Abstract
Tannins are polyphenols characterized by different molecular weights that plants are able to synthetize during their secondary metabolism. Macromolecules (proteins, structural carbohydrates and starch) can link tannins and their digestion can decrease. Tannins can be classified into two groups: hydrolysable tannins and condensed tannins. Tannins are polyphenols, which can directly or indirectly affect intake and digestion. Their ability to bind molecules and form complexes depends on the structure of polyphenols and on the macromolecule involved. Tannins have long been known to be an “anti-nutritional agent” in monogastric and poultry animals. Using good tannins’ proper application protocols helped the researchers observe positive effects on the intestinal microbial ecosystem, gut health, and animal production. Plant tannins are used as an alternative to in-feed antibiotics, and many factors have been described by researchers which contribute to the variability in their efficiencies. The objective of this study was to review the literature about tannins, their effects and use in ruminant nutrition.
1. Introduction
Tannins are polyphenolic compounds and the secondary metabolites of higher plants [1,2]. They are found naturally in the structures of plants and their molecular weight can vary between 300 and 5000 daltons. Higher-plant secondary metabolites have a significant polyphenolic structure which does not justify the specifications for placement in the tannin classification. Plant tannins have a phenolic effect (e.g., blue color, iron (III) chloride) and as a result of water-soluble polyphenols with molarities ranging from 300 to 3000, catalyzing alkaloids, gelatins, and other proteins [3]. Tannins are a diverse group of secondary plant metabolites that can be dissolved in polar solutions and are differentiated from polyphenols by their ability to dissolve in polarized solutions [4]. These compounds appear to have no function in plant metabolisms such as biosynthesis and energy exchange. Still, they are responsible for various biological activities. Tannins can prevent ruminal nitrogen metabolism by decreasing proteolytic bacteria [5,6]. Moreover, they can reduce the ruminal bio-hydrogenation process and increase the flow of unsaturated fatty acids to the duodenum [7]. Additionally, phenolic compounds and tannins, through selective activity on ruminal bacteria, can alter the process of ruminal bio-hydrogenation and increase the amount of conjugated linoleic acid in meat and milk production [8], but this effect is dosage-dependent. As a result, tannins improve the efficiency of nitrogen use by retaining more nitrogen in the body [6,9,10].
Ruminants, both intended for meat and milk production, are of significant economic interest in the world, with different production systems. Moreover, their production has been based on tannin-rich feed (Table 1) since ancient times, and actually, whether an extensive or intensive technique is applied, forages rich in tannins (Table 2) or rations with the addition of vegetable co-products rich in tannins (Table 3) are used in order to obtain positive effects on production, quality and animal welfare [11]. As it is clear, the potential sources of tannins for ruminants are plentiful.

Table 1.
Potential tannins’ source for animal feeding reported with their scientific name, common name, part of the plant richest in tannins and tannins’ chemical profile. Traditional and ancient vegetable tannins source used for ruminants [12,13,14,15].

Table 2.
Potential tannins’ source for animal feeding reported with their scientific name, common name, part of the plant richest in tannins and tannins’ chemical profile. Fodder and plants tannins source used for ruminants [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50].

Table 3.
Potential tannins’ source for animal feeding reported with their scientific name, common name, part of the plant richest in tannins and tannins’ chemical profile. Vegetal industry co-products tannins source used for ruminants [49,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72].
Several factors can modulate tannins effect as negative, positive or indifferent. This can be due to their different chemical composition or type, the administered quantity, the animal species involved and the diet to which tannins are added [73]. For these reasons, there are many aspects that could have affected the apparently inconsistent findings that can be found in literature. They are characterized by great chemical structural diversity, with results that cannot be easily extrapolated from one type of tannin to others [74].
Tannins may have important effects, both adverse and beneficial, on animal performance and on their production quality, due to chemical structure but also to their content in the diet; both aspects are intrinsically linked to animal physiology [75,76]. Some adverse effects may be listed as a reduction of feed intake, fiber digestibility and consequently animal performance [77], but some other researchers showed how tannins may enhance protein utilization, control internal parasites and act also on production (growth performance, milk, meat, wool) [75,78] and animal welfare [79,80]. Moreover, it has been shown that they may play an important role in improving antioxidant and immunity status of animals [57,81].
Recent studies have seen an increasing interest in this topic, minding that they can be used in nutritional strategies, but limits on their practical application are linked to potential adverse effects.
With a focus on ruminants, the main objective of this paper is to review the current knowledge about dietary tannins use and effects on animal production and performance (both milk and meat). Of course, tannins’ chemistry and their chemical properties will be discussed.
2. Classification of the Tannins
The tannins have several structures that can provide a systematic classification system with their properties and chemical structural basis for further analysis [82,83]. Many tannins can be hydrolyzed, and these are classified as “hydrolyzable tannins” (HT). Polymeric proanthocyanidins and non-hydrolyzable oligomeric were classified as condensed tannins [84,85]. The ‘HT’ included both the gallotannins and the ellagitannins [86,87]. Due to a different C–C interaction for polyphenolic waste and polyol, ellagitannins may not be hydrolyzed but classified as HT for specific purposes. In addition to hexahydroxydiphenoyl (HHDP), the first tannins were described in 1985 as having the C-glycosidic catechin units (the structural and functional component of isomeric HT). Because the only component of the C–C relationship between their catechin and glycosidic entities is partially hydrolyzable, these tannins were previously categorized as “non-classified.” The terms ‘complex tannins’ and flavanoellagitannins have been established over the following years to properly merge these ‘non-classified tannins’ [88,89]. Hydrolyzable and non-hydrolyzable or condensed tannins are the two types of tannins that can be found. However, they are not structurally diverse [90,91]. Tannins have catechin units flavanotannins or condensed flavonoids that characterize the tannins’ [92,93]. Paired flavan-3-ol (catechin) units are used in condensed tannins—polymer flavanotannins (oligomeric and polymeric proanthocyanidins). On a structural level, tannins are split into four categories: gallotannins, ellagitannins, complicated tannins, and condensed tannins (Figure 1) [94,95].
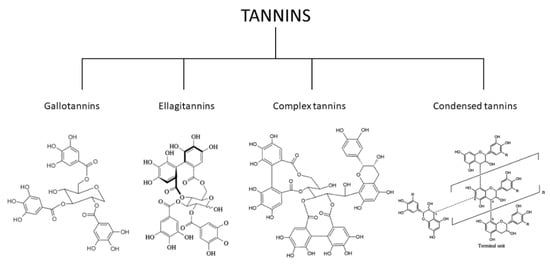
Figure 1.
Classification of the tannins [96,97].
- (1)
- Gallotannins tannins are derivatives of diverse polyol, catechin, or triterpenoid units of galloyl or their meta-depends [98].
- (2)
- Ellagitannins tannins are two C-C galloyls and have no glycosidically-related catechin unit attached to one another [99].
- (3)
- Complex tannins are bound glycosidically by a catechin component of gallotannine or ellagitannin [100].
- (4)
- Condensed tannins are all proanthocyanidins oligomeric and polymeric formed by the similarity between C-4 and one C-8 or C-6 of one catechin and the next catechin monomeric [101].
3. Chemical Properties of Tannins
Tannins are polyphenolic compounds also known as tannic acids. It is the name given to amorphous (amorphous) substances in the form of a light yellow-brown powder, flake or spongy mass obtained from plants. Tannins are solid compounds in phenol structure and are soluble in water. They are usually found in the roots, wood, bark, leaves and fruits of plants [102,103].
Tannins are chemically divided into hydrolyzable tannins (HT) and condensed tannins (CT). The HT yield water-soluble compounds such as gallic acid, pyrocatechic acid and sugar as hydrolysis in the presence of an acid or enzyme. It is slightly soluble in water, well soluble in alcohol and acetone. One of the best-known examples of HT is gallotanins. The CT, which are a much larger group, cannot be hydrolyzed. They form dark red insoluble compounds called flobafen with strong acids or some oxidizing agents against heat [102].
Hydrolyzable tannins contain hydroxyl groups esterified with carbohydrate (usually D glucose) and phenolic groups in the center. They decompose into carbohydrates and phenolic acids as a result of hydrolysis by weak acids, weak bases, hot water or enzymes such as tannase [104]. They are usually found in fruit seeds, in low amounts. They are degraded by the enzymatic functions of the ruminal microflora and by gastric digestion and turn into low molecular weight, absorbable toxic metabolites. As a result of degradation, gallic acid, pyrogallol, phloroglucinol and finally acetate and butyrate are formed as a result of successive enzyme activities [104,105].
Proanthocyanidins, also known as condensed tannins due to their chemical structure, are the most common tannin group found in trees and shrubs used as fodder plants [103]. These do not carry carbohydrates in the core; they are oligomers or polymers of carbon-carbon bonded flavonoid units (e.g., flavan-3-ol) resistant to degradation by hydrolysis [105]. Depending on the chemical structure and degree of polymerization of proanthocyanidins, their solubility in aqueous organic solvents varies. The term proanthocyanidin derives from the acid that catalyzes the oxidation reaction that results in the formation of red anthocyanidins by heating proanthocyanidins in acidic alcohol solutions. The most well-known anthocyanidins produced are cyanidin and delphinidin. Catechic tannins, which are not hydrolyzed by the action of enzymes or dilute acids, are the condensation product of catechin and turn into pyrocatechol by dry distillation [106].
The high affinity of protein tannins depends on how many phenolic compounds bind many places with carbonyl peptide groups [107,108,109].
The formation of these complexes is specific to the level of affinity between the participating molecules, which are based on each tannin’s chemical characteristics and the protein concerned [110,111,112]. For tannins, their relatively high molecular weight and chemical structure flexibility are some of the factors that support complexes’ development [113,114,115]. In addition, it was reported that the addition of condensed tannin (CT) extract to a forage-based diet can contribute to the decrease in CH4 emission by ruminants [10,82,116,117]. However, many types of condensed tannin with different biological activities, such as CH4 emissions, may impact animal responses [115]. The importance of the condensed tannin source and the dietary level must be understood for a better understanding of CH4 emission by ruminants and for the implementation of effective feeder methods that take benefit of such implications.
The small-statured and hydrophobic proteins have an open, flexible structure and are rich in proline [118,119,120]. Generally, tannins and proteins have very unstable bonds, which break and re-form continually. Kumar and Singh [121] showed that the formation of these structures might well involve four types of bonds.
The hydrogen linkages (reversible and pH-dependent) between phenolic hydroxyl radicals and amide oxygen in peptide-protein bonds are the same. The aromatic ring of the phenols interacts hydrophobically with hydrophobic regions of the protein (reversible and dependent on pH). By forming ionic (reversible) bonds between the phenolic ion and the cationic region of the protein (exclusive to HT). Covalent (irreversible) connection to quinones with oxidation and subsequent condensation of polyphenols with nucleophile protein groups. Hydrogen bonds were involved in forming tannin protein complexes for a long period, but later research demonstrated the significance of hydrophobic interactions.
4. Effect of Tannins on Ruminant Nutrition
It is important to monitor the feed consumption, the structure and molecular weight of the compound, and the physiology of consuming species to understand the beneficial and damaging impact of tannins on ruminants [116,122]. Distinct analytical methods, especially different standards, may yield different results (e.g., Equitrac, tannic acid, catechin, cyanidin, delphinidin, or the plant itself internal standards, etc.) [123]. Tannins are abundant in forages, trees, shrubs, and legumes often ingested by ruminants, particularly condensed tannins. As a result, the effects of condensed tannin on ruminant nutrition, health, and productivity have been extensively researched [73,115,124]. The beneficial or adverse effects of condensed tannins, particularly the concentration of crude protein (CP) in the diet, on ruminants are based on their amount, type, chemical structure, and feed composition [114] (Figure 2).
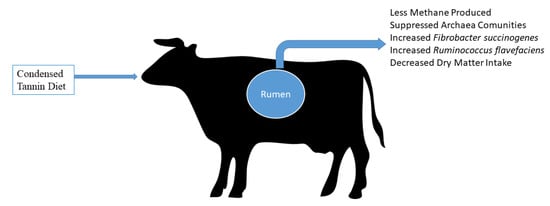
Figure 2.
Main effects of dietary inclusion of condensed tannins.
