Toward High-Energy-Density Aqueous Lithium-Ion Batteries Using Silver Nanowires as Current Collectors
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of Electrolytes
2.2. Preparation of AgNW Films
2.3. Fabrication of Electrodes
2.4. Electrochemical Measurements
2.5. Characterization
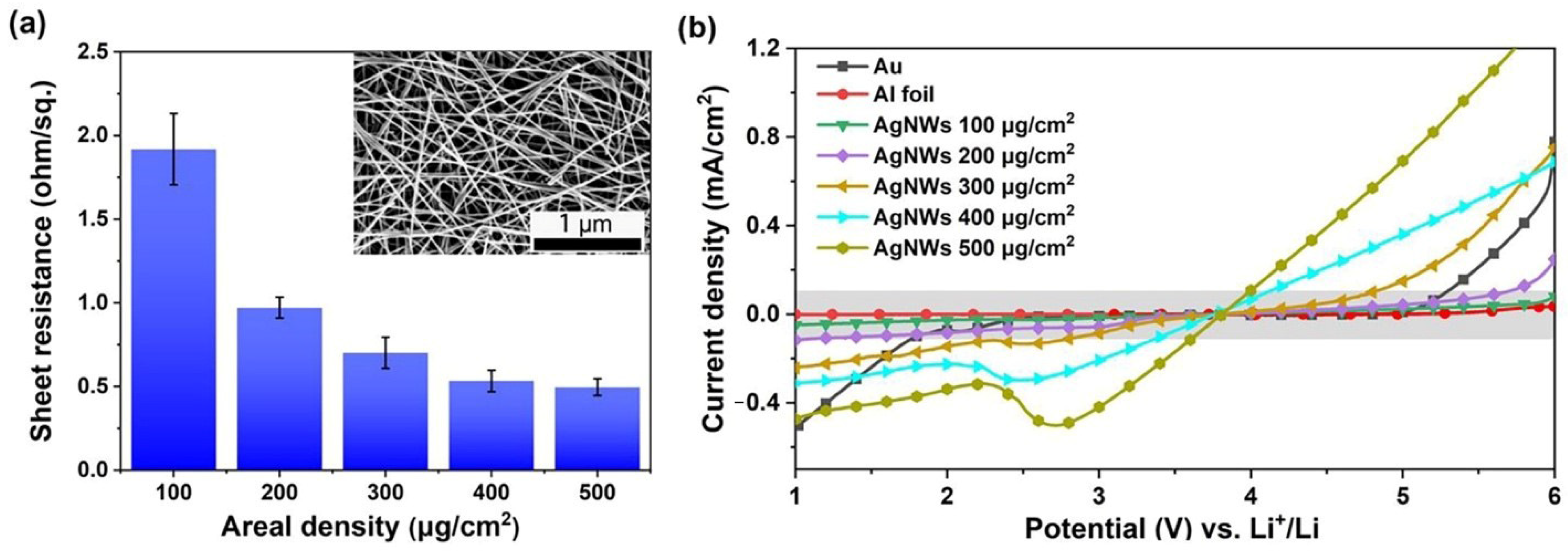
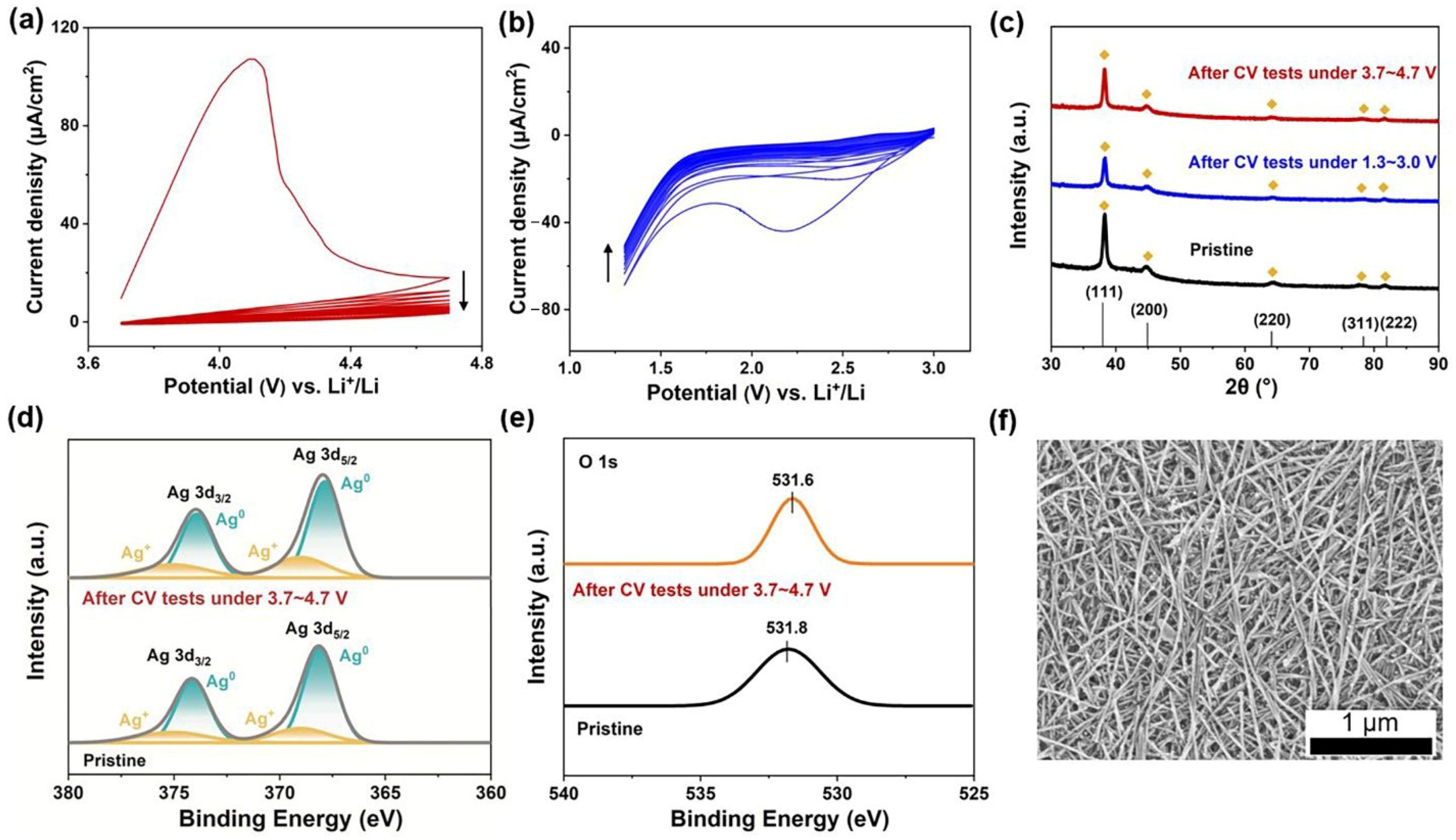
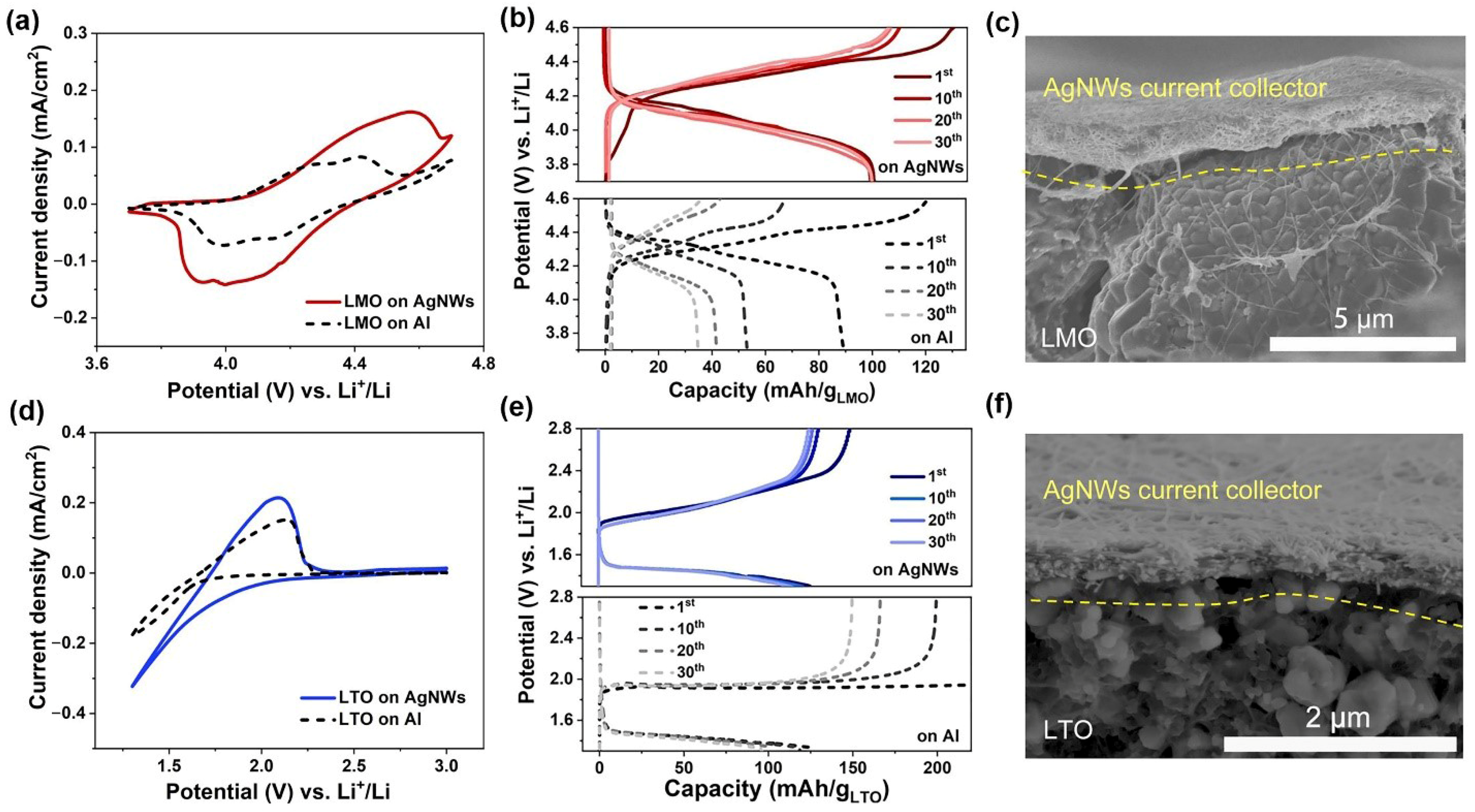
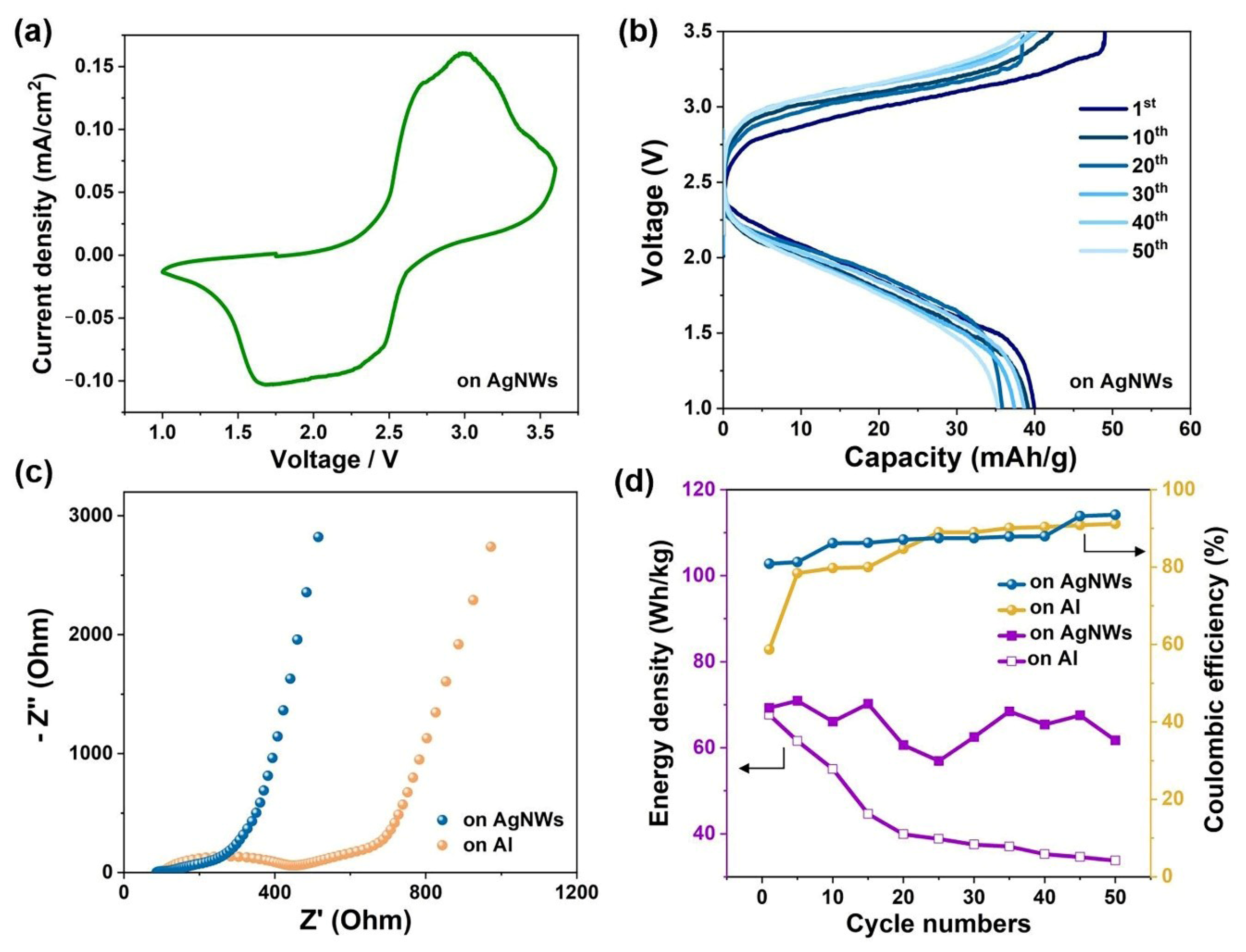
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, J.; Xia, Y. Recent Progress in Aqueous Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 830–840. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, K.Y.; Kim, H.; Kim, S.W.; Kang, K. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 2014, 114, 11788–11827. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.; Mohamad, A.A. Advances of aqueous rechargeable lithium-ion battery: A review. J. Power Sources 2015, 274, 237–251. [Google Scholar] [CrossRef]
- Bin, D.; Wen, Y.; Wang, Y.; Xia, Y. The development in aqueous lithium-ion batteries. J. Energy Chem. 2018, 27, 1521–1535. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced High-Voltage aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angew. Chem. Int. Ed. Engl. 2016, 55, 7136–7141. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z.A. Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
- Yang, C.; Suo, L.; Borodin, O.; Wang, F.; Sun, W.; Gao, T.; Fan, X.; Hou, S.; Ma, Z.; Amine, K.; et al. Unique aqueous Li-ion/sulfur chemistry with high energy density and reversibility. Proc. Natl. Acad. Sci. USA 2017, 114, 6197–6202. [Google Scholar] [CrossRef]
