History of Cobaltabis(dicarbollide) in Potentiometry, No Need for Ionophores to Get an Excellent Selectivity
Abstract
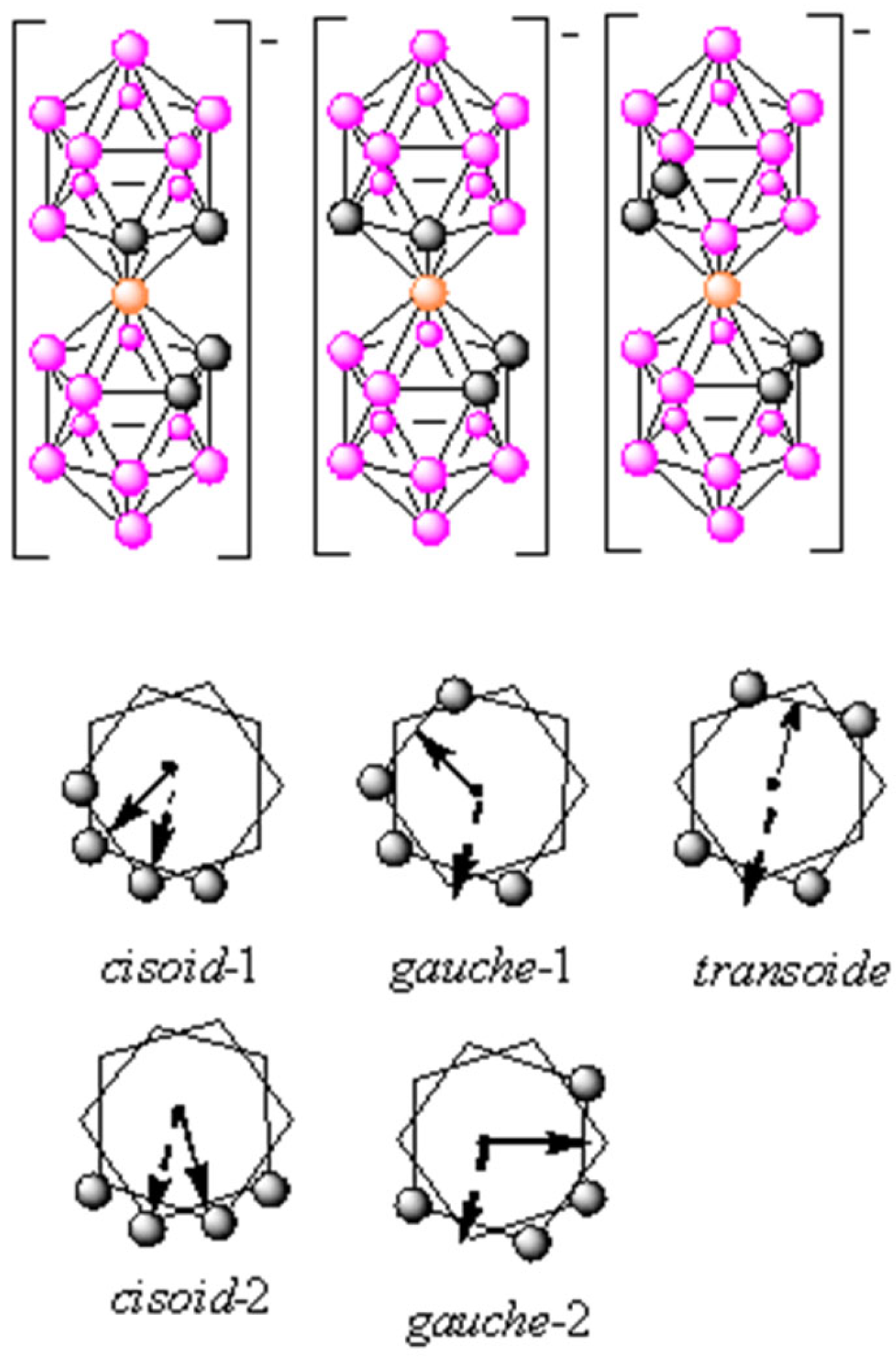
1. Introduction, Objectives and Characteristics of Closo-Borates and Metallabis(dicarbollides)
2. Generalities of Ion Selective Electrodes (ISEs) and First Steps in the Use of Closo Borates and Metallabis(dicarbollides) as ISEs
