Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use
Abstract
1. Introduction
2. Results
2.1. The Average Yield and Volatile Content in the Peel Essential Oil of Citrus limetta
2.2. Anti-Radical Activities
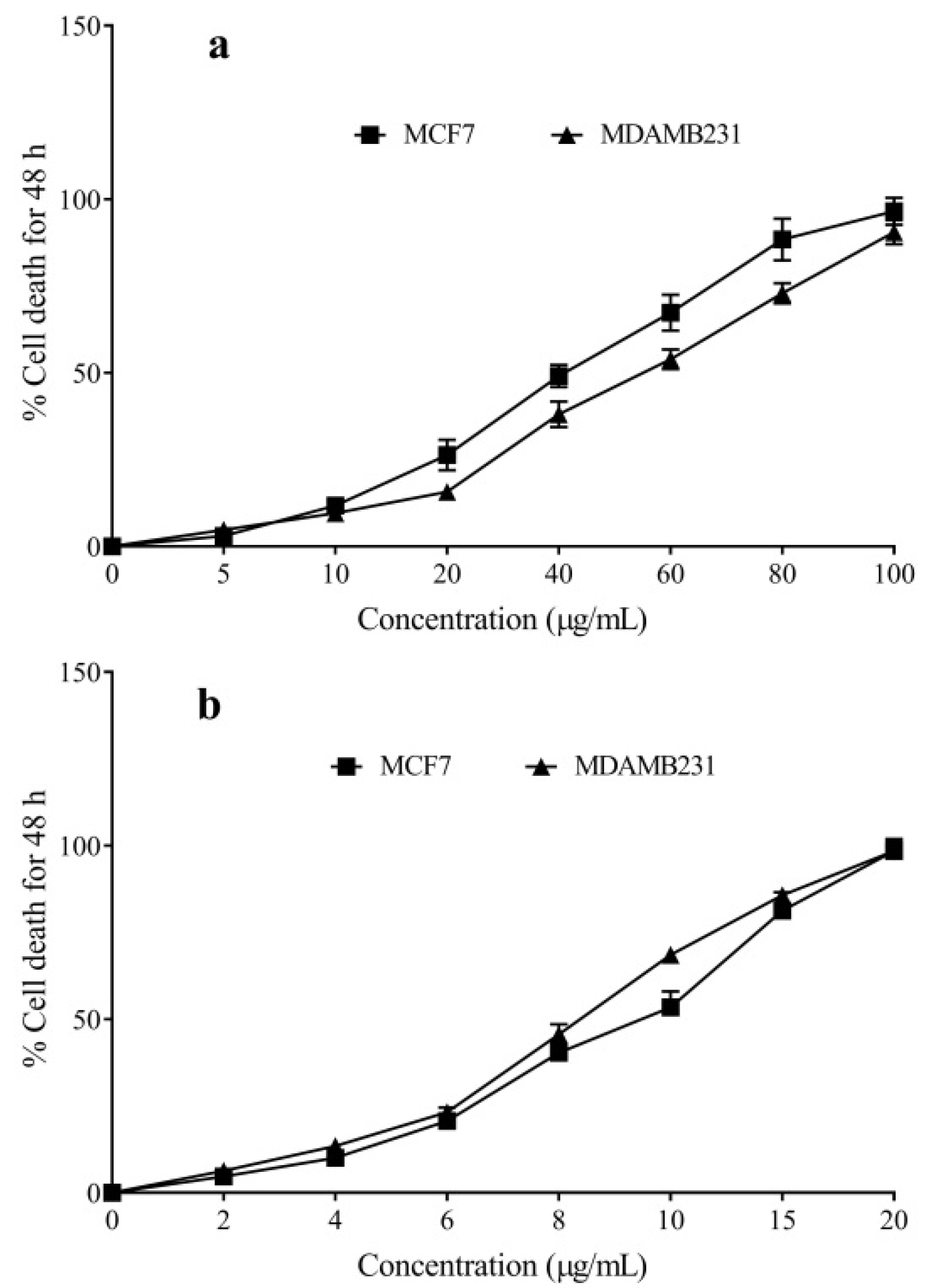
2.3. Cytotoxicity of the C. limetta Essential Oil, D-Limonene, and Cyclophosphamide
2.4. Bactericidal Properties of the CLEO
3. Discussion
4. Materials and Methods
4.1. Essential Oil Extraction from the Peel Waste of Citrus limetta
4.2. Analysis of the Component Chemicals in CLEO
4.3. Quenching Abilities of Citrus limetta Peel Essential Oil against Various Free Radicals
4.4. Anti-Proliferative Effect of the Citrus limetta Peel Essential Oil
4.5. Effect of the CLEO on ROS Level and Cytochrome C Release
4.6. Analysis of Antibacterial Activity
4.6.1. Bacterial Maintenance
4.6.2. Inhibition Zone Formation by C. limetta Essential Oil Treatment
4.6.3. C. limetta Essential Oil Minimum Inhibitory Concentrations (MIC)
4.6.4. Analysis of Biofilm Formation Inhibition by the Citrus limetta Essential Oil
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- Wikandari, R.; Nguyen, H.; Millati, R.; Niklasson, C.; Taherzadeh, M.J. Improvement of Biogas Production from Orange Peel Waste by Leaching of Limonene. Biomed. Res. Int. 2015, 2015, 494182. [Google Scholar] [CrossRef] [PubMed]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Mizuki, E.; Akao, T.; Saruwatari, T. Inhibitory effect of Citrus unshu peel on anaerobic digestion. Biol. Wastes 1990, 33, 161–168. [Google Scholar] [CrossRef]
- Millati, R.; Permanasari, E.D.; Sari, K.W.; Cahyanto, M.N.; Niklasson, C.; Taherzadeh, M.J. Anaerobic digestion of citrus waste using two-stage membrane bioreactor. IOP Conf. Ser. Mater. Sci. Eng. 2018, 316, 012063. [Google Scholar] [CrossRef]
- Kumar Sarangi, P.; Subudhi, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Prasad Shadangi, K.; Srivastava, R.K.; Pattnaik, B.; Arya, R.K. Utilization of agricultural waste biomass and recycling toward circular bioeconomy. Environ. Sci. Pollut. Res. Int. 2022, 13, 022–20669. [Google Scholar] [CrossRef]
