Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Compounds
2.2. Antiradical Potentials of Various Synthetic Bis-Chalcones
2.3. Synthetic Bis-Chalcones as Antimicrobial Agents
2.4. Larvicidal Activity of Synthetic Bis-Chalcones
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instruments Used for the Study
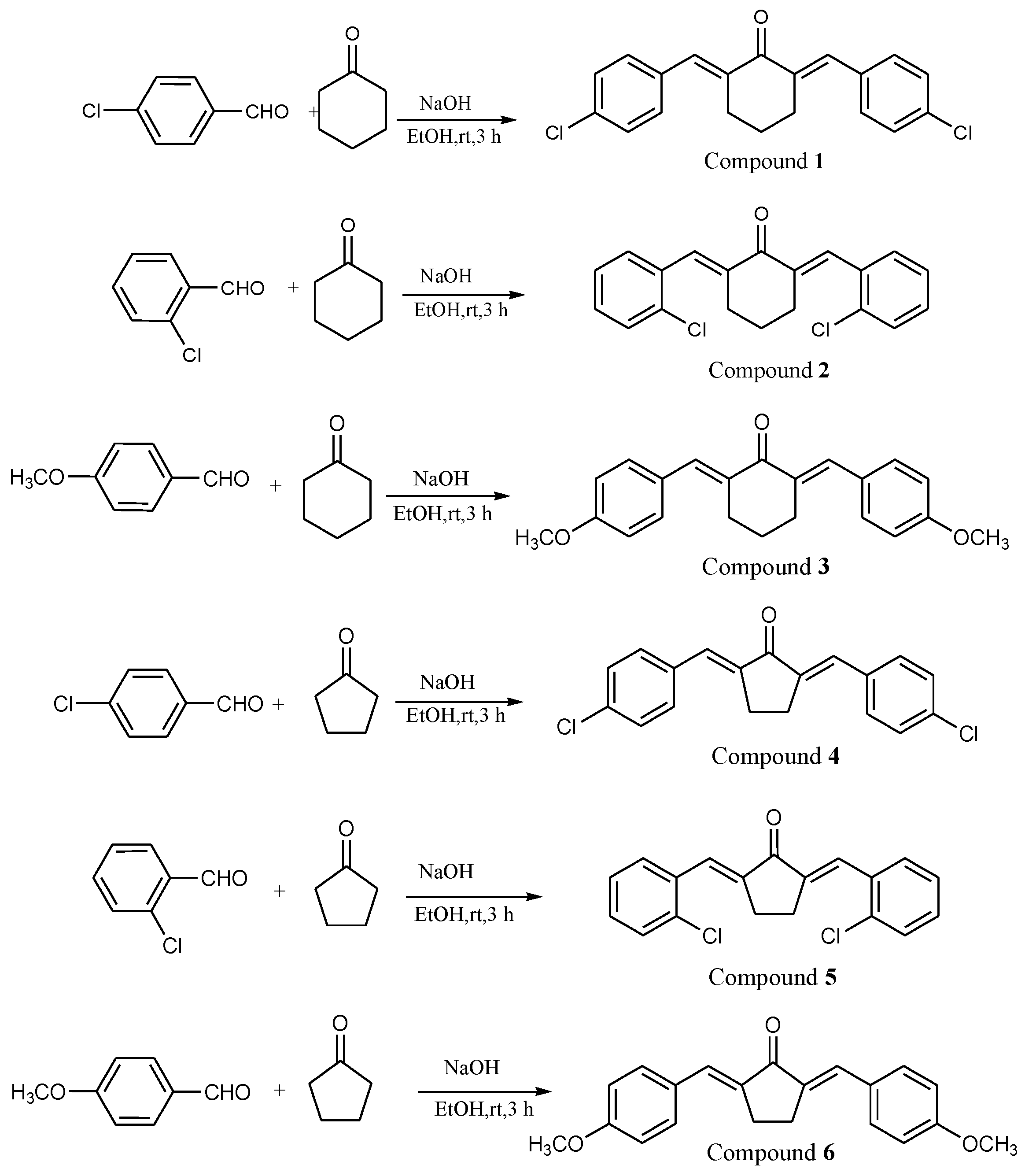
3.3. Synthesis and Characterization of Bis-Chalcones
3.4. Radical Generation Inhibition and Reducing Potential of the Synthetic Bis-Chalcones
3.5. Antibacterial Activity of the Synthetic Bis-Chalcones by Disc Diffusion Method
3.6. Analysis of the Larvicidal Activity of Synthetic Bis-Chalcones
3.7. Cytotoxicity Analysis of Synthetic Bis-Chalcones
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nieto, G. How Are Medicinal Plants Useful When Added to Foods? Medicines 2020, 7, 58. [Google Scholar] [CrossRef]
- Vatansever, R.; Ozyigit, I.; Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: A Review. Appl. Biochem. Biotechnol. 2017, 181, 464–482. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.K.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytosc. 2021, 7, 52. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Biological Potential and Medical Use of Secondary Metabolites. Medicines 2019, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 11, 497. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes. Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z.; Zha, D. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537. [Google Scholar] [CrossRef] [PubMed]
- Oldoni, T.L.C.; Cabral, I.S.R.; d’Arce, M.A.B.R.; Rosalen, P.L.; Ikegaki, M.; Nascimento, A.M.; Alencar, S.M. Isolation and analysis of bioactive isoflavonoids and chalcone from a new type of Brazilian propolis. Sep. Purif. Technol. 2011, 77, 208–213. [Google Scholar] [CrossRef]
- Escobar-Ramos, A.; Lobato-Garcia, C.E.; Zamilpa, A.; Gomez-Rivera, A.; Tortoriello, J.; Gonzalez-Cortazar, M. Homoisoflavonoids and Chalcones Isolated from Haematoxylum campechianum L. with Spasmolytic Activity. Molecules 2017, 22, 1405. [Google Scholar] [CrossRef]