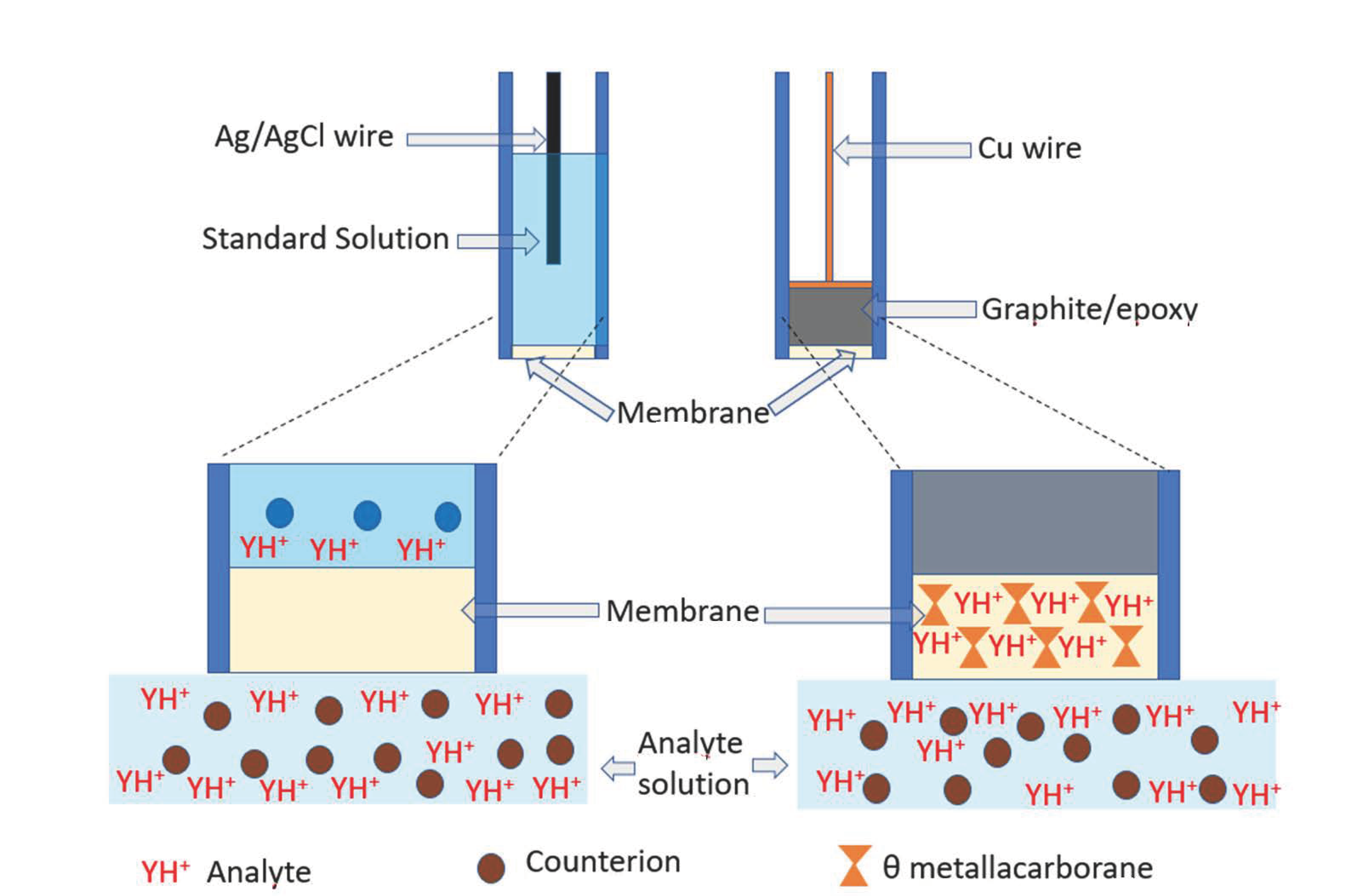
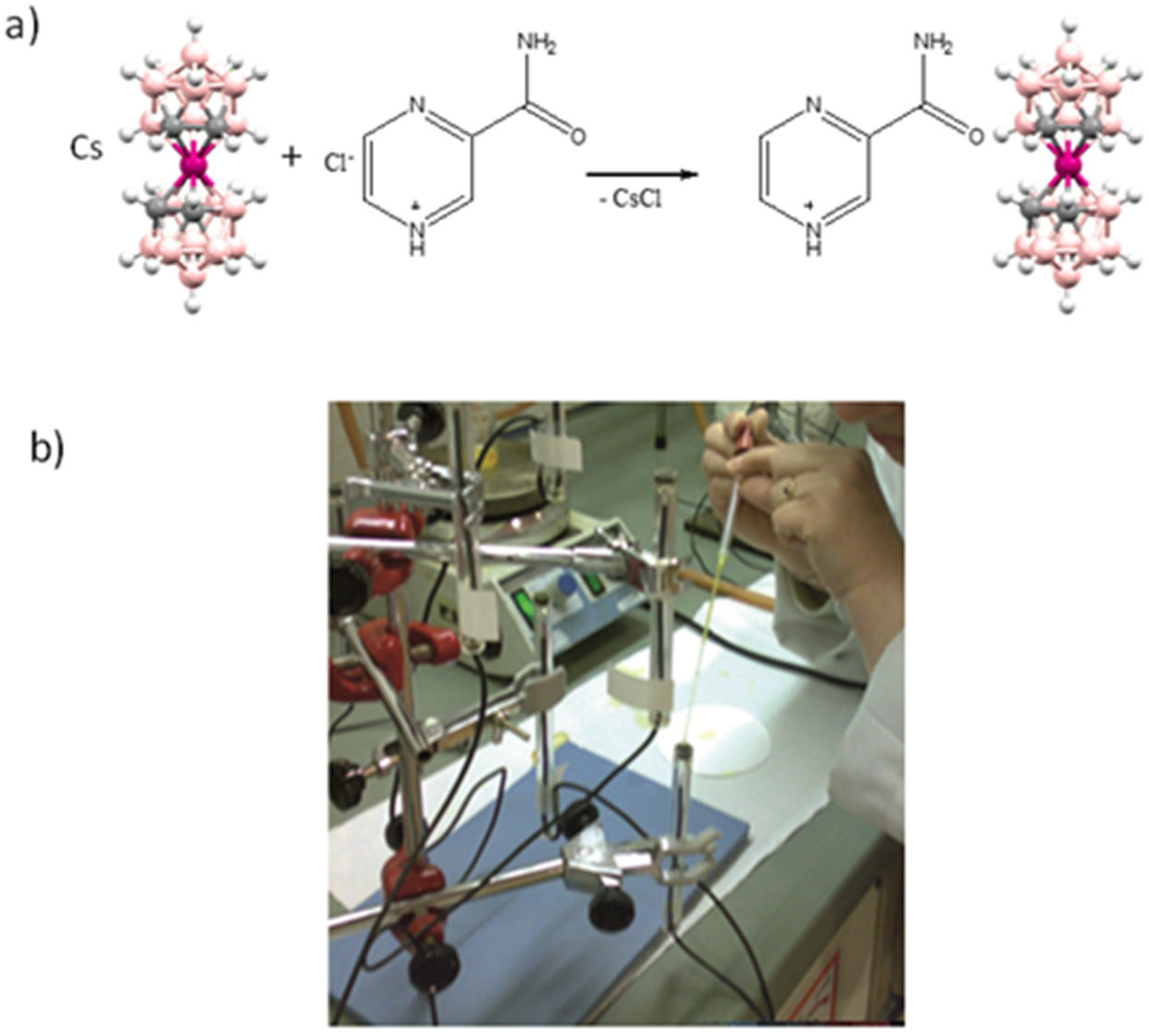
3. The Metallabis(dicarbollide) [3,3′-Co(1,2-C2B9H11)2]− as an Active Component of Membrane Solid State ISE
4. Results
5. Why Do These Membranes with Metallabis(dicarbollides), Being So Simple in Their Composition, Give Such Excellent Results?
