Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya
Abstract
1. Introduction
2. Results and Discussion
2.1. NADES Characterization
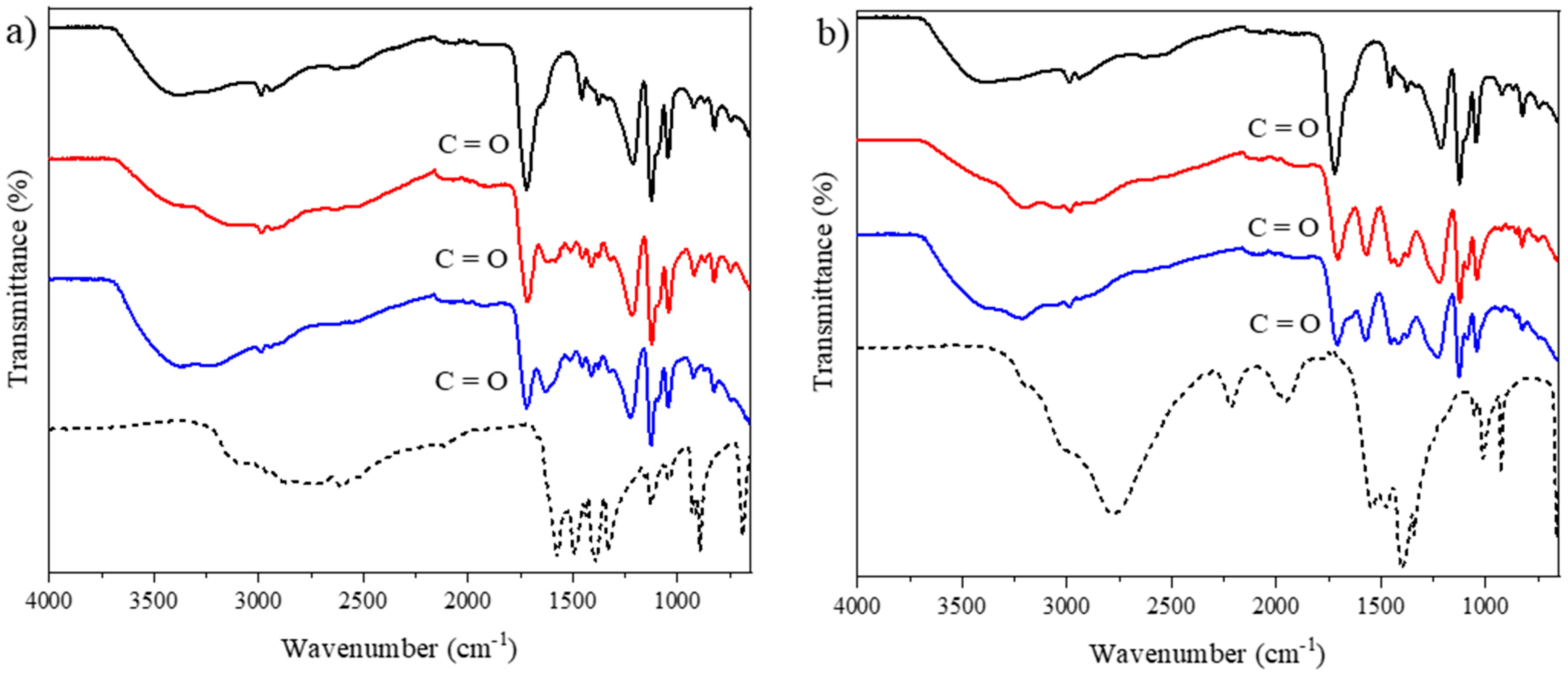
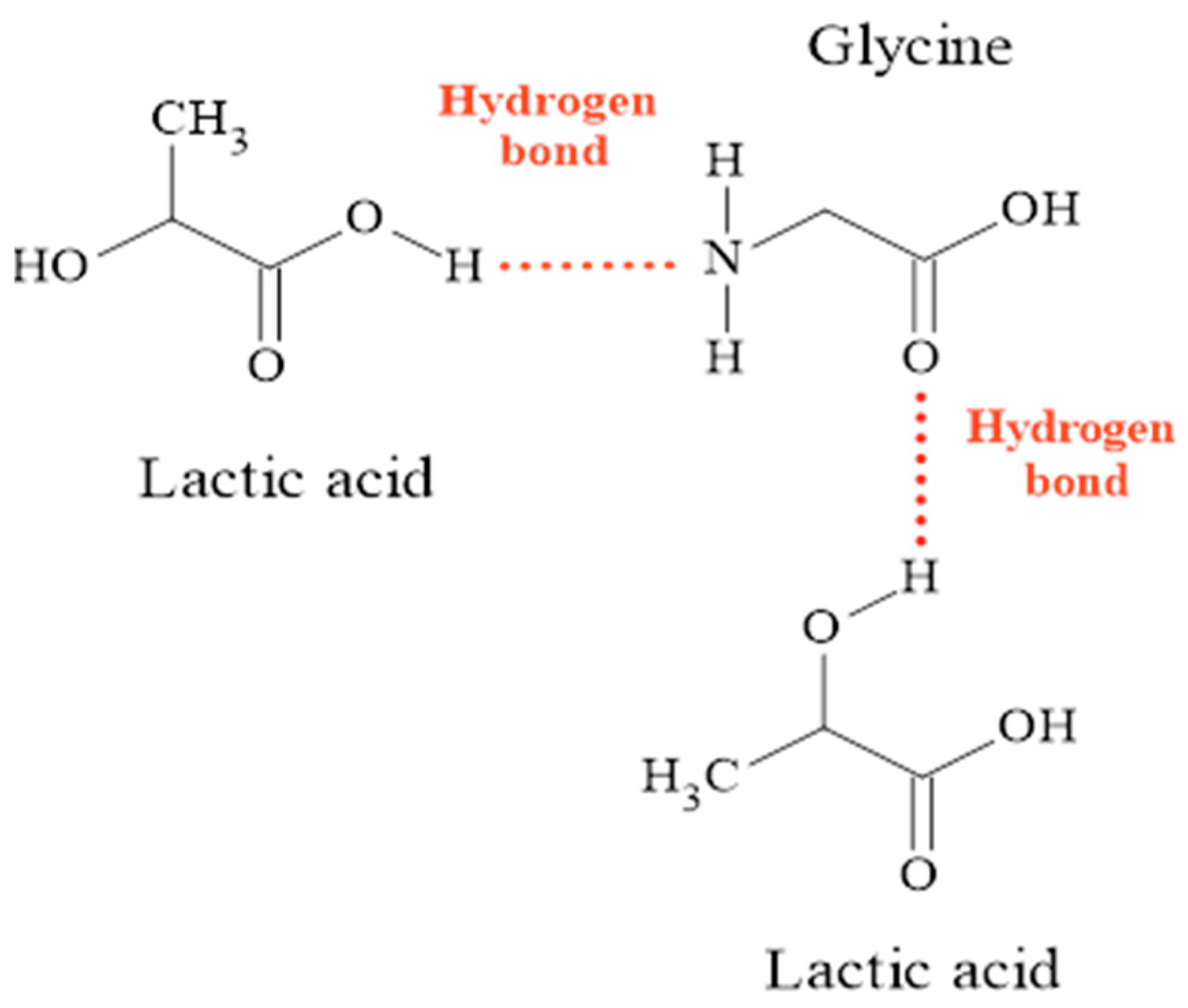
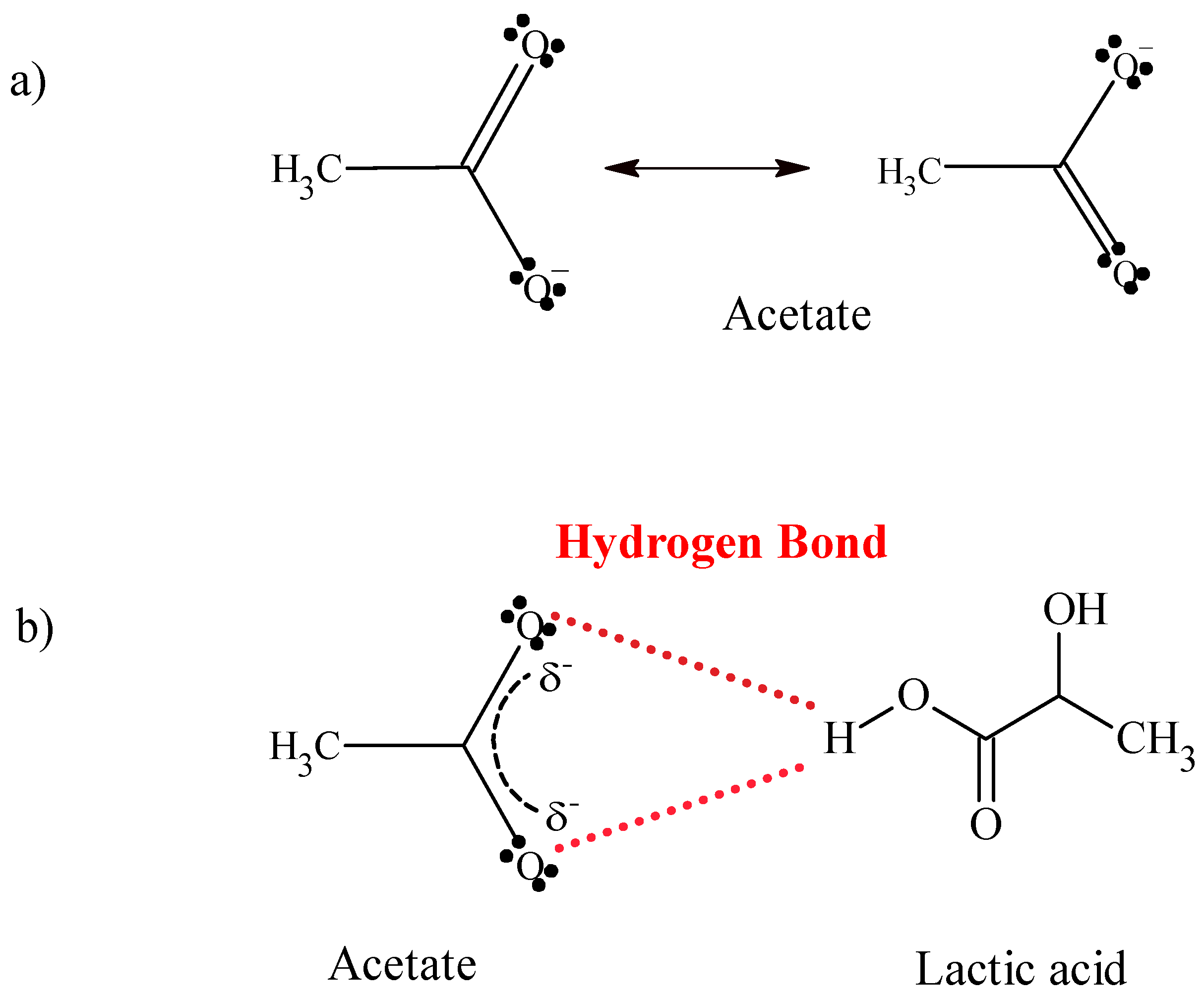
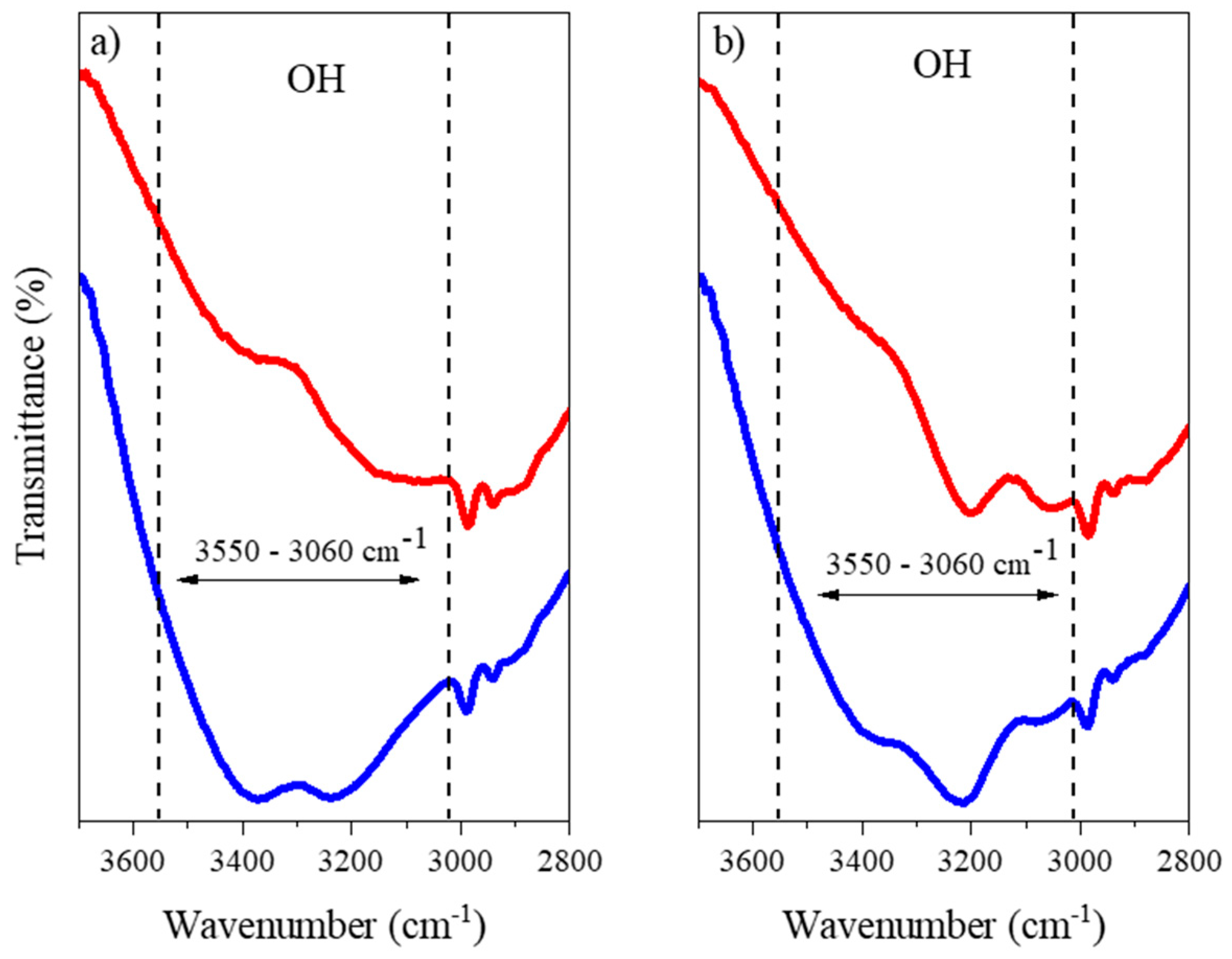
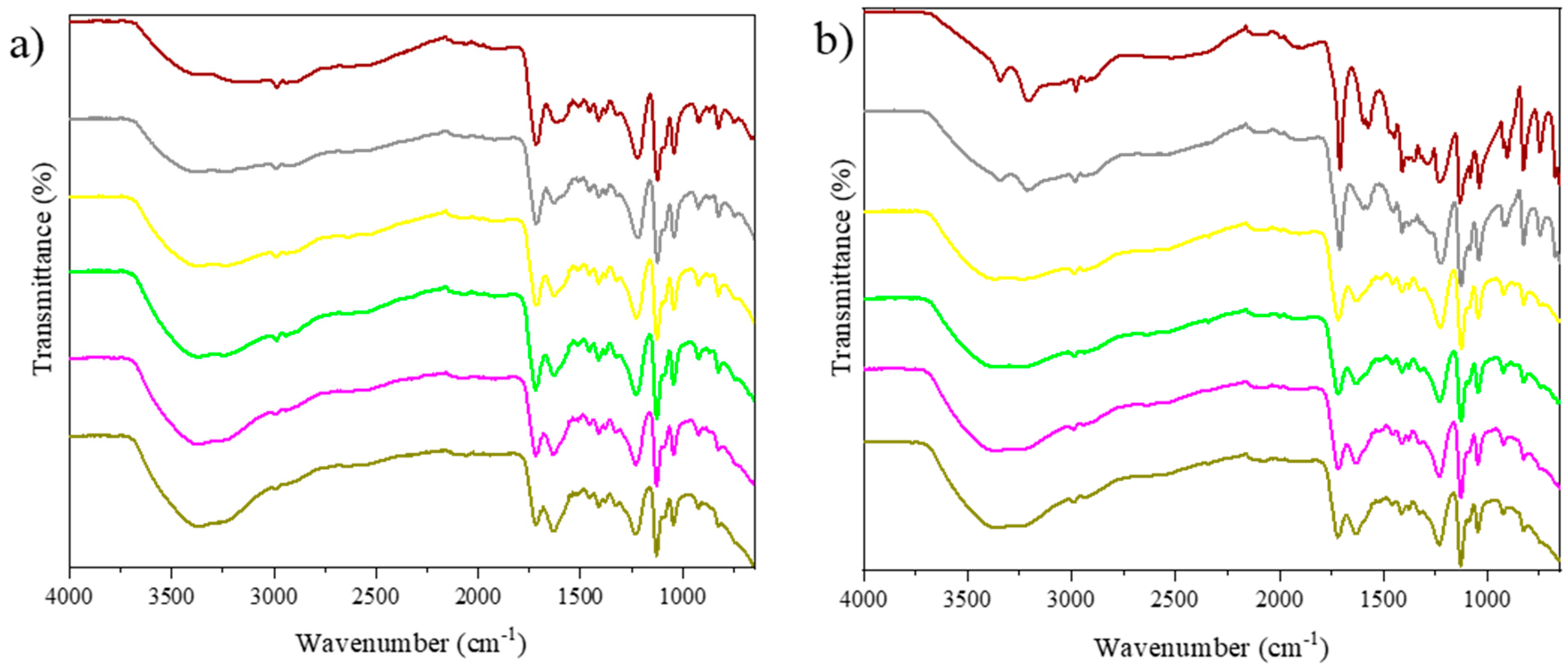
2.1.1. FTIR
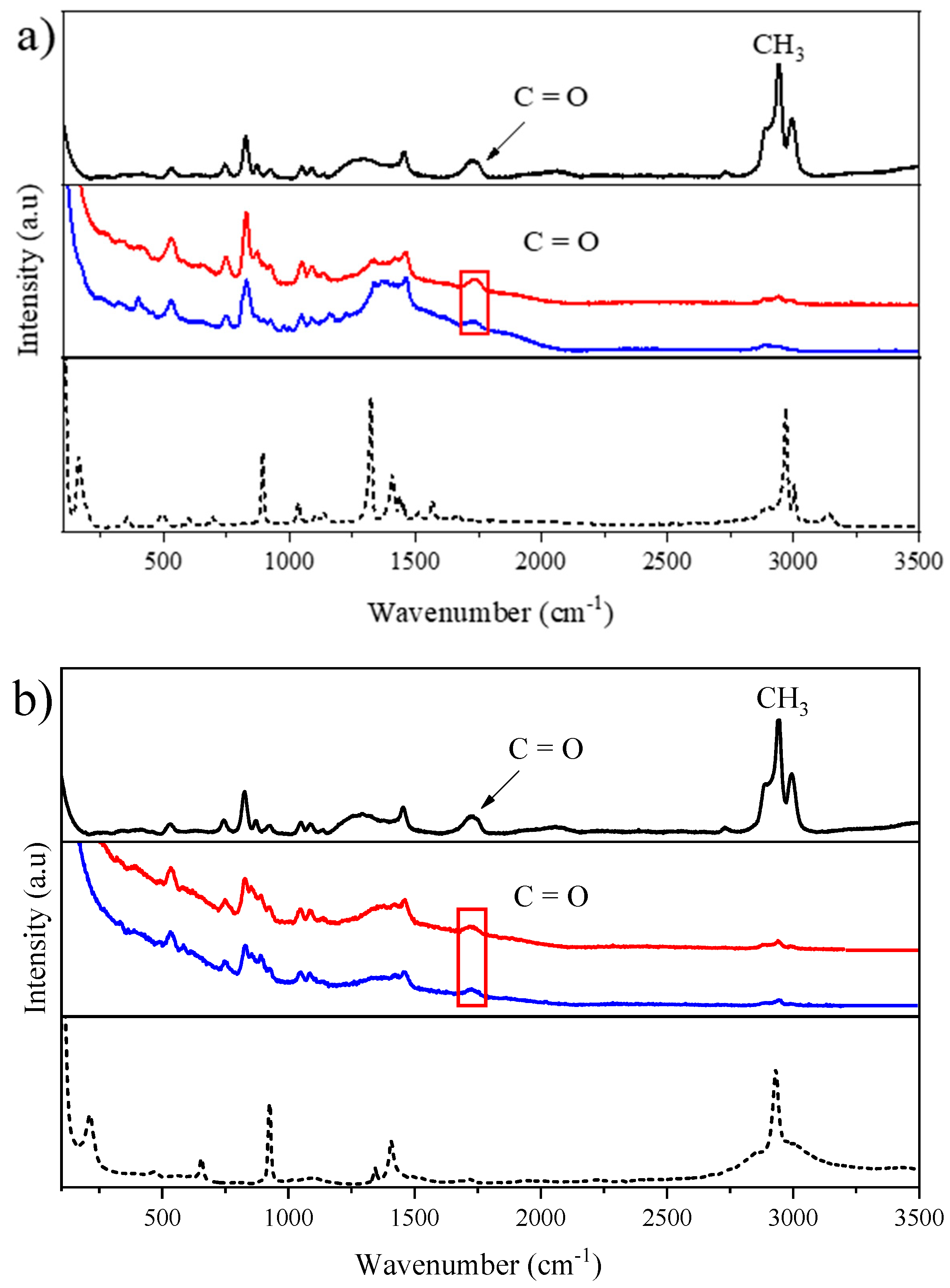
2.1.2. Raman
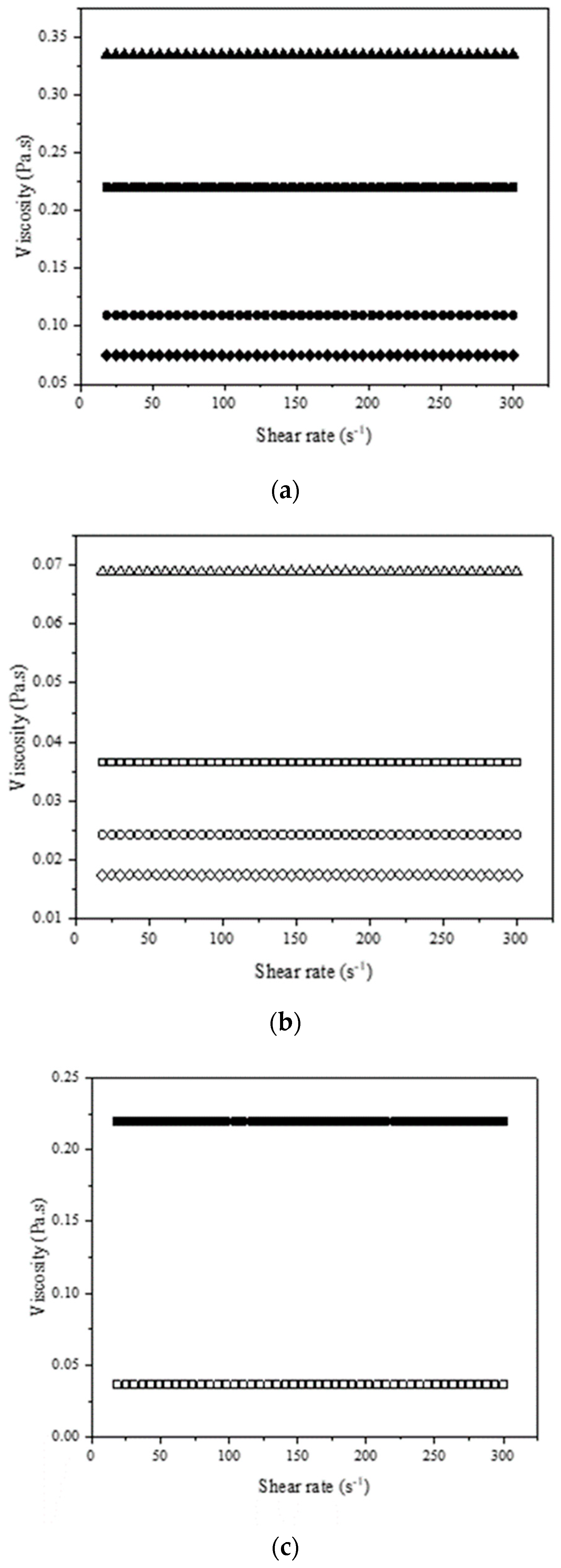
2.1.3. Rheological Behavior and Density
2.2. Extraction Process Applied to Dragon Fruit
Ultrasound-Assisted Extraction
3. Methods of Analyses
3.1. Reagents
3.2. Preparation of NADESs
3.3. NADESs Characterization
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.2. Raman Spectroscopy
3.3.3. Rheological Behavior—Viscosity
3.3.4. Density
3.4. Application of NADESs in the Extraction Process
Ultrasound-Assisted Extraction Process
3.5. Antioxidant Capacity and Bioactive Compounds
3.5.1. DPPH Analysis
3.5.2. Total Phenolic Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cicci, A.; Sed, G.; Bravi, M. Potential of choline chloride—Based natural deep eutectic solvents (NADES) in the extraction of microalgal metabolites. Chem. Eng. Trans. 2017, 57, 61–66. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Faggian, M.; Sut, S.; Perissutti, B.; Baldan, V.; Iztok, G.; Dall’Acqua, S. Natural deep eutectic solvents (NADES) as a tool for bioavailability improvement: Pharmacokinetics of rutin dissolved in proline/glycine after oral administration in rats: Possible application in nutraceuticals. Molecules 2016, 21, 1531. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhao, J.; Duan, H.; Guan, Y.; Zhao, L. Green and efficient extraction of four bioactive flavonoids from Pollen Typhae by ultrasound-assisted deep eutectic solvents extraction. J. Pharmaceut. Biomed. 2018, 161, 246–253. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches Extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Oomen, W.W.; Begines, P.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvent Extraction of Flavonoids of Scutellaria baicalensis as a Replacement for Conventional Organic Solvents. Molecules 2020, 25, 617. [Google Scholar] [CrossRef]