- Park, J.Y.; Ko, J.A.; Kim, D.W.; Kim, Y.M.; Kwon, H.J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzym. Inhib. Med. Chem. 2016, 31, 23–30. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, Y.; Luo, Q.; Wang, H.; Chen, R.; Liu, Y.; Li, Y. Antiradical Activity and Mechanism of Coumarin–Chalcone Hybrids: Theoretical Insights. J. Phys. Chem. A 2018, 122, 8520–8529. [Google Scholar] [CrossRef]
- Shin, J.E.; Choi, E.J.; Jin, Q.; Jin, H.G.; Woo, E.R. Chalcones isolated from Angelica keiskei and their inhibition of IL-6 production in TNF-alpha-stimulated MG-63 cell. Arch. Pharmacal Res. 2011, 34, 437–442. [Google Scholar] [CrossRef]
- Enoki, T.; Ohnogi, H.; Nagamine, K.; Kudo, Y.; Sugiyama, K.; Tanabe, M.; Kobayashi, E.; Sagawa, H.; Kato, I. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 2007, 55, 6013–6017. [Google Scholar] [CrossRef]
- Jantan, I.; Mohd Yasin, Y.H.; Jamil, S.; Sirat, H.; Basar, N. Effect of prenylated flavonoids and chalcones isolated from Artocarpus species on platelet aggregation in human whole blood. J. Nat. Med. 2010, 64, 365–369. [Google Scholar] [CrossRef]
- Ngameni, B.; Touaibia, M.; Patnam, R.; Belkaid, A.; Sonna, P.; Ngadjui, B.T.; Annabi, B.; Roy, R. Inhibition of MMP-2 secretion from brain tumor cells suggests chemopreventive properties of a furanocoumarin glycoside and of chalcones isolated from the twigs of Dorstenia turbinata. Phytochemistry 2006, 67, 2573–2579. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Okolo, E.N.; Ugwu, D.I.; Ezema, B.E.; Ndefo, J.C.; Eze, F.U.; Ezema, C.G.; Ezugwu, J.A.; Ujam, O.T. New chalcone derivatives as potential antimicrobial and antioxidant agent. Sci. Rep. 2021, 11, 21781. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D.K. Chalcone derivatives as potential antifungal agents: Synthesis, and antifungal activity. J. Adv. Pharm. Technol. Res. 2015, 6, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Upadhyay, S.P.; Suo, W.Z.; Singh, V.; Gurung, P.; Lee, E.S.; Sharma, R.; Sharma, M. Chalcone and its analogs: Therapeutic and diagnostic applications in Alzheimer’s disease. Bioorg. Chem. 2021, 108, 29. [Google Scholar] [CrossRef]
- Kucharíková, A.; Kusari, S.; Sezgin, S.; Spiteller, M.; Čellárová, E. Occurrence and Distribution of Phytochemicals in the Leaves of 17 In vitro Cultured Hypericum spp. Adapted to Outdoor Conditions. Front. Plant Sci. 2016, 7, 1616. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed]
