Highly Effective Thermally Activated Delayed Fluorescence Emitters Based on Symmetry and Asymmetry Nicotinonitrile Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Density Functional Theory (DFT) Calculations
2.3. Photophysical Properties
2.4. Electroluminescence (EL) Performance
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Dias, F.B.; Bourdakos, K.N.; Jankus, V.; Moss, K.C.; Kamtekar, K.T.; Bhalla, V.; Santos, J.; Bryce, M.R.; Monkman, A.P. Triplet Harvesting with 100% Efficiency by Way of Thermally Activated Delayed Fluorescence in Charge Transfer OLED Emitters. Adv. Mater. 2013, 25, 3707–3714. [Google Scholar] [CrossRef]
- Schwartz, G.; Pfeiffer, M.; Reineke, S.; Walzer, K.; Leo, K. Harvesting Triplet Excitons from Fluorescent Blue Emitters in White Organic Light-Emitting Diodes. Adv. Mater. 2007, 19, 3672–3676. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.; Ma, D.; Cheng, Y.; Wang, L.; Jing, X.; Wang, F. Harvesting Excitons via Two Parallel Channels for Efficient White Organic LEDs with Nearly 100% Internal Quantum Efficiency: Fabrication and Emission-Mechanism Analysis. Adv. Funct. Mater. 2009, 19, 84–95. [Google Scholar] [CrossRef]
- Sun, Y.R.; Giebink, N.C.; Kanno, H.; Ma, B.W.; Thompson, M.E.; Forrest, S.R. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Forrest, S.R. Electroluminescence mechanisms in organic light emitting devices employing a europium chelate doped in a wide energy gap bipolar conducting host. J. Appl. Phys. 2000, 87, 8049–8055. [Google Scholar] [CrossRef]
