (td, J = 9.2, 2.3 Hz, 1H), 7.04 (d, J = 9.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ159.6 (d, J = 232.3 Hz), 150.0, 148.1, 147.3, 140.1, 131.0, 129.5, 129.3, 128.4, 127.9, 126.2, 115.1, 112.7, 118.0 (d, J = 20.2 Hz), 108.3, 105.6, 105.3. 19F NMR (376 MHz, DMSO-d6) δ −119.35. ESI-HRMS: m/z calcd for C20H14FN2O3 [M + H]+: 349.0983, found: 349.0992.
- 5-Chloro-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ha): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 74%, red solid, mp 198 °C. IR: 3366, 1588, 1500, 1384, 1325, 1234, 1109. 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.10 (d, J = 9.1 Hz, 2H), 7.67 (d, J = 2.0 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.60 (d, J = 7.4 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.37 (td, J = 6.3, 5.5, 2.7 Hz, 2H), 7.05 (d, J = 9.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 149.9, 149.6, 147.9, 140.1, 130.8, 129.9, 129.6, 128.5, 128.5, 128.0, 126.2, 124.4, 118.9, 115.1, 113.2, 107.6. ESI-HRMS: m/z calcd for C20H14ClN2O3 [M + H]+: 365.0687, found: 365.0678.
- 5-Bromo-N-(4-nitrophenyl)-3-phenylbenzofuran-2-amine (3ia): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 80%, red solid, mp 210 °C. IR: 3367, 1585, 1504, 1468, 1324, 1232, 1109. 1H NMR (400 MHz, DMSO-d6) δ 10.08 (s, 1H), 8.09 (d, J = 9.1 Hz, 2H), 7.88 (d, J =1.2 Hz, 1H), 7.59 (d, J = 7.3 Hz, 3H), 7.54–7.44 (m, 3H), 7.36 (t, J = 7.3 Hz, 1H), 7.04 (d, J = 9.1 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 149.9, 149.9, 147.7, 140.1, 130.8, 130.5, 129.6, 128.5, 128.0, 127.1, 126.2, 121.8, 116.4, 115.1, 113.6, 107.4. ESI-HRMS: m/z calcd for C20H13BrN2NaO3 [M + Na]+: 431.0002, found: 430.9998.
- N-(4-Nitrophenyl)-3-(o-tolyl)benzofuran-2-amine (3ja): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 93%, red solid, mp 145 °C. IR: 3347, 2925, 1593, 1503, 1327, 1248, 1169, 1112. 1H NMR (400 MHz, CDCl3) δ 8.17–8.11 (m, 2H), 7.54–7.49 (m, 1H), 7.37–7.27 (m, 7H), 7.08–7.02 (m, 2H), 6.44 (s, 1H), 2.24 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 151.0, 147.6, 145.8, 141.1, 137.6, 130.9, 130.5, 129.8, 129.1, 128.4, 126.4, 125.9, 123.8, 123.4, 119.6, 114.7, 110.9, 106.2, 20.2. ESI-HRMS: m/z calcd for C21H17N2O3 [M + H]+: 345.1234, found: 345.1234.
- N-(4-Nitrophenyl)-3-(p-tolyl)benzofuran-2-amine (3ka): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 91%, red solid, mp 138 °C. IR: 3359, 2920, 1591, 1524, 1384, 1248, 1175, 1109. 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 9.1 Hz, 2H), 7.72–7.64 (m, 1H), 7.49 (d, J = 7.4 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.37–7.30 (m, 2H), 7.28 (d, J = 7.9 Hz, 2H), 6.99 (d, J = 9.1 Hz, 2H), 6.58 (s, 1H), 2.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 151.2, 148.4, 145.0, 141.0, 137.5, 130.0, 128.1, 128.1, 128.1, 126.0, 124.2, 123.5, 119.6, 114.5, 111.1, 108.3, 21.3. ESI-HRMS: m/z calcd for C21H16N2NaO3 [M + Na]+: 367.1053, found: 367.1054.
- 3-(3,5-Dimethylphenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3la): Petroleum ether: ethyl acetate = 12:1, Rf = 0.6, yield: 89%, red solid, mp 92 °C. IR: 3342, 2922, 1592, 1502, 1384, 1326, 1182, 1111. 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 9.1 Hz, 2H), 7.67 (dd, J = 6.0, 2.7 Hz, 1H), 7.49 (dd, J = 6.5, 2.2 Hz, 1H), 7.33 (dt, J = 6.6, 4.7 Hz, 2H), 7.16 (s, 2H), 7.03 (d, J = 9.2 Hz, 3H), 6.64 (s, 1H), 2.37 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 151.1, 148.2, 145.3, 141.0, 138.9, 131.0, 129.4, 128.2, 126.0, 124.0, 123.5, 119.6, 114.7, 111.0, 107.7, 21.5. ESI-HRMS: m/z calcd for C22H18N2NaO3 [M + Na]+: 381.1210, found: 381.1206.
- 3-(2-Isopropylphenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3ma): Petroleum ether: ethyl acetate = 12:1, Rf = 0.6, yield: 73%, red solid, mp 170 °C. IR: 3319, 2961, 1642, 1592, 1499, 1384, 1323, 1306, 1237, 1186, 1112. 1H NMR (400 MHz, Chloroform-d) δ 8.14 (d, J = 9.0 Hz, 2H), 7.52 (d, J = 8.0 Hz, 1H), 7.50–7.40 (m, 2H), 7.32–7.28 (m, 5H), 7.05 (d, J = 9.0 Hz, 2H), 6.49 (s, 1H), 3.02 (hept, J = 6.6 Hz, 1H), 1.18 (d, J = 6.8 Hz, 3H), 1.06 (d, J = 6.8 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 150.9, 148.9, 147.9, 146.0, 141.1, 130.9, 129.8, 129.0, 128.3, 126.3, 126.3, 125.9, 123.8, 123.5, 119.3, 114.6, 110.9, 106.3, 30.1, 24.5, 24.1. ESI-HRMS: m/z calcd for C23H21N2O3 [M + H]+: 373.1547, found: 373.1547.