- Liargkova, T.; Hadjipavlou-Litina, D.J.; Koukoulitsa, C.; Voulgari, E.; Avgoustakis, C. Simple chalcones and bis-chalcones ethers as possible pleiotropic agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 302–313. [Google Scholar] [CrossRef]
- Tajudeen Bale, A.; Mohammed Khan, K.; Salar, U.; Chigurupati, S.; Fasina, T.; Ali, F.; Wadood, A.; Taha, M.; Sekhar Nanda, S.; Ghufran, M.; et al. Chalcones and bis-chalcones: As potential α-amylase inhibitors; synthesis, in vitro screening, and molecular modelling studies. Bioorg. Chem. 2018, 79, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Xu, M.; Wu, P.; Shen, F.; Ji, J.; Rakesh, K.P. Chalcone derivatives and their antibacterial activities: Current development. Bioorg. Chem. 2019, 91, 103133. [Google Scholar] [CrossRef] [PubMed]
- Božić, D.D.; Milenković, M.; Ivković, B.; Ćirković, I. Antibacterial activity of three newly-synthesized chalcones & synergism with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. Indian J. Med. Res. 2014, 140, 130–137. [Google Scholar] [PubMed]
- Koudokpon, H.; Armstrong, N.; Dougnon, T.V.; Fah, L.; Hounsa, E.; Bankolé, H.S.; Loko, F.; Chabrière, E.; Rolain, J.M. Antibacterial Activity of Chalcone and Dihydrochalcone Compounds from Uvaria chamae Roots against Multidrug-Resistant Bacteria. BioMed Res. Int. 2018, 2018, 1453173. [Google Scholar] [CrossRef] [PubMed]
- Satokata, A.A.C.; Souza, J.H.; Silva, L.L.O.; Santiago, M.B.; Ramos, S.B.; Assis, L.R.; Theodoro, R.S.; Oliveira, L.R.; Regasini, L.O.; Martins, C.H.G. Chalcones with potential antibacterial and antibiofilm activities against periodontopathogenic bacteria. Anaerobe 2022, 76, 102588. [Google Scholar] [CrossRef]
- Kunthalert, D.; Baothong, S.; Khetkam, P.; Chokchaisiri, S.; Suksamrarn, A. A chalcone with potent inhibiting activity against biofilm formation by nontypeable Haemophilus influenzae. Microbiol. Immunol. 2014, 58, 581–589. [Google Scholar] [CrossRef]
- Bozic, D.D.; Milenkovic, M.; Ivkovic, B.; Cirkovic, I. Newly-synthesized chalcones-inhibition of adherence and biofilm formation of methicillin-resistant Staphylococcus aureus. Braz. J. Microbiol. 2014, 45, 263–270. [Google Scholar] [CrossRef]
- Uchil, A.; Murali, T.S.; Nayak, R. Escaping ESKAPE: A chalcone perspective. Results Chem. 2021, 3, 100229. [Google Scholar] [CrossRef]