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Zadsirjana, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, A.J.; Zhang, R.; Bian, X.; Chen, D.; Hu, X. Application of Electrochemical Methods for Pharmaceutical and Drug Analysis. Curr. Pharm. Anal. 2009, 5, 144–155. [Google Scholar] [CrossRef]
- Mostafa, I.M.; Meng, C.; Dong, Z.; Lou, B.; Xu, G. Potentiometric Sensors for the Determination of Pharmaceutical Drugs. Anal. Sci. 2022, 38, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kharitonov, S.V. Ion-Selective Electrodes in Medicinal Drug Determination. Russ. Chem. Rev. 2007, 76, 361. [Google Scholar] [CrossRef]
- Tong, H.Y.; Meng, J.; Liang, J.Y.; Li, J.P. Molecularly Imprinted Electrochemical Luminescence Sensor Based on Core-Shell Magnetic Particles with ZIF-8 Imprinted Material. Sens. Actuators B Chem. 2021, 330, 129405. [Google Scholar] [CrossRef]
- Li, X.H.; Kuang, X.J.; Sun, J.L. Rare Earth Elements Based Oxide Ion Conductors. Inorg. Chem. Front. 2021, 8, 1374. [Google Scholar] [CrossRef]
- Yao, M.M.; Huang, J.K.; Deng, Z.H.; Jin, W.Y.; Yuan, Y.L.; Nie, J.F.; Wang, H.; Du, F.Y.; Zhang, Y. Transforming Glucose into Fluorescent Graphene Quantum Dots Via Microwave Radiation for Sensitive Detection of Al3+ Ions Based on Aggregation-Induced Enhanced Emission. Analyst 2020, 145, 6981. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Andrews, T.D. Carborane Analogues of Cobalticinium Ion. Chem. Commun. 1965, 19, 443–444. [Google Scholar] [CrossRef]
- Lever, A.B.P. Electrochemical Parametrization of Metal Complex Redox Potentials, using the Ruthenium(III)/Ruthenium(II) Couple to Generate a Ligand Electrochemical Series. Inorg. Chem. 1990, 29, 1271–1285. [Google Scholar] [CrossRef]
- Morris, J.H.; Gysling, H.J.; Reed, D. Electrochemistry of Boron Compounds. Chem. Rev. 1985, 85, 51–76. [Google Scholar] [CrossRef]
- Núñez, R.; Tarrés, M.; Ferrer-Ugalde, F.; de Biani, F.F.; Teixidor, F. Electrochemistry and Photoluminescence of Icosahedral Carboranes, Boranes, Metallacarboranes, and their Derivatives. Chem. Rev. 2016, 116, 14307–14378. [Google Scholar] [CrossRef]
- Bauduin, P.; Prevost, S.; Farras, P.; Teixidor, F.; Diat, O.; Zemb, T. A Theta-Shaped Amphiphilic Cobaltabisdicarbollide Anion: Transition from Monolayer Vesicles to Micelles. Angew. Chem. Int. Ed. 2011, 50, 5298–5300. [Google Scholar] [CrossRef]
- Malaspina, D.C.; Viñas, C.; Teixidor, F.; Faraudo, J. Atomistic Simulations of COSAN: Amphiphiles without a Head-and-Tail Design Display “Head and Tail” Surfactant Behavior. Angew. Chem. Int. Ed. 2020, 59, 3088–3092. [Google Scholar] [CrossRef]
- Juárez-Pérez, E.J.; Núñez, R.; Viñas, C.; Sillanpää, R.; Teixidor, F. The Role of C–H···H–B Interactions in Establishing Rotamer Configurations in Metallabis (dicarbollide) Systems. Eur. J. Inorg. Chem. 2010, 16, 2385–2392. [Google Scholar] [CrossRef]
- Farràs, P.; Juárez-Pérez, E.J.; Lepšík, M.; Luque, R.; Núñez, R.; Teixidor, F. Metallacarboranes and their Interactions: Theoretical Insights and their Applicability. Chem. Soc. Rev. 2012, 41, 3445–3463. [Google Scholar] [CrossRef]
- Verdiá-Báguena, C.; Alcaraz, A.; Aguilella, V.M.; Cioran, A.M.; Tachikawa, S.; Nakamura, H.; Teixidor, F.; Viñas, C. Amphiphilic COSAN and I2-COSAN Crossing Synthetic Lipid Membranes: Planar Bilayers and Liposomes. Chem. Commun. 2014, 50, 6700–6703. [Google Scholar] [CrossRef]
- Fuentes, I.; Pujols, J.; Viñas, C.; Ventura, S.; Teixidor, F. Dual Binding Mode of Metallacarborane Produces a Robust Shield on Proteins. Chem. Eur. J. 2019, 25, 12820–12829. [Google Scholar] [CrossRef]
- de Marco, R.; Clarke, G.; Pejcic, B. Ion-Selective Electrode Potentiometry in Environmental Analysis. Electroanalysis 2007, 19, 1987–2001. [Google Scholar] [CrossRef]
- Mikhelson, K.N. Ion-Selective Electrodes; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bakker, E.; Pretsch, E. The New Wave of Ion-Selective Electrodes. Anal. Chem. 2007, 26, 46–51. [Google Scholar]