- Pope, M.; Kallmann, H.P.; Magnante, P. Electroluminescence in Organic Crystals. J. Chem. Phys. 1963, 38, 2042–2043. [Google Scholar] [CrossRef]
- Kim, K.-H.; Moon, C.-K.; Lee, J.-H.; Kim, S.-Y.; Kim, J.-J. Highly Efficient Organic Light-Emitting Diodes with Phosphorescent Emitters Having High Quantum Yield and Horizontal Orientation of Transition Dipole Moments. Adv. Mater. 2014, 26, 3844–3847. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Liu, D.; Wang, F.; Zhang, S. Bipolar host materials for high-efficiency blue phosphorescent and delayed-fluorescence OLEDs. J. Meter. Chem. C 2015, 3, 12529–12538. [Google Scholar] [CrossRef]
- Reineke, S.; Walzer, K.; Leo, K. Triplet-exciton quenching in organic phosphorescent light-emitting diodes with Ir-based emitters. Phys. Rev. B 2007, 75, 125328. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, C.H.; Adachi, C.; Lee, S.Y. Molecular design of highly effective thermally activated delayed fluorescence emitters based on ortho-substituted donor-acceptor-donor pyridinecarbonitrile derivatives and their application for high-performance OLEDs. Dyes Pigm. 2019, 171, 107775. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, B.; Huang, S.; Nomura, H.; Tanaka, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Adachi, C. Third-generation organic electroluminescence materials. Jpn. J. Appl. Phys. 2014, 53, 060101. [Google Scholar] [CrossRef]
- Hirata, S.; Sakai, Y.; Masui, K.; Tanaka, H.; Lee, S.Y.; Nomura, H.; Nakamura, N.; Yasumatsu, M.; Nakanotani, H.; Zhang, Q.; et al. Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat. Mater. 2015, 14, 330–336. [Google Scholar] [CrossRef]