- Hellewell, L.; Bhakta, S. Chalcones, stilbenes and ketones have anti-infective properties via inhibition of bacterial drug-efflux and consequential synergism with antimicrobial agents. Access Microbiol. 2020, 2, acmi000105. [Google Scholar] [CrossRef]
- Goncalves Mendes Neto, A.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy, R., III; Bhargav, R.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2021, 93, 1489–1495. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 022–01056. [Google Scholar] [CrossRef] [PubMed]
- Nakao, J.H.; Talkington, D.; Bopp, C.A.; Besser, J.; Sanchez, M.L.; Guarisco, J.; Davidson, S.L.; Warner, C.; McIntyre, M.G.; Group, J.P.; et al. Unusually high illness severity and short incubation periods in two foodborne outbreaks of Salmonella Heidelberg infections with potential coincident Staphylococcus aureus intoxication. Epidemiol. Infect. 2018, 146, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Anal, A.K. Prevalence and antibiogram study of Salmonella and Staphylococcus aureus in poultry meat. Asian Pac. J. Trop. Biomed. 2013, 3, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Targanski, S.K.; Sousa, J.R.; de Pádua, G.M.; de Sousa, J.M.; Vieira, L.C.; Soares, M.A. Larvicidal activity of substituted chalcones against Aedes aegypti (Diptera: Culicidae) and non-target organisms. Pest Manag. Sci. 2021, 77, 325–334. [Google Scholar] [CrossRef]
- Begum, N.A.; Roy, N.; Laskar, R.A.; Roy, K. Mosquito larvicidal studies of some chalcone analogues and their derived products: Structure–activity relationship analysis. Med. Chem. Res. 2011, 20, 184–191. [Google Scholar] [CrossRef]
- Pasquale, G.; Romanelli, G.P.; Autino, J.C.; García, J.; Ortiz, E.V.; Duchowicz, P.R. Quantitative Structure–Activity Relationships of Mosquito Larvicidal Chalcone Derivatives. J. Agric. Food Chem. 2012, 60, 692–697. [Google Scholar] [CrossRef]
- Weetman, D.; Kamgang, B.; Badolo, A.; Moyes, C.L.; Shearer, F.M.; Coulibaly, M.; Pinto, J.; Lambrechts, L.; McCall, P.J. Aedes Mosquitoes and Aedes-Borne Arboviruses in Africa: Current and Future Threats. Int. J. Environ. Res. Public Health 2018, 15, 220. [Google Scholar] [CrossRef]