- González, C.G.; Mustafa, N.R.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Application of natural deep eutectic solvents for the “green” extraction of vanillin from vanilla pods. Flavour Fragr. J. 2017, 33, 91–96. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.d.l.A.; Gomez, F.J.V.; Silva, M.F. Natural Designer Solvents for Greening Analytical Chemistry. TrAC Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An eco-friendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Ind. Crops Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Craveiro, R.; Aroso, I.; Flammia, V.; Carvalho, T.; Viciosa, M.T.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Properties and thermal behavior of natural deep eutectic solvents. J. Mol. Liq. 2016, 215, 534–540. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as ecofriendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 325, 113761. [Google Scholar] [CrossRef]
- Aroso, I.M.; Paiva, A.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents from choline chloride and betaine—Physicochemical properties. J. Mol. Liq. 2017, 241, 654–661. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hayyan, M.; Alsaadi, M.A.; Ibrahim, S.; Hayyan, A.; Hashim, M.A. Physical properties of ethylene glycol-based deep eutectic solvents. J. Mol. Liq. 2019, 276, 794–800. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Physicochemical and storage properties of chitosan-based films plasticized with deep eutectic solvent. Food Hydrocoll. 2020, 108, 106007. [Google Scholar] [CrossRef]
- Silva, D.R.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef]
- Lanari, D.; Zadra, C.; Negro, F.; Njem, R.; Marcotullio, M.C. Influence of choline chloride-based NADES on the composition of Myristica fragrans Houtt. essential oil. Heliyon 2022, 8, e09531. [Google Scholar] [CrossRef] [PubMed]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency inthe ultrasound-assisted extraction of antioxidant polyphenols fromcommon native Greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Zang, Y.-Y.; Yang, X.; Chen, Z.-G.; Wu, T. One-pot preparation of quercetin using natural deep eutectic solvents. Process Biochem. 2020, 89, 193–198. [Google Scholar] [CrossRef]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Alrugaibah, M.; Yagiz, Y.; Gu, L. Use natural deep eutectic solvents as efficient green reagents to extract procyanidins and anthocyanins from cranberry pomace and predictive modeling by RSM and artificial neural networking. Sep. Purif. Technol. 2021, 255, 117720. [Google Scholar] [CrossRef]