- Ravindran, R.; Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. A Review on Bioconversion of Agro-Industrial Wastes to Industrially Important Enzymes. Bioengineering 2018, 5, 93. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Pontoni, L.; Porqueddu, I.; Greco, R.; Pirozzi, F.; Malpei, F. Effect of the concentration of essential oil on orange peel waste biomethanization: Preliminary batch results. Waste Manag. 2016, 48, 440–447. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Citrus essential oils and their influence on the anaerobic digestion process: An overview. Waste Manag. 2014, 34, 2063–2079. [Google Scholar] [CrossRef]
- USDA Foreign Agricultural Service. Citrus: World Markets and Trade; USDA Foreign Agricultural Service: Washington, DC, USA, 2020. [Google Scholar]
- Deng, M.; Jia, X.; Dong, L.; Liu, L.; Huang, F.; Chi, J.; Ma, Q.; Zhao, D.; Zhang, M.; Zhang, R. Structural elucidation of flavonoids from Shatianyu (Citrus grandis L. Osbeck) pulp and screening of key antioxidant components. Food Chem. 2022, 366, 130605. [Google Scholar] [CrossRef]
- Somanathan Karthiga, R.; Sukhdeo, S.V.; Madhugiri Lakshminarayan, S.; Mysuru Nanjarajurs, S. Efficacy of Citrus maxima fruit segment supplemented paranthas in STZ induced diabetic rats. J. Food Sci. 2021, 86, 2091–2102. [Google Scholar] [CrossRef]
- Tsai, M.L.; Lin, C.D.; Khoo, K.A.; Wang, M.Y.; Kuan, T.K.; Lin, W.C.; Zhang, Y.N.; Wang, Y.Y. Composition and Bioactivity of Essential Oil from Citrus grandis (L.) Osbeck ‘Mato Peiyu’ Leaf. Molecules 2017, 22, 2154. [Google Scholar] [CrossRef]
- Yabalak, E.; Erdogan Eliuz, E.A.; Nazli, M.D. Evaluation of Citrus reticulata essential oil: Chemical composition and antibacterial effectiveness incorporated gelatin on E. coli and S. aureus. Int. J. Environ. Health Res. 2022, 32, 1261–1270. [Google Scholar] [CrossRef]
- Song, X.; Liu, T.; Wang, L.; Liu, L.; Li, X.; Wu, X. Antibacterial Effects and Mechanism of Mandarin (Citrus reticulata L.) Essential Oil against Staphylococcus aureus. Molecules 2020, 25, 4956. [Google Scholar] [CrossRef]
- Tao, N.; Jia, L.; Zhou, H. Anti-fungal activity of Citrus reticulata Blanco essential oil against Penicillium italicum and Penicillium digitatum. Food Chem. 2014, 153, 265–271. [Google Scholar] [CrossRef]
- Ishfaq, M.; Akhtar, B.; Muhammad, F.; Sharif, A.; Akhtar, M.F.; Hamid, I.; Sohail, K.; Muhammad, H. Antioxidant and Wound Healing Potential of Essential Oil from Citrus reticulata Peel and Its Chemical Characterization. Curr. Pharm. Biotechnol. 2021, 22, 1114–1121. [Google Scholar] [CrossRef]
