Sustainable and Efficacy Approach of Green Synthesized Cobalt Oxide (Co3O4) Nanoparticles and Evaluation of Their Cytotoxicity Activity on Cancerous Cells
Abstract
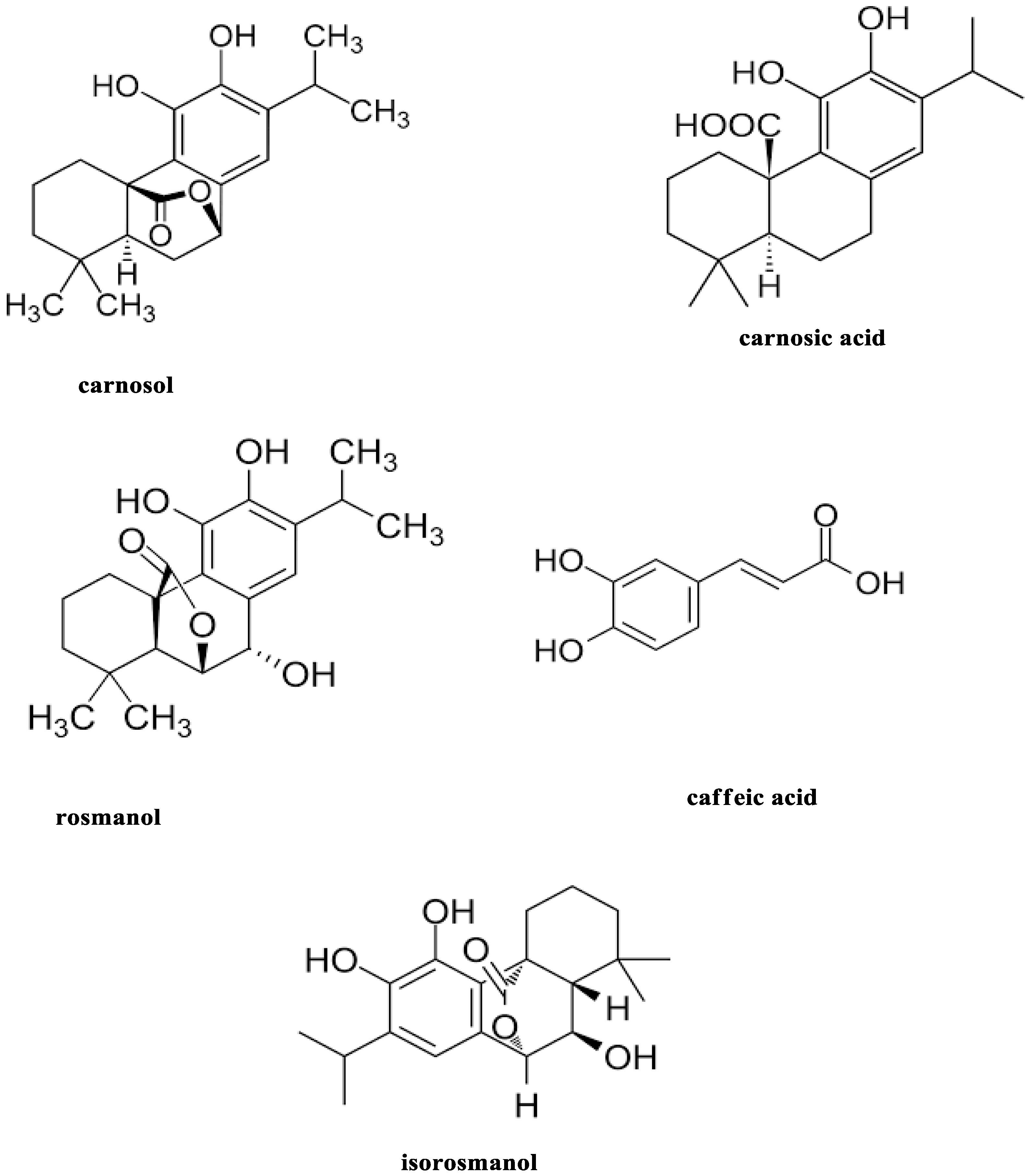
1. Introduction
2. Results and Discussion
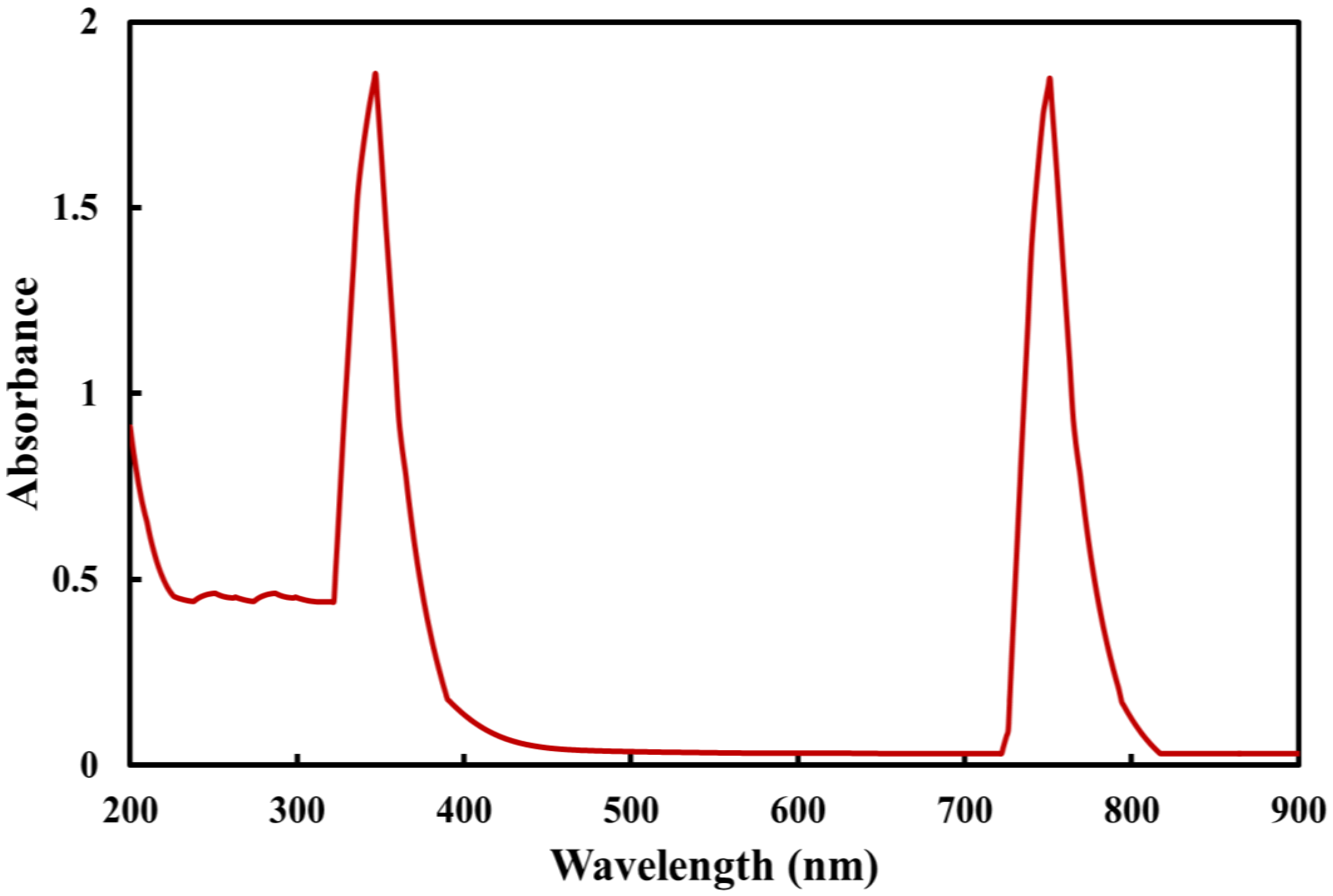
2.1. UV Spectroscopy
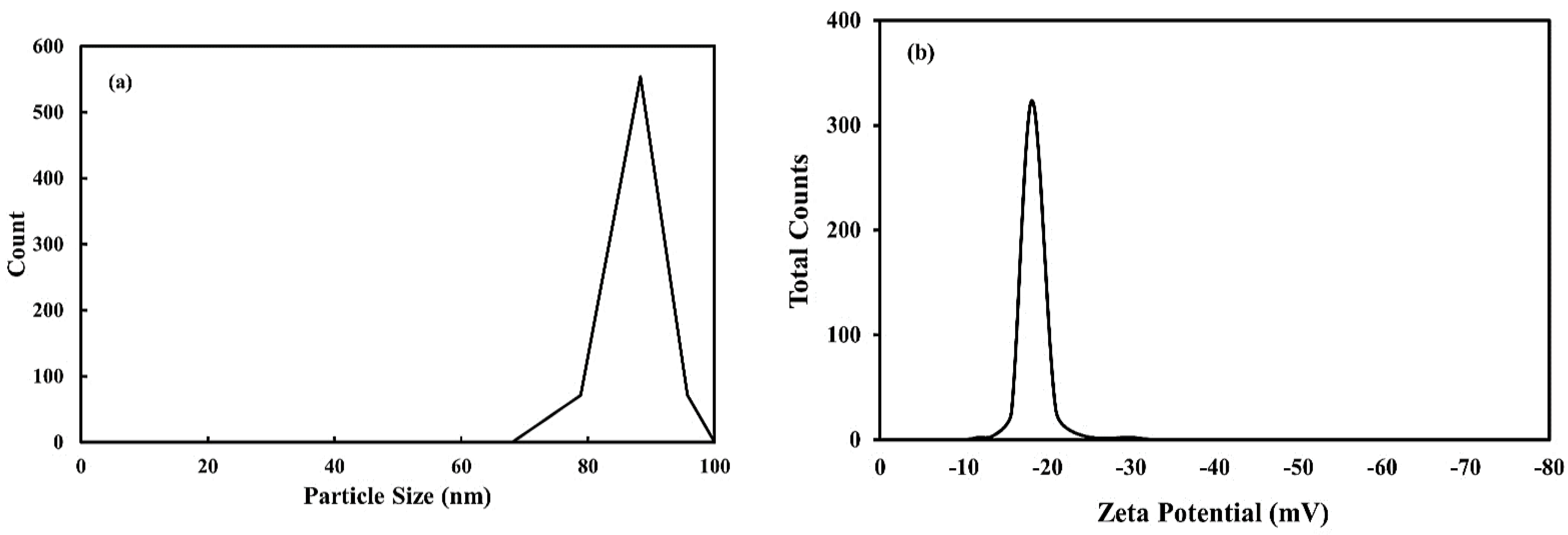