- 3-(4-Methoxyphenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3na): Petroleum ether: ethyl acetate = 10:1, Rf = 0.4, yield: 74%, red solid, mp 177 °C. IR: 3358, 2950, 1594, 1502, 1307, 1231, 1152, 1114. 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 9.0 Hz, 2H), 7.70–7.63 (m, 1H), 7.48 (t, J = 7.1 Hz, 3H), 7.37–7.28 (m, 2H), 6.99 (dd, J = 11.2, 8.9 Hz, 4H), 6.50 (s, 1H), 3.85 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.1, 151.2, 148.5, 144.7, 141.0, 129.4, 128.2, 126.0, 124.3, 123.4, 123.3, 119.6, 114.7, 114.4, 111.1, 108.4, 55.4. ESI-HRMS: m/z calcd for C21H17N2O4 [M + H]+: 361.1183, found: 361.1183.
- 3-(3-Methoxyphenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3oa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.4, yield: 77%, red solid, mp 107 °C. IR: 3315, 2920, 1591, 1501, 1325, 1309, 1239, 1183, 1110. 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 9.1 Hz, 2H), 7.69 (dd, J = 6.5, 2.2 Hz, 1H), 7.50 (dd, J = 6.9, 1.9 Hz, 1H), 7.39 (t, J = 7.9 Hz, 1H), 7.36–7.28 (m, 2H), 7.13 (d, J = 7.7 Hz, 1H), 7.10 (t, J = 2.0 Hz, 1H), 7.03 (d, J = 9.1 Hz, 2H), 6.91 (dd, J = 8.2, 2.3 Hz, 1H), 6.63 (s, 1H), 3.81 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.2, 151.1, 148.1, 145.4, 141.1, 132.5, 130.4, 128.0, 126.0, 124.2, 123.6, 120.5, 119.5, 114.7, 114.0, 112.8, 111.1, 107.5, 55.3. ESI-HRMS: m/z calcd for C21H16N2NaO4 [M + Na]+: 383.1002, found: 383.1004.
- 3-(Naphthalen-1-yl)-N-(4-nitrophenyl)benzofuran-2-amine (3pa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 58%, red solid, mp 194 °C. IR: 3309, 2920, 1637, 1587, 1498, 1322, 1305, 1242, 1183, 1110. 1H NMR (400 MHz, CDCl3) δ 8.11 (d, J = 9.1 Hz, 2H), 7.99–7.91 (m, 2H), 7.83 (d, J = 8.4 Hz, 1H), 7.51–7.61 (m, 4H), 7.44 (t, J = 7.5 Hz, 1H), 7.36–7.31 (m, 1H), 7.29 (d, J = 6.7 Hz, 1H), 7.24 (d, J = 7.4 Hz, 1H), 7.06 (d, J = 9.1 Hz, 2H), 6.46 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 151.0, 147.4, 146.8, 141.1, 134.1, 131.7, 129.6, 128.8, 128.8, 128.2, 128.1, 126.7, 126.4, 125.8, 125.8, 125.4, 123.7, 123.6, 119.7, 114.9, 110.9, 104.1. ESI-HRMS: m/z calcd for C24H17N2O3 [M + H]+: 381.1234, found: 381.1225.
- 3-(4-Fluorophenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3qa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 81%, red solid, mp 126 °C. IR: 3332, 2920, 1587, 1500, 1472, 1325, 1108. 1H NMR (400 MHz, DMSO-d6) δ 9.93 (s, 1H), 8.10 (d, J = 9.2 Hz, 2H), 7.72–7.58 (m, 4H), 7.40–7.28 (m, 4H), 7.00 (d, J = 9.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 161.7 (d, J = 252.5 Hz), 151.2, 150.5, 146.3, 139.9, 130.5, 130.4, 128.0, 127.8, 126.3, 124.8, 124.0, 119.7, 116.4 (d, J = 20.2 Hz), 114.8, 111.6, 108.0. 19F NMR (376 MHz, DMSO-d6) δ -114.37. ESI-HRMS: m/z calcd for C20H14FN2O3 [M + H]+: 349.0983, found: 349.0977.
- 3-(4-Chlorophenyl)-N-(4-nitrophenyl)benzofuran-2-amine (3ra): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 70%, red solid, mp 177 °C. IR: 3312, 2920, 1638, 1588, 1491, 1390, 1308, 1241, 1114. 1H NMR (400 MHz, CDCl3) δ 8.15 (d, J = 9.0 Hz, 2H), 7.64 (d, J = 7.1 Hz, 1H), 7.53–7.47 (m, 3H), 7.44 (d, J = 8.4 Hz, 2H), 7.39–7.29 (m, 2H), 7.00 (d, J = 9.0 Hz, 2H), 6.54 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 151.2, 148.0, 145.3, 141.3, 133.5, 129.7, 129.5, 129.5, 127.7, 126.0, 124.5, 123.7, 119.3, 114.6, 111.2, 107.3. ESI-HRMS: m/z calcd for C20H14ClN2O3 [M + H]+: 365.0687, found: 365.0687.