- Bakker, E.; Pretsch, E. Modern Potentiometry. Angew. Chem. Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef]
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalysis 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Bloch, R.; Shatkay, A.; Saroff, H.A. Fabrication and Evaluation of Membranes as Specific Electrodes for Calcium Ions. Biophys. J. 1967, 7, 865–877. [Google Scholar] [CrossRef]
- Moody, G.J.; Oke, R.B.; Thomas, J.D.R. A Calcium-Sensitive Electrode Based on a Liquid Ion Exchanger in a Poly (Vinyl Chloride) Matrix. Analyst 1970, 95, 910–918. [Google Scholar] [CrossRef]
- Stefanac, Z.; Simon, W. Ion Specific Electrochemical Behavior of Macrotetrolides in Membranes. Microchem. J. 1967, 12, 125–132. [Google Scholar] [CrossRef]
- Viñas, C.; Gómez, S.; Bertran, J.; Barron, J.; Teixidor, F.; Dozol, J.-F.; Rouquette, H.; Kivekkäs, R.; Sillanpää, R. C-substituted bis (dicarbollide) metal compounds as sensors and extractants of radionuclides from nuclear wastes. J. Organomet. Chem. 1999, 581, 188–193. [Google Scholar] [CrossRef]
- Kopytin, A.V.; Zhizhin, K.Y.; Urusov, Y.I.; Mustyatsa, V.N.; Kokunov, Y.V.; Kuznetsov, N.T. Potentiometric Sensors with Membranes Based on Ionic Liquid Tetradecylammonium Triethylammonio-closo-Dodecaborate. J. Anal. Chem. 2012, 67, 168–171. [Google Scholar] [CrossRef]
- Kubasov, A.S.; Turishev, E.S.; Kopytin, A.V.; Shpigun, L.K.; Zhizhin, K.Y.; Kuznetsov, N.T. Sulfonium closo-hydridodecaborate Anions as Active Components of a Potentiometric Membrane Sensor for Lidocaine Hydrochloride. Inorg. Chim. Acta. 2021, 514, 119992. [Google Scholar] [CrossRef]
- Peper, S.; Telting-Diaz, M.; Almond, P.; Albrecht-Schmitt, T.; Bakker, A. Perbrominated closo-Dodecacarborane Anion, [1-HCB11Br11]−, as an Ion Exchanger in Cation-Selective Chemical Sensors. Anal. Chem. 2002, 74, 1327–1332. [Google Scholar] [CrossRef]
- Bakker, E.; Nägele, M.; Schaller, U.; Pretsch, E. Applicability of the Phase Boundary Potential Model to the Mechanistic Understanding of Solvent Polymeric Membrane-Based Ion-Selective Electrodes. Electroanalysis 1995, 7, 817–822. [Google Scholar] [CrossRef]
- Karpfen, F.M.; Randles, J.E.B. Ionic Equilibria and Phase-Boundary Potentials in Oil-Water Systems. Trans. Faraday Soc. 1953, 49, 823–831. [Google Scholar] [CrossRef]
- Núñez, R.; Romero, I.; Teixidor, F.; Viñas, C. Icosahedral Boron Clusters: A Perfect Tool for the Enhancement of Polymer Features. Chem. Soc. Rev. 2016, 45, 5147–5173. [Google Scholar] [CrossRef] [PubMed]
- Peper, S.; Qin, Y.; Almond, P.; McKee, M.; Telting-Diaz, M.; Albrecht-Schmitt, T.; Bakker, E. Ion-Pairing Ability, Chemical Stability, and Selectivity Behavior of Halogenated Dodecacarborane Cation Exchangers in Neutral Carrier-Based Ion-Selective Electrodes. Anal. Chem. 2003, 75, 2131–2139. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Lipophilicity of Tetraphenylborate Derivatives as Anionic Sites in Neutral Carrier-Based Solvent Polymeric Membranes and Lifetime of Corresponding Ion-Selective Electrochemical and Optical Sensors. Anal. Chim. Acta 1995, 309, 7–17. [Google Scholar] [CrossRef]
- Ortuno, J.A.; Rodenas, V.; Garcıa, M.S.; Albero, M.I.; Sanchez-Pedreno, C.A. New Tiapride Selective Electrode and Its Clinical Application. Sensors 2007, 7, 400–409. [Google Scholar] [CrossRef]
- Malongo, K.; Blankert, B.; Kambu, O.; Amighi, K.; Nsangu, J.; Kauffmann, J.M. Amodiaquine Polymeric Membrane Electrode. J. Pharm. Biomed. Anal. 2006, 41, 70–76. [Google Scholar] [CrossRef]
- Aboul-Enein, H.Y.; Sun, X.X.; Sun, C.J. Ion Selective PVC Membrane Electrode for the Determination of Methacycline Hydrochloride in Pharmaceutical Formulation. Sensors 2002, 2, 424–431. [Google Scholar] [CrossRef]