- Xie, J.; Liang, Z.; Lu, Y.C. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 2020, 19, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Kuhnel, R.S.; Reber, D.; Remhof, A.; Figi, R.; Bleiner, D.; Battaglia, C. “Water-in-salt” electrolytes enable the use of cost-effective aluminum current collectors for aqueous high-voltage batteries. Chem. Commun. 2016, 52, 10435–10438. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ouyang, Z.; Gu, M.; Cheng, W. Mechanically strong, optically transparent, giant metal superlattice nanomembranes from ultrathin gold nanowires. Adv. Mater. 2013, 25, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jiang, F.; Yang, C.; Fu, K.K.; Hayden, J.; Lin, C.F.; Xie, H.; Jiao, M.; Yang, C.; Wang, Y.; et al. Epitaxial Welding of Carbon Nanotube Networks for Aqueous Battery Current Collectors. ACS Nano 2018, 12, 5266–5273. [Google Scholar] [CrossRef]
- Huo, D.; Kim, M.J.; Lyu, Z.; Shi, Y.; Wiley, B.J.; Xia, Y. One-Dimensional Metal Nanostructures: From Colloidal Syntheses to Applications. Chem. Rev. 2019, 119, 8972–9073. [Google Scholar] [CrossRef]
- Jian, M.Q.; Wang, C.Y.; Wang, Q.; Wang, H.M.; Xia, K.L.; Yin, Z.; Zhang, M.C.; Liang, X.P.; Zhang, Y.Y. Advanced carbon materials for flexible and wearable sensors. Sci. China Mater. 2017, 60, 1026–1062. [Google Scholar] [CrossRef]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced Carbon for Flexible and Wearable Electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, S.; Zhao, H. Advances in constructing silver nanowire-based conductive pathways for flexible and stretchable electronics. Nanoscale 2022, 14, 11484–11511. [Google Scholar] [CrossRef]