- Chemat, F.; Huma, Z.-E.; Khan, M.K. Applications of ultrasund in food technology: Processing, preservation and extraction. Ultras. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Singh, B.S.; Lobo, H.R.; Pinjari, D.V.; Jarag, K.J.; Pandit, A.B.; Shankarling, G.S. Ultrasound and deep eutectic solvent (DES): A novel blend of techniques for rapid and energy efficient synthesis of oxazoles. Ultras. Sonochem. 2013, 20, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; Xiao, J.; Smeriglio, A.; Trombetta, D.; Burlando, B. Emerging Exotic Fruits: New Functional Foods in the European Market. eFood 2020, 1, 126–139. [Google Scholar] [CrossRef]
- Freitas, S.T.; Mitcham, E.J. Quality of pitaia fruit (Hylocereus Undatus) as influenced by storage temperature and packaging. Sci. Agr. 2013, 70, 257–262. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Wang, L. Structure characteristics of a water-soluble polysaccharide purified from dragon fruit (Hylocereus undatus) pulp. Carbohydr. Polym. 2016, 146, 224–230. [Google Scholar] [CrossRef] [PubMed]
- García-Cruz, L.; Valle-Guadarrama, S.; Salinas-Moreno, Y.; Joaquín-Cruz, E. Physical, Chemical, and Antioxidant Activity Characterization of Pitaya (Stenocereus pruinosus) Fruits. Plant Foods Hum. Nutr. 2013, 68, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Long, Q.; Gao, F.; Han, C.; Jin, P.; Zeng, Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Posthavest Biol. Tec. 2017, 124, 1–7. [Google Scholar] [CrossRef]
- Wybraniec, S.; Mizrahi, Y. Fruit flesh betacyanin pigments in Hylocereus Cacti. J. Agric. Food Chem. 2002, 50, 6086–6089. [Google Scholar] [CrossRef] [PubMed]
- Nurliyana, R.; Zahir, S.I.; Suleiman, M.K.; Aisyah, M.R.; Rahim, K.K. Antioxidant study of pulps and peels of dragon fruits: A comparative study. Int. Food Res. J. 2010, 17, 367–375. [Google Scholar]
- Tsai, Y.; Lin, C.-G.; Chen, W.-L.; Huang, Y.-C.; Chen, C.-Y.; Huang, K.-F.; Yang, C.-H. Evaluation of the antioxidant and wound-healing properties of extracts from different parts of Hylocereus polyrhizus. Agronomy 2009, 9, 27. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Uslu, N.; Özcan, M.M. The effect of ultrasound-vacuum-assisted extraction on bioactive properties of pitaya (Hylocereus undatus). Int. J. Food Sci. Technol. 2021, 56, 6618–6625. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, W.; Li, X.; Shu, C.; Jiang, W.; Cao, J. Nutrition, phytochemical profile, bioactivities and applications in food industry of pitaya (Hylocereus spp.) peels: A comprehensive review. Trends Food Sci. Technol. 2021, 116, 199–217. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, A.K.; Woo, E.M.; Katiyar, V. Effects of Amphiphilic Chitosan on Stereocomplexation and Properties of Poly (lactic acid) Nano-biocomposite. Sci. Rep. 2019, 8, 4351. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.Z.A.; Dillip, G.R.; Raghavaiah, P.; Mallikarjuna, K.; Raju, B.D.P. Spectroscopic and thermal studies of γ-glycine crystal grown from potassium bromide for optoelectronic applications. Arab. J. Chem. 2013, 6, 429–433. [Google Scholar] [CrossRef]