- Tanaka, H.; Shizu, K.; Miyazaki, H.; Adachi, C. Efficient green thermally activated delayed fluorescence (TADF) from a phenoxazine–triphenyltriazine (PXZ–TRZ) derivative. Chem. Commun. 2012, 48, 11392–11394. [Google Scholar] [CrossRef]
- Braveenth, R.; Lee, H.; Kim, S.; Raagulan, K.; Kim, S.; Kwon, J.H.; Chai, K.Y. High efficiency green TADF emitters of acridine donor and triazine acceptor D–A–D structures. J. Mater. Chem. C 2019, 7, 7672–7680. [Google Scholar] [CrossRef]
- Xiang, S.; Lv, X.; Sun, S.; Zhang, Q.; Huang, Z.; Guo, R.; Gu, H.; Liu, S.; Wang, L. To improve the efficiency of thermally activated delayed fluorescence OLEDs by controlling the horizontal orientation through optimizing stereoscopic and linear structures of indolocarbazole isomers. J. Mater. Chem. C 2018, 6, 5812–5820. [Google Scholar] [CrossRef]
- Liang, X.; Wang, Z.; Wang, L.; Hanif, M.; Hu, D.; Su, S.; Xie, Z.; Gao, Y.; Yang, B.; Ma, Y. Tailoring Excited State Properties and Energy Levels Arrangement via Subtle Structural Design on D-π-A Materials. Chin. J. Chem. 2017, 35, 1559–1568. [Google Scholar] [CrossRef]
- Miralles, N.; Romero, R.M.; Fernández, E.; Muñiz, K. A mild carbon–boron bond formation from diaryliodonium salts. Chem. Commun. 2015, 51, 14068–14071. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Liu, D.; Li, J.; Wang, J. Accelerating PLQY and RISC rates in deep-blue TADF materials with the acridin-9(10H)-one acceptor by tuning the peripheral groups on carbazole donors. J. Mater. Chem. C 2022, 43, 16524–16535. [Google Scholar] [CrossRef]