- Aragão, C.F.; Pinheiro, V.C.S.; Nunes Neto, J.P.; Silva, E.; Pereira, G.J.G.; Nascimento, B.; Castro, K.D.S.; Maia, A.M.; Catete, C.P.; Martins, L.C.; et al. Natural Infection of Aedes aegypti by Chikungunya and Dengue type 2 Virus in a Transition Area of North-Northeast Brazil. Viruses 2019, 11, 1126. [Google Scholar] [CrossRef]
- Gloria-Soria, A.; Armstrong, P.M.; Powell, J.R.; Turner, P.E. Infection rate of Aedes aegypti mosquitoes with dengue virus depends on the interaction between temperature and mosquito genotype. Proc. Biol. Sci. 2017, 284, 1864. [Google Scholar]
- Mourya, D.T.; Gokhale, M.D.; Majumdar, T.D.; Yadav, P.D.; Kumar, V.; Mavale, M.S. Experimental Zika virus infection in Aedes aegypti: Susceptibility, transmission & co-infection with dengue & chikungunya viruses. Indian J. Med. Res. 2018, 147, 88–96. [Google Scholar]
- Hegde, S.M.; Kumar, M.N.; Kavya, K.; Kumar, K.M.; Nagesh, R.; Patil, R.H.; Babu, R.L.; Ramesh, G.T.; Sharma, S.C. Interplay of nuclear receptors (ER, PR, and GR) and their steroid hormones in MCF-7 cells. Mol. Cell. Biochem. 2016, 422, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Fog, C.K.; Christensen, I.J.; Lykkesfeldt, A.E. Characterization of a human breast cancer cell line, MCF-7/RU58R-1, resistant to the pure antiestrogen RU 58,668. Breast Cancer Res. Treat. 2005, 91, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Leung, E.; Kannan, N.; Krissansen, G.W.; Findlay, M.P.; Baguley, B.C. MCF-7 breast cancer cells selected for tamoxifen resistance acquire new phenotypes differing in DNA content, phospho-HER2 and PAX2 expression, and rapamycin sensitivity. Cancer Biol. Ther. 2010, 9, 717–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef]
- Al-Bader, M.; Ford, C.; Al-Ayadhy, B.; Francis, I. Analysis of estrogen receptor isoforms and variants in breast cancer cell lines. Exp. Ther. Med. 2011, 2, 537–544. [Google Scholar] [CrossRef]
- Kalinina, T.S.; Kononchuk, V.V.; Gulyaeva, L.F. Expression of estrogen-, progesterone-, and androgen-responsive genes in MCF-7 and MDA-MB-231 cells treated with o,p’-DDT, p,p’-DDT, or endosulfan. J. Biochem. Mol. Toxicol. 2021, 35, 1–8. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Lalhminghlui, K.; Jagetia, G.C. Evaluation of the free-radical scavenging and antioxidant activities of Chilauni, Schima wallichii Korth in vitro. Future Sci. OA 2018, 4, FSO272. [Google Scholar] [CrossRef]