- Agasti, N.; Kaushik, N.K. One Pot Synthesis of Crystalline Silver Nanoparticles. Am. J. Nanomater. 2014, 2, 4–7. [Google Scholar] [CrossRef]
- Başkan, M.H.; Aydin, M.; Çanakçi, D.; Osmanoğlu, Ş. Electron paramagnetic resonance and FT-IR spectroscopic studies of DL-2-aminoadipic acid and ammonium acetate powders. Radiat. Eff. Defect. Solids 2013, 169, 256–264. [Google Scholar] [CrossRef]
- Delgado-Mellado, N.; Larriba, M.; Navarro, P.; Rigual, V.; Ayuso, M.; García, J.; Rodríguez, F. Thermal stability of choline chloride deep eutectic solvents by TGA/FTIR-ATR analysis. J. Mol. Liq. 2018, 260, 37–43. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, B.; Yang, H.; Wang, B.; Zhang, N.; Dou, H.; Wei, G.; Sun, Y.; Zhang, L. Investigation of glycerol-derived binary and ternary systems in CO2 capture process. Fuel 2017, 210, 836–843. [Google Scholar] [CrossRef]
- Selvanathan, V.; Azzahari, A.D.; Halim, A.A.A.; Yahya, R. Ternary natural deep eutectic solvent (NADES) infused phthaloyl starch as cost efficient quasi-solid gel polymer electrolyte. Carbohydr. Polym. 2017, 167, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.P.R.; Mora-Vargas, J.A.; Guimarães, T.G.S.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods. J. Mol. Liq. 2019, 293, 111452. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.-B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chu, K.; Li, H.; Su, L.; Yang, K.; Wang, Y.; Li, X. In situ Raman and synchrotron X-ray diffraction study on crystallization of Choline chloride/Urea deep eutectic solvent under high pressure. Chem. Phys. Lett. 2016, 661, 240–245. [Google Scholar] [CrossRef]
- Burikov, S.; Dolenko, T.; Patsaeva, S.; Starokurov, Y.; Yuzhakov, V. Raman and IR spectroscopy research on hydrogen bonding in water-ethanol systems. Mol. Phys. 2010, 108, 2427–2436. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, D.-W.; Zhu, Z. Development of natural deep eutectic solvents (NADESs) as anti-freezing agents for the frozen food industry: Water-tailoring effects, anti-freezing mechanisms and applications. Food Chem. 2022, 371, 131150. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, K.; Zhu, Y.; Zhu, R.; Ye, F.; Song, N.; Xu, Y. Physicochemical properties of deep eutectic solvents formed by choline chloride and phenolic compounds at T = (293.15 to 333.15) K: The influence of electronic effect of substitution group. J. Mol. Liq. 2017, 232, 182–187. [Google Scholar] [CrossRef]