- Huang, Q.; Zhu, Y. Patterning of Metal Nanowire Networks: Methods and Applications. ACS Appl. Mater. Interfaces 2021, 13, 60736–60762. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Cruz, M.A.; Yang, F.; Wiley, B.J. Accelerating electrochemistry with metal nanowires. Curr. Opin. Electrochem. 2019, 16, 19–27. [Google Scholar] [CrossRef]
- Tokuno, T.; Nogi, M.; Jiu, J.; Sugahara, T.; Suganuma, K. Transparent electrodes fabricated via the self-assembly of silver nanowires using a bubble template. Langmuir 2012, 28, 9298–9302. [Google Scholar] [CrossRef]
- Chen, G.; Bi, L.; Yang, Z.; Chen, L.; Wang, G.; Ye, C. Water-Based Purification of Ultrathin Silver Nanowires toward Transparent Conductive Films with a Transmittance Higher than 99. ACS Appl. Mater. Interfaces 2019, 11, 22648–22654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, T.; Shi, L.; Wen, N.; Wu, Z.; Sun, C.; Jiang, D.; Guo, Z. Recent progress for silver nanowires conducting film for flexible electronics. J. Nanostruct. Chem. 2021, 11, 323–341. [Google Scholar] [CrossRef]
- Najafi, M.; Abedini, A. The critical role of polymeric binders on AgO cathodes in high rate batteries. Thin Solid Films 2021, 721, 138532. [Google Scholar] [CrossRef]
- Wang, D.; Hua, H.; Liu, Y.; Tang, H.; Li, Y. Single Ag Nanowire Electrodes and Single Pt@Ag Nanowire Electrodes: Fabrication, Electrocatalysis, and Surface-Enhanced Raman Scattering Applications. Anal. Chem. 2019, 91, 4291–4295. [Google Scholar] [CrossRef]