- Park, I.S.; Lee, J.; Yasuda, T. High-performance blue organic light-emitting diodes with 20% external electroluminescence quantum efficiency based on pyrimidine-containing thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2016, 4, 7911–7916. [Google Scholar] [CrossRef]
- Nasu, K.; Nakagawa, T.; Lin, C.J.; Cheng, C.H.; Tseng, M.R.; Yasuda, T.; Adachi, C. A highly luminescent spiro-anthracenone-based organic light-emitting diode exhibiting thermally activated delayed fluorescence. Chem. Commun. 2013, 49, 10385–10387. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Monkman, A.P.; Penfold, T.J. The Importance of Vibronic Coupling for Efficient Reverse Intersystem Crossing in Thermally Activated Delayed Fluorescence Molecules. ChemPhysChem 2016, 17, 2956–2961. [Google Scholar] [CrossRef]
- Li, C.; Duan, C.; Han, C.; Xu, H. Secondary Acceptor Optimization for Full-Exciton Radiation: Toward Sky-Blue Thermally Activated Delayed Fluorescence Diodes with External Quantum Efficiency of ≈ 30%. Adv. Mater. 2018, 30, e1804228. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Higginbotham, H.F.; Matulaitis, T.; Danos, A.; Bismillah, A.N.; Haase, N.; Etherington, M.K.; Yufit, D.S.; McGonigal, P.R.; Gražulevičius, J.V.; et al. Revealing resonance effects and intramolecular dipole interactions in the positional isomers of benzonitrile-core thermally activated delayed fluorescence materials. J. Mater. Chem. C 2019, 7, 9184–9194. [Google Scholar] [CrossRef]