- Kulapina, E.G.; Barinova, O.V. Ion-Selective Electrodes for the Determination of Nitrogen-Containing Medicinal Substances. J. Anal. Chem. 2001, 56, 457–460. [Google Scholar] [CrossRef]
- Ortuño, J.A.; Hernández, J.; Sánchez-Pedreño, C. Ion-Selective Electrode for the Determination of Some Multidrug Resistance Reversers. Sens. Actuators B Chem. 2006, 119, 282–287. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E.; Bühlmann, P. Selectivity of Potentiometric Ion Sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Freiser, H. Ion-Selective Electrodes in Analytical Chemistry; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Bakker, E.; Chumbimuni-Torres, K. Modern Directions for Potentiometric Sensor. J. Braz. Chem. Soc. 2008, 19, 621–629. [Google Scholar] [CrossRef]
- Guggenheim, E.A. The Conceptions of Electrical Potential Difference between Two Phases and the Individual Activities of Ions. J. Phys. Chem. 1929, 33, 842–849. [Google Scholar] [CrossRef]
- Guggenheim, E.A. On the Conception of Electrical Potential Difference between two Phases. II. J. Phys. Chem. 1930, 34, 1540–1543. [Google Scholar] [CrossRef]
- Theorell, T. An Attempt to Formulate a Quantitative Theory of Membrane Permeability. Proc. Soc. Exp. Biol. Med. 1935, 33, 282–285. [Google Scholar] [CrossRef]
- Meyer, K.H.; Sievers, J.F. La Perméabilité des Membranes I. Théorie de la Perméabilité Ionique. Helv. Chim. Acta 1936, 19, 649–664. [Google Scholar] [CrossRef]
- Stoica, A.I.; Vinas, C.; Teixidor, F. Application of the Cobaltabisdicarbollide Anion to the Development of Ion Selective PVC Membrane Electrodes for Tuberculosis Drug Analysis. Chem. Commun. 2008, 48, 6492–6494. [Google Scholar] [CrossRef]
- Stoica, A.I.; Vinas, C.; Teixidor, F. Cobaltabisdicarbollide Anion Receptor for Enantiomer-Selective Membrane Electrodes. Chem. Commun. 2009, 33, 4988–4990. [Google Scholar] [CrossRef]
- Stoica, A.I.; Kleber, C.; Vinas, C.; Teixidor, F. Ion Selective Electrodes for Protonable Nitrogen Containing Analytes: Metallacarboranes as Active Membrane Components. Electrochim. Acta 2013, 113, 94–98. [Google Scholar] [CrossRef]
- Bliem, C.; Fruhmann, P.; Stoica, A.I.; Kleber, C. Development and Optimization of an Ion-selective Electrode for Serotonin Detection. Electroanalysis 2017, 29, 1635–1642. [Google Scholar] [CrossRef]
- Saini, A.; Gallardo-Gonzalez, J.; Baraket, A.; Fuentes, I.; Viñas, C.; Zine, N.; Bausells, J.; Teixidor, F.; Errachid, A. A Novel Potentiometric Microsensor for Real-Time Detection of Irgarol using the Ion-Pair Complex [Irgarol-H]+[Co(C2B9H11)2]−. Sens. Actuators B Chem. 2018, 268, 164–169. [Google Scholar] [CrossRef]
- Saini, A.; Fuentes, I.; Viñas, C.; Zine, N.; Bausells, J.; Errachid, A.; Teixidor, F. A Simple Membrane with the Electroactive [Sulfapyridine-H]+[Co(C2B9H11)2]− for the Easy Potentiometric Detection of Sulfonamides. J. Organomet. Chem. 2019, 893, 32–38. [Google Scholar] [CrossRef]
- Gallardo-Gonzalez, J.; Saini, A.; Baraket, A.; Boudjaoui, S.; Alcácer, A.; Strelas, A.; Teixidor, F.; Zine, N.; Bausells, J.; Errachid, A. A Highly Selective Potentiometric Amphetamine Microsensor Based on All-Solid-State Membrane Using a New Ion-Pair Complex, [3,3′-Co(1,2-closo-C2B9H11)2]−[C9H13NH]+. Sens. Actuators B Chem. 2018, 266, 823–829. [Google Scholar] [CrossRef]
- Mayerhuber, L.; Trattner, S.; Luger, S.; Weigelhofer, G.; Hametner, C.; Fruhmann, P. Development of Ion-Selective Electrodes for Antipyrine and its Derivatives as Potential Tool for Environmental Water Monitoring. J. Electroanal. Chem. 2021, 886, 115110–115117. [Google Scholar] [CrossRef]
- Thoma, A.P.; Cimerman, Z.; Fiedler, U.; Bedekovic, D.; Güggi, M.; Jordan, P.; May, K.; Pretsch, E.; Prelog, V.; Simon, W. Enantiomerenselektives Verhalten in Membranen Eines Chiralen Elektrisch Neutralen Ionophores. Chimia 1975, 29, 344–346. [Google Scholar]