Frothy bloat is a common gastrointestinal disease in ruminants, which is caused by the accumulation of gas in the rumen and reticulum, causes damage to the function of the gastrointestinal tract and respiratory tract, which can be reduced by using tannins [125]. Li et al. [126] reported that a bloat of 1.0 mg CT/g of dry matter (DM) efficiently prevents pasture. Alfalfa pasture bloat can be controlled by incorporating condensed tannin-containing fodder, such as sainfoin, into the crop. Tannins have also been reported to help ruminants control parasites in their digestive tracts [127]. Hoste et al. [128] have reported in vivo studies on significant anthemia effects in the gastrointestinal tract of sheep, goats and deer on tannins in sainfoin, sulla, Acacia nilotica, Sericea lespedeza, L. pedunculatus, and chicory.
4.1. Effect of Tannins on Voluntary Feed Intake
The researchers hypothesized that tannin ingestion would limit ruminant voluntary feed intake. As a result, researchers now have a lot more information and can make more accurate conclusions about tannins, their dosages, and their effects on the animals which consume them [129,130].
The voluntary feed intake of ruminants by providing plants with high condensed tannin (CT) content (usually >50 g/kg DM) can be reduced, with a moderate or low intake (<50g/kg DM) not affecting it [131]. On this side, Cabral Filho et al. [132] observed no effect on DM consumption of sheep.
Voluntary feed consumption in ruminants depends on the taste and degree of digestion of the feed. With the consumption of tannin-containing feeds, tannin forms a compound with the glycoprotein found in the saliva. A high amount of tannin reduces the flavor and consumption of the feed. Therefore, it causes a decrease in productivity in animals. Some animals can adapt to diets containing high amounts of tannin by increasing the quantity of proline-rich proteins in the saliva. These proline-rich proteins in saliva form bonds with tannin. Thus, it prevents tannin from making compounds with proteins in the diet [133]. Barry and McNabb [134] reported a detrimental effect on voluntary feeding in grazing sheep given Lotus pedunculatus (condensed tannin content > 50 g/kg DM), but no findings in L. corniculatus (condensed tannin content 34–44 g/kg DM)
The consumption of tannin-containing feeds for a long time causes enlargement of the salivary gland [135]. Not all animals can secrete proline-rich proteins in saliva. For example, animals such as humans, mice, deer, and gazelles secrete proline-rich protein in their saliva. However, this secretion is much less in sheep and cattle [136]. It has been reported by several researchers that feed consumption decreased in sheep fed diets containing high amounts of tannin. Waghorn, Shelton, McNabb and McCutcheon [131] reported that feed consumption decreased in sheep fed with feed containing 55 g of tannin per kilogram. It has been reported that the amount of tannin below this level has little or no effect on the feed consumption of the animal.
The effect of condensed tannin varies according to the ruminant species. They do not contain proline-rich protein in the saliva of domesticated sheep and cattle [136]. For this reason, it can be said that domesticated sheep and cattle may be more affected by tannin.
Hydrolyzable tannins have also had variable effects depending upon the consumed quantity. McSweeney et al. [137] observed that Terminalia oblongata having low HT (34 g kg-1 DM), when supplemented to sheep, shows no significant reduction in voluntary feed intake (34 g kg−1 DM) while Clidemia hirta, a shrub which is high with HT (>50 g kg−1 DM) shows a reduction in voluntary feed action in the same animal. According to Frutos et al. [138], voluntary food intake of soya bean treated sheep with HT (20.8 g HT/kg DM diet) did not decrease. However, in an experiment with sheep fed 8 g Tannic Acid per kg live weight, voluntary feed consumption fell considerably within 24 h (from 18 to 2.5 g DM/kg LW).
Feed palatability, sluggish digesting, and enhanced conditioned aversions are three recommendations for clarifying the harmful effects of excessive tannin concentrations on voluntary feed intake [138].
The reaction between salivary tannins and mucoproteins and the direct response to taste receptors can reduce the taste, an astringent sensation [121]. The saliva of many herbivore species consists of proline-rich tannins and proteins that are plant species components and can bind with tannins [139]. The tannin-proline-rich protein complexes, which are stable over the whole pH range of the digestive system, are responsible for building stable complexes other than protein-tannins, which have a negative influence on taste and feeding and promote digestion of tannin-rich diets [140].
Herbivores are thought to have evolved distinct adaptation mechanisms for tannin-rich plants through time [140,141]. All the time animals secrete proline-rich proteins, but sheep only produce them when they consume tannin-rich plants [136]. However, in cows, tannin consumption did not result in increased synthesis of these proteins, despite the presence of many proteins with high partiality in their saliva [142].
Narjisse, Elhonsali and Olsen [140] directly transplanted tannins into the rumen to test if non-rational causes were to blame for the voluntary feeding intake reduction. The digestive system’s discharge is harmed when dry matter digestion is slowed. This sends out signals that the animal is “overflowing” and gives the nerve center feedback. Researchers have determined that this might impact voluntary feed intake in ways other than diminished palatability [131].
Thirdly, the detrimental post-prandial effects of tannin consumption and the establishment of conditioned aversions are found [143]. Rumen microbiota plays a fundamental role in ruminant nutrition, and factors related to microbial fermentation are assumed to mediate the post-prandial consequences of ingestion of tannin-rich feeder [138].
4.2. Effect of Tannins on Digestibility of the Diet
There are many articles on tannins’ ability to reduce food digestibility. Tannins mainly affect proteins (Figure 3). Tannins were also found to make slight changes to different degrees in other components [121]. The effect of tannins on protein is based on hydrogen bonds that are stable between pH 3.5 and 8. Ruminal pH changes affect the complexes and provide information about the function of tannins in the gastrointestinal tract [103]. Cabral Filho et al. [132] investigated three levels of tannin in the diet of sheep and reported a different DM digestibility between high- and low-tannin cultivars. The low tannin content diet had a higher crude protein digestibility, and there were no significant differences between the medium tannin content and high tannin content diets. The highest values for low tannin content and high tannin content were found for the neutral detergent fiber (NDF) digestibility, and the average medium tannin content diet was 582 g/kg, which differed only a little from the low tannin content diet.
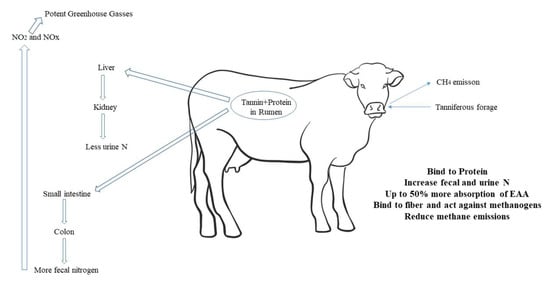
Figure 3.
Main effect of tannins binding proteins in rumen.
Tannin ingestion modifies digestibility by the correlation of the ruminal fermentation pattern. These effects are addressed in two sub-sections, but the repeated conclusion is that the higher dietary tenor of tannin reduces food digestibility by increasing fecal nitrogen excretion. In any case, increased release of endogenous proteins such as salivary glycoproteins, body fluids, and gastric catalysts, and increased descaling of intestinal cells, are detrimental consequences of tannin consumption [131]. This rise in fecal nitrogen might be due to an increase in metabolic fecal nitrogen, i.e., endogenous nitrogen that does not account for a decrease in the amount of protein ingested from the feed.
According to Besharati and Taghizadeh [144], increased dried grape by-products in diets shows a negative effect on CP digestibility. It was assumed that tannins could influence feed protein and rumen microbiota; the accessibility of rumen microbiota to such proteins has decreased because tannins bind with protein. The same authors [144] analyzed four diets, one as a basal diet (alfalfa) and the remainder as mixed diets (dried grape by-product with alfalfa). Table 4 shows the digestibility of the chemical components of the in vivo study. Increased products of dried grape in diets lead to lower digestibility, but no difference was found. Incremental grapes substituted by alfalfa levels resulted in the digestibility of DM by quadratic and cubic, but no linear effect was observed. Dry grape by-product supplementation has no impact on organic matter (OM) digestibility in experimental diets. An increase in dried grape by-products was associated with no significant response, and a quadratic response was found. In addition, the digestibility of CP was affected by dried grape by-product in basal diets, as well as an increase in the levels of supplementation of dried grapes by-product in CP digestibility in diet. There was no indication of a quadratic or cubic impact. In treatments, the treated and control group four had the greatest and lowest digestible CP, respectively. The addition of dried grape by-products to treatments reduces the digestibility of NDF and acid detergent fiber (ADF) with increased dried grape by-products to foods. A linear increase in the amount of additional dried by-products affected the digestibility of ADF and NDF in diets.

Table 4.
Statistical significance of perceived nutritional digestibility and ruminal states in sheep given dry grape by-products-based diets [144].
McSweeney et al. [137] investigated the effects of increasing condensed tannin in the diet in sheep, finding that increasing condensed tannin in the diet (from 6 to 65 g/kg DM) lowered N digestibility from 0.805 to 0.378 and raised excretory N in sheep feces from 4.3 to 9.7 g/d. Hagerman et al. [103] showed that tannins could reduce CP digestibility. The significant drop in N digestibility was reported to be due to the tannin presence, and the results were similar to those in Lotus pedunculatus-fed sheep and the ryegrass in Lotus pedunculatus with or without polyethanol glycol (PEG) [131].
If the drop in N digestibility was attributable to a decrease in protein digestion, the decreased protein digestibility might account for the overall reduction in tannin-related DM digestibility [144].
Yisehak et al. [145] demonstrated that PEG addition in the diet as a tannin binder enhanced digestion and performance in both species but with the largest effect size in sheep. Corrêa et al. [146] showed that tannins are useful to reduce intakes of feed but to increase daily feed time linearly, even though the number of meals did not change. Tannins also decreased DM, CP, OM, urinary urea, and TDN digestibility linearly, while reducing ADF and NDF was quadratic. Tannins reduced rumen loss by slowing transit and digesting rates in a linear fashion. They concluded that tannins might have a significant effect in lowering nitrogen excretion in urine.
4.3. Effect of Tannins on Ruminal Microbiome and Fermentation
The rumen microbiome had great importance, because through rumination, fibrous and non-fibrous plant material can be transformed into valuable products such as meat and milk, thanks to the rumen microbiome activity [147]. Rumen inhabits various microbes like bacteria, protozoa, fungi, archaea, and bacteriophages [148]. The rumen microbiome and host had symbiotic relationships, each one provide substances to the other: first of all, mastication and rumination are processes that permit to expand feed surface area to microbial attachment, mixing constantly digesta and yielding fermentation products as volatile fatty acids compounds (VFA); moreover, rumen permits the elimination of useful and toxic substances maintaining the best condition for microbial growth and activity, returning urea for microbial development (Figure 4) [147].
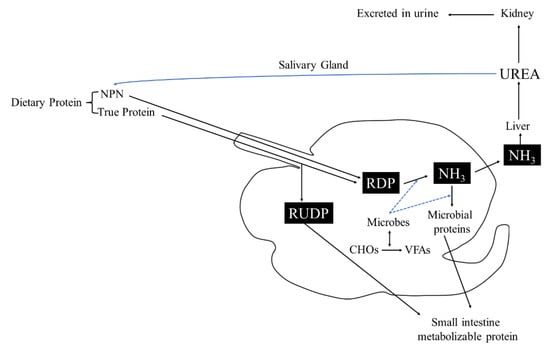
Figure 4.
Urea cycle in rumen. RUDP: rumen undegradable protein; RDP: rumen degradable protein.
Rumen microbiome is mainly made up of bacteria, with a population that exceeds one thousand cells per gram of rumen content and includes over 200 species [148]. Their main role is the metabolism of carbohydrates and nitrogen taken with the diet. Methane emission is mainly dependent on H2-producing bacteria [149].
Fungi represents a range of 5 to 20% of the total ruminal microbiota [150,151]. The anaerobic ones play key role in lignocellulosic fiber breakdown [152], considered one of the most able fiber-degrading agents [153], they produces a lot of enzymes (cellulases, xylanases, mannanases, esterases, glucosidases, and glucanases) that are important for plant polymers breakdown; but they are also characterized by amylolytic [154] and proteolytic activities [155].
Ruminal protozoa, commonly divided in flagellates and ciliates, represent about 20–50% of the total rumen microbiome [156]. Instead, that of ciliates is particularly important because it engulfs carbohydrates and do not allow abnormal rumen fermentations which could alter the pH and make the rumen environment uncomfortable for the microbiota [157]. They have a positive correlation with CH4 production because they are involved in H2 production that is subsequently converted to CH4 by the methanogens through the hydrogenotrophic pathway [158], so their defaunation is able to reduce CH4 emission [157,159]. Archea, the third rumen domain, constitutes about the 20% of rumen microbiome [160]. They are involved in methanogenesis [160,161], starting by different substrates as formate, or acetate, methanol, H2, methylamines, and CO2 (71).
