Study on Asymmetric Vibrational Coherent Magnetic Transitions and Origin of Fluorescence in Symmetric Structures
Abstract
:1. Introduction
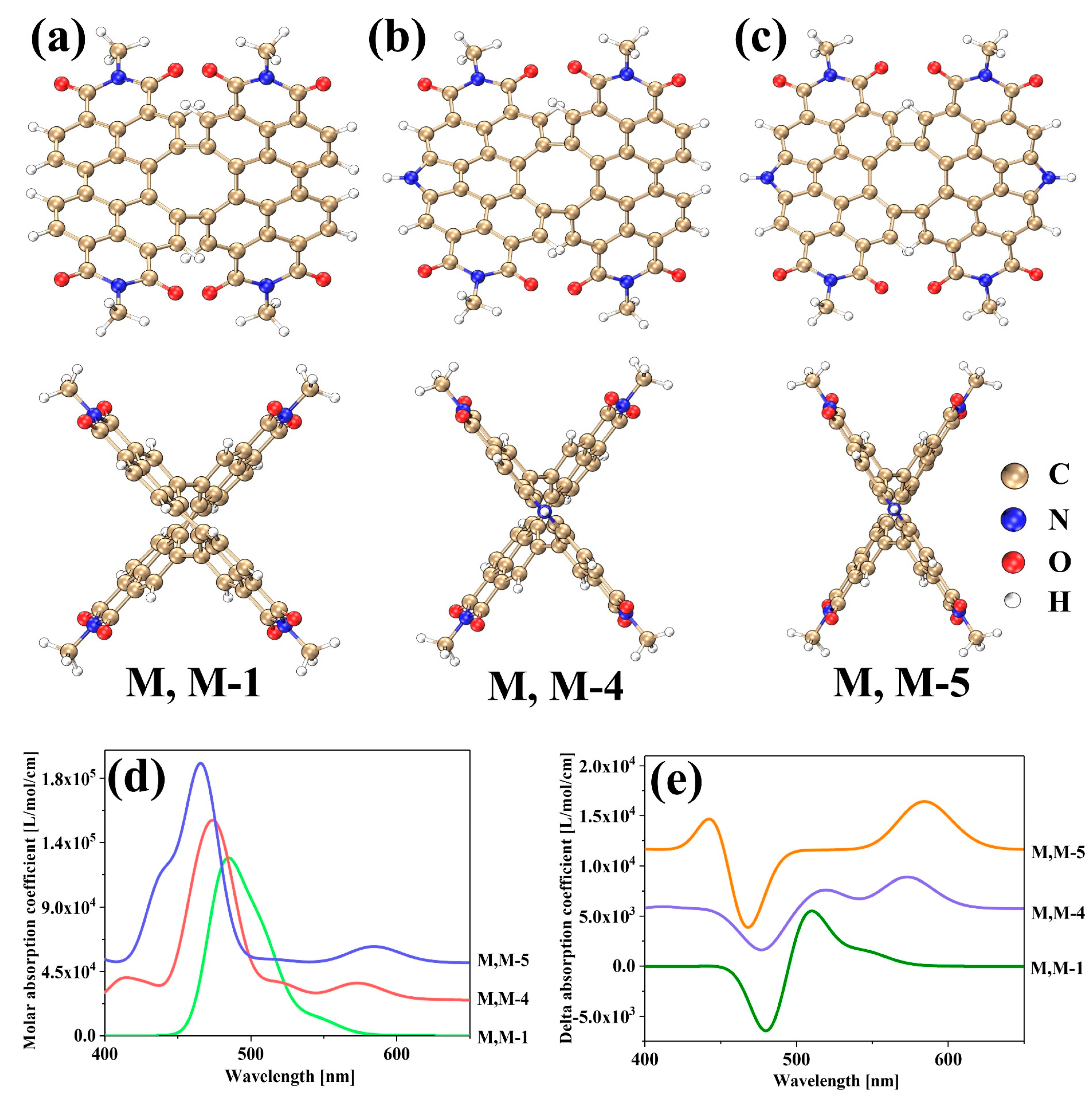
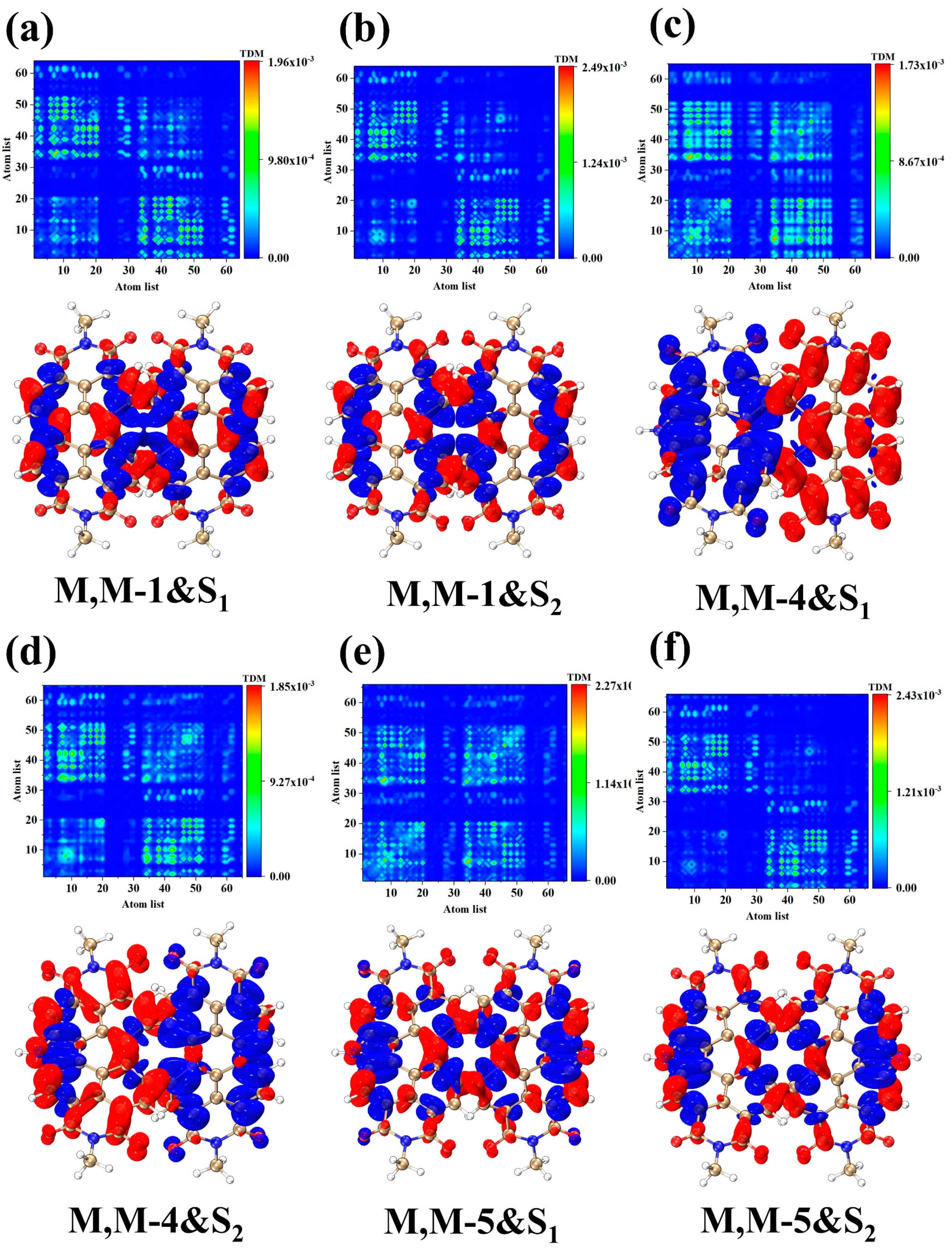
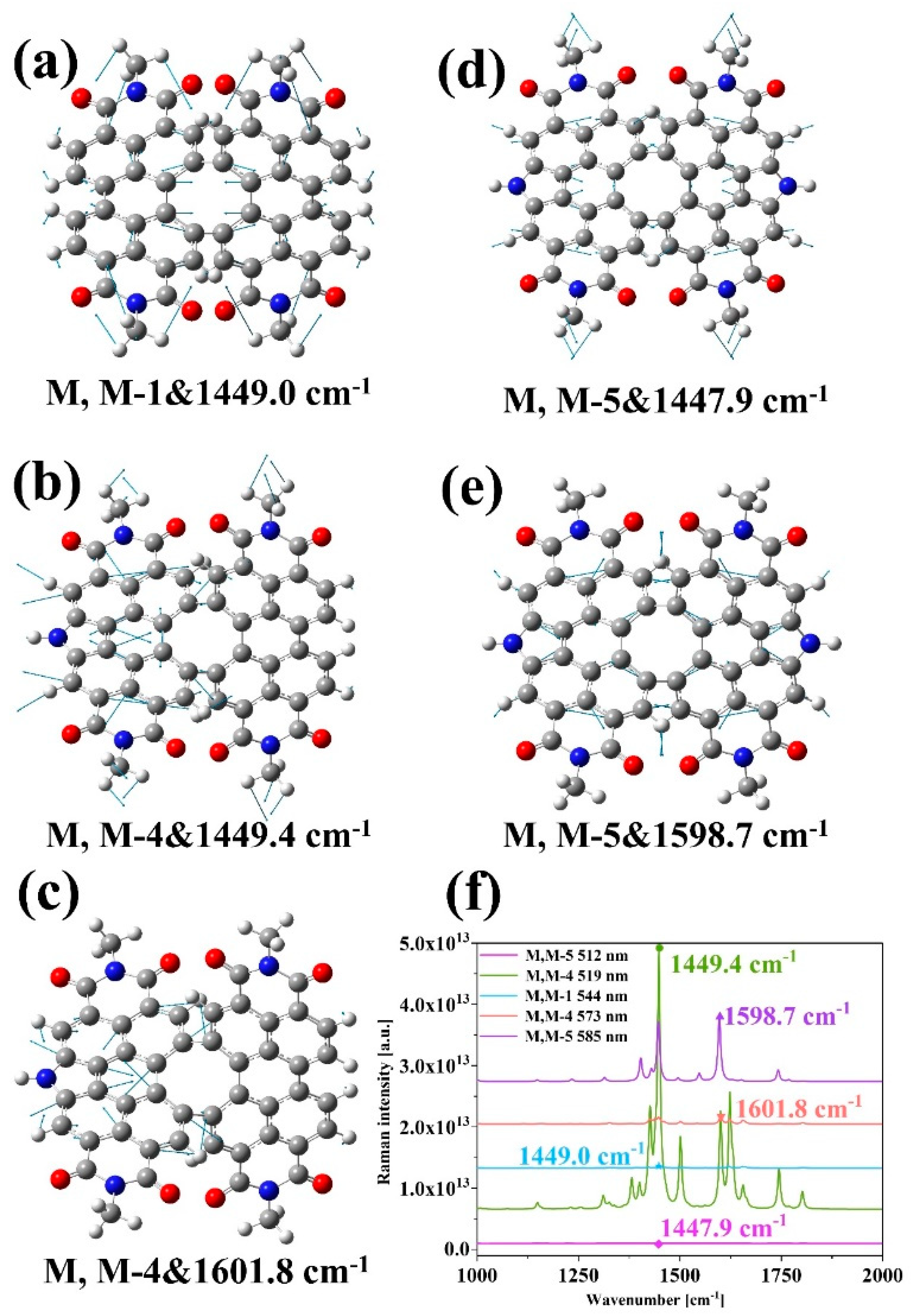
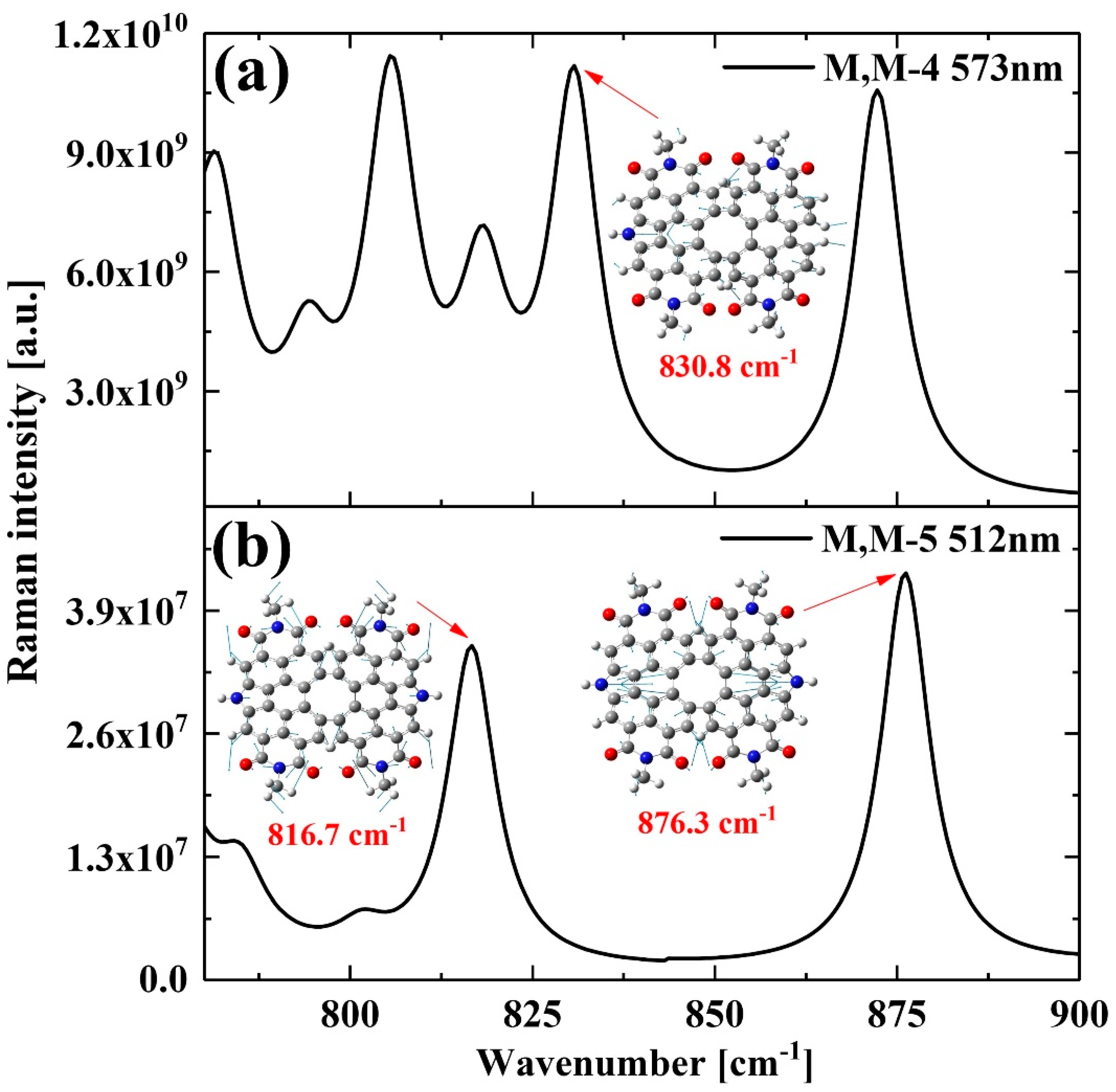
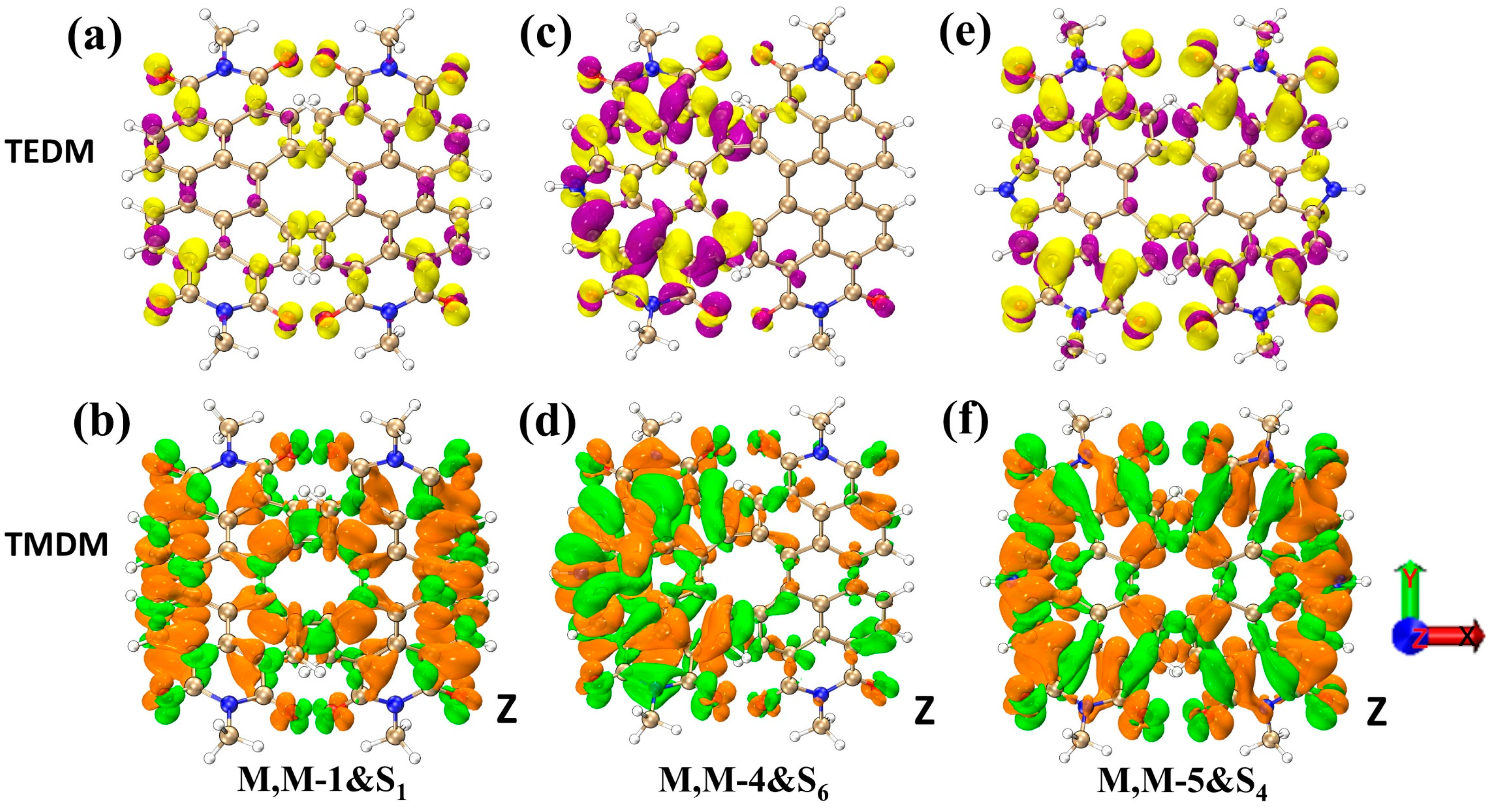
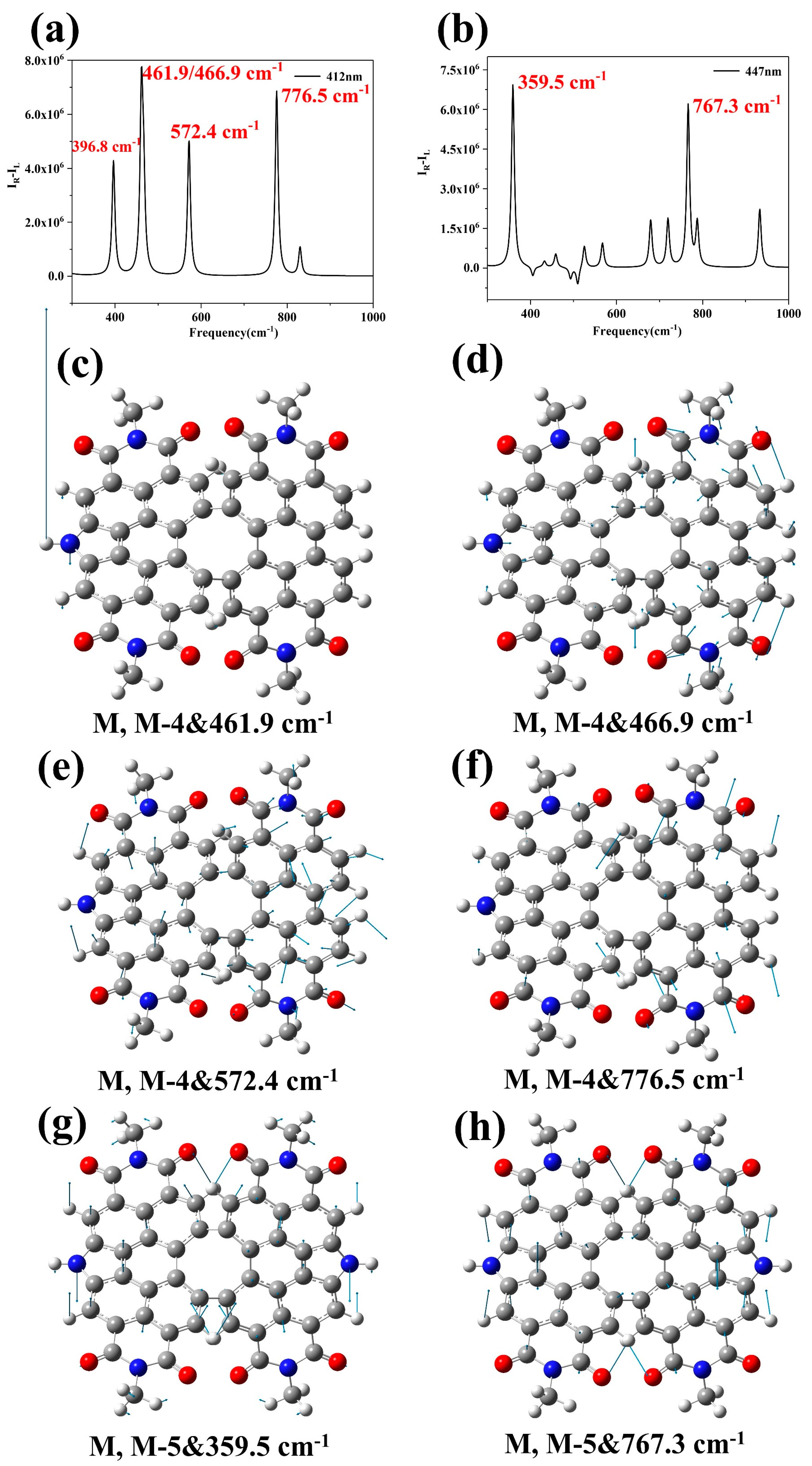
2. Results and Discussion
3. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mu, X.; Wang, X.; Jun, Q.; Sun, M. Photoinduced Charge Transfer in Donor-Bridge-Acceptor in One- and Two-Photon Absorption: Sequential and Super-Exchange Mechanisms. J. Phys. Chem. C 2020, 124, 4968–4981. [Google Scholar] [CrossRef]
- Wang, J.; Mu, X.; Sun, M.; Mu, T. Optoelectronic properties and applications of graphene-based hybrid nanomaterials and van der Waals heterostructures. Appl. Mater. 2019, 16, 1–20. [Google Scholar] [CrossRef]
- Mu, X.; Sun, M. The linear and non-linear optical absorption and asymmetrical electromagnetic interaction in chiral twisted bilayer graphene with hybrid edges. Mater. Today Phys. 2020, 14, 100222. [Google Scholar] [CrossRef]