- Trojanowicz, M. Enantioselective Electrochemical Sensors and Biosensors: A Mini-Review. Electrochem. Commun. 2014, 38, 47–52. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Kaniewska, M. Electrochemical Chiral Sensors and Biosensors. Electroanalysis 2009, 21, 229–238. [Google Scholar] [CrossRef]
- Yasaka, Y.; Yamamoto, T.; Kimura, K.; Shono, T. Simple Evaluation of Enantiomer-Selectivity of Crown Ether Using Membrane Electrode. Chem. Lett. 1980, 9, 769–772. [Google Scholar] [CrossRef]
- Maruyama, K.; Sohmiya, H.; Tsukube, H. New Chiral Host Molecules Derived from Naturally Occurring Monensin Ionophore. J. Chem. Soc. Chem. Commun. 1989, 864–865. [Google Scholar] [CrossRef]
- Maruyama, K.; Sohmiya, H.; Tsukube, H. Enantiomer Recognition of Organic Ammonium Salts by Podand and Crown-e Monensin Amides: New Synthetic Strategy for Chiral Receptors. Tetrahedron 1992, 48, 805–818. [Google Scholar] [CrossRef]
- Kaniewska, M.; Sikora, T.; Kataky, R.; Trojanowicz, M. Enantioselectivity of Potentiometric Sensors with Application of Different Mechanisms of Chiral Discrimination. J. Biochem. Biophys. Methods 2008, 70, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Qu, L.; Wang, Z.; Li, G.; Feng, W.; Yang, G. A facile electrochemical chiral sensor for tryptophan enantiomers based on multiwalled carbon nanotube/hydroxypropyl-β-cyclodextrin functionalized carboxymethyl cellulose. Microchem. J. 2022, 175, 107133. [Google Scholar] [CrossRef]
- Liua, N.; Yang, B.; Yin, Z.-Z.; Cai, W.; Li, J.; Kong, Y. A chiral sensing platform based on chiral metal-organic framework for enantiodiscrimination of the isomers of tyrosine and tryptophan. J. Electroanal. Chem. 2022, 918, 116445. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, L.; Meng, Q.; Jiang, Y.; Xie, L. A biomolecule chiral interface base on BSA for electrochemical recognition of amine enantiomers. Chirality 2021, 33, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.; Mayerhuber, L.; Weigelhofer, G.; Hein, T.; Holzer, B.; Hametner, C.; Fruhmann, P. Development of Ion-selective Electrodes for Tropane, Atropine, and Scopolamine—A Concept for the Analysis of Tropane Alkaloids. Electroanalysis 2022, 34, 1579–1586. [Google Scholar] [CrossRef]
- Analytical Chemistry Division, Commission on Analytical Nomenclature. Recommendations for Nomenclature of Ion-Selective Electrodes, “Recommendations–1975”. Pure Appl. Chem. 1976, 48, 127–132. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for Nomenclature of Ion-Selective Electrodes IUPAC Recommendations 1994. Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Poater, J.; Viñas, C.; Bennour, I.; Gordils, S.E.; Sola, M.; Teixidor, F. Too Persistent to Give Up: Aromaticity in Boron Clusters Survives Radical Structural Changes. J. Am. Chem. Soc. 2020, 142, 9396–9407. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: New York, NY, USA, 2016. [Google Scholar]
- Plesek, J. Potential applications of the boron cluster compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- Tarrés, M.; Canetta, E.; Paul, E.; Forbes, J.; Azzouni, K.; Viñas, C.; Teixidor, F.; Harwood, A.J. Biological interaction of living cells with COSAN-based synthetic vesicles. Sci. Rep. 2015, 5, 7804. [Google Scholar] [CrossRef]
- Fuentes, I.; García-Mendiola, T.; Sato, S.; Pita, M.; Nakamura, H.; Lorenzo, E.; Teixidor, F.; Marques, F.; Viñas, C. Metallacarboranes on the Road to Anticancer Therapies: Cellular Uptake, DNA Interaction, and Biological Evaluation of Cobaltabisdicarbollide [COSAN]−. Chem. Eur. J. 2018, 24, 17239–17254. [Google Scholar] [CrossRef] [PubMed]
- Bennour, I.; Ramos, M.N.; Nuez-Martínez, M.; Xavier, J.A.M.; Buades, A.B.; Sillanpää, R.; Teixidor, F.; Choquesillo-Lazarte, D.; Romero, I.; Martinez-Medina, M.; et al. Water soluble organometallic small molecules as promising antibacterial agents: Synthesis, physical–chemical properties and biological evaluation to tackle bacterial infections. Dalton Trans. 2022, 51, 7188–7209. [Google Scholar] [CrossRef]