- Lee, H.L.; Lee, K.H.; Lee, J.Y.; Lee, H.J. Molecular design opening two emission pathways for high efficiency and long lifetime of thermally activated delayed fluorescent organic light-emitting diodes. J. Meter. Chem. C 2021, 9, 7328–7335. [Google Scholar] [CrossRef]
- Chou, H.-H.; Cheng, C.-H. A Highly Efficient Universal Bipolar Hos for Blue, Green and Red Phosphorescent OLEDs. Adv. Mater. 2010, 22, 2468–2471. [Google Scholar] [CrossRef]
- Lee, S.Y.; Adachi, C.; Yasuda, T. High-Efficiency Blue Organic Light-Emitting Diodes Based on Thermally Activated Delayed Fluorescence from Pheoxaphosphine and Phenoxathiin Derivatives. Adv. Mater. 2016, 28, 4626–4631. [Google Scholar] [CrossRef]
- Lee, C.H.; Choi, S.H.; Oh, S.J.; Lee, J.H.; Shim, J.W.; Adachi, C.; Lee, S.Y. Highly effective organic light-emitting diodes containing thermally activated delayed fluorescence emitters with horizontal molecular orientation. RSC Adv. 2020, 10, 42897–42902. [Google Scholar] [CrossRef] [PubMed]
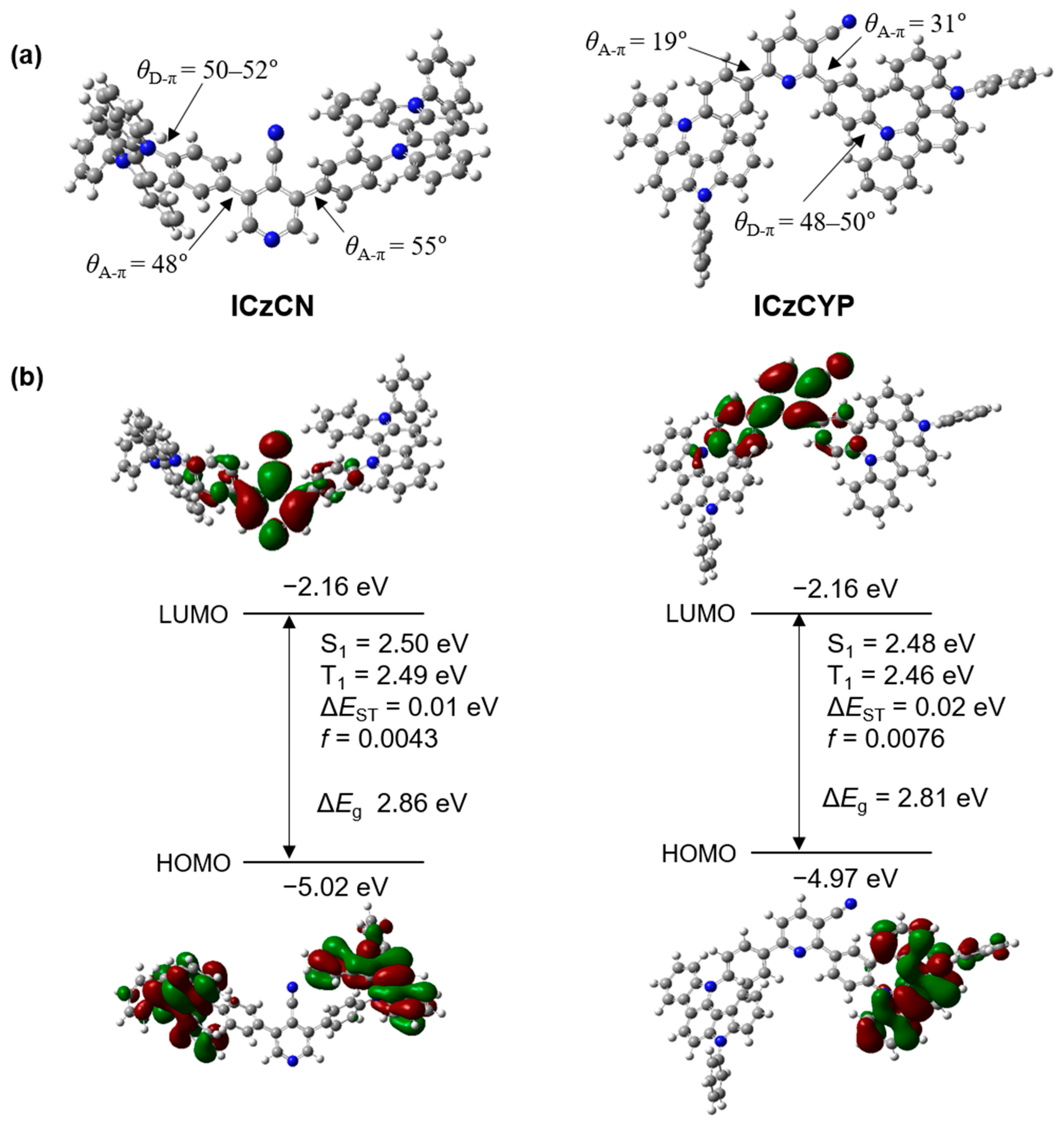
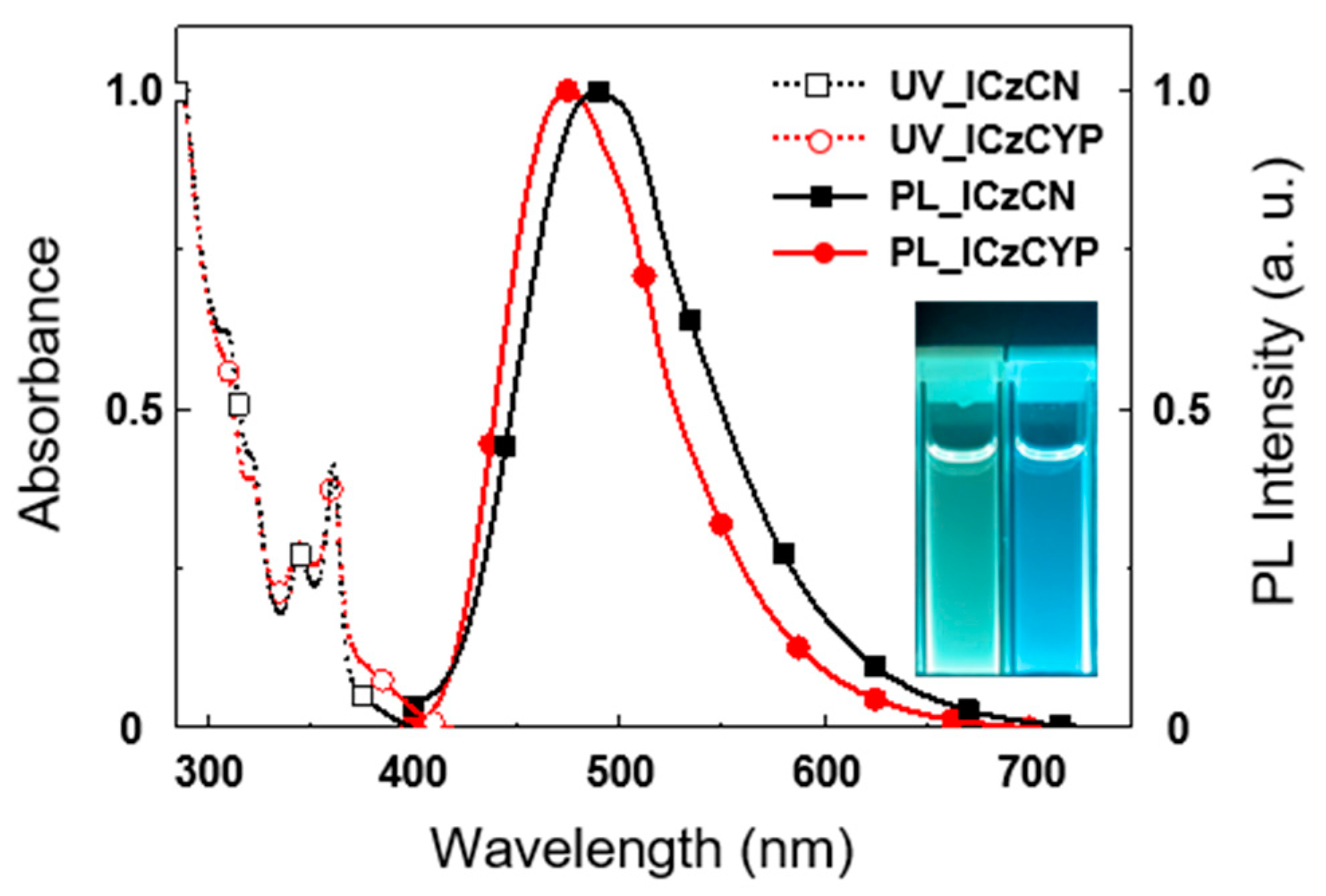
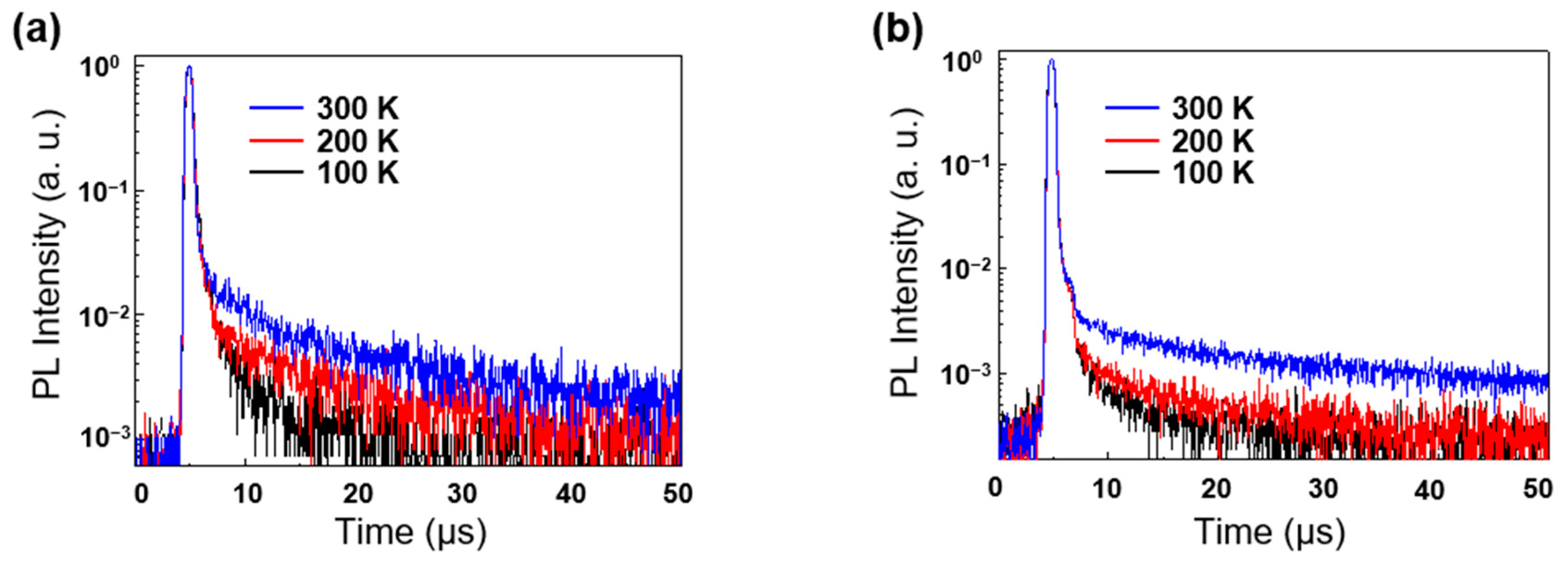
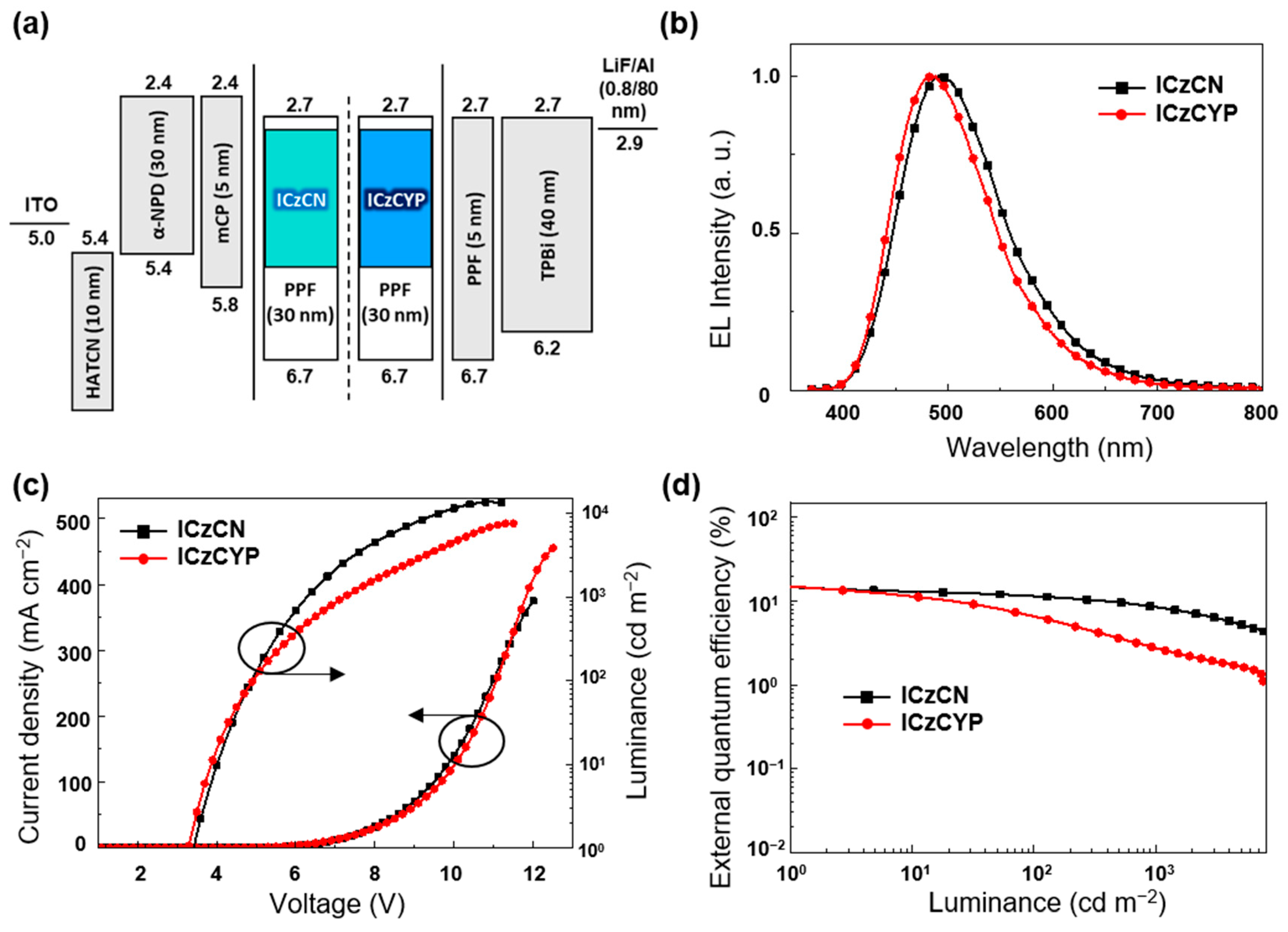
| Emitter | λabs1 (nm) | λPL [nm] sol 1/Film 2 | ΦPL [%] sol 1/Film 2 | τp3/Φp4 (ns/%) | τd3/Φd4 (μs/%) | Ip/Ea/Eg5 (eV) | ES/ET/ΔEST6 (eV) |
|---|---|---|---|---|---|---|---|
| ICzCN | 371 | 489/491 | 63/76 | 56/30 | 14/46 | 5.80/2.93/2.87 | 2.85/2.79/0.06 |
| ICzCYP | 370 | 475/484 | 52/58 | 42/27 | 31/ 31 | 5.86/2.95/2.91 | 2.88/2.83/0.05 |
| Emitter | krS (s−1) | kd (s−1) | knr, T (s−1) | kISC (s−1) | kRISC (s−1) | ΦISC (%) | ΦRISC (%) |