- N-(4-Nitrophenyl)-3-(4-(trifluoromethyl)phenyl)benzofuran-2-amine (3sa): Petroleum ether: ethyl acetate = 10:1, Rf = 0.6, yield: 74%, red solid, mp 226 °C. IR: 3311, 2967, 1590, 1311, 1307, 1272, 1239, 1112, 1066. 1H NMR (400 MHz, DMSO-d6) δ 10.09 (s, 1H), 8.13 (d, J = 9.2 Hz, 2H), 7.85 (s, 4H), 7.75 (dd, J = 6.6, 2.2 Hz, 1H), 7.63 (dd, J = 6.9, 1.8 Hz, 1H), 7.43–7.32 (m, 2H), 7.08 (d, J = 9.2 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 151.1, 149.9, 147.5, 140.1, 136.0, 129.1, 128.0, 127.7, 127.6, 126.4, 126.3, 126.3, 126.1, 124.8, 124.2, 123.4, 119.5, 115.2, 111.7, 106.5. 19F NMR (376 MHz, DMSO-d6) δ −60.99. ESI-HRMS: m/z calcd for C21H13F3N2NaO3 [M + Na]+: 421.0770, found: 421.0766.
- N-(4-Chlorophenyl)-3-phenylbenzofuran-2-amine (4ab): Petroleum ether: ethyl acetate = 15:1, Rf = 0.6, yield: 83%, white solid, mp 102 °C. IR: 3371, 1636, 1594, 1479, 1384, 1238, 1183. 1H NMR (400 MHz, CDCl3) δ 7.62–7.67 (m, 1H), 7.58 (d, J = 7.2 Hz, 2H), 7.51–7.44 (m, 3H), 7.35 (t, J = 7.4 Hz, 1H), 7.28 (t, J = 3.6 Hz, 1H), 7.25 (d, J = 5.0 Hz, 1H), 7.25–7.20 (m, 2H), 7.00–6.93 (m, 2H), 6.13 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 150.9, 147.9, 140.8, 132.0, 129.3, 129.2, 128.6, 128.1, 127.1, 126.1, 123.3, 123.2, 118.8, 117.3, 110.8, 104.5. ESI-HRMS: m/z calcd for C20H15ClNO [M + H]+: 320.0837, found: 320.0822.
- N-(4-Bromophenyl)-3-phenylbenzofuran-2-amine (4ac): Petroleum ether: ethyl acetate = 15:1, Rf = 0.6, yield: 74%, white solid, mp 137 °C. IR: 3360, 1636, 1590, 1489, 1379, 1241, 1174. 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J = 5.4, 3.3 Hz, 1H), 7.57 (d, J = 7.4 Hz, 2H), 7.51–7.43 (m, 3H), 7.40–7.32 (m, 3H), 7.30–7.26 (m, 2H), 6.91 (d, J = 8.6 Hz, 2H), 6.12 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 150.9, 147.7, 141.3, 132.2, 132.0, 129.12, 128.5, 128.1, 127.1, 123.3, 123.2, 118.8, 117.7, 113.3, 110.8, 104.7. ESI-HRMS: m/z calcd for C20H15BrNO [M + H]+: 364.0332, found: 364.0323.
- N-(3-Chlorophenyl)-3-phenylbenzofuran-2-amine (4ad): Petroleum ether: ethyl acetate = 15:1, Rf = 0.6, yield: 81%, white solid, mp 134 °C. IR: 3359, 1637, 1593, 1492, 1378, 1242, 1173. 1H NMR (400 MHz, Chloroform-d) δ 7.68 (dd, J = 6.1, 2.9 Hz, 1H), 7.59 (d, J = 7.2 Hz, 2H), 7.48–7.52 (m, 3H), 7.37 (t, J = 7.4 Hz, 1H), 7.32–7.29 (m, 2H), 7.20 (t, J = 8.1 Hz, 1H), 7.05 (t, J = 1.9 Hz, 1H), 6.99–6.86 (m, 2H), 6.15 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 151.0, 147.3, 143.6, 135.1, 131.9, 130.4, 129.2, 128.4, 128.2, 127.2, 123.4, 123.3, 121.1, 119.0, 115.9, 114.1, 110.9, 105.5. ESI-HRMS: m/z calcd for C20H13ClNO [M − H]−: 318.0680, found: 318.0685.
- 3-Phenyl-N-(p-tolyl)benzofuran-2-amine (4ae): Petroleum ether: ethyl acetate = 15:1, Rf = 0.6, yield: 56%, white solid, mp 92 °C. IR: 3380, 2925, 1608, 1517, 1384, 1196, 1071. 1H NMR (400 MHz, Chloroform-d) δ 7.69–7.56 (m, 3H), 7.52–7.42 (m, 3H), 7.33 (t, J = 7.4 Hz, 1H), 7.29–7.22 (m, 2H), 7.11 (d, J = 8.2 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.12 (s, 1H), 2.33 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 150.8, 149.2, 139.5, 132.5, 129.9, 129.2, 128.9, 128.1, 126.8, 123.1, 122.6, 118.4, 116.7, 110.7, 102.6, 20.7. ESI-HRMS: m/z calcd for C21H18NO [M + H]+: 300.1383, found: 300.1380.