- Mu, X.; Chen, X.; Wang, J.; Sun, M. Visualizations of Electric and Magnetic Interactions in Electronic Circular Dichroism and Raman Optical Activity. J. Phys. Chem. A 2019, 23, 8071–8081. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Chen, P.; Sheng, H.; Li, C.; Wang, J. Physical mechanism on linear spectrum and nonlinear spectrum in double helical carbon nanomolecule-infinitene. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 282, 121674. [Google Scholar] [CrossRef]
- Wang, J.; Ma, F.; Liang, W.; Sun, M. Electrical properties and applications of graphene, hexagonal boron nitride (h-BN), and graphene/h-BN heterostructures. Mater. Today Phys. 2017, 2, 6–34. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Li, H.; Jiang, S.; Wang, J.; Zhang, W.; Song, X.; Jia, B.; Qiu, J.; Ma, T. Engineering local environment of ruthenium by defect-tuned SnO2 over carbon cloth for neutral-media N2 electroreduction. Carbon 2022, 195, 199–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, J. Chiral helical polymer materials derived from achiral monomers and their chiral applications. Polym. Chem. 2020, 11, 5407–5423. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Zhou, L.; Liu, N.; Wu, Z.Q. Asymmetric Living Supramolecular Polymerization: Precise Fabrication of One-Handed Helical Supramolecular Polymers. Angew. Chem. Int. Ed. 2022, 61, e202207028. [Google Scholar] [CrossRef]
- Scanga, R.A.; Reuther, J.F. Helical polymer self-assembly and chiral nanostructure formation. Polym. Chem. 2021, 12, 1857–1897. [Google Scholar] [CrossRef]
- Zhou, L.; He, K.; Liu, N.; Wu, Z.Q. Recent advances in asymmetric organocatalysis based on helical polymers. Polym. Chem. 2022, 13, 3967–3974. [Google Scholar] [CrossRef]
- Nagata, Y.; Takeda, R.; Suginome, M. Asymmetric catalysis in chiral solvents: Chirality transfer with amplification of homochirality through a helical macromolecular scaffold. ACS Cent. Sci. 2019, 5, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.D.; Mastai, Y. Chiral polymers and polymeric particles for enantioselective crystallization. Isr. J. Chem. 2018, 58, 1330–1337. [Google Scholar] [CrossRef]