- Gautam, R.; Kumar, N.; Lynam, J.G. Theoretical and experimental study of choline chloride-carboxylic acid deep eutectic solvents and their hydrogen bonds. J. Mol. Struct. 2020, 1222, 128849. [Google Scholar] [CrossRef]
- Samsudin, N.A.; Low, F.W.; Yusoff, Y.; Shakeri, M.; Tan, X.Y.; Lai, C.W.; Amin, N.; Oon, C.S.; Newaz, K.S.; Tiong, S.K.; et al. Effect of temperature on synthesis of cellulose nanoparticles via ionic liquid hydrolysis process. J. Mol. Liq. 2020, 308, 113030. [Google Scholar] [CrossRef]
- Botta, R.; Chindaudon, P.; Eiamchai, P.; Horprathum, M.; Limwichean, S.; Chananonnawathorn, C.; Patthanasettakul, V.; Jomphoak, A.; Nuntawong, N. Detection and classification of volatile fatty acids using surface-enhanced Raman scattering and density functional theory calculations. J. Raman Spectrosc. 2019, 50, 1817–1828. [Google Scholar] [CrossRef]
- Sjöberg, B.; Foley, S.; Cardey, B.; Enescu, M. An experimental and theoretical study of the amino acid side chain Raman bands in proteins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 28, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Khnykina, K.A.; Kireev, V.V.; Krunina, N.V.; Kundikova, N.D.; Verina, E.V. Influence of Environment on Raman Spectra of Glycine and Prospect of Their Use for Functional Diagnostics of Human Beings. In Proceedings of the Global Smart Industry Conference (GloSIC), Chelyabinsk, Russia, 13–15 November 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Frost, R.L.; Kloprogge, J.T. Raman spectroscopy of the acetates of sodium, potassium and magnesium at liquid nitrogen temperature. J. Mol. Struct. 2000, 526, 131–141. [Google Scholar] [CrossRef]
- Rajeswari, N.; Selvasekarapandian, S.; Karthikeyan, S.; Sanjeeviraja, C.; Iwai, Y.; Kawamura, J. Structural, vibrational, thermal, and electrical properties of PVA/PVP biodegradable polymer blend electrolyte with CH3COONH4. Ionics 2013, 19, 105–1113. [Google Scholar] [CrossRef]
- Araujo, C.F.; Coutinho, J.A.P.; Nolasco, M.M.; Parker, S.F.; Ribeiro-Claro, P.J.A.; Rudić, S.; Soares, B.I.G.; Vaz, P.D. Inelastic neutron scattering study of reline: Shedding light on the hydrogen bonding network of deep eutectic solvents. Phys. Chem. Chem. Phys. 2017, 19, 17998–18009. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic acid-based deep natural eutectic solvents for the extraction of bioactive metabolites of Humulus lupulus L.: Supramolecular organization, phytochemical profiling and biological activity. Sep. Purif. Technol. 2021, 264, 1180391. [Google Scholar] [CrossRef]