- Thavanapong, N.; Wetwitayaklung, P.; Charoenteeraboon, J. Comparison of Essential Oils Compositions of Citrus maxima Merr. Peel Obtained by Cold Press and Vacuum Stream Distillation Methods and of Its Peel and Flower Extract Obtained by Supercritical Carbon Dioxide Extraction Method and Their Antimicrobial Activity. J. Essent. Oil Res. 2010, 22, 71–77. [Google Scholar] [CrossRef]
- Visakh, N.U.; Pathrose, B.; Narayanankutty, A.; Alfarhan, A.; Ramesh, V. Utilization of Pomelo (Citrus maxima) Peel Waste into Bioactive Essential Oils: Chemical Composition and Insecticidal Properties. Insects 2022, 13, 480. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, R.; Prakash, B.; Kumar, A.; Singh, S.; Mishra, P.K.; Dubey, N.K. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem. Toxicol. 2010, 48, 1734–1740. [Google Scholar] [CrossRef]
- Kumar, S.; Warikoo, R.; Mishra, M.; Seth, A.; Wahab, N. Larvicidal efficacy of the Citrus limetta peel extracts against Indian strains of Anopheles stephensi Liston and Aedes aegypti L. Parasitol. Res. 2012, 111, 173–178. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and Anticancer Activities of Essential Oil from Gannan Navel Orange Peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Yousefian Rad, E.; Homayouni Tabrizi, M.; Ardalan, P.; Seyedi, S.M.R.; Yadamani, S.; Zamani-Esmati, P.; Haghani Sereshkeh, N. Citrus lemon essential oil nanoemulsion (CLEO-NE), a safe cell-depended apoptosis inducer in human A549 lung cancer cells with anti-angiogenic activity. J. Microencapsul. 2020, 37, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef]
- Sharma, K.; Garg, V.K. Vermicomposting of Waste: A Zero-Waste Approach for Waste Management. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–164. [Google Scholar] [CrossRef]
- Gomes-Araújo, R.; Martínez-Vázquez, D.G.; Charles-Rodríguez, A.V.; Rangel-Ortega, S.; Robledo-Olivo, A. Bioactive Compounds from Agricultural Residues, Their Obtaining Techniques, and the Antimicrobial Effect as Postharvest Additives. Int. J. Food Sci. 2021, 2021, 9936722. [Google Scholar] [CrossRef]
- Vuuren, S.F.v.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Thakre, A.; Zore, G.; Kodgire, S.; Kazi, R.; Mulange, S.; Patil, R.; Shelar, A.; Santhakumari, B.; Kulkarni, M.; Kharat, K.; et al. Limonene inhibits Candida albicans growth by inducing apoptosis. Med. Mycol. 2017, 56, 565–578. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene against Listeria monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Zhou, J.; Azrad, M.; Kong, L. Effect of Limonene on Cancer Development in Rodent Models: A Systematic Review. Front. Sustain. Food Syst. 2021, 5, 407. [Google Scholar] [CrossRef]