- Rahman, M.; Almalki, W.H.; Afzal, O.; Altamimi, A.S.A.; Ullah, S.N.M.N.; Barkat, M.A.; Beg, S. Chiral-engineered supraparticles: Emerging tools for drug delivery. Drug Discov. Today 2022, 28, 103420. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wu, Q.L.; Zhou, L.; Hou, X.H.; Liu, N.; Wu, Z.Q. Chiral recognition and resolution based on helical polymers. Chin. J. Polym. Sci. 2021, 39, 1521–1527. [Google Scholar] [CrossRef]
- Ikai, T.; Okuda, S.; Aizawa, M.; Yashima, E. Chiral and Achiral Pendant-Bound Poly (biphenylylacetylene)s Bearing Amide and/or Carbamate Groups: One-Handed Helix Formations and Chiral Recognition Abilities. Macromolecules 2022, 55, 7023–7031. [Google Scholar] [CrossRef]
- Liu, B.; Böckmann, M.; Jiang, W.; Doltsinis, N.L.; Wang, Z. Perylene diimide-embedded double [8] helicenes. J. Am. Chem. Soc. 2020, 142, 7092–7099. [Google Scholar] [CrossRef]
- Xu, L.; Wu, Y.J.; Gao, R.T.; Li, S.Y.; Liu, N.; Wu, Z.Q. Visible Helicity Induction and Memory in Polyallene toward Circularly Polarized Luminescence, Helicity Discrimination, and Enantiomer Separation. Angew. Chem. Int. Ed. 2023, 62, e202217234. [Google Scholar] [CrossRef]
- Alizada, M.; Gul, A.; Oguz, M.; Kursunlu, A.N.; Yilmaz, M. Ion sensing of sister sensors based-on calix[4]arene in aqueous medium and their bioimaging applications. Dye. Pigment. 2021, 184, 108741. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Oguz, M.; Yilmaz, M. On/off rhodamine-bodipy-based fluorimetric/colorimetric sensor for detection of mercury (II) in half-aqueous medium. IEEE Sens. J. 2019, 19, 2009–2015. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Fan, M.; Zheng, X.; Guan, J.; Yin, M. Chirality of Perylene Diimides: Design strategies and applications. Angew. Chem. Int. Ed. 2022, 134, e202202532. [Google Scholar] [CrossRef]
- Kardos, M. Über einige Aceanthrenchinon-und 1.9-Anthracen-Derivate. Berichte Dtsch. Chem. Ges. 1913, 46, 2086–2091. [Google Scholar] [CrossRef]