- Ahmadi, R.; Hemmateenejad, B.; Safavi, A.; Shojaeifard, Z.; Shahsavar, A.; Mohajeri, A.; Heydari Dokoohaki, M.; Zolghadr, A.R. Deep eutectic–water binary solvent associations investigated by vibrational spectroscopy and chemometrics. Phys. Chem. Chem. Phys. 2018, 20, 18463–18473. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Boumediene, M.; Haddad, B.; Paolone, A.; Assenine, M.A.; Villemin, D.; Rahmouni, M.; Bresson, S. Synthesis, conformational studies, vibrational spectra and thermal properties, of new 1,4-(phenylenebis(methylene) bis(methylimidazolium) ionic liquids. J. Mol. Struct. 2020, 1220, 128731. [Google Scholar] [CrossRef]
- Hayyan, A.; Mjalli, F.S.; Alnashef, I.M.; Al-Wahaibi, T.; Al-Wahaibi, Y.M.; Hashim, M.A. Fruit sugar-based deep eutectic solvents and their physical properties. Thermochim. Acta 2012, 541, 70–75. [Google Scholar] [CrossRef]
- Savi, L.K.; Carpiné, D.; Waszczynskyj, N.; Ribani, R.H.; Haminiuk, C.W.I. Influence of temperature, water content and type of organic acid on the formation, stability and properties of a functional natural deep eutectic solvents. Fluid Ph. Equilibria 2019, 488, 40–47. [Google Scholar] [CrossRef]
- Cai, G.; Yang, S.; Wang, X.; Zhou, Q.; Xu, J.; Lu, X. Densities and Viscosities of Binary Mixtures Containing the Polyhydric Protic Ionic Liquid (2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate) and Water or Alcohols. J. Solut. Chem. 2020, 49, 423–427. [Google Scholar] [CrossRef]
- AlOmar, M.K.; Hayyan, M.; Alsaadi, M.A.; Akib, S.; Hayyan, A.; Hashim, M.A. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2016, 215, 98–103. [Google Scholar] [CrossRef]
- Tamrakar, A.; Gunadi, A.; Piccione, P.M.; Ramachandran, R. Dynamic agglomeration profiling during the drying phase in an agitated filter dyer: Parametric investigation and regime map studies. Powder Technol. 2016, 303, 109–123. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.d.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhou, T.; Lai, T.; Zhang, H. Optimization of the Microwave-Assisted Extraction of Polyphenols from Red Pitaya Peel using Response Surface Methodology. J. Sci. Ind Res. 2018, 77, 419–424. [Google Scholar]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of Conventional and Ultrasonic-Assisted Extraction on Phenolic Contents, Betacyanin Contents, and Antioxidant Capacity of Red Dragon Fruit (Hylocereus polyrhizus). Sci. World J. 2014, 2014, 964731. [Google Scholar] [CrossRef] [PubMed]