- N-(Naphthalen-2-yl)-3-phenylbenzofuran-2-amine (4af): Petroleum ether: ethyl acetate = 15:1, Rf = 0.6, yield: 73%, White solid, mp 124 °C. IR: 3377, 1629, 1600, 1454, 1381, 1220, 1184. 1H NMR (400 MHz, DMSO-d6) δ 9.19 (s, 1H), 7.77 (d, J = 6.2 Hz, 1H), 7.75 (d, J = 5.3 Hz, 1H), 7.72–7.69 (m, 1H), 7.68–7.65 (m, 2H), 7.62 (d, J = 8.2 Hz, 1H), 7.56–7.60 (m, 1H), 7.45 (t, J = 7.7 Hz, 2H), 7.39–7.34 (m, 1H), 7.33–7.27 (m, 3H), 7.27–7.24 (m, 1H), 7.22–7.25 (m, 1H), 7.19 (d, J = 2.0 Hz, 1H). ESI-HRMS: m/z calcd for C24H16NO [M − H]−: 334.1226, found: 334.1239.
- Ethyl-(3-phenylbenzofuran-2-yl) glycinate (4ag): Petroleum ether: ethyl acetate = 6:1, Rf = 0.5, yield: 46%, White solid, mp 96 °C. IR: 3349, 2927, 1734, 1612, 1463, 1393, 1206, 1122. 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 7.2 Hz, 2H), 7.45–7.55 (m, 3H), 7.33 (d, J = 8.0 Hz, 1H), 7.30 (t, J = 7.5 Hz, 1H), 7.19 (t, J = 7.4 Hz, 1H), 7.12–7.06 (m, 1H), 5.05 (t, J = 5.6 Hz, 1H), 4.24 (q, J = 7.1 Hz, 2H), 4.18 (d, J = 5.9 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 170.7, 153.7, 150.0, 133.2, 130.2, 129.2, 127.6, 126.0, 123.1, 120.6, 117.1, 109.9, 93.9, 61.6, 45.4, 14.2. ESI-HRMS: m/z calcd for C18H18NO3 [M + H]+: 296.1281, found: 296.1281.
- N-(tert-butyl)-3-phenylbenzofuran-2-amine (4ah): Petroleum ether: ethyl acetate = 20:1, Rf = 0.6, yield: 85%, White solid, mp 108 °C. IR: 3367, 2967, 1606, 1458, 1379, 1210, 1015. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 7.0 Hz, 2H), 7.51 –7.45 (m, 3H), 7.37 (d, J = 7.9 Hz, 1H), 7.30 (t, J = 7.2 Hz, 1H), 7.18 (t, J = 7.2 Hz, 1H), 7.10 (t, J =7.2 Hz, 1H), 4.39 (s, 1H), 1.43 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 155.2, 150.4, 133.7, 129.6, 129.2, 127.8, 125.9, 122.8, 120.6, 117.0, 110.0 97.0, 53.5, 30.6. ESI-HRMS: m/z calcd for C18H20NO [M + H]+: 266.1539, found: 266.1537.
3.3. X-ray Crystallographic Data of 3ia
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simonetti, S.O.; Larghi, E.L.; Bracca, A.B.J.; Kaufman, T.S. Angular Tricyclic Benzofurans and Related Natural Products of Fungal Origin. Isolation, Biological Activity and Synthesis. Nat. Prod. Rep. 2013, 30, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Radadiya, A.; Shah, A. Bioactive Benzofuran Derivatives: An Insight on Lead Developments, Radioligands and Advances of the Last Decade. Eur. J. Med. Chem. 2015, 97, 356–376. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Ojika, M.; Suzuki, S.; Murakami, M.; Sakagami, Y. Iantherans A and B, Unique Dimeric Polybrominated Benzofurans as Na, K-ATPase Inhibitors from a Marine Sponge, Ianthella sp. Bioorg. Med. Chem. 2001, 9, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Faridi, U.; Sisodia, B.S.; Darokar, M.P.; Luqman, S.; Khanuja, S.P.S. Synthesis of 1-(3′,4′,5′-Trimethoxy) Phenyl Naphtho[2,1b]Furan as a Novel Anticancer Agent. Bioorg. Med. Chem. Lett. 2006, 16, 911–914. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chethana, K.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An Emerging Scaffold for Antimicrobial Agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Ryu, C.-K.; Kim, Y.H.; Im, H.A.; Kim, J.Y.; Yoon, J.H.; Kim, A. Synthesis and antifungal activity of 6,7-bis(arylthio)-quinazoline-5,8-diones and furo[2,3-f]quinazolin-5-ols. Bioorg. Med. Chem. Lett. 2012, 22, 500–503. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, C.M.; Lin, H.C.; Huang, C.F.; Lee, C.Y.; Si Tou, T.C.; Hung, C.C.; Chang, C.S. Structure-activity relationship study of novel 2-aminobenzofuran derivatives as P-glycoprotein inhibitors. Eur. J. Med. Chem. 2017, 125, 1023–1035. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Nocentini, A.; Elsayed, Z.M.; Al-Warhi, T.; Aljaeed, N.; Alotaibi, O.J.; Al-Sanea, M.M.; Abdel-Aziz, H.A.; Supuran, C.T. Benzofuran-based carboxylic acids as carbonic anhydrase inhibitors and antiproliferative agents against breast cancer. ACS Med. Chem. Lett. 2020, 11, 1022–1027. [Google Scholar] [CrossRef]