In a large pH range (3.5-7), tannins can bind dietary proteins, starch and sugars creating strong complexes [162], and this can limit their digestibility if concentration is more than 5% [163]. Although many studies have been conducted, the real action of tannins in rumen is not yet clear [116]. In fact, although they possess bacteriostatic effects, their association with rumen microbiota is different due to different tannins nature. In fact, hydrolysable tannins resulted more hydrolysable by microbiome than condensed ones [137]. Tannins can inhibit methanogens activity directly, limiting the degree of microbial hydrolysis, and indirectly decreasing the H2 availability [164], although this can reduce fiber digestion. Their ability to modify the ruminal microbiome can result in a reduction in protein degradation, can reduce methanogenesis and inhibit the fatty acids biohydrogenation [165,166,167] (Figure 5).
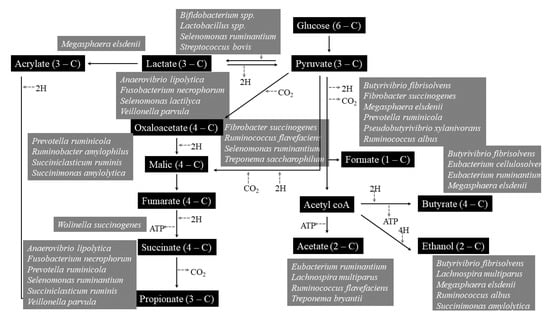
Figure 5.
Main microbes involved in rumen fermentation processes.
As expected, results about potential shifting of microbial population and rumen fermentation are variable depending on species, doses, and tannins source. Researchers reported different results about methane mitigation. Some studies reported that tannins can reduce the short-chain fatty acids and acetate production, increasing the diversity and abundance of butyrate-producing bacteria and other beneficial species (such as probiotic species), and enhancing the amino acid metabolic pathways [146]. Although these exhibit different effects, the mechanism action is not known. Tannin supplementations seems to reduce the crude protein digestion and methane production in beef [168], although other authors reported no effect on live and growth performance of beef, no effect on in vitro digestion traits, and no reduction in CH4 emission [57]. Different tannins sources have been used with the aim to mitigate methane emission without affecting, but improving, productive performance. For example, gallic acid has the potential to reduce the rumen greenhouses production without affecting performances [169]. The same results were found for tara, mimosa, gambier extracts [170] and legumes with condensed tannins [171]. However, dietary sources of tannins and their chemical composition can affect quiet differently rumen microbiota, in fact HT are considered more suitable than CT for methane mitigation [164]. The encapsulation can be considered a good solution for tannin extract due to the slow release of tannin and their more efficient utilization [172,173]. However, CT rich diets can reduce CH4 emissions in ruminants, with an effective inhibitory effect on ruminal protein degradation and CH4 emission, but this requires careful source selection for diet inclusion to avoid adverse effects on feed digestibility and efficiency [174]. Nevertheless, many other studies are necessary in order to fully understand the mechanism of action of tannins at the rumen level regarding the potential inhibitory effect of methanogens and protozoa, and above all to determine the best inclusion level to inhibit the production of methane without compromising the productions. The tannins are characterized by a powerful antioxidant effect as they can scavenge free radicals due to hydroxyl groups, degree of polymerization, and redox activities [175,176]. In fact, tannin supplementation improves ruminant’s antioxidant state [57,177,178,179]. Among the chemical characteristics, hydrolysable tannins are considered the most potent antioxidants [180].
Tannins are the main source for the degradation of the ruminal protein [103]. Tannins are intimately connected to these molecules, and the pH of the ruminal media encourages tannin protein complex synthesis. Protein degradation can be reduced as a result of lower ammonia nitrogen synthesis and more non-ammonia flow to the duodenums [131].
Tannins influence proteins and carbohydrates, particularly hemicelluloses, celluloses, starch, and pectins [181]. The tannins have long been a secondary anti-nutritional effect on fiber degradation [137,182].
The methods for minimizing ruminal damage to various dietary tannin components are unknown. The most widely established procedures are denial of nutrients, enzyme inhibition, and direct action on rumen bacteria [183,184]. Regarding the first of these, various authors have indicated that attachment of rumen microbes to plant cell walls is necessary for the breakdown process, but the presence of tannins in plant material prevents the plant material from adhering to rumen microorganisms [185,186]. Furthermore, complexes of proteins and carbohydrates decrease the effectiveness with which these nutrients may reach these microbes [187]. Tannins also limit the availability of several metal ions that are required for rumen microbial metabolism [184].
Tannins also inhibit the enzymatic activity of rumen microbes [137,186]. But the exact mechanism of enzyme inhibition activity of tannins is still poorly understood. Polyphenolics have been found to react to the cell wall of bacteria and to secrete extracellular enzymes. Any interaction will probably inhibit the transport of nutrients into the cell and delay the organism’s growth. Although the exact mechanism of how tannins bind to enzymes, whether bacterial or endogenous, does not indicate inhibition, some writers have reported that tannins modify bacterial proteolytic, cellulolytic, and other enzyme activity [142,188]. When fibrolytic enzymes were investigated, condensed tannin was more effective in inhibiting hemicellulases than cellulases [143]. This is since they relate to bacterial cell walls, whereas hemicellulases are extracellular and more susceptible [135]. This explains why most investigators have reported a greater decrease in hemicellulose degradability in tannin presence [131,182]. However, this can vary depending on the tannin desired [186].
Besharati and Taghizadeh [144] indicated tannin contents in the plant material reduce fermentation, resulting in major differences among VFA and NH3–N concentrations. Priolo et al. [189] reported that when PEG and tannin-feed was given to sheep, rapid rumen fermentation was observed by PEG, resulting in significant differences among VFA and NH3–N concentrations.
CT/bacterial cell reactions are less well-conceived than CT/feed protein reactions. However, the interactions between condensed tannin and the substrate appear to be different between bacterial cells and Rubisco. The CT-bacterial interaction was found much stronger than CT-protein interaction, or the interaction is of a different kind [190]. The condensed tannin most likely connects the bacterial cell surface to limit the action of cell-bound enzymes. The inhibition scope may vary with different condensed tannin types. However, the fact that we cannot quantify actual condensed tannin contents of the CT-bacterial interactions requires more research since digesta affects our knowledge of physical and chemical relationships between CT, rumen microbes, and plant protein [191].
Cabral Filho et al. [132] observed no significant differences between the treatment groups with diets containing three different concentrations of tannin. Aboagye, Oba, Castillo, Koenig, Iwaasa and Beauchemin [10] have shown that the combination of HT and condensed tannin at 1.5% dietary DM also tended to reduce CH4 emissions without adverse effects on performance. Sadarman et al. [192] found that adding tannin to both acacia and chestnuts had no significant influence on DM, OM degradation, total volatile fatty acids (tVFA), NH3, and pH. The accumulation of gas at 8, 12, and 24 h was influenced by acacia tannin. They concluded that adding tannins up to levels 2%, acacia, and chestnuts did not interfere with the fermentation of rumen and in vitro by-products of soy sauce. Toral et al. [193] observed that nutrition tannins boosted milk content by numerous FA ramification isomers of 18/1 and 18/2, but that they were not effective in changing milk FA composition in the long run, especially when combined with quebracho tannins in a linoleic acid-rich diet (Figure 6).
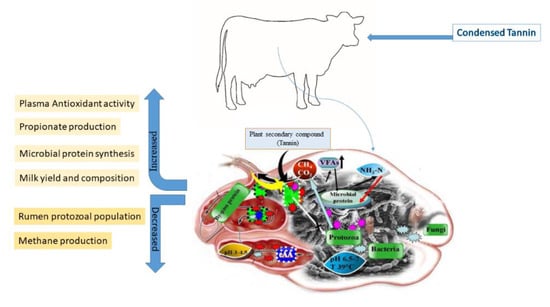
Figure 6.
Main positive effect of plant secondary compounds assumed by diet.
4.4. Effect of Tannins on Milk Production and Composition
While the daily milk yield was 16.5 kg in dairy cows fed L. corniculatus with a tannin content of 27 g/kg DM, a yield of 13.8 kg in animals fed L. corniculatus and polyethylen glycol (PEG) was found [194]. Dschaak, et al. [195] reported that cows fed a ration supplemented with condensed tannin extract (CTE) had a decrease in milk urea nitrogen and ruminal ammonia nitrogen concentrations, without any loss in milk protein yield. Dairy cows fed L. corniculatus produced milk 60 percent greater than perennial ryegrass-fed cows, with a 10 percent increase in milk protein. PEG responses showed that the action of condensed tannin could explain half of the lotus effect [78].
Condensed tannin of lactating L. corniculatus ewes had little effect on early lactate milk production but enhanced whole milk, lactose, and protein secretion rates by 21, 12, and 14 percent, respectively, during mid to late lactation [143]. Studies assumed that the increase in milk production is due to condensed tannin content, while the content does not affect the increased voluntary feed intake (VFI). In these studies, it is clearly seen that tannin has a positive effect on milk production. However, more studies are needed to determine at what levels tannin has a positive effect in the diet. It has been accepted that the nutritional value of milk fat in ruminants can be improved by manipulating rumen biohydrogenation by changing diet-related factors. In recent years, most of the research for modulators of ruminal biohydrogenation focused to secondary plant compounds such as tannins. Both in vitro and in vivo study results show that both CT and HT can affect rumen biohydrogenation. Most of them, or their metabolites, can be found in milk and cheese [196]. However, these results are also controversial. Some in vitro studies have reported accumulation of VA and a decrease in stearic acid (18:0, SA) in rumen fluid and rumen bacteria after incubations with tannin sources such as CT or HT at the ruminal biohydrogenation stage [197,198,199,200].
Sulla is one of the most studied tannin sources in terms of its effect on the FA profile of ruminant meat and milk. Addis, et al. [201] reported that milk from sulla-fed sheep had higher levels of short-chain FA and LNA and higher levels of short-chain FA and LNA than milk from sheep fed Lolium rididum Gaudin, Medicago polymorpha L. and Chrysanthemum coronarium L., it was observed that it showed an atherogenicity index, but the 18:1 cis-9 content was lower. It was observed that dietary supplementation with quebracho (Schinopsis balansae) tannin extract (150 g/day, 0.45% DM intake) had no effect on milk FA composition of dairy cows [202]. Studies on the effect of tannins on the milk FA profile in ruminants show a wide variation in ration sources and inclusion levels of tannins, supplementation time, ration composition and physiological stages of the animal, resulting in controversial results. Although tannins are heterogeneous compounds, their structures and sizes are variable. Moreover, their metabolism and activity would be expected to depend on the tannin type. This helps to explain the controversial results regarding the ability of ruminant-derived products to modulate the FA profile.
4.5. Effect of Tannins on Meat Production and Composition
Most of the research reported that the intramuscular fat content was not affected by the dietary treatment explaining this lack of differences with the diet formulation. In fact, all experimental diets that compared a control group to an experimental group with addition of tannins had isonitrogenous and isoenergetic diets. Differently, the intramuscular fatty acid composition was clearly affected by the inclusion of tannins in the diet in most of them.
In ruminants, muscle fatty acids deposition depend both on the intake of different fatty acids and on the extent of the ruminal biohydrogenation of the ingested PUFA [203]. Fatty acids found in ruminant meat can be derived from two main ways, direct uptake of preformed fatty acids then transported in plasma and a de novo synthesis directly in body tissue [204].
The preformed fatty acids derived directly by digestive tract absorption, so they have a dietary or ruminal origin [205]. Differently, there is a low contribution, if present, of body fat mobilization [206]. Once in the rumen, fat is hydrolyzed by lipases and free fatty acids are released [207]. After this, biohydrogenation by rumen microorganism occurred to saturate fatty acids and reduce their toxicity (Figure 7) [205]
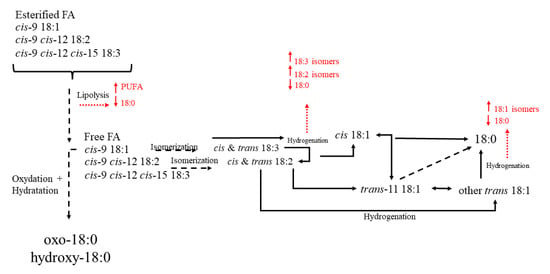
Figure 7.
Main biohydrogenation processes in the rumen.
However, it must be considered that the biohydrogenation process resulted incomplete and some intermediate metabolites can leave the rumen and be absorbed in the intestinal tract and then incorporated in meat, with relevant consequences for consumers [208]. This aspect is the most important for nutritionists because the possibility to modulate the biohydrogenation represents an opportunity to decrease the percentage of disappearance of the dietary PUFA Moreover, some rumen microorganisms have specific synthetases for fatty acids able to synthesize odd- and branched-chain fatty acids that we can find in meat [209].