- Chakroun, D.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Enhanced-performance extraction of olive (Olea europaea) leaf polyphenols using L-lactic acid/ammonium acetate deep eutectic solvent combined with β-cyclodextrin: Screening, optimisation, temperature effects and stability. Biomass Conv. Bioref. 2021, 11, 1125–1136. [Google Scholar] [CrossRef]
- Kaoui, S.; Chebli, B.; Baddi, G.A.; Basaid, K.; Mir, Y. Response surface modeling and optimization of the extraction conditions using lactic acid-based deep eutectic solvents as green alternative extraction media for Mentha pulegium. Phytochem. Anal. 2022, 33, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Toledo Hijo, A.A.C.; Maximo, G.J.; Costa, M.C.; Batista, E.A.C.; Meirelles, A.J.A. Applications of Ionic Liquids in the Food and Bioproducts Industries. ACS Sustain. Chem. Eng. 2016, 4, 5347–5369. [Google Scholar] [CrossRef]
- Silva, D.T.d.; Pauletto, R.; Cavalheiro, S.d.S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; Silva, C.d.B.d.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Handayani, R.; Bangun, A.; Deborah, P.D.; Mun’im, A. Optimization of microwave- and ultrasonic-assisted extraction of mahkota dewa (Phaleria macrocarpa [scheff.] Boerl.) Fruit pulp. Int. J. Appl. Pharm. 2020, 12, 32–37. [Google Scholar] [CrossRef]
- Yue, Y.; Huang, Q.; Fu, Y.; Chang, J. A quick selection of natural deep eutectic solvents for the extraction of chlorogenic acid from herba Artemisiae scopariae. RSC Adv. 2020, 1621, 23403–23409. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Blidi, S.; Bikaki, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: Apple waste peels as a case study. Waste Biomass Valorization 2015, 6, 1125–1133. [Google Scholar] [CrossRef]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box– Behnken experimental design and kinetics. Ind. Crop. Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Brand-Willians, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol Viticult. 1965, 16, 144–168. [Google Scholar]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenol sand vitamin C in plant-derived products. J. Agr. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef]
- Statsoft, Inc. Statistica (data analysis software system), version 7; Statsoft, Inc.: Tulsa, OK, USA, 2004. [Google Scholar]