- Oliva, P.; Romagnoli, R.; Manfredini, S.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; Giacomini, A.; Rruga, F.; et al. Design, synthesis, in vitro and in vivo biological evaluation of 2-amino-3-aroylbenzo[b]furan derivatives as highly potent tubulin polymerization inhibitors. Eur. J. Med. Chem. 2020, 200, 112448. [Google Scholar] [CrossRef]
- Ishikawa, T.; Miyahara, T.; Asakura, M.; Higuchi, S.; Miyauchi, Y.; Saito, S. One-Pot Multistep Synthesis of 4-Acetoxy-2-Amino-3-Arylbenzofurans from 1-Aryl-2-Nitroethylenes and Cyclohexane-1,3-Diones. Org. Lett. 2005, 7, 1211–1214. [Google Scholar] [CrossRef]
- Murai, M.; Miki, K.; Ohe, K. A New Route to 3-Acyl-2-Aminobenzofurans: Palladium-Catalysed Cycloisomerisation of 2-(Cyanomethyl)Phenyl Esters. Chem. Commun. 2009, 23, 3466–3468. [Google Scholar] [CrossRef] [PubMed]
- Borra, S.; Chandrasekhar, D.; Khound, S.; Maurya, R.A. Access to 1a, 6b-Dihydro-1H-Benzofuro[2, 3-b]Azirines and Benzofuran-2-Amines via Visible Light Triggered Decomposition of α-Azidochalcones. Org. Lett. 2017, 19, 5364–5367. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Jiang, K.; Cao, J.; Fu, L.; Yu, L.; Lai, G.; Cui, Y.; Hu, Z.; Wang, G. Synthesis of 3-Alkyl-or 3-Allenyl-2-Amidobenzofurans via Electrophilic Cyclization of o-Anisole-Substituted Ynamides with Carbocations. Org. Lett. 2013, 15, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Sattar, M.; Kumar, R.; Kumar, S. Synthesis of Unsymmetrical Diaryl Acetamides, Benzofurans, Benzophenones, and Xanthenes by Transition-Metal-Free Oxidative Cross-Coupling of sp3 and sp2 C-H Bonds. J. Org. Chem. 2016, 81, 9206–9218. [Google Scholar] [CrossRef]
- Luan, Y.; Schaus, S.E. Enantioselective Addition of Boronates to o-Quinone Methides Catalyzed by Chiral Biphenols. J. Am. Chem. Soc. 2012, 134, 19965–19968. [Google Scholar] [CrossRef]
- Saha, S.; Alamsetti, S.K.; Schneider, C. Chiral Brønsted Acid-Catalyzed Friedel–Crafts Alkylation of Electron-Rich Arenes with in situ-Generated ortho-Quinone Methides: Highly Enantioselective Synthesis of Diarylindolylmethanes and Triarylmethanes. Chem. Commun. 2015, 51, 1461–1464. [Google Scholar] [CrossRef]
- Wu, B.; Gao, X.; Yan, Z.; Chen, M.W.; Zhou, Y.G. C-H Oxidation/Michael Addition/Cyclization Cascade for Enantioselective Synthesis of Functionalized 2-Amino-4H-Chromenes. Org. Lett. 2015, 17, 6134–6137. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, D.Y. Enantioselective Decarboxylative Alkylation of β-Keto Acids to ortho-Quinone Methides as Reactive Intermediates: Asymmetric Synthesis of 2,4-Diaryl-1-Benzopyrans. Org. Lett. 2018, 20, 2944–2947. [Google Scholar] [CrossRef]
- Bai, W.J.; David, J.G.; Feng, Z.G.; Weaver, M.G.; Wu, K.L.; Pettus, T.R.R. The Domestication of ortho-Quinone Methides. Acc. Chem. Res. 2014, 47, 3655–3664. [Google Scholar] [CrossRef]
- Huang, H.M.; Wu, X.Y.; Leng, B.R.; Zhu, Y.L.; Meng, X.C.; Hong, Y.; Jiang, B.; Wang, D.C. Cu(ii)-Catalyzed formal [4 + 2] cycloaddition between quinone methides (QMs) and electron-poor 3-vinylindoles. Org. Chem. Front. 2020, 7, 414–419. [Google Scholar] [CrossRef]
- Jaworski, A.A.; Scheidt, K.A. Emerging Roles of in situ Generated Quinone Methides in Metal-Free Catalysis. J. Org. Chem. 2016, 81, 10145–10153. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Gao, S. Recent Advances in the Application of Diels–Alder Reactions Involving o-Quinodimethanes, aza-o-Quinone Methides and o-Quinone Methides in Natural Product Total Synthesis. Chem. Soc. Rev. 2018, 47, 7926–7953. [Google Scholar] [CrossRef] [PubMed]
- Alden-Danforth, E.; Scerba, M.T.; Lectka, T. Asymmetric Cycloadditions of o-Quinone Methides Employing Chiral Ammonium Fluoride Precatalysts. Org. Lett. 2008, 10, 4951–4953. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Liao, H.H.; Rueping, M. Enantio- and Diastereoselective Access to Distant Stereocenters Embedded within Tetrahydroxanthenes: Utilizing ortho-Quinone Methides as Reactive Intermediates in Asymmetric Brønsted Acid Catalysis. Angew. Chem. Int. Ed. 2014, 53, 13258–13263. [Google Scholar] [CrossRef]