- Cheng, Y.; Yan, M.; Jiang, Z. Electrochemical Behavior and Reduction Mechanism of High Valence Silver Oxide in Alkaline Solution. Electrochem. Solid-State Lett. 2007, 10, F5. [Google Scholar] [CrossRef]
- Huang, W.; Kang, X.; Xu, C.; Zhou, J.; Deng, J.; Li, Y.; Cheng, S. 2D PdAg Alloy Nanodendrites for Enhanced Ethanol Electroxidation. Adv. Mater. 2018, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Chu, G.; Luo, L.; Jin, J.; Zhao, B.; Tian, Y. Detection of 6-Mercaptopurine by silver nanowires-coated silicon wafer based on surface-enhanced Raman scattering spectroscopy. Colloid Surface Physicochem. Eng. Aspect. 2016, 508, 309–315. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Wang, K. Polyimide Aerogels Cross-Linked with Aminated Ag Nanowires: Mechanically Strong and Tough. Polymers 2017, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Tang, M.; Qin, L.; Kang, S.Z.; Li, X. Aluminum sheet induced flower-like carbon nitride anchored with silver nanowires for highly efficient SERS detection of trace malachite green. Environ. Res. 2022, 204, 112289. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.K.; Bhargav, P.B.; Ahmed, N.; Chandra, B.; Gnanapraksh, D.M.; Thyagarajan, N.; Racchana, R. All-solution Processed Highly Transparent Silver Nanowires/PEDOT:PSS Conducting Thin Films for Optoelectronic Applications. ChemistrySelect 2020, 5, 1370–1374. [Google Scholar] [CrossRef]
- Choo, D.C.; Kim, T.W. Degradation mechanisms of silver nanowire electrodes under ultraviolet irradiation and heat treatment. Sci. Rep. 2017, 7, 1696. [Google Scholar] [CrossRef] [PubMed]