- Masalles, C.; Llop, J.; Vinas, C.; Teixidor, F. Extraordinary overoxidation resistance increase in self-doped polypyrroles by using non-conventional low charge-density anions. Adv. Mater. 2002, 14, 826–829. [Google Scholar] [CrossRef]
- Goszczynski, T.M.; Fink, K.; Kowalski, K.; Lesnikowski, Z.J.; Boratynski, J. Interactions of Boron Clusters and their Derivatives with Serum Albumin. Sci. Rep. 2017, 7, 9800. [Google Scholar] [CrossRef] [PubMed]
- Cıgler, P.; Kozısek, M.; Rezacova, P.; Brynda, J.; Otwinowski, Z.; Pokorna, J.; Plesek, J.; Grüner, B.; Doleckova-Maresova, L.; Masa, M.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc. Natl. Acad. Sci. USA 2005, 102, 15394–15399. [Google Scholar] [CrossRef]
- Fanfrlık, J.; Brynda, J.; Rezac, J.; Hobza, P.; Lepsık, M. Interpretation of Protein/Ligand Crystal Structure using QM/MM Calculations: Case of HIV-1 Protease/Metallacarborane Complex. J. Phys. Chem. B 2008, 112, 15094–15102. [Google Scholar] [CrossRef]
- Matějíček, P.; Zedník, J.; Ušelová, K.; Pleštil, J.; Fanfrlík, J.; Nykänen, A.; Ruokolainen, J.; Hobza, P.; Procházka, K. Stimuli-Responsive Nanoparticles Based on Interaction of Metallacarborane with Poly (ethylene oxide). Macromolecules 2009, 42, 4829–4837. [Google Scholar] [CrossRef]
- Tarrés, M.; Viñas, C.; Gonzalez-Cardoso, P.; Hänninen, M.M.; Sillanpää, R.; Dordovic, V.; Uchman, M.; Teixidor, F.; Matejicek, P. Aqueous Self-Assembly and Cation Selectivity of Cobaltabisdicarbollide Dianionic Dumbbells. Chem. Eur. J. 2014, 20, 6786–6794. [Google Scholar] [CrossRef]
- Teixidor, F.; Pedrajas, J.; Rojo, I.; Viñas, C.; Kivekäs, R.; Sillanpää, R.; Sivaev, I.; Bregadze, V.; Sjöberg, S. Chameleonic Capacity of [3,3′-Co(1,2-C2B9H11)2]-in Coordination. Generation of the Highly Uncommon S (thioether)-Na Bond. Organometallics 2003, 22, 3414–3423. [Google Scholar] [CrossRef]
- Planas, J.G.; Viñas, C.; Teixidor, F.; Comas-Vives, A.; Ujaque, G.; Lledós, A.; Light, M.E.; Hursthouse, M.B. Self-Assembly of Mercaptane−Metallacarborane Complexes by an Unconventional Cooperative Effect: A C−H···S−H···H−B Hydrogen/Dihydrogen Bond Interaction. J. Am. Chem. Soc. 2005, 127, 15976–15982. [Google Scholar] [CrossRef]

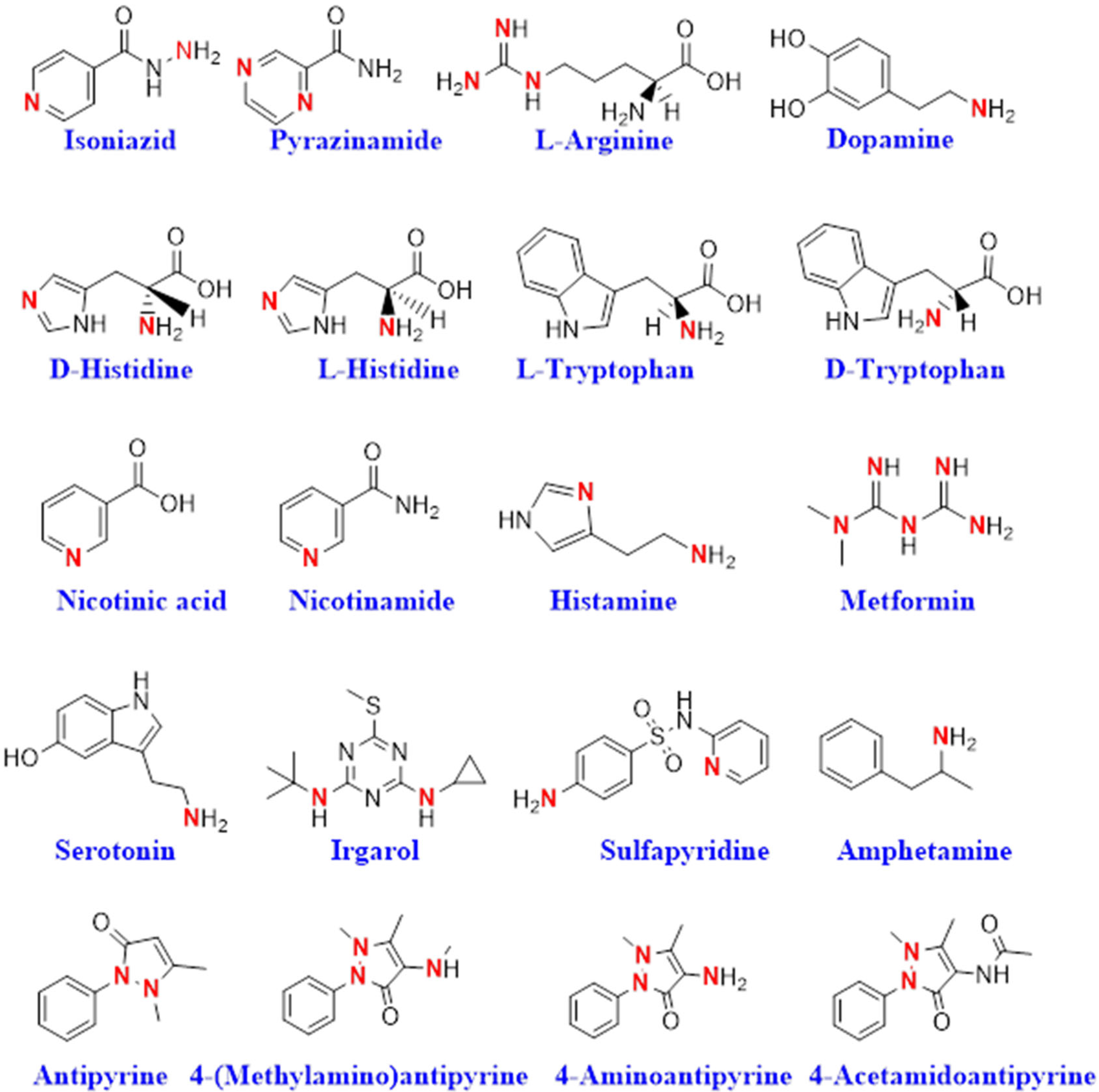
| Samples | Ion Pair Complex Formula | Plasticizer | Slope (mV·Decade−1) | Concentration Range (mol·dm−3) | Detection Limit (mol·dm−3) | Reference |
|---|---|---|---|---|---|---|
| Isoniazid | [H3INH][o-COSAN]3 | NPOE | 52.37 | 1.00 × 10−4– 1.00 × 10−1 | 5.00 × 10−5 | [49] |
| Isoniazid | [H3INH][o-COSAN]3 | DOP | 47.80 | 1.00 × 10−4– 1.00 × 10−1 | 5.80 × 10−5 | [49] |
| Pyrazinamide | H[HPZA]2[o-COSAN]3 | NPOE | 56.98 | 5.00 × 10−4– 1.00 × 10−1 | 3.00 × 10−5 | [49] |
| Pyrazinamide | H[HPZA]2[o-COSAN]3 | DOP | 46.70 | 5.00 × 10−5– 1.00 × 10−1 | 1.00 × 10−5 | [49] |
| L-Arginine | [HArg][o-COSAN] | DBP | 45.80 | 5.00 × 10−6– 1.00 × 10−1 | 3.00 × 10−6 | [50] |
| L-Arginine | [HArg][o-COSAN] | DEHP | 37.70 | 1.00 × 10−5– 1.00 × 10−1 | 5.00 × 10−5 | [50] |
| D-Histidine | [H2His][o-COSAN]2 | DBP | 36.50 | 1.00 × 10−5– 1.00 × 10−1 | 8.00 × 10−6 | [50] |
| D-Histidine | [H2His][o-COSAN]2 | DEHP | 42.40 | 5.00 × 10−5– 1.00 × 10−1 | 2.00 × 10−5 | [50] |
| L-Histidine | [H2His][o-COSAN]2 | DBP | 47.40 | 5.00 × 10−6– 1.00 × 10−1 | 1.00 × 10−6 | [50] |
| L-Histidine | [H2His][o-COSAN]2 | DEHP | 48.50 | 5.00 × 10−4– 1.00 × 10−1 | 1.00 × 10−4 | [50] |
| D-Tryptophan | [HTry][o-COSAN] | DBP | 60.50 | 5.00 × 10−7– 1.00 × 10−1 | 2.00 × 10−7 | [50] |
| L-Tryptophan | [HTry][o-COSAN] | DBP | 62.60 | 5.00 × 10−7– 1.00 × 10−1 | 1.00 × 10−7 | [50] |
| Dopamine | [HDA][o-COSAN] | Dibutylsebacate | 44.97 ± 1.23 | 1.00 × 10−6– 1.00 × 10−2 | 0.80 × 10−6 | [51] |