- Lee, A.; Scheidt, K.A. N-Heterocyclic Carbene-Catalyzed Enantioselective Annulations: A Dual Activation Strategy for a Formal [4+2] Addition for Dihydrocoumarins. Chem. Commun. 2015, 51, 3407–3410. [Google Scholar] [CrossRef]
- Alamsetti, S.K.; Spanka, M.; Schneider, C. Synergistic Rhodium/Phosphoric Acid Catalysis for the Enantioselective Addition of Oxonium Ylides to ortho-Quinone Methides. Angew. Chemie Int. Ed. 2016, 55, 2392–2396. [Google Scholar] [CrossRef]
- Lv, H.; Jia, W.Q.; Sun, L.H.; Ye, S. N-Heterocyclic Carbene Catalyzed [4+3] Annulation of Enals and o-Quinone Methides: Highly Enantioselective Synthesis of Benzo-ε-Lactones. Angew. Chemie Int. Ed. 2013, 52, 8607–8610. [Google Scholar] [CrossRef]
- Chen, M.W.; Cao, L.L.; Ye, Z.S.; Zhou, Y.G.; Jiang, G.F. A Mild Method for Generation of o-Quinone Methides under Basic Conditions. The Facile Synthesis of Trans-2,3-Dihydrobenzofurans. Chem. Commun. 2013, 49, 1660–1662. [Google Scholar] [CrossRef]
- Wu, B.; Chen, M.W.; Ye, Z.S.; Yu, C.B.; Zhou, Y.G. A Streamlined Synthesis of 2,3-Dihydrobenzofurans via the ortho-Quinone Methides Generated from 2-Alkyl-Substituted Phenols. Adv. Synth. Catal. 2014, 356, 383–387. [Google Scholar] [CrossRef]
- Meisinger, N.; Roiser, L.; Monkowius, U.; Himmelsbach, M.; Robiette, R.; Waser, M. Asymmetric Synthesis of 2,3-Dihydrobenzofurans by a [4 + 1] Annulation Between Ammonium Ylides and In Situ Generated o-Quinone Methides. Chem. Eur. J. 2017, 23, 5137–5142. [Google Scholar] [CrossRef]
- Rodriguez, K.X.; Vail, J.D.; Ashfeld, B.L. Phosphorus(III)-Mediated Stereoconvergent Formal [4 + 1]-Cycloannulation of 1,2-Dicarbonyls and o-Quinone Methides: A Multicomponent Assembly of 2,3-Dihydrobenzofurans. Org. Lett. 2016, 18, 4514–4517. [Google Scholar] [CrossRef] [PubMed]
- Osyanin, V.A.; Osipov, D.V.; Klimochkin, Y.N. Reactions of o-Quinone Methides with Pyridinium Methylides: A Diastereoselective Synthesis of 1,2-Dihydronaphtho[2,1-b]Furans and 2,3-Dihydrobenzofurans. J. Org. Chem. 2013, 78, 5505–5520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Fang, Z.Q.; Li, W.J.; Li, P.F. Phosphine-mediated enantioselective [4 + 1] annulations between ortho-quinone methides and Morita–Baylis–Hillman carbonates. Org. Chem. Front. 2018, 5, 2728–2733. [Google Scholar] [CrossRef]
- Jiang, X.L.; Liu, S.J.; Gu, Y.Q.; Mei, G.J.; Shi, F. Catalytic Asymmetric [4 + 1] Cyclization of ortho-Quinone Methides with 3-Chlorooxindoles. Adv. Synth. Catal. 2017, 359, 3341–3346. [Google Scholar] [CrossRef]
- Liang, P.; Pan, Y.; Ma, X.; Jiao, W.; Shao, H. A Facile Method for the Synthesis of Fused Perhydropyrano[2,3-b]Pyrans Promoted by Yb(OTf)3. Chem. Commun. 2018, 54, 3763–3766. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Liang, P.; Pang, L.; Ma, X.; Jiao, W.; Shao, H. Oxadiazepine Synthesis by Formal [4+3] Cycloaddition of o-Chloromethyl Arylsulfonamides with Nitrones Promoted by NaHCO3. Adv. Synth. Catal. 2018, 360, 3015–3019. [Google Scholar] [CrossRef]
- Ma, X.; Tang, Q.; Ke, J.; Zhang, J.; Wang, C.; Wang, H.; Li, Y.; Shao, H. Straightforward and Highly Diastereoselective Synthesis of 2,2-Di-Substituted Perhydrofuro[2,3-b]Pyran (and Furan) Derivatives Promoted by BiCl3. Chem. Commun. 2013, 49, 7085–7087. [Google Scholar] [CrossRef][Green Version]
- Ma, X.; Tang, Q.; Ke, J.; Yang, X.; Zhang, J.; Shao, H. InCl3 Catalyzed Highly Diastereoselective [3+2] Cycloaddition of 1,2-Cyclopropanated Sugars with Aldehydes: A Straightforward Synthesis of Persubstituted Bis -Tetrahydrofurans and Perhydrofuro[2,3-b]Pyrans. Org. Lett. 2013, 15, 5170–5173. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Tang, Q.; Ke, J.; Zou, W.; Shao, H. Stereospecific [3+2] Cycloaddition of 1,2-Cyclopropanated Sugars and Ketones Catalyzed by SnCl4: An Efficient Synthesis of Multi-Substituted Perhydrofuro[2,3-b]Furans and Perhydrofuro[2,3-b]Pyrans. Chem. Commun. 2014, 50, 3505–3508. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Liang, P.; Ma, X.; Jiao, W.; Shao, H. An Effective Method for the Synthesis of 1,3-Dihydro-2H-Indazoles via N-N Bond Formation. Adv. Synth. Catal. 2019, 361, 5552–5557. [Google Scholar] [CrossRef]