- Zhou, H.; Tao, N.; Jia, L. Antifungal activity of citral, octanal and α-terpineol against Geotrichum citri-aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Zielińska, A.; Martins-Gomes, C.; Ferreira, N.R.; Silva, A.M.; Nowak, I.; Souto, E.B. Anti-inflammatory and anti-cancer activity of citral: Optimization of citral-loaded solid lipid nanoparticles (SLN) using experimental factorial design and LUMiSizer®. Int. J. Pharm. 2018, 553, 428–440. [Google Scholar] [CrossRef]
- Oliveira, A.; Fernandes, C.C.; Santos, L.S.; Candido, A.; Magalhaes, L.G.; Miranda, M.L.D. Chemical composition, in vitro larvicidal and antileishmanial activities of the essential oil from Citrus reticulata Blanco fruit peel. Braz. J. Biol. = Rev. Brasleira Biol. 2021, 83, e247539. [Google Scholar] [CrossRef]
- Ambrosio, C.M.S.; Diaz-Arenas, G.L.; Agudelo, L.P.A.; Stashenko, E.; Contreras-Castillo, C.J.; da Gloria, E.M. Chemical Composition and Antibacterial and Antioxidant Activity of a Citrus Essential Oil and Its Fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Changes in chemical composition and biological activity of essential oil from Thomson navel orange (Citrus sinensis L. Osbeck) peel under freezing, convective, vacuum, and microwave drying methods. Food Sci. Nutr. 2020, 8, 124–138. [Google Scholar] [CrossRef]
- Abdel-Kawy, M.A.; Michel, C.G.; Kirollos, F.N.; Hussien, R.A.A.; Al-Mahallawi, A.M.; Sedeek, M.S. Chemical composition and potentiation of insecticidal and fungicidal activities of Citrus trifoliata L. fruits essential oil against Spodoptera littoralis, Fusarium oxysporum and Fusarium solani via nano-cubosomes. Nat. Prod. Res. 2021, 35, 2438–2443. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova-Kostova, R.; Goranov, B.; Hristova-Ivanova, Y.; Slavchev, A.; Denkova, Z.; Kostov, G. Chemical composition, antioxidant activity and antimicrobial activity of essential oil from Citrus aurantium L zest against some pathogenic microorganisms. Z. Naturforschung. C J. Biosci. 2019, 74, 105–111. [Google Scholar] [CrossRef]