|---|---|---|---|---|---|---|---|
| ICzCN | 1.3 × 107 | 1.3 × 105 | 4.4 × 104 | 3.0 × 107 | 2.8 × 105 | 54 | 84 |
| ICzCYP | 6.7 × 106 | 1.1 × 105 | 6.4 × 104 | 1.8 × 107 | 1.8 × 105 | 69 | 45 |
| Emitter | λEL 1 (nm) | Von2 (V) | Lmax (cd m−2) | ηc3 (cd A−1) | ηp3 (lm W−1) | ηext3 (%) | CIEx,y 4 (x,y) |
|---|---|---|---|---|---|---|---|
| ICzCN | 507 | 3.4 | 13742 | 42.1 | 44.1 | 14.8 | (0.26, 0.47) |
| ICzCYP | 497 | 3.3 | 7627 | 46.5 | 50.3 | 14.9 | (0.23, 0.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.G.; Lee, C.H.; Adachi, C.; Lee, S.Y. Highly Effective Thermally Activated Delayed Fluorescence Emitters Based on Symmetry and Asymmetry Nicotinonitrile Derivatives. Molecules 2022, 27, 8274. https://doi.org/10.3390/molecules27238274
Choi MG, Lee CH, Adachi C, Lee SY. Highly Effective Thermally Activated Delayed Fluorescence Emitters Based on Symmetry and Asymmetry Nicotinonitrile Derivatives. Molecules. 2022; 27(23):8274. https://doi.org/10.3390/molecules27238274
Chicago/Turabian StyleChoi, Min Gyeong, Chan Hee Lee, Chihaya Adachi, and Sae Youn Lee. 2022. "Highly Effective Thermally Activated Delayed Fluorescence Emitters Based on Symmetry and Asymmetry Nicotinonitrile Derivatives" Molecules 27, no. 23: 8274. https://doi.org/10.3390/molecules27238274
APA StyleChoi, M. G., Lee, C. H., Adachi, C., & Lee, S. Y. (2022). Highly Effective Thermally Activated Delayed Fluorescence Emitters Based on Symmetry and Asymmetry Nicotinonitrile Derivatives. Molecules, 27(23), 8274. https://doi.org/10.3390/molecules27238274