- Youn, J.S.; Kim, Y.J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2018, 28, 201–207. [Google Scholar] [CrossRef]
- Walia, S.; Mukhia, S.; Bhatt, V.; Kumar, R.; Kumar, R. Variability in chemical composition and antimicrobial activity of Tagetes minuta L. essential oil collected from different locations of Himalaya. Ind. Crops Prod. 2020, 150, 112449. [Google Scholar] [CrossRef]
- Khanapure, S.; Jagadale, M.; Bansode, P.; Choudhari, P.; Rashinkar, G. Anticancer activity of ruthenocenyl chalcones and their molecular docking studies. J. Mol. Struct. 2018, 1173, 142–147. [Google Scholar] [CrossRef]
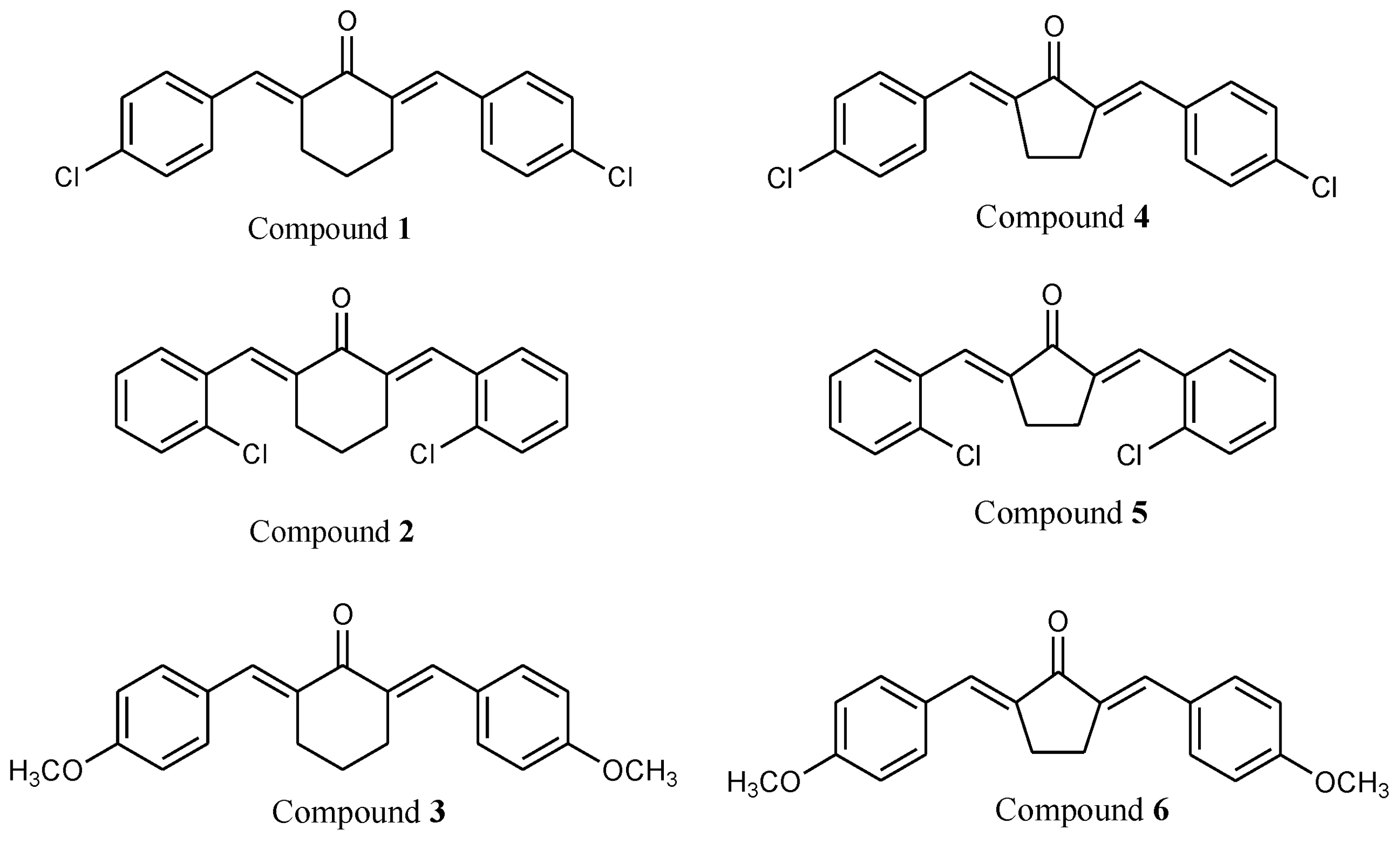
| DPPH (IC50 µg/mL) | ABTS (IC50 µg/mL) | Nitric Oxide (IC50 µg/mL) | FRAP (EC50 µg/mL) | |
|---|---|---|---|---|
| (2E,6E)-2,6-bis(4-methoxybenzylidene) cyclohexanone (compound 1) | 18.41 ± 1.45 | 18.63 ± 1.41 | 28.87 ± 1.49 | 1.35 ± 0.10 |
| (2E,6E)-2,6-bis(4-chlorobenzylidene) cyclohexanone (compound 2) | 19.92 ± 1.52 | 21.57 ± 1.55 | 26.04 ± 1.61 | 5.24 ± 0.21 |
| (2E,6E)-2,6-bis(2-chlorobenzylidene) cyclohexanone (compound 3) | 27.75 ± 2.50 | 26.47 ± 1.42 | 34.30 ± 2.55 | 12.40 ± 0.20 |
| (2E,5E)-2,5-bis(4-(tetrahydro-2H-pyran-2-yloxy) benzylidene) cyclopentanone (compound 4) | 25.42 ± 1.39 | 22.18 ± 1.29 | 29.15 ± 1.72 | 4.34 ± 0.11 |
| 2,5-bis(4-hydroxybenzylidene) cyclopentanone (compound 5) | 36.49 ± 1.55 | 42.10 ± 2.27 | 45.67 ± 3.04 | 15.61 ± 0.30 |
| 4-(tetrahydro-2H-pyran-2-yloxy) benzaldehyde (compound 6) | 35.47 ± 1.64 | 46.17 ± 3.23 | 49.09 ± 3.11 | 16.20 ± 0.24 |
| Bacteria | Compounds with Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Escherichia coli | 18.4 ± 0.1 | 22.5 ± 0.2 * | 16.7 ± 0.3 | 20.6 ± 0.3 * | 17.7 ± 0.3 | 16.4 ± 0.1 |
| Pseudomonas aeruginosa | 19.8 ± 0.2 * | 19.1 ± 0.2 * | 14.7 ± 0.2 | 20.1 ± 0.3 | 16.9 ± 0.2 | 15.0 ± 0.3 |
| Staphylococcus aureus | 18.5 ± 0.1 | 20.1 ± 0.3 * | 13.7 ± 0.1 | 19.5 ± 0.2 * | 15.1 ± 0.2 | 14.6 ± 0.3 |
| Salmonella enteritidis | 18.2 ± 0.1 | 19.0 ± 0.3 * | 15.4 ± 0.2 | 18.6 ± 0.1 * | 16.0 ± 0.2 | 17.4 ± 0.1 |
| Compound | LC50 (µg/mL) |
|---|---|
| (2E,6E)-2,6-bis(4-methoxybenzylidene) cyclohexanone (compound 1) | 45.27 ± 2.34 |
| (2E,6E)-2,6-bis(4-chlorobenzylidene) cyclohexanone (compound 2) | 59.81 ± 2.09 |