The adipose tissue is a great active site of fatty acids de novo synthesis. The main substrates derived from carbohydrates rumen fermentation and are represented by acetate and lactate [204] and they give origin to the formation of fatty acids with up to 16 carbons, with the predominant production of palmitic acid (16:0) [210], that can be after desaturated or elongated [211]. The stearoyl-CoA desaturase represented the most active desaturase [212]. A great proportion of 16:0 is converted to stearic acid (18:0) [210]. The 18:0 can be than desaturated to cis-9 18:1 (oleic acid) [212] in palmitoleic acid (trans-9 16:1) and to cis-9 trans-11 CLA. [213]
It is evident that tannins act at rumen level modulating the lipid metabolism and affecting the meat fatty acid profile [198,214]. This could probably be due to changes that tannins can induce in microbial rumen population, although it is not well known which are really involved in which specific microorganism are involved in ruminal biohydrogenation of dietary PUFA [215,216]. Different studies have tried to examine tannins effect on fatty acids deposition in meat, but different results have been observed probably due to diversity of tannins, concentration, species, and time of diet assumption. However, is evident a reduction in the biohydrogenation extent of dietary PUFA, with most of the results reporting an increase in them. Most studies reported increases in 18:2n-6 and 18:3n-3 concentrations in meat [8,200]. However, low levels of tannin addition in diet can also lack in affecting dietary PUFA proportions in meat [217,218,219,220,221]. However, the mechanisms explaining the abovementioned effects remain uncertain, although it seems to be assumed that the rumen microbiota modulation represents the best way to mediate meat fatty acid profile [222].
Tannins can also modify the concentration of some biohydrogenation intermediates. In fact, tannins can inhibit the saturation of vaccenic acid [223], although the capacity to increase trans-11 18:1 and cis-9 trans-11 CLA concentration is still controversial. In some studies, vaccenic acid was increased with no effect on cis-9 trans-11 CLA [200,221], while other studies reported increased cis-9 trans-11 CLA with no effect on vaccenic acid [223,224].
It must be considered that lipid diet level result important to have magnification of tannins effect on biohydrogenation. With low lipid diet, the most used in experimental trials, there is poor biohydrogenation substrate that can put in evidence the tannins effect on the process. The effects of tannins on de novo synthesized fatty acids are not well studied; nevertheless it is reported that tannins can be able to mitigate the inhibition of de novo fatty acids synthesis in meat [225].
4.6. Effect of Tannins on Wool Production
Access to sulfur-containing amino acids has a very effective role in wool production. Clean wool is mostly protein with a high cysteine concentration. Studies demonstrated that activation of condensed tannin in L. pedunculatus and L. corniculatus enhanced the rate of irreversible cystine loss from blood plasma, owing to lower sulfur-containing amino acids breakdown in the rumen [226,227].
Wool growth responses to condensed tannin impact vary on both the concentration and type of CT, with L. corniculatus growth exceeding 10% in the 22-38 g CT/kg DM range. When the condensed tannin level rose over 50 g/kg DM, the reactions seemed to be negative effects, particularly for L. pedunculatus and sulla. However, when condensed tannin levels were below 22 g CT/kg DM, the response to wool growth varied. Therefore, positive CT-effects for wool production in L. corniculatus occur between 22 and 38 g CT/kg DM [78].
4.7. Effect of Tannins on Reproduction
One of the important factors affecting reproduction in sheep is nutrition. Ovulation rates in sheep grazing L. corniculatus was found to be 22% higher on average than those grazing meadow grass and clover. This probably was due to condensed tannin content of L. corniculatus, determined with PEG, that is able to increase the lambing rate due to the increased fertilized rate of oocytes [78].
Downing and Scaramuzzi [228] found that the ovulation rate (OR) in sheep is influenced by nutritional levels, although the mechanism(s) through which nutrition affects OR remain unclear. The OR depends on the animal nutrition that probably relates to the ovary’s metabolic, hormonal control [228]. Changes in the plasma concentrations of growth hormone, insulin, and insulin-like are compatible with changes in biosphere energy and protein balance and muscle protein synthesis produced by nutrition. These factors can directly or by modulating ovarian gonadotropin function affect ovarian function [229]. More studies have shown that increases in ovulation rate are associated with the branched-chain plasma amino acid (BCAA) and essential amino acids (EAA) levels [143]. At the same time, intravenous infusion branched-chain plasma amino acid was increased. These findings are supported by those of Al-Gubory et al. [230], who found that the BCAAT cytosolic enzyme may directly stimulate the ovaries, raising ovulation rate through an unknown mechanism, as a result, it reduces the growth of specific BCAAA activity in the skeletal muscles of sheep [229]. BCAA is also highly sensitive to insulin in muscle protein [231]. Furthermore, only the ovaries found BCAA transferase (BCAAT) iso-enzyme [232]. These findings are confirmed by the results from Al-Gubory, Garrel, Faure and Sugino [230], which show that the developmental reduction in specific BCAAA activity in the skeletal muscles of the sheep is due to the BCAAT cytosolic enzyme, which can directly stimulate the ovaries, increasing the ovulation rate by an unknown mechanism.
4.8. Effect of Tannin on Parasites in the Digestive System
Parasites in the digestive system of grazing animals are one of the factors that cause significant losses in ruminants. In animals contaminated with parasites, reductions in growth, milk yield, fleece yield and reproduction occur [233]. Anthelmintic drugs are generally used to control parasites in the digestive system. The presence of these drug residues in animal products makes consumers think [234]. Therefore, the inclusion of plant species that reduce the number and effects of parasites in the diet reduces the use of these drugs, e.g.; it is known that lantana camara (Sage tea) is an important herb in the control of parasites and nematodes in the digestive system. Eucalyptus species are reported to have anthelmintic effects in goats [235].
It has been reported that condensed tannins exert their antiparasitic effects by directly inhibiting the larval development of digestive tract parasites and indirectly by binding to proteins in the rumen and preventing microbial degradation. As a result, it is thought that they improve the immunity of the host animal by providing the passage of amino acids into the duodenum and increasing protein digestion. The consumption of rations containing tannin in lambs and sheep infected with parasites increased the live weight gain and caused a decrease in the number of parasite eggs laid with manure [78]. The number of eggs in the manure obtained from sheep consuming feeds containing condensed tannins decreased by approximately 20–50% [78]. Condensed tannin is thought to either act as an anthelmintic in these plants or cause a suitable environment for microorganisms. More studies are needed to determine the amount of tannin that should be present in the diet to take advantage of the negative effects of tannins on parasites.
Plants rich in condensed tannins with demonstrated activity against gastrointestinal nematodes in sheep and goats include Lespedeza cuneata (sericea lespedeza), Onobrychis viciifolia (sainfoin), Hedysarum coronarium (sulla), Lotus pedunculatus (big trefoil), and Lotus corniculatus (birdsfoot trefoil). These plants can be used to reduce fecundity of nematodes but did not offer a control of them [236].
5. Conclusions
Tannin is a phenolic compound that tends to form compounds with nutrients found in feeds. Tannin, which is found in various proportions in plants, has negative and positive effects on animals.
Condensed tannin and feed sources containing tannin can be added to the ration at certain rates in order to enable ruminants to make better use of proteins and to reduce losses caused by excessive fragmentation of proteins in the rumen. This ratio should not negatively affect microbial protein synthesis in the rumen. In addition, the amount of tannin added to the ration should be at a level that will not have a toxic effect on animals. The determination of the tannin content of the feeds to be added to the ration has an important place in determining the amount of tannin to be added. In order to reduce the negative effects of tannin on animals and rumen microorganisms, compounds such as PEG can be added to the diets containing high levels of tannin.
Regarding tannin type and concentration, its effect can differ in animal performance. In contrast, hydrolyzable tannin can efficiently increase bypass protein, resulting in high performance in ruminants such as milk yield and milk production. Additionally, tannin can be controlled by bloat problems and intestinal parasites in grazing ruminants. However, more access to information about the effects of tannin in animal nutrition requires further investigation.
Author Contributions
Conceptualization, M.B., A.M. and V.P.; methodology, A.K.; software, M.J. and H.E.; validation, A.M., J.M.L. and P.D.P.; investigation, M.B. and V.P.; resources, J.M.L.; data curation, M.B. and V.P.; writing—original draft preparation, A.M., M.B. and V.P.; writing—review and editing, A.M., P.D.P. and J.M.L.; visualization, A.M., P.D.P. and J.M.L.; supervision, A.M., P.D.P. and J.M.L.; funding acquisition, J.M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bocco, R.; Gandonou, C.; Gbaguidi, F.; Ahouansou, A. Phytochemical screening and quantitative variation of some secondary metabolites in five cultivated rice varieties. J. Appl. Biosci. 2017, 113, 11146–11157. [Google Scholar] [CrossRef]
- Herrera, R.; Verdecia, D.; Ramírez, J.; García, M.; Cruz, A.M. Secondary metabolites of Leucaena leucochephala. Their relationship with some climate elements, different expressions of digestibility and primary metabolites. Cuba. J. Agric. Sci. 2017, 51, 107–116. [Google Scholar]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. 1-Overview of polyphenols and their properties. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 3–44. [Google Scholar]
- Halvorson, J.J.; Schmidt, M.A.; Hagerman, A.E.; Gonzalez, J.M.; Liebig, M.A. Reduction of soluble nitrogen and mobilization of plant nutrients in soils from U.S northern Great Plains agroecosystems by phenolic compounds. Soil Biol. Biochem. 2016, 94, 211–221. [Google Scholar] [CrossRef]
- Arzola-Alavarez, C.; Castillo-Castillo, Y.; Anderson, R.; Hume, M.; Ruiz-Barrera, O.; Min, B.; Arzola-Rubio, A.; Beier, R.; Salinas-Chavira, J. Influence of Pine Bark Tannin on Bacterial Pathogens Growth and Nitrogen Compounds on Changes in Composted Poultry Litter. Braz. J. Poult. Sci. 2020, 22, 1–5. [Google Scholar] [CrossRef]
- Herremans, S.; Vanwindekens, F.; Decruyenaere, V.; Beckers, Y.; Froidmont, E. Effect of dietary tannins on milk yield and composition, nitrogen partitioning and nitrogen use efficiency of lactating dairy cows: A meta-analysis. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1209–1218. [Google Scholar] [CrossRef]
- Abo-Donia, F.M.; Yang, L.Y.; Hristov, A.N.; Wang, M.; Tang, S.X.; Zhou, C.S.; Han, X.F.; Kang, J.H.; Tan, Z.L.; He, Z.X. Effects of tannins on the fatty acid profiles of rumen fluids and milk from lactating goats fed a total mixed ration containing rapeseed oil. Livest. Sci. 2017, 204, 16–24. [Google Scholar] [CrossRef]