- Winkler, J.D.; Asselin, S.M. Synthesis of Novel Heterocyclic Structures via Reaction of Isocyanides with S-trans-Enones. Org. Lett. 2006, 8, 3975–3977. [Google Scholar] [CrossRef] [PubMed]
- Masdeu, C.; Gómez, E.; Williams, N.A.O.; Lavilla, R. Double Insertion of Isocyanides into Dihydropyridines: Direct Access to Substituted Benzimidazolium Salts. Angew. Chem. Int. Ed. 2007, 46, 3043–3046. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhou, P.; Liu, F.; Hao, W.J.; Yao, C.; Jiang, B.; Tu, S.J. Cobalt(II)/Silver Relay Catalytic Isocyanide Insertion/Cycloaddition Cascades: A New Access to Pyrrolo[2,3-b]Indoles. Chem. Commun. 2015, 51, 9519–9522. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Wu, Y.N.; Chen, Z.Z.; Hao, W.J.; Li, G.; Tu, S.J.; Jiang, B. Synthesis of 3-Iminoindol-2-Amines and Cyclic Enaminones via Palladium-Catalyzed Isocyanide Insertion-Cyclization. J. Org. Chem. 2015, 80, 5764–5770. [Google Scholar] [CrossRef]
- Prasad, B.; Nallapati, S.B.; Kolli, S.K.; Sharma, A.K.; Yellanki, S.; Medisetti, R.; Kulkarni, P.; Sripelly, S.; Mukkanti, K.; Pal, M. Pd-Catalyzed Isocyanide Insertion/Nucleophilic Attack by Indole C-3/Desulfonylation in the Same Pot: A Direct Access to Indoloquinolines of Pharmacological Interest. RSC Adv. 2015, 5, 62966–62970. [Google Scholar] [CrossRef]
- Senadi, G.C.; Hu, W.P.; Boominathan, S.S.K.; Wang, J.J. Palladium(0)-Catalyzed Single and Double Isonitrile Insertion: A Facile Synthesis of Benzofurans, Indoles, and Isatins. Chem. Eur. J. 2015, 21, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- ITO, Y.; Kobayashi, K.; Maeno, M.; Saegusa, T. A New Synthetic Method for Preparation of 1,3,4,5-Tetrahydro-2H-1-Benzazepin-2-One Derivatives. Chem. Lett. 1980, 9, 487–490. [Google Scholar] [CrossRef]
- Liang, M.; Zhang, S.; Jia, J.; Tung, C.H.; Wang, J.; Xu, Z. Synthesis of Spiroketals by Synergistic Gold and Scandium Catalysis. Org. Lett. 2017, 19, 2526–2529. [Google Scholar] [CrossRef]
- Liu, S.; Chen, K.; Lan, X.C.; Hao, W.J.; Li, G.; Tu, S.J.; Jiang, B. Synergistic Silver/Scandium Catalysis for Divergent Synthesis of Skeletally Diverse Chromene Derivatives. Chem. Commun. 2017, 53, 10692–10695. [Google Scholar] [CrossRef]
- Thirupathi, N.; Tung, C.H.; Xu, Z. Scandium (III)-Catalyzed Cycloaddition of in Situ Generated ortho-Quinone Methides with Vinyl Azides: An Efficient Access to Substituted 4H-Chromenes. Adv. Synth. Catal. 2018, 360, 3585–3589. [Google Scholar] [CrossRef]
- Wang, L.; Ferguson, J.; Zeng, F. Palladium-Catalyzed Direct Coupling of 2-Vinylanilines and Isocyanides: An Efficient Synthesis of 2-Aminoquinolines. Org. Biomol. Chem. 2015, 13, 11486–11491. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug- Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. AdmetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yang, H. ADMET-Score—A Comprehensive Scoring Function for Evaluation of Chemical Drug-Likeness. Med. Chem. Commun. 2019, 10, 148–157. [Google Scholar] [CrossRef]
- Wang, H.; Tang, S.; Zhang, G.; Pan, Y.; Jiao, W.; Shao, H. Synthesis of N-Substituted Iminosugar C-Glycosides and Evaluation as Promising a-Glucosidase Inhibitors. Molecules 2022, 27, 5517. [Google Scholar] [CrossRef]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the Interaction Landscape of Bioactive Molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef]
- Antoine, D.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucl. Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Z. Facile Construction of Functionalized 4H-Chromene via Tandem Benzylation and Cyclization. Chem. Commun. 2008, 42, 5381–5383. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Entry | Promoter | Solvent | Yield b (%) |
| 1 | TsOH | CH2Cl2 | 18 |
| 2 | TfOH | CH2Cl2 | 15 |
| 3 | benzoic acid | CH2Cl2 | trace |