| Dopamine | [HDA][o-COSAN] | Bis (2-ethyl hexyl) phthalate | 53.23 ± 1.75 | 1.00 × 10−5– 1.00 × 10−2 | 7.20 × 10−6 | [51] |
| Dopamine | [HDA][o-COSAN] | NPOE | 58.17 ± 1.44 | 5.00 × 10−6– 1.00 × 10−2 | 1.00 × 10−6 | [51] |
| Dopamine | [HDA][o-COSAN] | DOP | 55.96 ± 0.85 | 5.00 × 10−6– 1.00 × 10−2 | 1.00 × 10−6 | [51] |
| Nicotinamide | [HNAmd][o-COSAN] | DOP | 52.11 ± 1.17 | 5.00 × 10−6– 1.00 × 10−2 | 1.00 × 10−6 | [51] |
| Nicotinic acid | [HNA][o-COSAN] | DOP | 57.55 ± 0.88 | 1.00 × 10−6– 1.00 × 10−2 | 0.70 × 10−6 | [51] |
| Histamine | [H2His][o-COSAN]2 | NPOE | 31.62 ± 0.43 | 5.00 × 10−6– 1.00 × 10−2 | 0.80 × 10−6 | [51] |
| Metformin | [H2Met][o-COSAN]2 | NPOE | 25.82 ± 1.91 | 1.00 × 10−5– 1.00 × 10−2 | 6.00 × 10−6 | [51] |
| Serotonin | [HSer][o-COSAN] | DBS | 50.50 ± 0.50 | 2.25 × 10−5– 1.00 × 10−2 | 4.51 × 10−6 | [52] |
| Serotonin | [HSer][o-COSAN] | DBP | 50.60 ± 0.50 | 2.25 × 10−5– 1.00 × 10−2 | 1.17 × 10−5 | [52] |
| Serotonin | [HSer][o-COSAN] | TBP | 60.50 ± 0.30 | 2.25 × 10−5– 1.00 × 10−2 | 1.56 × 10−5 | [52] |
| Serotonin | [HSer][o-COSAN] | NPOE | 51.10 ± 0.10 | 2.25 × 10−5– 1.00 × 10−2 | 1.70 × 10−5 | [52] |
| Irgarol | [Irg-H][o-COSAN] | DOP | 56.67 ± 2.30 | 1.00 × 10−5– 1.00 × 10−1 | 3.00 × 10−6 | [53] |
| Irgarol | [Irg-H][o-COSAN] | DOS | 57.17 ± 1.70 | 1.00 × 10−5– 1.00 × 10−1 | 2.00 × 10−6 | [53] |
| Irgarol | [Irg-H][o-COSAN] | NPOE | 48.21 ± 6.40 | 1.00 × 10−5– 1.00 × 10−1 | 4.00 × 10−6 | [53] |
| Sulfapyridine | A-H][o-COSAN] | NPOE | 47.69 | 1.00 × 10−6– 1.00 × 10−3 | 4.00 × 10−6 | [54] |
| Sulfapyridine | [A-H][o-COSAN] | DOS | 61.29 | 1.00 × 10−6– 1.00 × 10−3 | 1.00 × 10−6 | [54] |
| Sulfapyridine | [A-H][o-COSAN] | DOP | 61.26 | 1.00 × 10−6– 1.00 × 10−3 | 1.00 × 10−5 | [54] |
| Amphetamine | [Amph-H][o-COSAN] | DBP | 60 | 1.00 × 10−5– 1.00 × 10−3 | 12.00 × 10−6 | [55] |
| Amphetamine | [Amph-H][o-COSAN] | DOP | 42 | 1.00 × 10−5– 1.00 × 10−3 | 8.00 × 10−6 | [55] |
| Amphetamine | [Amph-H][o-COSAN] | DOS | 53 | 1.00 × 10−5– 1.00 × 10−3 | 4.00 × 10−5 | [55] |
| Amphetamine | [Amph-H][o-COSAN] | NPOE | 45 | 1.00 × 10−5– 1.00 × 10−3 | 2.00 × 10−5 | [55] |
| Antipyrine | [AP][o-COSAN] | NPOE | 79.6 ± 4.9 | 1.00 × 10−5– 1.00 × 10−2 | 70.8 × 10−6 ± 9.3 | [56] |
| Antipyrine | [AP][o-COSAN] | DBS | 57.0 ± 1.4 | 1.00 × 10−5– 1.00 × 10−2 | 29.8 × 10−6 ± 2.2 | [56] |
| 4-(methylamino) antipyrine | [MAAP][o-COSAN] | NPOE | 33.9 ± 1.0 | 1.00 × 10−5– 1.00 × 10−2 | 27.3 × 10−6 ± 1.5 | [56] |
| 4-(methylamino)antipyrine | [MAAP][o-COSAN] | DBS | 48.2 ± 1.0 | 1.00 × 10−5– 1.00 × 10−2 | 279.5 × 10−6 ± 7.0 | [56] |
| 4-aminoantipyrine | [AAP][o-COSAN] | NPOE | 54.6 ± 3.8 | 1.00 × 10−5– 1.00 × 10−2 | 88.2 × 10−6 ± 14.8 | [56] |
| 4-aminoantipyrine | [AAP][o-COSAN] | DBS | 71.2 ± 6.1 | 1.00 × 10−5– 1.00 × 10−2 | 342.0 × 10−6 ± 27.2 | [56] |
| 4-acetamidoantipyrine | [AAAP][o-COSAN] | NPOE | 57.0 ± 2.0 | 1.00 × 10−5– 1.00 × 10−2 | 252.2 × 10−6 ± 18.7 | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoica, A.-I.; Viñas, C.; Teixidor, F. History of Cobaltabis(dicarbollide) in Potentiometry, No Need for Ionophores to Get an Excellent Selectivity. Molecules 2022, 27, 8312. https://doi.org/10.3390/molecules27238312
Stoica A-I, Viñas C, Teixidor F. History of Cobaltabis(dicarbollide) in Potentiometry, No Need for Ionophores to Get an Excellent Selectivity. Molecules. 2022; 27(23):8312. https://doi.org/10.3390/molecules27238312
Chicago/Turabian StyleStoica, Anca-Iulia, Clara Viñas, and Francesc Teixidor. 2022. "History of Cobaltabis(dicarbollide) in Potentiometry, No Need for Ionophores to Get an Excellent Selectivity" Molecules 27, no. 23: 8312. https://doi.org/10.3390/molecules27238312
APA StyleStoica, A.-I., Viñas, C., & Teixidor, F. (2022). History of Cobaltabis(dicarbollide) in Potentiometry, No Need for Ionophores to Get an Excellent Selectivity. Molecules, 27(23), 8312. https://doi.org/10.3390/molecules27238312