- Kamel, H.E.M.; Al-Dobaib, S.N.; Salem, A.Z.M.; López, S.; Alaba, P.A. Influence of dietary supplementation with sunflower oil and quebracho tannins on growth performance and meat fatty acid profile of Awassi lambs. Anim. Feed Sci. Technol. 2018, 235, 97–104. [Google Scholar] [CrossRef]
- Norris, A.B.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. AFST: Influence of quebracho tannin extract fed at differing rates within a high-roughage diet on the apparent digestibility of dry matter and fiber, nitrogen balance, and fecal gas flux. Anim. Feed Sci. Technol. 2020, 260, 114365. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolyzable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Jerónimo, E.; Pinheiro, C.; Lamy, E.; Dentinho, M.T.; Sales-Baptista, E.; Lopes, O.; Silva, F. Tannins in Ruminant Nutrition: Impact on Animal Performance and Quality of Edible Products; Combs, C.A., Ed.; Nova Science Publisher Inc.: Hauppauge, NY, USA, 2016; pp. 121–168. [Google Scholar]
- Procter, H.R. A Text-Book of Tanning: A Treatise on the Conversion of Skins into Leather, Both Practical and Theoretical; E. & FN Spon: New York, NY, USA, 1885. [Google Scholar]
- Villon, A.-M. Traité pratique de la fabrication des cuirs et du travail des peaux; Librairie Polytechnique: Paris, France, 1889. [Google Scholar]
- Howes, F.N. Vegetable Tanning Materials; Butterworths Scientific Publications: London, UK, 1953. [Google Scholar]
- Falcão, L.; Araújo, M.E.M. Vegetable Tannins Used in the Manufacture of Historic Leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef]
- Xiong, J.; Grace, M.H.; Esposito, D.; Wang, F.; Lila, M.A. Phytochemical Characterization and Anti-inflammatory Properties of Acacia mearnsii Leaves. Nat. Prod. Commun. 2016, 11, 1934578. [Google Scholar] [CrossRef]
- dos Santos, C.; Vargas, Á.; Fronza, N.; dos Santos, J.H.Z. Structural, textural and morphological characteristics of tannins from Acacia mearnsii encapsulated using sol-gel methods: Applications as antimicrobial agents. Colloids Surf. B Biointerfaces 2017, 151, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rather, L.J.; Shahid ul, I.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Brizi, C.; Santulli, C.; Micucci, M.; Budriesi, R.; Chiarini, A.; Aldinucci, C.; Frosini, M. Neuroprotective Effects of Castanea sativa Mill. Bark Extract in Human Neuroblastoma Cells Subjected to Oxidative Stress. J. Cell. Biochem. 2016, 117, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and Spiny Burs of Castanea Sativa from an Experimental Chestnut Grove: Metabolomic Analysis and Anti-Neuroinflammatory Activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Saletti, R.; Giovando, S.; Tringali, C. A mass spectrometry and 1H NMR study of hypoglycemic and antioxidant principles from a Castanea sativa tannin employed in oenology. Food Chem. 2018, 268, 585–593. [Google Scholar] [CrossRef]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Štumpf, S.; Hostnik, G.; Primožič, M.; Leitgeb, M.; Salminen, J.-P.; Bren, U. The Effect of Growth Medium Strength on Minimum Inhibitory Concentrations of Tannins and Tannin Extracts against E. coli. Molecules 2020, 25, 2947. [Google Scholar] [CrossRef] [PubMed]
- Reggi, S.; Giromini, C.; Dell’Anno, M.; Baldi, A.; Rebucci, R.; Rossi, L. In Vitro Digestion of Chestnut and Quebracho Tannin Extracts: Antimicrobial Effect, Antioxidant Capacity and Cytomodulatory Activity in Swine Intestinal IPEC-J2 Cells. Animals 2020, 10, 195. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) Burs Extracts and Functional Compounds: UHPLC-UV-HRMS Profiling, Antioxidant Activity, and Inhibitory Effects on Phytopathogenic Fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Papoutsi, Z.; Kassi, E.; Chinou, I.; Halabalaki, M.; Skaltsounis, L.A.; Moutsatsou, P. Walnut extract (Juglans regia L.) and its component ellagic acid exhibit anti-inflammatory activity in human aorta endothelial cells and osteoblastic activity in the cell line KS483. Br. J. Nutr. 2008, 99, 715–722. [Google Scholar] [CrossRef]
- Bonelli, F.; Turini, L.; Sarri, G.; Serra, A.; Buccioni, A.; Mele, M. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet. Res. 2018, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, G.; Li, D.; Meng, H.; Zhu, Z.; Zhao, Y.; Qi, Y.; Zhang, X. Walnut (Juglans regia L.) Kernel Extracts Protect Against Isoproterenol-Induced Myocardial Infarction in Rats. Rejuvenation Res. 2019, 22, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Meagher, L.P.; Lane, G.; Sivakumaran, S.; Tavendale, M.H.; Fraser, K. Characterization of condensed tannins from Lotus species by thiolytic degradation and electrospray mass spectrometry. Anim. Feed Sci. Technol. 2004, 117, 151–163. [Google Scholar] [CrossRef]
- Hedqvist, H.; Mueller-Harvey, I.; Reed, J.D.; Krueger, C.G.; Murphy, M. Characterisation of tannins and in vitro protein digestibility of several Lotus corniculatus varieties. Anim. Feed Sci. Technol. 2000, 87, 41–56. [Google Scholar] [CrossRef]
- Liu, C.; Cai, D.; Zhang, L.; Tang, W.; Yan, R.; Guo, H.; Chen, X. Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antivir. Res. 2016, 134, 97–107. [Google Scholar] [CrossRef]
- Min, B.R.; Fernandez, J.M.; Barry, T.N.; McNabb, W.C.; Kemp, P.D. The effect of condensed tannins in Lotus corniculatus upon reproductive efficiency and wool production in ewes during autumn. Anim. Feed Sci. Technol. 2001, 92, 185–202. [Google Scholar] [CrossRef]
- Raitanen, J.-E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy—Sustainable Preservative and Aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef]
- Arunkumar, J.; Rajarajan, S. Study on antiviral activities, drug-likeness and molecular docking of bioactive compounds of Punica granatum L. to Herpes simplex virus-2 (HSV-2). Microb. Pathog. 2018, 118, 301–309. [Google Scholar] [CrossRef]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French Oak Wood Extract Robuvit on Markers of Oxidative Stress and Activity of Antioxidant Enzymes in Healthy Volunteers: A Pilot Study. Oxidative Med. Cell. Longev. 2014, 2014, 639868. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Leoni, G.; Maldini, M.; Natarelli, L.; Comitato, R.; Schonlau, F.; Virgili, F.; Canali, R. Absorption, Metabolism, and Effects at Transcriptome Level of a Standardized French Oak Wood Extract, Robuvit, in Healthy Volunteers: Pilot Study. J. Agric. Food Chem. 2014, 62, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Khalilpour, S.; Sangiovanni, E.; Piazza, S.; Fumagalli, M.; Beretta, G.; Dell’Agli, M. In vitro evidences of the traditional use of Rhus coriaria L. fruits against skin inflammatory conditions. J. Ethnopharmacol. 2019, 238, 111829. [Google Scholar] [CrossRef] [PubMed]
- Isik, S.; Tayman, C.; Cakir, U.; Koyuncu, I.; Taskin Turkmenoglu, T.; Cakir, E. Sumac (Rhus coriaria) for the prevention and treatment of necrotizing enterocolitis. J. Food Biochem. 2019, 43, e13068. [Google Scholar] [CrossRef]
- Monforte, M.T.; Smeriglio, A.; Germanò, M.P.; Pergolizzi, S.; Circosta, C.; Galati, E.M. Evaluation of antioxidant, antiinflammatory, and gastroprotective properties of Rubus fruticosus L. fruit juice. Phytotherapy Res. 2018, 32, 1404–1414. [Google Scholar] [CrossRef]
- Mirazi, N.; Hosseini, A. Attenuating properties of Rubus fruticosus L. on oxidative damage and inflammatory response following streptozotocin-induced diabetes in the male Wistar rats. J. Diabetes Metab. Disord. 2020, 19, 1311–1316. [Google Scholar] [CrossRef]
- Venter, P.B.; Sisa, M.; van der Merwe, M.J.; Bonnet, S.L.; van der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 1: The chemical composition of quebracho (Schinopsis lorentzii and Schinopsis balansae) heartwood extract. Phytochemistry 2012, 73, 95–105. [Google Scholar] [CrossRef]
- Cardullo, N.; Muccilli, V.; Cunsolo, V.; Tringali, C. Mass Spectrometry and 1H-NMR Study of Schinopsis lorentzii (Quebracho) Tannins as a Source of Hypoglycemic and Antioxidant Principles. Molecules 2020, 25, 3257. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; De Mello, A.S. Effects of the Incorporation of Tannin Extract from Quebracho Colorado Wood on Color Parameters, Lipid Oxidation, and Sensory Attributes of Beef Patties. Foods 2020, 9, 667. [Google Scholar] [CrossRef]
- Yang, M.H.; Ali, Z.; Khan, I.A.; Khan, S.I. Anti-inflammatory Activity of Constituents Isolated from Terminalia chebula. Nat. Prod. Commun. 2014, 9, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; García-Villanova, B.; Guerra-Hernández, E.; Verardo, V. Grape Seeds Proanthocyanidins: An Overview of In Vivo Bioactivity in Animal Models. Nutrients 2019, 11, 2435. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant–herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review of Tannins’ Biological Activities and Their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, P.; Maggiolino, A.; Albano, C.; De Palo, P.; Blando, F. Ensiling Grape Pomace With and Without Addition of a Lactiplantibacillus plantarum Strain: Effect on Polyphenols and Microbiological Characteristics, in vitro Nutrient Apparent Digestibility, and Gas Emission. Front Vet. Sci. 2022, 9, 808293. [Google Scholar] [CrossRef] [PubMed]
- Wong-Paz, J.E.; Guyot, S.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B.; Contreras-Esquivel, J.C.; Aguilar, C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-HPLC-ESI-MS. Food Chem. 2021, 340, 127830. [Google Scholar] [CrossRef]
- Hayder, Z.; Elfalleh, W.; Othman, K.B.; Benabderrahim, M.A.; Hannachi, H. Modeling of polyphenols extraction from pomegranate by-product using rotatable central composite design of experiments. Acta Ecol. Sin. 2021, 41, 150–156. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut shells (Italian cultivar “Marrone di Roccadaspide” PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MSn rationalization of tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef]
- Saad, H.; Charrier-El Bouhtoury, F.; Pizzi, A.; Rode, K.; Charrier, B.; Ayed, N. Characterization of pomegranate peels tannin extractives. Ind. Crops Prod. 2012, 40, 239–246. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Priya, B.; Gurumoorthi, P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J. Food Sci. Technol. 2015, 52, 92–104. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Quiñones, J.; Latorre, M.A.; Blando, F.; Centoducati, G.; Dahl, G.E.; De Palo, P. Effects of dietary supplementation with Pinus taeda hydrolyzed lignin on in vivo performances, in vitro nutrient apparent digestibility, and gas emission in beef steers. Anim. Feed Sci. Technol. 2019, 255, 114217. [Google Scholar] [CrossRef]
- Maggiolino, A.; Lorenzo, J.M.; Salzano, A.; Faccia, M.; Blando, F.; Serrano, M.P.; Latorre, M.A.; Quiñones, J.; De Palo, P. Effects of aging and dietary supplementation with polyphenols from Pinus taeda hydrolysed lignin on quality parameters, fatty acid profile and oxidative stability of beef. Anim. Prod. Sci. 2020, 60, 713–724. [Google Scholar] [CrossRef]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Politi, F.A.S.; Mello, J.C.P.d.; Migliato, K.F.; Nepomuceno, A.L.A.; Moreira, R.R.D.; Pietro, R.C.L.R. Antimicrobial, Cytotoxic and Antioxidant Activities and Determination of the Total Tannin Content of Bark Extracts Endopleura uchi. Int. J. Mol. Sci. 2011, 12, 2757–2768. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of bioactive compounds from spruce bark waste using sub- and supercritical fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Han, F.; Kang, X.; Gu, H.; Yang, L. Microwave-assisted method for simultaneous hydrolysis and extraction in obtaining ellagic acid, gallic acid and essential oil from Eucalyptus globulus leaves using Brönsted acidic ionic liquid [HO3S(CH2)4mim]HSO4. Ind. Crops Prod. 2016, 81, 152–161. [Google Scholar] [CrossRef]