| 4 | BF3·Et2O | CH2Cl2 | 40 |
| 5 | InCl3 | CH2Cl2 | 23 |
| 6 | Sc(OTf)3 | CH2Cl2 | 53 |
| 7 | Sc(OTf)3 | THF | 29 |
| 8 | Sc(OTf)3 | MeCN | 37 |
| 9 | Sc(OTf)3 | DCE | 50 |
| 10 | Sc(OTf)3 | toluene | 60 |
| 11 c | Sc(OTf)3 | toluene | 75 |
| 12 d | Sc(OTf)3 | toluene | 71 |
| 13 c,e | Sc(OTf)3 | toluene | 81 |
| 14 c,f | Sc(OTf)3 | toluene | 69 |
| 15 c,e,g | Sc(OTf)3 | toluene | 87 |
| Compound Name | MW | nHetero Atoms | Rotatable Bonds | H-Bond Acceptor | H-Bond Donor | TPSA (Å sqr) | MlogP |
|---|---|---|---|---|---|---|---|
| 3aa | 330.34 | 5 | 4 | 3 | 1 | 70.99 | 4.03 |
| 3ba | 344.37 | 5 | 4 | 3 | 1 | 70.99 | 3.44 |
| 3ca | 344.37 | 5 | 4 | 3 | 1 | 70.99 | 3.44 |
| 3da | 344.37 | 5 | 4 | 3 | 1 | 70.99 | 3.44 |
| 3ea | 386.45 | 5 | 5 | 3 | 1 | 70.99 | 4.90 * |
| 3fa | 360.37 | 6 | 5 | 4 | 1 | 80.22 | 3.71 |
| 3ga | 348.33 | 6 | 4 | 4 | 1 | 70.99 | 3.60 |
| 3ha | 364.79 | 6 | 4 | 3 | 1 | 70.99 | 3.71 |
| 3ia | 409.24 | 6 | 4 | 3 | 1 | 70.99 | 3.82 |
| 3ja | 344.37 | 5 | 4 | 3 | 1 | 70.99 | 3.44 |
| 3ka | 344.37 | 5 | 4 | 3 | 1 | 70.99 | 3.44 |
| 3la | 358.40 | 5 | 4 | 3 | 1 | 70.99 | 3.66 |
| 3ma | 372.42 | 5 | 5 | 3 | 1 | 70.99 | 4.69 * |
| 3na | 360.37 | 6 | 5 | 4 | 1 | 80.22 | 3.71 |
| 3oa | 360.37 | 6 | 5 | 4 | 1 | 80.22 | 3.71 |
| 3pa | 380.40 | 5 | 4 | 3 | 1 | 70.99 | 4.73 * |
| 3qa | 348.33 | 6 | 4 | 4 | 1 | 70.99 | 3.60 |
| 3ra | 364.79 | 6 | 4 | 3 | 1 | 70.99 | 3.71 |
| 3sa | 398.34 | 8 | 5 | 6 | 1 | 70.99 | 4.04 |
| 4ab | 319.79 | 3 | 3 | 1 | 1 | 25.17 | 4.79 * |
| 4ac | 364.24 | 3 | 3 | 1 | 1 | 25.17 | 4.90 * |
| 4ad | 319.79 | 3 | 3 | 1 | 1 | 25.17 | 4.79 * |
| 4ae | 299.37 | 2 | 3 | 1 | 1 | 25.17 | 4.52 * |
| 4af | 335.31 | 2 | 3 | 1 | 1 | 25.17 | 4.99 * |
| 4ag | 295.34 | 4 | 6 | 3 | 1 | 51.47 | 2.80 |
| 4ah | 265.36 | 2 | 3 | 1 | 1 | 25.17 | 3.79 |
| Compound Name | Human Intestinal Absorption | Blood Brain Barrier | Caco-2 Permeability | Ames Mutagenesis | Carcinogenicity | Acute Oral Toxicity | Average Score |
|---|---|---|---|---|---|---|---|
| 3ba | 0.9868 | 0.7500 | 0.7184 | 0.7400 | 0.5000 | 0.5118 | 0.7012 |
| 3fa | 0.9848 | 0.7500 | 0.7869 | 0.7400 | 0.6020 | 0.5636 | 0.7379 |
| 3ga | 0.9874 | 0.7500 | 0.7567 | 0.6800 | 0.5381 | 0.4706 | 0.6971 |
| 3ha | 0.9860 | 0.7500 | 0.6451 | 0.7600 | 0.5881 | 0.5396 | 0.7115 |
| 3ia | 0.9835 | 0.7500 | 0.5890 | 0.7000 | 0.5371 | 0.5325 | 0.6820 |
| 3ka | 0.9868 | 0.7500 | 0.8320 | 0.7900 | 0.5000 | 0.5118 | 0.7284 |
| 3na | 0.9848 | 0.7500 | 0.7356 | 0.7700 | 0.6020 | 0.5636 | 0.7343 |
| 3qa | 0.9874 | 0.7500 | 0.7716 | 0.7600 | 0.5381 | 0.4706 | 0.7130 |
| 3ra | 0.9860 | 0.7500 | 0.5674 | 0.8100 | 0.5881 | 0.5396 | 0.7069 |
| 4ab | 0.9939 | 0.9000 | 0.6813 | 0.5500 | 0.5419 | 0.5439 | 0.7018 |
| 4ac | 0.9925 | 0.9000 | 0.6529 | 0.6300 | 0.5929 | 0.5220 | 0.7151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Tang, S.; Pan, Y.; Liang, P.; Ma, X.; Jiao, W.; Shao, H. A Novel Method to Construct 2-Aminobenzofurans via [4 + 1] Cycloaddition Reaction of In Situ Generated Ortho-Quinone Methides with Isocyanides. Molecules 2022, 27, 8538. https://doi.org/10.3390/molecules27238538
Lin H, Tang S, Pan Y, Liang P, Ma X, Jiao W, Shao H. A Novel Method to Construct 2-Aminobenzofurans via [4 + 1] Cycloaddition Reaction of In Situ Generated Ortho-Quinone Methides with Isocyanides. Molecules. 2022; 27(23):8538. https://doi.org/10.3390/molecules27238538
Chicago/Turabian StyleLin, Huaxin, Senling Tang, Yang Pan, Peng Liang, Xiaofeng Ma, Wei Jiao, and Huawu Shao. 2022. "A Novel Method to Construct 2-Aminobenzofurans via [4 + 1] Cycloaddition Reaction of In Situ Generated Ortho-Quinone Methides with Isocyanides" Molecules 27, no. 23: 8538. https://doi.org/10.3390/molecules27238538
APA StyleLin, H., Tang, S., Pan, Y., Liang, P., Ma, X., Jiao, W., & Shao, H. (2022). A Novel Method to Construct 2-Aminobenzofurans via [4 + 1] Cycloaddition Reaction of In Situ Generated Ortho-Quinone Methides with Isocyanides. Molecules, 27(23), 8538. https://doi.org/10.3390/molecules27238538