- Schaack, C.; Evans, A.M.; Ng, F.; Steigerwald, M.L.; Nuckolls, C. High-Performance Organic Electronic Materials by Contorting Perylene Diimides. J. Am. Chem. Soc. 2021, 144, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, W.; Xu, L.; Zhu, Y. High Photocatalytic Oxygen Evolution via Strong Built-In Electric Field Induced by High Crystallinity of Perylene Imide Supramolecule. Adv. Mater. 2022, 34, 2102354. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Nowak-Król, A.; Nagler, O.; Kraus, F.; Zhu, N.; Zheng, N.; Müller, M.; Schmidt, D.; Xie, Z.; Würthner, F. Tetrahydroxy-perylene bisimide embedded in a zinc oxide thin film as an electron-transporting layer for high-performance non-fullerene organic solar cells. Angew. Chem. Int. Ed. 2019, 131, 13185–13189. [Google Scholar] [CrossRef]
- Yang, H.; Ma, S.; Zhao, B.; Deng, J. Brightening up Full-Color and White Circularly Polarized Luminescence through Chiral Induction and Circularly Polarized Light Excitation. ACS Appl. Mater. Interfaces 2023, 15, 13668–13677. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, W.; Wang, Z. Fulvalene-Embedded Perylene Diimide and Its Stable Radical Anion. Angew. Chem. Int. Ed. 2020, 59, 752–757. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Z.; Wang, Z.; Jiang, W. Boosting Circularly Polarized Luminescence Performance by a Double π-Helix and Heteroannulation. J. Am. Chem. Soc. 2022, 144, 11397–11404. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Fox, D.J. Gaussian 16, Revision A 3; Gaussian. Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Density functional theory with London dispersion corrections. Wires Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
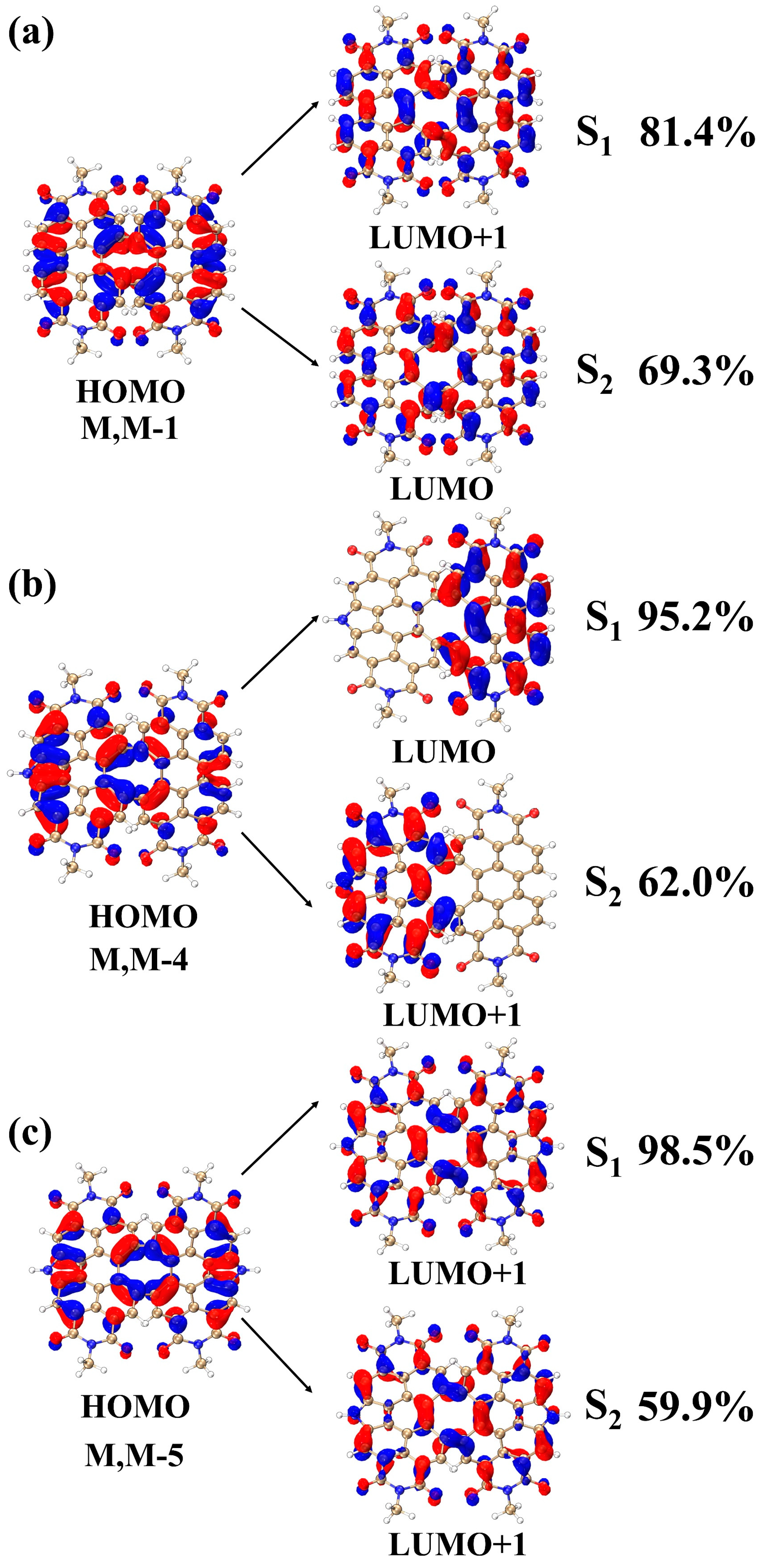
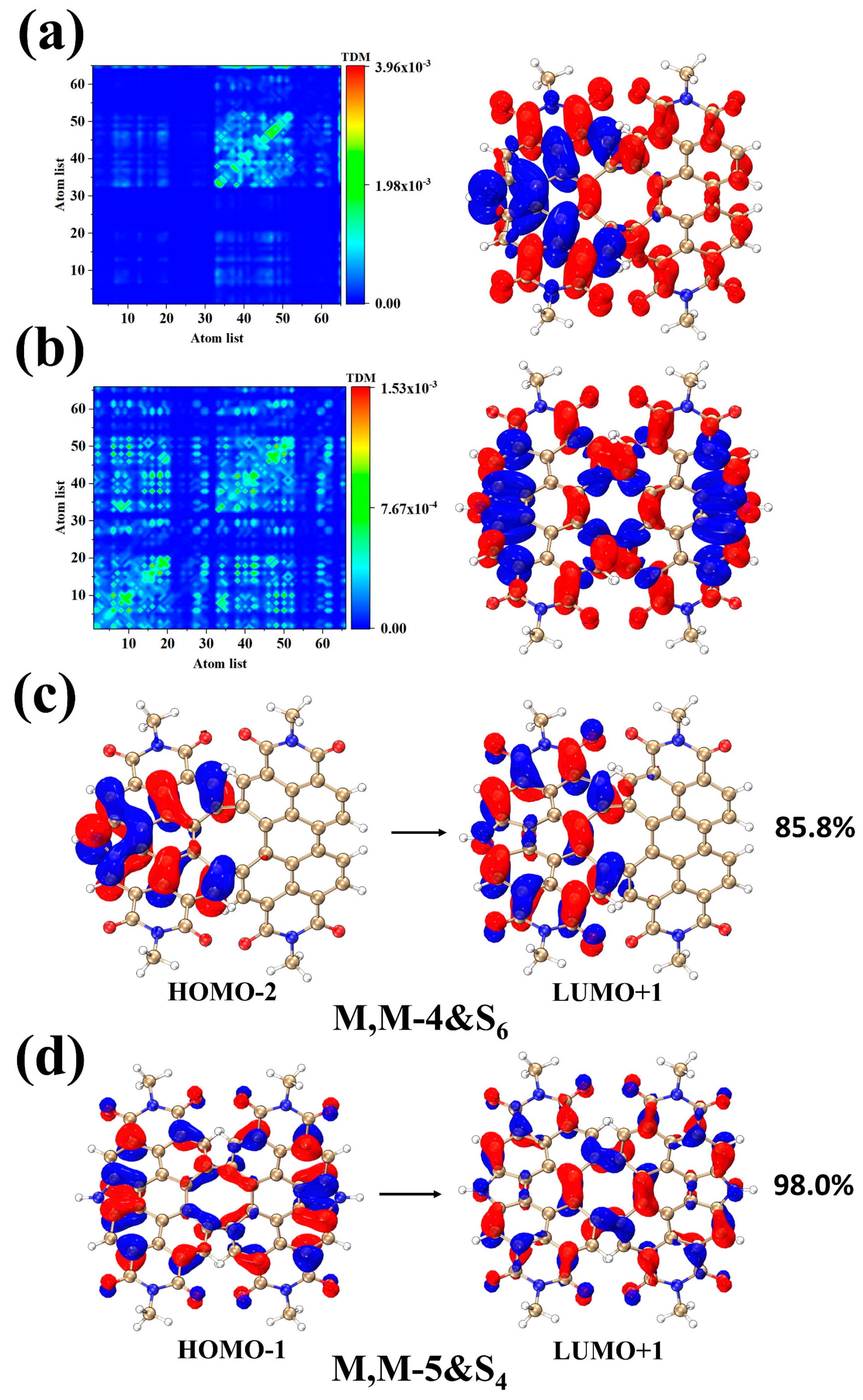
| Excited States | MOs | Contribution Ratio | |
|---|---|---|---|
| M, M-1 | S1 | HOMO→LUMO+1 | 81.4% |
| HOMO−1→LUMO | 17.5% | ||
| S2 | HOMO→LUMO | 69.3% | |
| HOMO−1→LUMO+1 | 29.6% | ||
| M, M-4 | S1 | HOMO→LUMO | 95.2% |
| HOMO→LUMO+1 | 1.88% | ||
| HOMO−1→LUMO | 1.67% | ||
| S2 | HOMO→LUMO+1 | 62.0% | |
| HOMO−1→LUMO | 31.3% | ||
| HOMO−1→LUMO+1 | 5.2% | ||