- Leong, L.P.; Shui, G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Wang, H.-W.; Liu, Y.-Q.; Wei, S.-L.; Yan, Z.-J.; Lu, K. Comparison of Microwave-Assisted and Conventional Hydrodistillation in the Extraction of Essential Oils from Mango (Mangifera indica L.) Flowers. Molecules 2010, 15, 7715–7723. [Google Scholar] [CrossRef]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-carotene, lycopene, and alpha-tocopherol contents of selected Thai fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Mahattanatawee, K.; Manthey, J.A.; Luzio, G.; Talcott, S.T.; Goodner, K.; Baldwin, E.A. Total Antioxidant Activity and Fiber Content of Select Florida-Grown Tropical Fruits. J. Agric. Food Chem. 2006, 54, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Opara, L.U.; Al-Ani, M.R.; Al-Shuaibi, Y.S. Physico-chemical Properties, Vitamin C Content, and Antimicrobial Properties of Pomegranate Fruit (Punica granatum L.). Food Bioprocess Technol. 2009, 2, 315–321. [Google Scholar] [CrossRef]
- Dabbou, S.; Dabbou, S.; Flamini, G.; Pandino, G.; Gasco, L.; Helal, A.N. Phytochemical Compounds from the Crop Byproducts of Tunisian Globe Artichoke Cultivars. Chem. Biodivers. 2016, 13, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Besharati, M.; Palangi, V.; Salem, A.Z.M.; De Palo, P.; Lorenzo, J.M.; Maggiolino, A. Substitution of raw lucerne with raw citrus lemon by-product in silage: In vitro apparent digestibility and gas production. Front Vet. Sci 2022, 9. [Google Scholar] [CrossRef]
- Quiñones, J.; Maggiolino, A.; Bravo, S.; Muñoz, E.; Lorenzo, J.M.; Cancino, D.; Díaz, R.; Saenz, C.; Sepúlveda, N.; De Palo, P. Effect of canola oil on meat quality and fatty acid profile of Araucano creole lambs during fattening period. Anim. Feed Sci. Technol. 2019, 248, 20–26. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Rodríguez, R.; de la Fuente, G.; Gómez, S.; Fondevila, M. Biological effect of tannins from different vegetal origin on microbial and fermentation traits in vitro. J. Anim. Prod. Sci. 2014, 54, 1039–1046. [Google Scholar] [CrossRef]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2014, 69, 32–48. [Google Scholar] [CrossRef]
- Waghorn, G. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production—Progress and challenges. Anim. Feed Sci. Technol. 2008, 147, 116–139. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Francis, G.; Becker, K. Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 2007, 1, 1371–1391. [Google Scholar] [CrossRef]
- Min, B.R.; Barry, T.N.; Attwood, G.T.; McNabb, W.C. The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: A review. Anim. Feed Sci. Technol. 2003, 106, 3–19. [Google Scholar] [CrossRef]
- Salzano, A.; Damiano, S.; D’Angelo, L.; Ballistreri, G.; Claps, S.; Rufrano, D.; Maggiolino, A.; Neglia, G.; De Palo, P.; Ciarcia, R. Productive Performance and Meat Characteristics of Kids Fed a Red Orange and Lemon Extract. Animals 2021, 11, 809. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Longobardi, C.; Salzano, A.; D’Angelo, L.; Amenta, M.; Maggiolino, A.; De Palo, P.; Claps, S.; Rufrano, D.; Iannaccone, F.; et al. Red orange and lemon extract preserve from oxidative stress, DNA damage and inflammatory status in lambs. Ital. J. Anim. Sci. 2022, 21, 934–942. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Francavilla, M.; Neglia, G.; Esposito, L.; Caroprese, M. Extracts from Microalga Chlorella sorokiniana Exert an Anti-Proliferative Effect and Modulate Cytokines in Sheep Peripheral Blood Mononuclear Cells. Animals 2019, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Naumann, H.D.; Tedeschi, L.O.; Zeller, W.E.; Huntley, N.F. The role of condensed tannins in ruminant animal production: Advances, limitations and future directions. Rev. Bras. De Zootec. 2017, 46, 929–949. [Google Scholar] [CrossRef]
- Bhanu, P.A.; Mohan Krishna Reddy, M.; Sadhana Reddy, N.; Kesava Rao, B. Isolation and Characterization of Pharmacologically Active Tannins from Stem Bark of Syzygium samarangense. In Medicinal Plants: Biodiversity, Sustainable Utilization and Conservation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 549–561. [Google Scholar]
- Bartzoka, E.D.; Lange, H.; Mosesso, P.; Crestini, C. Synthesis of nano- and microstructures from proanthocyanidins, tannic acid and epigallocatechin-3-O-gallate for active delivery. Green Chem. 2017, 19, 5074–5091. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition and antioxidant potential of grain legume seeds: A review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and their Metabolites-The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Navarro, J.; Cazals, G.; Enjalbal, C.; Izquierdo-Cañas, P.M.; Gómez-Alonso, S.; Saucier, C. Flavanol Glycoside Content of Grape Seeds and Skins of Vitis vinifera Varieties Grown in Castilla-La Mancha, Spain. Molecules 2019, 24, 4001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Wang, L.-H.; Zeng, X.-A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13. [Google Scholar] [CrossRef]
- Guo, X.; Wang, H.; Ni, J.; Liang, Z.; Wu, X.; Xue, J.; Wang, X. Geraniin selectively promotes cytostasis and apoptosis in human colorectal cancer cells by inducing catastrophic chromosomal instability. Mutagenesis 2018, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Ningrat, R.; Eun, J.; Min, B. Effects of supplemental virgin coconut oil and condensed tannin extract from pine bark in lactation dairy diets on ruminal fermentation in a dual-flow continuous culture system. Adv. Dairy Res. 2016, 1–6. [Google Scholar]
- Sebestyén, Z.; Jakab, E.; Badea, E.; Barta-Rajnai, E.; Şendrea, C.; Czégény, Z. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J. Anal. Appl. Pyrolysis 2019, 138, 178–187. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Santiago-Medina, F.J.; Al-Marzouki, F.M.; Abdalla, S. Isocyanate-Free Polyurethanes by Coreaction of Condensed Tannins with Aminated Tannins. J. Renew. Mater. 2017, 5, 21–29. [Google Scholar] [CrossRef]
- Çakar, S.; Güy, N.; Özacar, M.; Fındık, F. Investigation of Vegetable Tannins and Their Iron Complex Dyes for Dye Sensitized Solar Cell Applications. Electrochim. Acta 2016, 209, 407–422. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Duran, L.V.; Buenrostro-Figueroa, J.J.; Sepúlveda-Torre, L.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Tannin Degrading Enzymes: Catalytic Properties and Technological Perspectives. In Enzymes in Food Technology: Improvements and Innovations; Kuddus, M., Ed.; Springer: Singapore, 2018; pp. 125–141. [Google Scholar]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and Definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- Ferreira, D.; Nel, R.J.J.; Bekker, R. 3.19-Condensed Tannins. In Comprehensive Natural Products Chemistry; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: Oxford, UK, 1999; pp. 747–797. [Google Scholar]
- Muñoz, R.; de las Rivas, B.; López de Felipe, F.; Reverón, I.; Santamaría, L.; Esteban-Torres, M.; Curiel, J.A.; Rodríguez, H.; Landete, J.M. Chapter 4—Biotransformation of Phenolics by Lactobacillus plantarum in Fermented Foods. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 63–83. [Google Scholar]
- Mcdougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Silanikove, N.; Perevolotsky, A.; Provenza, F.D. Use of tannin-binding chemicals to assay for tannins and their negative postingestive effects in ruminants. Anim. Feed Sci. Technol. 2001, 91, 69–81. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Robbins, C.T.; Weerasuriya, Y.; Wilson, T.C.; McArthur, C. Tannin chemistry in relation to digestion. Rangel. Ecol. Manag. J. Range Manag. Arch. 1992, 45, 57–62. [Google Scholar] [CrossRef]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins—A current perspective. Biodegradation 1998, 9, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Puniya, A.K.; Aguilar, C.N.; Singh, K. Interaction of gut microflora with tannins in feeds. Naturwissenschaften 2005, 92, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.-P.; Green, R.J. Ellagitannins with Glucopyranose Cores Have Higher Affinities to Proteins than Acyclic Ellagitannins by Isothermal Titration Calorimetry. J. Agric. Food Chem. 2019, 67, 12730–12740. [Google Scholar] [CrossRef]
- Ma, W.; Waffo-Teguo, P.; Jourdes, M.; Li, H.; Teissedre, P.-L. Chemical affinity between tannin size and salivary protein binding abilities: Implications for wine astringency. PLoS ONE 2016, 11, e0161095. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, P.; Wang, H.; Cai, S.; Liao, Y.; Mo, Z.; Xu, X.; Ding, C.; Zhao, C.; Li, J. Antibacterial and anti-biofouling coating on hydroxyapatite surface based on peptide-modified tannic acid. Colloids Surf. B Biointerfaces 2017, 160, 136–143. [Google Scholar] [CrossRef]
- Prasetiyono, B.W.H.E.; Subrata, A.; Tampoebolon, B.I.M.; Surono; Widiyanto. In Vitro Ruminal Degradability of Soybean Meal Protein Protected with Natural Tannin. IOP Conf. Ser. Earth Environ. Sci. 2018, 119, 012016. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic–Protein Interactions: Effects on Food Properties and Health Benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Bunglavan, S.; Dutta, N. Use of tannins as organic protectants of proteins in digestion of ruminants. J. Livest. Sci. 2013, 4, 67–77. [Google Scholar]
- Engström, M.T.; Arvola, J.; Nenonen, S.; Virtanen, V.T.J.; Leppä, M.M.; Tähtinen, P.; Salminen, J.P. Structural Features of Hydrolyzable Tannins Determine Their Ability to Form Insoluble Complexes with Bovine Serum Albumin. J. Agric. Food Chem. 2019, 67, 6798–6808. [Google Scholar] [CrossRef]
- Ropiak, H.M.; Lachmann, P.; Ramsay, A.; Green, R.J.; Mueller-Harvey, I. Identification of structural features of condensed tannins that affect protein aggregation. PLoS ONE 2017, 12, e0170768. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Chemical composition and biological activity of staghorn sumac (Rhus typhina). Food Chem. 2017, 237, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Beauchemin, K.A. Potential of Molecular Weight and Structure of Tannins to Reduce Methane Emissions from Ruminants: A Review. Animals 2019, 9, 856. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Méndez, C.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 2017, 45, 199–203. [Google Scholar] [CrossRef]
- Delimont, N.M.; Rosenkranz, S.K.; Haub, M.D.; Lindshield, B.L. Salivary proline-rich protein may reduce tannin-iron chelation: A systematic narrative review. Nutr. Metab. 2017, 14, 47. [Google Scholar] [CrossRef]
- Girard, A.L.; Teferra, T.; Awika, J.M. Effects of condensed vs hydrolysable tannins on gluten film strength and stability. Food Hydrocoll. 2019, 89, 36–43. [Google Scholar] [CrossRef]
- Silva, M.S.; García-Estévez, I.; Brandão, E.; Mateus, N.; de Freitas, V.; Soares, S. Molecular Interaction Between Salivary Proteins and Food Tannins. J. Agric. Food Chem. 2017, 65, 6415–6424. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. Effect of Tannin and Species Variation on In vitro Digestibility, Gas, and Methane Production of Tropical Browse Plants. Asian-Australas J. Anim. Sci. 2015, 28, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.A.; El-Din, A.N.M.N.; El-Zarkouny, S.Z.; El-Zaiat, H.M.; Zeitoun, M.M.; Sallam, S.M.A. Impact of Quebracho Tannins Supplementation on Productive and Reproductive Efficiency of Dairy Cows. J. Open J. Anim. Sci. 2016, 6, 21. [Google Scholar] [CrossRef][Green Version]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Li, Y.-G.; Tanner, G.; Larkin, P. The DMACA–HCl Protocol and the Threshold Proanthocyanidin Content for Bloat Safety in Forage Legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Sottie, E.T.; Acharya, S.N.; McAllister, T.; Thomas, J.; Wang, Y.; Iwaasa, A. Alfalfa Pasture Bloat Can Be Eliminated by Intermixing with Newly-Developed Sainfoin Population. Agron. J. 2014, 106, 1470–1478. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 22, 253–261. [Google Scholar] [CrossRef]