- Goncalves Mendes Neto, A.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy, R., 3rd; Bhargav, R.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2021, 93, 1489–1495. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Fancello, F.; Petretto, G.L.; Marceddu, S.; Venditti, T.; Pintore, G.; Zara, G.; Mannazzu, I.; Budroni, M.; Zara, S. Antimicrobial activity of gaseous Citrus limon var pompia leaf essential oil against Listeria monocytogenes on ricotta salata cheese. Food Microbiol. 2020, 87, 103386. [Google Scholar] [CrossRef] [PubMed]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Raspo, M.A.; Vignola, M.B.; Andreatta, A.E.; Juliani, H.R. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Narang, N.; Jiraungkoorskul, W. Anticancer Activity of Key Lime, Citrus aurantifolia. Pharm. Rev. 2016, 10, 118–122. [Google Scholar]
- Hero, T.; Buhler, H.; Kouam, P.N.; Priesch-Grzeszowiak, B.; Lateit, T.; Adamietz, I.A. The Triple-negative Breast Cancer Cell Line MDA-MB 231 Is Specifically Inhibited by the Ionophore Salinomycin. Anticancer Res. 2019, 39, 2821–2827. [Google Scholar] [CrossRef]
- Shi, Y.; Ye, P.; Long, X. Differential Expression Profiles of the Transcriptome in Breast Cancer Cell Lines Revealed by Next Generation Sequencing. Cell. Physiol. Biochem. 2017, 44, 804–816. [Google Scholar] [CrossRef]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018, 38, BSR20181253. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef]
- White, B.; Evison, A.; Dombi, E.; Townley, H.E. Improved delivery of the anticancer agent citral using BSA nanoparticles and polymeric wafers. Nanotechnol. Sci. Appl. 2017, 10, 163–175. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Cui, H.F.; Cao, C.Y.; Zhang, Y.B. 4-Terpineol exhibits potent in vitro and in vivo anticancer effects in Hep-G2 hepatocellular carcinoma cells by suppressing cell migration and inducing apoptosis and sub-G1 cell cycle arrest’. J. B.U.ON. Off. J. Balkan Union Oncol. 2021, 26, 294. [Google Scholar]
- Hassan, S.B.; Gali-Muhtasib, H.; Goransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-kappaB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar]
- House, N.C.; Puthenparampil, D.; Malayil, D.; Narayanankutty, A. Variation in the polyphenol composition, antioxidant, and anticancer activity among different Amaranthus species. S. Afr. J. Bot. 2020, 135, 408–412. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. Hydrogen Peroxide Scavenging Activity of Novel Coumarins Synthesized Using Different Approaches. PLoS ONE 2015, 10, e0132175. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Gopinath, M.K.; Vakayil, M.; Ramavarma, S.K.; Babu, T.D.; Raghavamenon, A.C. Non-enzymatic conversion of primary oxidation products of Docosahexaenoic acid into less toxic acid molecules. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 203, 222–228. [Google Scholar] [CrossRef]