| NADESs | Without Water | With Water | ||||||
|---|---|---|---|---|---|---|---|---|
| (Pa s) | ρ (g mL−1) b,* | R2 | χ² | (Pa·s) | ρ (g mL−1) b,* | R2 | χ² | |
| LA:glycine | 0.220 | 1.272 ± 0.002 a | 0.99942 | 0.19631 | 0.037 | 1.220 ± 0.001 e | 0.98648 | 0.32691 |
| LA:ammonium acetate | 0.109 | 1.208 ± 0.001 b | 0.99474 | 0.40818 | 0.024 | 1.179 ± 0.000 f | 0.98744 | 0.21744 |
| LA:sodium acetate | 0.335 | 1.288 ± 0.001 c | 0.99966 | 0.12422 | 0.069 | 1.244 ± 0.002 g | 0.99885 | 0.20942 |
| LA:choline chloride | 0.075 | 1.188 ± 0.002 d | 0.99042 | 0.3181 | 0.017 | 1.159 ± 0.002 h | 0.98501 | 0.26056 |
| Solvents | Phenolics (mg/100g d.b.) * | DPPH (g fruit/g DPPH db) |
|---|---|---|
| Lactic acid:glycine | 193.18 ± 1.26 b | 1765.46 ± 47.43 a |
| Lactic acid:ammonium acetate | 186.08 ± 4.70 b | 1623.96 ± 78.50 a |
| Lactic acid:sodium acetate | 157.43 ± 5.37 c | 1872.55 ± 169.88 a |
| Ethanol | 450.41 ± 1.74 a | 1562.45 ± 185.34 a |
| NADESs | |||
|---|---|---|---|
| Hydrogen Donor | Hydrogen Acceptor | Molar | Water (%) |
| Ratio | |||
| Lactic acid | Glycine | 4:1 | - |
| Lactic acid | Ammonium acetate | 3:1 | - |
| Lactic acid | Sodium acetate | 3:1 | - |
| Lactic acid | Choline chloride | 3:1 | - |
| Lactic acid | Glycine | 4:1 | 24 |
| Lactic acid | Ammonium acetate | 3:1 | 20 |
| Lactic acid | Sodium acetate | 3:1 | 20 |
| Lactic acid | Choline chloride | 3:1 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, I.V.; Sakurai, Y.C.N.; Ferreira, N.R.; Moreira, S.G.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules 2022, 27, 8310. https://doi.org/10.3390/molecules27238310
Pires IV, Sakurai YCN, Ferreira NR, Moreira SGC, da Cruz Rodrigues AM, da Silva LHM. Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules. 2022; 27(23):8310. https://doi.org/10.3390/molecules27238310
Chicago/Turabian StylePires, Ianê Valente, Yasmin Caroline Nóvoa Sakurai, Nelson Rosa Ferreira, Sanclayton Geraldo Carneiro Moreira, Antonio Manoel da Cruz Rodrigues, and Luiza Helena Meller da Silva. 2022. "Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya" Molecules 27, no. 23: 8310. https://doi.org/10.3390/molecules27238310
APA StylePires, I. V., Sakurai, Y. C. N., Ferreira, N. R., Moreira, S. G. C., da Cruz Rodrigues, A. M., & da Silva, L. H. M. (2022). Elaboration and Characterization of Natural Deep Eutectic Solvents (NADESs): Application in the Extraction of Phenolic Compounds from pitaya. Molecules, 27(23), 8310. https://doi.org/10.3390/molecules27238310