- Méndez-Ortiz, F.A.; Sandoval-Castro, C.A.; Ventura-Cordero, J.; Sarmiento-Franco, L.A.; Torres-Acosta, J.F.J. Condensed tannin intake and sheep performance: A meta-analysis on voluntary intake and live weight change. Anim. Feed Sci. Technol. 2018, 245, 67–76. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Banerjee, P.S.; Goswami, T.K.; Sharma, K. Effect of condensed tannins supplementation through leaf meal mixture on voluntary feed intake, immune response and worm burden in Haemonchus contortus infected sheep. J. Parasit. Dis. 2016, 40, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Waghorn, G.C.; Shelton, I.D.; McNabb, W.C.; McCutcheon, S.N. Effects of condensed tannins in Lotus pedunculatus on its nutritive value for sheep. 2. Nitrogenous aspects. J. Agric. Sci. 1994, 123, 109–119. [Google Scholar] [CrossRef]
- Cabral Filho, S.; Abdalla, A.; Bueno, I.; Oliveira, A. Effect of sorghum tannins in sheep fed with high-concentrate diets. Arq. Bras. De Med. Veterinária E Zootec. 2013, 65, 1759–1766. [Google Scholar] [CrossRef]
- Butter, N.; Dawson, J.; Buttery, P. Effects of dietary tannins on ruminants. Second. Plant Prod. Antinutritional Benef. Actions Anim. Feed. 1999, 51–70. [Google Scholar]
- Barry, T.N.; McNabb, W.C. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 1999, 81, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; Cornell University Press: Ithaca, NY, USA, 1994; p. 2. [Google Scholar]
- Austin, P.J.; Suchar, L.A.; Robbins, C.T.; Hagerman, A.E. Tannin-binding proteins in saliva of deer and their absence in saliva of sheep and cattle. J. Chem. Ecol. 1989, 15, 1335–1347. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Palmer, B.; McNeill, D.M.; Krause, D.O. Microbial interactions with tannins: Nutritional consequences for ruminants. Anim. Feed Sci. Technol. 2001, 91, 83–93. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.; Mantecón, A. Tannins and ruminant nutrition, Review. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- McArthur, C.; Sanson, G.D.; Beal, A.M. Salivary proline-rich proteins in mammals: Roles in oral homeostasis and counteracting dietary tannin. J. Chem. Ecol. 1995, 21, 663–691. [Google Scholar] [CrossRef]
- Narjisse, H.; Elhonsali, M.A.; Olsen, J.D. Effects of oak (Quercus ilex) tannins on digestion and nitrogen balance in sheep and goats. Small Rumin. Res. 1995, 18, 201–206. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Rice, M.E.; Ritchard, N.T. Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16 (4→8) Catechin (Procyanidin). J. Agric. Food Chem. 1998, 46, 2590–2595. [Google Scholar] [CrossRef]
- Makkar, P.; Dawra, R.; Singh, B. Determination of Both Tannin and Protein in a Tannin-Protein Complex. J. Agric. Food Chem. 1988, 36, 523–525. [Google Scholar] [CrossRef]
- Wang, Y.; Douglas, G.B.; Waghorn, G.C.; Barry, T.N.; Foote, A.G. Effect of condensed tannins in Lotus corniculatus upon lactation performance in ewes. J. Agric. Sci. 1996, 126, 353–362. [Google Scholar] [CrossRef]
- Besharati, M.; Taghizadeh, A. Evaluation of dried grape by-product as a tanniniferous tropical feedstuff. Anim. Feed Sci. Technol. 2009, 152, 198–203. [Google Scholar] [CrossRef]
- Yisehak, K.; Kibreab, Y.; Taye, T.; Ribeiro Alves Lourenço, M.; Janssens, G.P.J. Response to dietary tannin challenges in view of the browser/grazer dichotomy in an Ethiopian setting: Bonga sheep versus Kaffa goats. Trop. Anim. Health Prod. 2016, 48, 125–131. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Mendes, L.W.; Lemos, L.N.; Crouzoulon, P.; Niderkorn, V.; Hoste, H.; Costa-Júnior, L.M.; Tsai, S.M.; Faciola, A.P.; Abdalla, A.L.; et al. Tannin supplementation modulates the composition and function of ruminal microbiome in lambs infected with gastrointestinal nematodes. FEMS Microbiol. Ecol. 2020, 96, fiaa024. [Google Scholar] [CrossRef]
- Millen, D.D.; Arrigoni, M.D.B.; Pacheco, R.D.L. Rumenology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- McSweeney, C.S.; Denman, S.E.; Mackie, R.I. Rumen bacteria. In Methods in Gut Microbial Ecology for Ruminants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 23–37. [Google Scholar]
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Rezaeian, M.; Beakes, G.W.; Parker, D.S. Distribution and estimation of anaerobic zoosporic fungi along the digestive tracts of sheep. Mycol. Res. 2004, 108, 1227–1233. [Google Scholar] [CrossRef]
- Elekwachi, C.O.; Wang, Z.; Wu, X.; Rabee, A.; Forster, R.J. Total rRNA-Seq Analysis Gives Insight into Bacterial, Fungal, Protozoal and Archaeal Communities in the Rumen Using an Optimized RNA Isolation Method. Front. Microbiol. 2017, 8, 1814. [Google Scholar] [CrossRef]
- Lee, S.S.; Ha, J.K.; Cheng, K.-J. Relative Contributions of Bacteria, Protozoa, and Fungi to In Vitro Degradation of Orchard Grass Cell Walls and Their Interactions. Appl. Environ. Microbiol. 2000, 66, 3807–3813. [Google Scholar] [CrossRef]
- Solomon, K.V.; Haitjema, C.H.; Henske, J.K.; Gilmore, S.P.; Borges-Rivera, D.; Lipzen, A.; Brewer, H.M.; Purvine, S.O.; Wright, A.T.; Theodorou, M.K.; et al. Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science 2016, 351, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.L.R.; Phillips, M.W. The role of anaerobic gut fungi in ruminants. Nutr. Res. Rev. 1998, 11, 133–168. [Google Scholar] [CrossRef] [PubMed]
- Gruninger, R.J.; Puniya, A.K.; Callaghan, T.M.; Edwards, J.E.; Youssef, N.; Dagar, S.S.; Fliegerova, K.; Griffith, G.W.; Forster, R.; Tsang, A.; et al. Anaerobic fungi (phylum Neocallimastigomycota): Advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol. Ecol. 2014, 90, 1–17. [Google Scholar] [CrossRef]
- Williams, A.G.; Coleman, G.S. The rumen protozoa. In The Rumen Microbial Ecosystem; Springer: Berlin/Heidelberg, Germany, 1997; pp. 73–139. [Google Scholar]
- Newbold, C.J.; de la Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.; Wein, T.; Levy, B.; Eshed, S.; Dror, R.; Reiss, V.; Zehavi, T.; Furman, O.; Mizrahi, I.; Jami, E. Protozoa populations are ecosystem engineers that shape prokaryotic community structure and function of the rumen microbial ecosystem. ISME J. 2022, 16, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Rathahao-Paris, E.; Popova, M.; Boccard, J.; Nielsen, K.F.; Boudra, H. Rumen microbial communities influence metabolic phenotypes in lambs. Front. Microbiol. 2015, 6, 1060. [Google Scholar] [CrossRef]
- Kim, M.; Morrison, M.; Yu, Z. Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 2011, 76, 49–63. [Google Scholar] [CrossRef]
- Paul, K.; Nonoh, J.O.; Mikulski, L.; Brune, A. “Methanoplasmatales,” Thermoplasmatales-Related Archaea in Termite Guts and Other Environments, Are the Seventh Order of Methanogens. Appl. Environ. Microbiol. 2012, 78, 8245–8253. [Google Scholar] [CrossRef]
- Szumacher-Strabel, M.; Cieślak, A. Potential of phytofactors to mitigate rumen ammonia and methane production. J. Anim. Feed Sci. 2010, 19, 319–337. [Google Scholar] [CrossRef]
- Aliyu, A.; Olusola, O.O.; Gilead, E.F.; Abdullahi, S.U.; Michael, D.M. Anti-nutritional and phytochemical profile of some plants grazed upon by ruminants in North Central Nigeria during the dry season (January to April). Int. J. Livest. Prod. 2016, 7, 19–23. [Google Scholar] [CrossRef]
- Hassan, F.-u.; Arshad, M.A.; Ebeid, H.M.; Rehman, M.S.-u.; Khan, M.S.; Shahid, S.; Yang, C. Phytogenic Additives Can Modulate Rumen Microbiome to Mediate Fermentation Kinetics and Methanogenesis Through Exploiting Diet–Microbe Interaction. Front Vet. Sci 2020, 7, 575801. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Min, B.-R.; Saxena, J. Dietary Tannins on Microbial Ecology of the Gastrointestinal Tract in Ruminants. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 237–262. [Google Scholar]
- Canul-Solis, J.; Campos-Navarrete, M.; Piñeiro-Vázquez, A.; Casanova-Lugo, F.; Barros-Rodríguez, M.; Chay-Canul, A.; Cárdenas-Medina, J.; Castillo-Sánchez, L. Mitigation of Rumen Methane Emissions with Foliage and Pods of Tropical Trees. Animals 2020, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Besharati, M.; Giannenas, I.; Palangi, V.; Ayasan, T.; Noorian, F.; Maggiolino, A.; Lorenzo, J.M. Chitosan/Calcium–Alginate Encapsulated Flaxseed Oil on Dairy Cattle Diet: In Vitro Fermentation and Fatty Acid Biohydrogenation. Animals 2022, 12, 1400. [Google Scholar] [PubMed]
- Yang, K.; Wei, C.; Zhao, G.Y.; Xu, Z.W.; Lin, S.X. Effects of dietary supplementing tannic acid in the ration of beef cattle on rumen fermentation, methane emission, microbial flora and nutrient digestibility. J. Anim. Physiol. Anim. Nutr. 2017, 101, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.A.; Oba, M.; Koenig, K.M.; Zhao, G.Y.; Beauchemin, K.A. Use of gallic acid and hydrolyzable tannins to reduce methane emission and nitrogen excretion in beef cattle fed a diet containing alfalfa silage1,2. J. Anim. Sci. 2019, 97, 2230–2244. [Google Scholar] [CrossRef]
- Salami, S.A.; Valenti, B.; Bella, M.; O’Grady, M.N.; Luciano, G.; Kerry, J.P.; Jones, E.; Priolo, A.; Newbold, C.J. Characterisation of the ruminal fermentation and microbiome in lambs supplemented with hydrolysable and condensed tannins. FEMS Microbiol. Ecol. 2018, 94, fiy061. [Google Scholar] [CrossRef]
- Saminathan, M.; Kumari Ramiah, S.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W.; Idrus, Z. In vitro study on the effects of condensed tannins of different molecular weights on bovine rumen fungal population and diversity. Ital. J. Anim. Sci. 2019, 18, 1451–1462. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Akanmu, A.M. Effect of Lipid-Encapsulated Acacia Tannin Extract on Feed Intake, Nutrient Digestibility and Methane Emission in Sheep. Animals 2019, 9, 863. [Google Scholar] [CrossRef]
- Adejoro, F.A.; Hassen, A.; Thantsha, M.S. Characterization of starch and gum arabic-maltodextrin microparticles encapsulating acacia tannin extract and evaluation of their potential use in ruminant nutrition. Asian-Australas J. Anim Sci 2019, 32, 977–987. [Google Scholar] [CrossRef]
- Min, B.R.; Solaiman, S.; Waldrip, H.M.; Parker, D.; Todd, R.W.; Brauer, D. Dietary mitigation of enteric methane emissions from ruminants: A review of plant tannin mitigation options. Anim. Nutr. 2020, 6, 231–246. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A 2016, 33, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Nawab, A.; Li, G.; An, L.; Nawab, Y.; Zhao, Y.; Xiao, M.; Tang, S.; Sun, C. The Potential Effect of Dietary Tannins on Enteric Methane Emission and Ruminant Production, as an Alternative to Antibiotic Feed Additives—A Review. Ann. Anim. Sci. 2020, 20, 355–388. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Jin, L.; Xu, Z.; Barbieri, L.R.; Acharya, S.; Hu, T.M.; McAllister, T.A.; Stanford, K.; Wang, Y. Effects of purple prairie clover (Dalea purpurea Vent.) on feed intake, nutrient digestibility and faecal shedding of Escherichia coli O157:H7 in lambs. Anim. Feed Sci. Technol. 2015, 207, 51–61. [Google Scholar] [CrossRef]
- Peng, K.; Shirley, D.C.; Xu, Z.; Huang, Q.; McAllister, T.A.; Chaves, A.V.; Acharya, S.; Liu, C.; Wang, S.; Wang, Y. Effect of purple prairie clover (Dalea purpurea Vent.) hay and its condensed tannins on growth performance, wool growth, nutrient digestibility, blood metabolites and ruminal fermentation in lambs fed total mixed rations. Anim. Feed Sci. Technol. 2016, 222, 100–110. [Google Scholar] [CrossRef]
- Chambi, F.; Chirinos, R.; Pedreschi, R.; Betalleluz-Pallardel, I.; Debaste, F.; Campos, D. Antioxidant potential of hydrolyzed polyphenolic extracts from tara (Caesalpinia spinosa) pods. Ind. Crops Prod. 2013, 47, 168–175. [Google Scholar] [CrossRef]