| M, M-5 | S1 | HOMO→LUMO+1 | 98.5% |
| S2 | HOMO→LUMO+1 | 59.9% | |
| HOMO−1→LUMO | 38.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Li, N.; Ma, J.; Wang, J. Study on Asymmetric Vibrational Coherent Magnetic Transitions and Origin of Fluorescence in Symmetric Structures. Molecules 2023, 28, 6645. https://doi.org/10.3390/molecules28186645
Sun L, Li N, Ma J, Wang J. Study on Asymmetric Vibrational Coherent Magnetic Transitions and Origin of Fluorescence in Symmetric Structures. Molecules. 2023; 28(18):6645. https://doi.org/10.3390/molecules28186645
Chicago/Turabian StyleSun, Lulu, Ning Li, Ji Ma, and Jingang Wang. 2023. "Study on Asymmetric Vibrational Coherent Magnetic Transitions and Origin of Fluorescence in Symmetric Structures" Molecules 28, no. 18: 6645. https://doi.org/10.3390/molecules28186645
APA StyleSun, L., Li, N., Ma, J., & Wang, J. (2023). Study on Asymmetric Vibrational Coherent Magnetic Transitions and Origin of Fluorescence in Symmetric Structures. Molecules, 28(18), 6645. https://doi.org/10.3390/molecules28186645