- Kim, Y.O.; Narayanankutty, A.; Kuttithodi, A.M.; Kim, H.-J.; Na, S.W.; Kunnath, K.; Rajagopal, R.; Alfarhan, A. Azima tetracantha Leaf Methanol Extract Inhibits Gastric Cancer Cell Proliferation through Induction of Redox Imbalance and Cytochrome C Release. Appl. Sci. 2022, 12, 120. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Walia, S.; Mukhia, S.; Bhatt, V.; Kumar, R.; Kumar, R. Variability in chemical composition and antimicrobial activity of Tagetes minuta L. essential oil collected from different locations of Himalaya. Ind. Crops Prod. 2020, 150, 112449. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). E.C.f.A.S.T.E.o.t.E.S.o.C.M.a.I.D. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Aljeldah, M.M. Antioxidant and Antimicrobial Potencies of Chemically-Profiled Essential Oil from Asteriscus graveolens against Clinically-Important Pathogenic Microbial Strains. Molecules 2022, 27, 3539. [Google Scholar] [CrossRef]
- Selim, S.; Almuhayawi, M.S.; Alqhtani, H.; Al Jaouni, S.K.; Saleh, F.M.; Warrad, M.; Hagagy, N. Anti-Salmonella and Antibiofilm Potency of Salvia officinalis L. Essential Oil against Antibiotic-Resistant Salmonella enterica. Antibiotics 2022, 11, 489. [Google Scholar] [CrossRef]
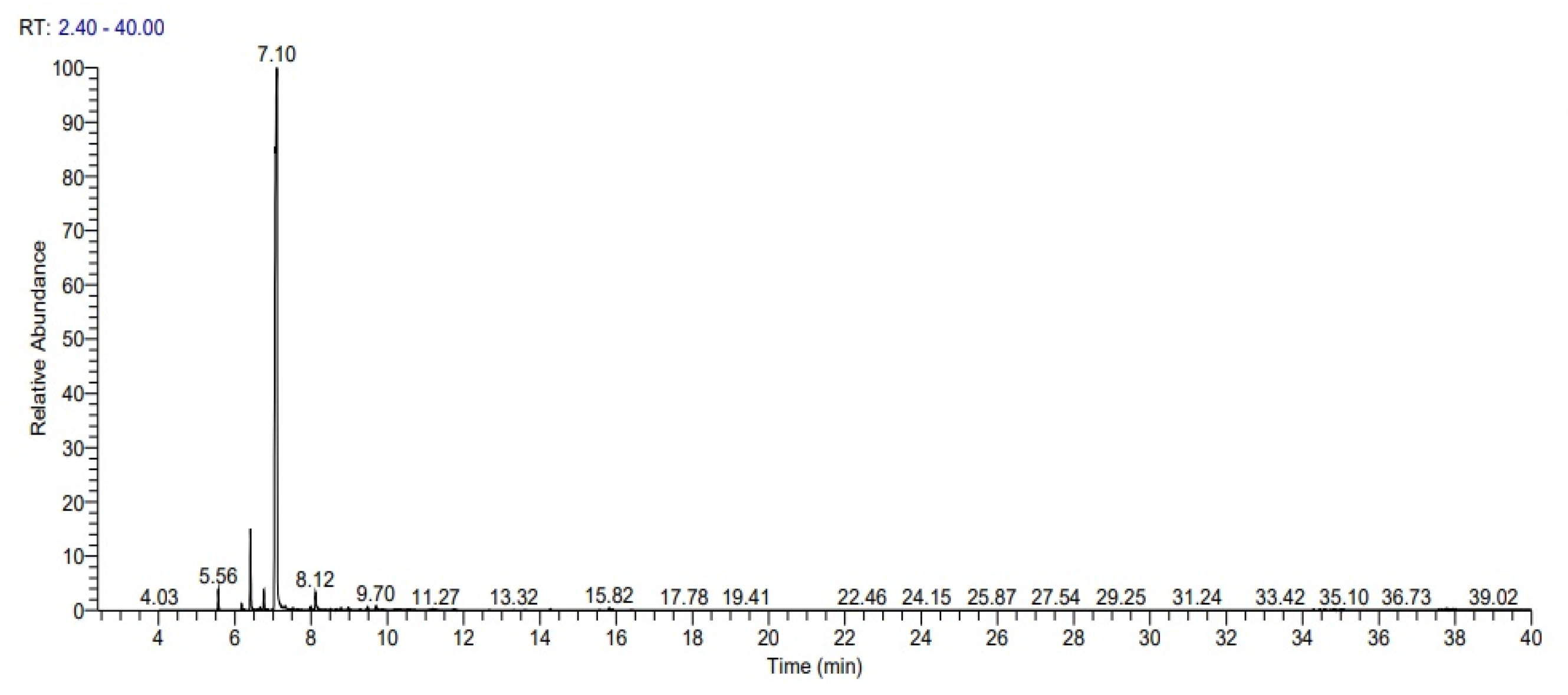

| Peak No. | Retention Time | Component | Retention Index | %Relative Area a | |
|---|---|---|---|---|---|
| Calculated | Library | ||||
| 1 | 5.56 | α-pinene | 939 | 938 | 1.17 ± 0.96 |
| 2 | 6.41 | α-myrcene | 985 | 983 | 4.85 ± 0.67 |
| 3 | 6.76 | 3-carene | 1011 | 1012 | 1.20 ± 0.08 |
| 4 | 7.09 | D-limonene | 1029 | 1029 | 85.71 ± 0.41 |
| 5 | 8.12 | Linalyl acetate | 1237 | 1133 | 1.64 ± 0.37 |
| 6 | 8.97 | Citronellal | 1153 | 1141 | 0.36 ± 0.06 |
| 7 | 9.48 | Terpinen-4-ol | 1175 | 1176 | 0.36 ± 0.28 |
| 8 | 9.70 | α-Terpineol | 1178 | 1180 | 0.44 ± 0.03 |
| 9 | 10.31 | cis-p-mentha-1(7),8-dien-2-ol | 1190 | 1195 | 0.43 ± 0.43 |
| 10 | 15.82 | Guaia-1(10),11-diene | 1490 | 1488 | 0.32 ± 0.91 |
| DPPH Radical Scavenging | ABTS Radical Scavenging | H2O2 Radical Scavenging | Ferric Reducing Antioxidant Power | Lipid Peroxidation Inhibition | |
|---|---|---|---|---|---|
| CLEO | 11.35 ± 0.51 * | 10.36 ± 0.55 * | 8.28 ± 0.35 * | 8.67 ± 0.21 | 30.19 ± 0.27 * |