- Ben Salem, H.; Nefzaoui, A.; Ben Salem, L.; Tisserand, J.L. Intake, digestibility, urinary excretion of purine derivatives and growth by sheep given fresh, air-dried or polyethylene glycol-treated foliage of Acacia cyanophylla Lindl. Anim. Feed Sci. Technol. 1999, 78, 297–311. [Google Scholar] [CrossRef]
- Hervás, G.; Frutos, P.; Javier Giráldez, F.; Mantecón, Á.R.; Álvarez Del Pino, M.a.C. Effect of different doses of quebracho tannins extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.-J. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Chiquette, J.; Cheng, K.J.; Costerton, J.W.; Milligan, L.P. Effect of tannins on the digestibility of two isosynthetic strains of birdsfoot trefoil (Lotus corniculatus L.) using in vitro and in sacco techniques. Can. J. Plant Sci. 1988, 68, 751–760. [Google Scholar] [CrossRef]
- McAllister, T.A.; Bae, H.D.; Jones, G.A.; Cheng, K.-J. Microbial attachment and feed digestion in the rumen. J. Anim. Sci. 1994, 72, 3004–3018. [Google Scholar] [CrossRef] [PubMed]
- Mangan, J.L. Nutritional Effects of Tannins in Animal Feeds. Nutr. Res. Rev. 1988, 1, 209–231. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, L.; Brooker, J.D. Effect of hydrolysable and condensed tannins on growth, morphology and metabolism of Streptococcus gallolyticus (S. caprinus) and Streptococcus bovis. Microbiology 2001, 147, 1025–1033. [Google Scholar] [CrossRef]
- Priolo, A.; Waghorn, G.C.; Lanza, M.; Biondi, L.; Pennisi, P. Polyethylene glycol as a means for reducing the impact of condensed tannins in carob pulp: Effects on lamb growth performance and meat quality. J. Anim. Sci. 2000, 78, 810–816. [Google Scholar] [CrossRef]
- Molan, P.C. Honey as a topical antibacterial agent for treatment of infected wounds. World Wide Wounds 2001, 10, 1–13. [Google Scholar]
- Terrill, T.H.; Waghorn, G.C.; Woolley, D.J.; McNabb, W.C.; Barry, T.N. Assay and digestion of 14C-labelled condensed tannins in the gastrointestinal tract of sheep. Br. J. Nutr. 1994, 72, 467–477. [Google Scholar] [CrossRef]
- Sadarman, S.; Ridla, M.; Nahrowi, N.; Ridwan, R.; Harahap, R.; Nurfitriani, R.; Jayanegara, A. Kualitas Fisik Silase Ampas Kecap Dengan Aditif Tanin Akasia (Acacia Mangium Wild.) Dan Aditif Lainnya. J. Peternak. 2019, 16, 66–75. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Belenguer, A.; Bichi, E.; Frutos, P. Effect of the inclusion of quebracho tannins in a diet rich in linoleic acid on milk fatty acid composition in dairy ewes. J. Dairy Sci. 2013, 96, 431–439. [Google Scholar] [CrossRef]
- Harris, S.; Clark, D.; Laboyrie, P. Birdsfoot trefoil-an alternative legume for New Zealand dairy pastures. NZ Grassland Assoc. 1998, 60, 99–103. [Google Scholar] [CrossRef]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows1. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef] [PubMed]
- Faccia, M.; Maggiolino, A.; Natrella, G.; Zizzadoro, C.; Mazzone, A.; Poulopoulou, I.; Bragaglio, A.; De Palo, P. Ingested versus inhaled limonene in sheep: A pilot study to explore potential different transfer to the mammary gland and effects on milk and Caciotta cheese aroma. J. Dairy Sci. 2022, 105, 8143–8157. [Google Scholar] [CrossRef] [PubMed]
- Durmic, Z.; McSweeney, C.S.; Kemp, G.W.; Hutton, P.; Wallace, R.J.; Vercoe, P.E. Australian plants with potential to inhibit bacteria and processes involved in ruminal biohydrogenation of fatty acids. Anim. Feed Sci. Technol. 2008, 145, 271–284. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Bryner, S.F.; Scheeder, M.R.L.; Wettstein, H.R.; Leiber, F.; Kreuzer, M.; Soliva, C.R. Evidence for the inhibition of the terminal step of ruminal α-linolenic acid biohydrogenation by condensed tannins. J. Dairy Sci. 2009, 92, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Minieri, S.; Rapaccini, S.; Antongiovanni, M.; Mele, M. Effect of chestnut and quebracho tannins on fatty acid profile in rumen liquid- and solid-associated bacteria: An in vitro study. Animal 2011, 5, 1521–1530. [Google Scholar] [CrossRef]
- Vasta, V.; Mele, M.; Serra, A.; Scerra, M.; Luciano, G.; Lanza, M.; Priolo, A. Metabolic fate of fatty acids involved in ruminal biohydrogenation in sheep fed concentrate or herbage with or without tannins1. J. Anim. Sci. 2009, 87, 2674–2684. [Google Scholar] [CrossRef]
- Addis, M.; Cabiddu, A.; Pinna, G.; Decandia, M.; Piredda, G.; Pirisi, A.; Molle, G. Milk and Cheese Fatty Acid Composition in Sheep Fed Mediterranean Forages with Reference to Conjugated Linoleic Acid cis-9,trans-11. J. Dairy Sci. 2005, 88, 3443–3454. [Google Scholar] [CrossRef]
- Benchaar, C.; Chouinard, P.Y. Short communication: Assessment of the potential of cinnamaldehyde, condensed tannins, and saponins to modify milk fatty acid composition of dairy cows1. J. Dairy Sci. 2009, 92, 3392–3396. [Google Scholar] [CrossRef]
- Maggiolino, A.; Faccia, M.; Holman, B.W.B.; Hopkins, D.L.; Bragaglio, A.; Natrella, G.; Mazzone, A.; De Palo, P. The effect of oral or respiratory exposure to limonene on goat kid performance and meat quality. Meat Sci. 2022, 191, 108865. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Natalello, A.; Luciano, G.; Fondevila, M.; Priolo, A.; Toral, P.G. Ability of tannins to modulate ruminal lipid metabolism and milk and meat fatty acid profiles. Anim. Feed Sci. Technol. 2020, 269, 114623. [Google Scholar] [CrossRef]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. BOARD-INVITED REVIEW: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem1. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Biohydrogenation then and now. Eur. J. Lipid Sci. Technol. 2007, 109, 737–739. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Ross, R.P.; Devery, R.; Stanton, C. The Health Promoting Properties of the Conjugated Isomers of α-Linolenic Acid. Lipids 2011, 46, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J. Lipid Metabolism; CAB International: Wallingford, CT, USA; Oxford, UK, 2000; pp. 97–119. [Google Scholar]
- Shingfield, K.; Bonnet, M.; Scollan, N. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal 2013, 7, 132–162. [Google Scholar] [CrossRef]
- Bernard, L.; Leroux, C.; Chilliard, Y. Expression and Nutritional Regulation of Stearoyl-CoA Desaturase Genes in the Ruminant Mammary Gland: Relationship with Milk Fatty Acid Composition. In Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Ntambi, P.D.J.M., Ed.; Springer: New York, NY, USA, 2013; pp. 161–193. [Google Scholar]
- Kadegowda, A.K.G.; Burns, T.A.; Miller, M.C.; Duckett, S.K. Cis-9, trans-11 conjugated linoleic acid is endogenously synthesized from palmitelaidic (C16:1 trans-9) acid in bovine adipocytes1. J. Anim. Sci. 2013, 91, 1614–1623. [Google Scholar] [CrossRef]
- Maggiolino, A.; Sgarro, M.F.; Natrella, G.; Lorenzo, J.; Colucci, A.; Faccia, M.; De Palo, P. Dry-Aged Beef Steaks: Effect of Dietary Supplementation with Pinus taeda Hydrolyzed Lignin on Sensory Profile, Colorimetric and Oxidative Stability. Foods 2021, 10, 1080. [Google Scholar] [CrossRef]
- Enjalbert, F.; Combes, S.; Zened, A.; Meynadier, A. Rumen microbiota and dietary fat: A mutual shaping. J. Appl. Microbiol. 2017, 123, 782–797. [Google Scholar] [CrossRef]
- Carreño, D.; Toral, P.G.; Pinloche, E.; Belenguer, A.; Yáñez-Ruiz, D.R.; Hervás, G.; McEwan, N.R.; Newbold, C.J.; Frutos, P. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Sci. Rep. 2019, 9, 11857. [Google Scholar] [CrossRef]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Campidonico, L.; Toral, P.G.; Priolo, A.; Luciano, G.; Valenti, B.; Hervás, G.; Frutos, P.; Copani, G.; Ginane, C.; Niderkorn, V. Fatty acid composition of ruminal digesta and longissimus muscle from lambs fed silage mixtures including red clover, sainfoin, and timothy12. J. Anim. Sci. 2016, 94, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Dohme-Meier, F.; Silacci, P.; Ampuero Kragten, S.; Kreuzer, M.; Bee, G. Forage legumes rich in condensed tannins may increase n-3 fatty acid levels and sensory quality of lamb meat. J. Sci. Food Agric. 2016, 96, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Lobón, S.; Joy, M.; Sanz, A.; Álvarez-Rodríguez, J.; Blanco, M. The fatty acid composition of ewe milk or suckling lamb meat can be used to discriminate between ewes fed different diets. J. Anim. Prod. Sci. 2019, 59, 1108–1118. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jerónimo, E. Inclusion of the aerial part and condensed tannin extract from Cistus ladanifer L. in lamb diets—Effects on growth performance, carcass and meat quality and fatty acid composition of intramuscular and subcutaneous fat. Meat Sci. 2020, 160, 107945. [Google Scholar] [CrossRef]
- Mannelli, F.; Daghio, M.; Alves, S.P.; Bessa, R.J.B.; Minieri, S.; Giovannetti, L.; Conte, G.; Mele, M.; Messini, A.; Rapaccini, S.; et al. Effects of Chestnut Tannin Extract, Vescalagin and Gallic Acid on the Dimethyl Acetals Profile and Microbial Community Composition in Rumen Liquor: An In Vitro Study. Microorganisms 2019, 7, 202. [Google Scholar] [CrossRef]
- Rana, M.S.; Tyagi, A.; Hossain, S.A.; Tyagi, A.K. Effect of tanniniferous Terminalia chebula extract on rumen biohydrogenation, Δ9-desaturase activity, CLA content and fatty acid composition in longissimus dorsi muscle of kids. Meat Sci. 2012, 90, 558–563. [Google Scholar] [CrossRef]
- Gravador, R.S.; Luciano, G.; Jongberg, S.; Bognanno, M.; Scerra, M.; Andersen, M.L.; Lund, M.N.; Priolo, A. Fatty acids and oxidative stability of meat from lambs fed carob-containing diets. Food Chem. 2015, 182, 27–34. [Google Scholar] [CrossRef]
- van Leeuwen, K.A.; Camin, F.; Jerónimo, E.; Vasta, V.; Prenzler, P.D.; Ryan, D.; Bessa, R.J.B. Dietary Effects on Stable Carbon Isotope Composition of Fatty Acids in Polar and Neutral Fractions of Intramuscular Fat of Lambs. J. Agric. Food Chem. 2017, 65, 9404–9411. [Google Scholar] [CrossRef]
- McNabbl, W.C.; Waghorn, G.C.; Barry, T.N.; Shelton, I.D. The effect of condensed tannins in Lotus pedunculatus on the digestion and metabolism of methionine, cystine and inorganic sulphur in sheep. Br. J. Nutr. 1993, 70, 647–661. [Google Scholar] [CrossRef]
- Wang, Y.; Waghorn, G.C.; Barry, T.N.; Shelton, I.D. The effect of condensed tannins in Lotus corniculatus on plasma metabolism of methionine, cystine and inorganic sulphate by sheep. Br. J. Nutr. 1994, 72, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Scaramuzzi, R.J. Nutrient effects on ovulation rate, ovarian function and the secretion of gonadotrophic and metabolic hormones in sheep. J. Reprod. Fertil. Suppl. 1991, 43, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.A.; Joss, J.; Scaramuzzi, R.J. Ovulation rate and the concentrations of gonadotrophins and metabolic hormones in ewes infused with glucose during the late luteal phase of the oestrous cycle. J. Endocrinol. 1995, 146, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. BioMedicine Online 2012, 25, 551–560. [Google Scholar] [CrossRef]
- Heesom, K.J.; Harbeck, M.; Kahn, C.R.; Denton, R.M. Insulin action on metabolism. Diabetologia 1997, 40, B3. [Google Scholar] [CrossRef] [PubMed]
- Saus, J.; Wieslander, J.; Langeveld, J.P.; Quinones, S.; Hudson, B.G. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J. Biol. Chem. 1988, 263, 13374–13380. [Google Scholar] [CrossRef]
- Parkins, J.J.; Holmes, P.H. Effects of Gastrointestinal Helminth Parasites on Ruminant Nutrition. Nutr. Res. Rev. 1989, 2, 227–246. [Google Scholar] [CrossRef]
- Jackson, F. Anthelmintic resistance—The state of play. Br. Vet. J. 1993, 149, 123–138. [Google Scholar] [CrossRef]
- Bennet-Jenkins, E.; Bryant, C. Novel sources of anthelmintics. Int. J. Parasitol. 1996, 26, 937–947. [Google Scholar] [CrossRef]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 186, 18–27. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).