- Kitta, M.; Kuratani, K.; Tabuchi, M.; Takeichi, N.; Akita, T.; Kiyobayashi, T.; Kohyama, M. Irreversible structural change of a spinel Li4Ti5O12 particle via Na insertion-extraction cycles of a sodium-ion battery. Electrochim. Acta 2014, 148, 175–179. [Google Scholar] [CrossRef]
- Lee, H.W.; Muralidharan, P.; Ruffo, R.; Mari, C.M.; Cui, Y.; Kim, D.K. Ultrathin spinel LiMn2O4 nanowires as high power cathode materials for Li-ion batteries. Nano Lett. 2010, 10, 3852–3856. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhan, C.; Wu, T.; Wen, J.; Lei, Y.; Kropf, A.J.; Wu, H.; Miller, D.J.; Elam, J.W.; Sun, Y.K.; et al. Effectively suppressing dissolution of manganese from spinel lithium manganate via a nanoscale surface-doping approach. Nat. Commun. 2014, 5, 5693. [Google Scholar] [CrossRef]
- Selvamani, V.; Phattharasupakun, N.; Wutthiprom, J.; Sawangphruk, M. High-performance spinel LiMn2O4@carbon core–shell cathode materials for Li-ion batteries. Sustain. Energy Fuels 2019, 3, 1988–1994. [Google Scholar] [CrossRef]
- Seki, H.; Yoshima, K.; Yamashita, Y.; Matsuno, S.; Takami, N. Aqueous lithium-ion battery of Li4Ti5O12/LiMn2O4 using a lithium-ion conductive solid electrolytes separator. J. Power Sources 2021, 482, 228950. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Han, W. Recycling and management of waste lead-acid batteries: A mini-review. Waste Manag. Res. 2016, 34, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cui, W.J.; He, P.; Xia, Y.Y. Raising the cycling stability of aqueous lithiumion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Zhu, Y.; Sun, H.; Wu, Y.; Zhu, K. An aqueous rechargeable lithium battery of excellent rate capability based on a nanocomposite of MoO3 coated with PPy and LiMn2O4. Energy Environ. Sci. 2012, 5, 6909–6913. [Google Scholar] [CrossRef]