| D-limonene | 48.49 ± 0.22 | 41.22 ± 0.13 | 20.67 ± 0.34 | 19.08 ± 0.33 | 58.16 ± 0.43 |
| Ascorbic acid | 9.57 ± 0.75 * | 11.08 ± 2.11 * | 19.62 ± 1.60 | 3.41 ± 0.29 * | 65.98 ± 1.95 |
| MCF-7 | MDAMB231 | |
|---|---|---|
| CLEO | 47.31 ± 3.11 | 55.11 ± 4.62 |
| D-Limonene | 392.57 ± 5.29 | 428.33 ± 4.61 |
| Cyclophosphamide | 10.02 ± 0.38 | 9.37 ± 0.25 |
| Strain | Zone of Inhibition (mm) | ||
|---|---|---|---|
| CLEO | D-Limonene | GM | |
| Escherichia coli | 13.5 ± 0.4 | 16.7 ± 0.2 | 22.5 ± 0.1 |
| Pseudomonas aeruginosa | 16.8 ± 0.2 | 18.7 ± 0.2 | 19.5 ± 0.2 |
| Staphylococcus aureus | 17.1 ± 0.5 | 20.9 ± 0.3 | 23.0 ± 0.1 |
| Salmonella enterica | 15.9 ± 0.3 | 17.1 ± 0.4 | 19.5 ± 0.3 |
| Bacteria | MIC Concentration (mg/mL) | ||
|---|---|---|---|
| CLEO | D-Limonene | GM | |
| Escherichia coli | 0.50 ± 0.03 * | 0.0625 ± 0.02 | 0.0312 ± 0.01 |
| Pseudomonas aeruginosa | 0.75 ± 0.03 | 0.0312 ± 0.01 | 0.0312 ± 0.01 |
| Staphylococcus aureus | 0.50 ± 0.02 | 1.25 ± 0.1 | 1.5 ± 0.3 |
| Salmonella enterica | 0.625 ± 0.03 * | 0.0312 ± 0.00 | 0.0312 ± 0.01 |
| Percentage Inhibition | |||
|---|---|---|---|
| CLEO | D-Limonene | GM | |
| Escherichia coli | 90.6 ± 1.6 | 100 | 100 |
| Pseudomonas aeruginosa | 92.19 ± 1.2 | 100 | 100 |
| Staphylococcus aureus | 95.6 ± 2.1 | 100 | 100 |
| Salmonella enterica | 93.8 ± 1.5 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Pathrose, B.; Olatunji, O.J.; Al-Ansari, A.; Alfarhan, A.; Ramesh, V. Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use. Molecules 2022, 27, 8329. https://doi.org/10.3390/molecules27238329
Narayanankutty A, Visakh NU, Sasidharan A, Pathrose B, Olatunji OJ, Al-Ansari A, Alfarhan A, Ramesh V. Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use. Molecules. 2022; 27(23):8329. https://doi.org/10.3390/molecules27238329
Chicago/Turabian StyleNarayanankutty, Arunaksharan, Naduvilthara U. Visakh, Anju Sasidharan, Berin Pathrose, Opeyemi Joshua Olatunji, Abdullah Al-Ansari, Ahmed Alfarhan, and Varsha Ramesh. 2022. "Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use" Molecules 27, no. 23: 8329. https://doi.org/10.3390/molecules27238329
APA StyleNarayanankutty, A., Visakh, N. U., Sasidharan, A., Pathrose, B., Olatunji, O. J., Al-Ansari, A., Alfarhan, A., & Ramesh, V. (2022). Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use. Molecules, 27(23), 8329. https://doi.org/10.3390/molecules27238329