| (2E,6E)-2,6-bis(2-chlorobenzylidene) cyclohexanone (compound 3) | 99.04 ± 2.18 |
| (2E,5E)-2,5-bis(4-(tetrahydro-2H-pyran-2-yloxy) benzylidene) cyclopentanone (compound 4) | 56.46 ± 3.07 |
| 2,5-bis(4-hydroxybenzylidene) cyclopentanone (compound 5) | 89.22 ± 3.12 |
| 4-(tetrahydro-2H-pyran-2-yloxy) benzaldehyde (compound 6) | 79.18 ± 2.69 |
| Compound | LC50 (µg/mL) | |
|---|---|---|
| MCF-7 | MDA-MB-231 | |
| (2E,6E)-2,6-bis(4-methoxybenzylidene) cyclohexanone (compound 1) | 86.13 ± 3.45 | 128.66 ± 3.62 |
| (2E,6E)-2,6-bis(4-chlorobenzylidene) cyclohexanone (compound 2) | 79.51 ± 2.85 | 97.64 ± 3.15 |
| (2E,6E)-2,6-bis(2-chlorobenzylidene) cyclohexanone (compound 3) | 132.49 ± 3.71 | 160.54 ± 5.22 |
| (2E,5E)-2,5-bis(4-(tetrahydro-2H-pyran-2-yloxy) benzylidene) cyclopentanone (compound 4) | 71.09 ± 2.34 | 89.62 ± 2.18 |
| 2,5-bis(4-hydroxybenzylidene) cyclopentanone (compound 5) | 103.56 ± 2.48 | 141.05 ± 4.84 |
| 4-(tetrahydro-2H-pyran-2-yloxy) benzaldehyde (compound 6) | 109.82 ± 4.10 | 155.32 ± 5.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuttithodi, A.M.; Nikhitha, D.; Jacob, J.; Narayanankutty, A.; Mathews, M.; Olatunji, O.J.; Rajagopal, R.; Alfarhan, A.; Barcelo, D. Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones. Molecules 2022, 27, 8209. https://doi.org/10.3390/molecules27238209
Kuttithodi AM, Nikhitha D, Jacob J, Narayanankutty A, Mathews M, Olatunji OJ, Rajagopal R, Alfarhan A, Barcelo D. Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones. Molecules. 2022; 27(23):8209. https://doi.org/10.3390/molecules27238209
Chicago/Turabian StyleKuttithodi, Aswathi Moothakoottil, Divakaran Nikhitha, Jisha Jacob, Arunaksharan Narayanankutty, Manoj Mathews, Opeyemi Joshua Olatunji, Rajakrishnan Rajagopal, Ahmed Alfarhan, and Damia Barcelo. 2022. "Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones" Molecules 27, no. 23: 8209. https://doi.org/10.3390/molecules27238209
APA StyleKuttithodi, A. M., Nikhitha, D., Jacob, J., Narayanankutty, A., Mathews, M., Olatunji, O. J., Rajagopal, R., Alfarhan, A., & Barcelo, D. (2022). Antioxidant, Antimicrobial, Cytotoxicity, and Larvicidal Activities of Selected Synthetic Bis-Chalcones. Molecules, 27(23), 8209. https://doi.org/10.3390/molecules27238209