- Dong, X.; Chen, L.; Su, X.; Wang, Y.; Xia, Y. Flexible Aqueous Lithium-Ion Battery with High Safety and Large Volumetric Energy Density. Angew. Chem. Int. Ed. Engl. 2016, 55, 7474–7477. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Han, F.; Fan, X.; Liu, H.; Xu, K.; Wang, C. “Water-in-Salt” electrolytes enable green and safe Li-ion batteries for large scale electric energy storage applications. J. Mater. Chem. A. 2016, 4, 6639–6644. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.; Wang, Y.; Wu, Y.; Zhang, L.; Gong, M.; Lin, X.; Wang, D. Toward High-Energy-Density Aqueous Lithium-Ion Batteries Using Silver Nanowires as Current Collectors. Molecules 2022, 27, 8207. https://doi.org/10.3390/molecules27238207
Kong J, Wang Y, Wu Y, Zhang L, Gong M, Lin X, Wang D. Toward High-Energy-Density Aqueous Lithium-Ion Batteries Using Silver Nanowires as Current Collectors. Molecules. 2022; 27(23):8207. https://doi.org/10.3390/molecules27238207
Chicago/Turabian StyleKong, Jingyi, Yangyang Wang, Ying Wu, Liang Zhang, Min Gong, Xiang Lin, and Dongrui Wang. 2022. "Toward High-Energy-Density Aqueous Lithium-Ion Batteries Using Silver Nanowires as Current Collectors" Molecules 27, no. 23: 8207. https://doi.org/10.3390/molecules27238207
APA StyleKong, J., Wang, Y., Wu, Y., Zhang, L., Gong, M., Lin, X., & Wang, D. (2022). Toward High-Energy-Density Aqueous Lithium-Ion Batteries Using Silver Nanowires as Current Collectors. Molecules, 27(23), 8207. https://doi.org/10.3390/molecules27238207