2.2. Dynamic Light Scattering (DLS) and Zeta Potential
2.3. TEM
2.4. SEM
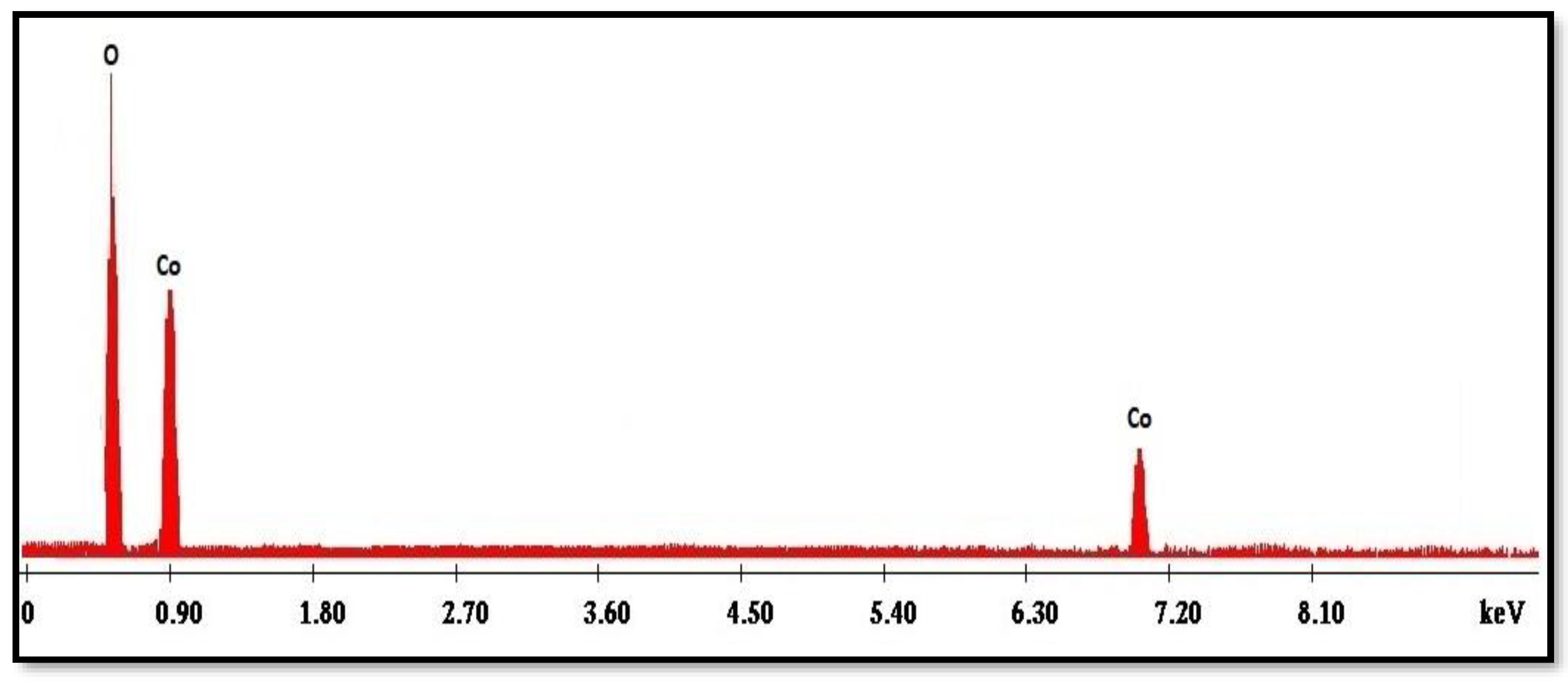
2.5. EDX Spectrum
2.6. Crystalline Structure
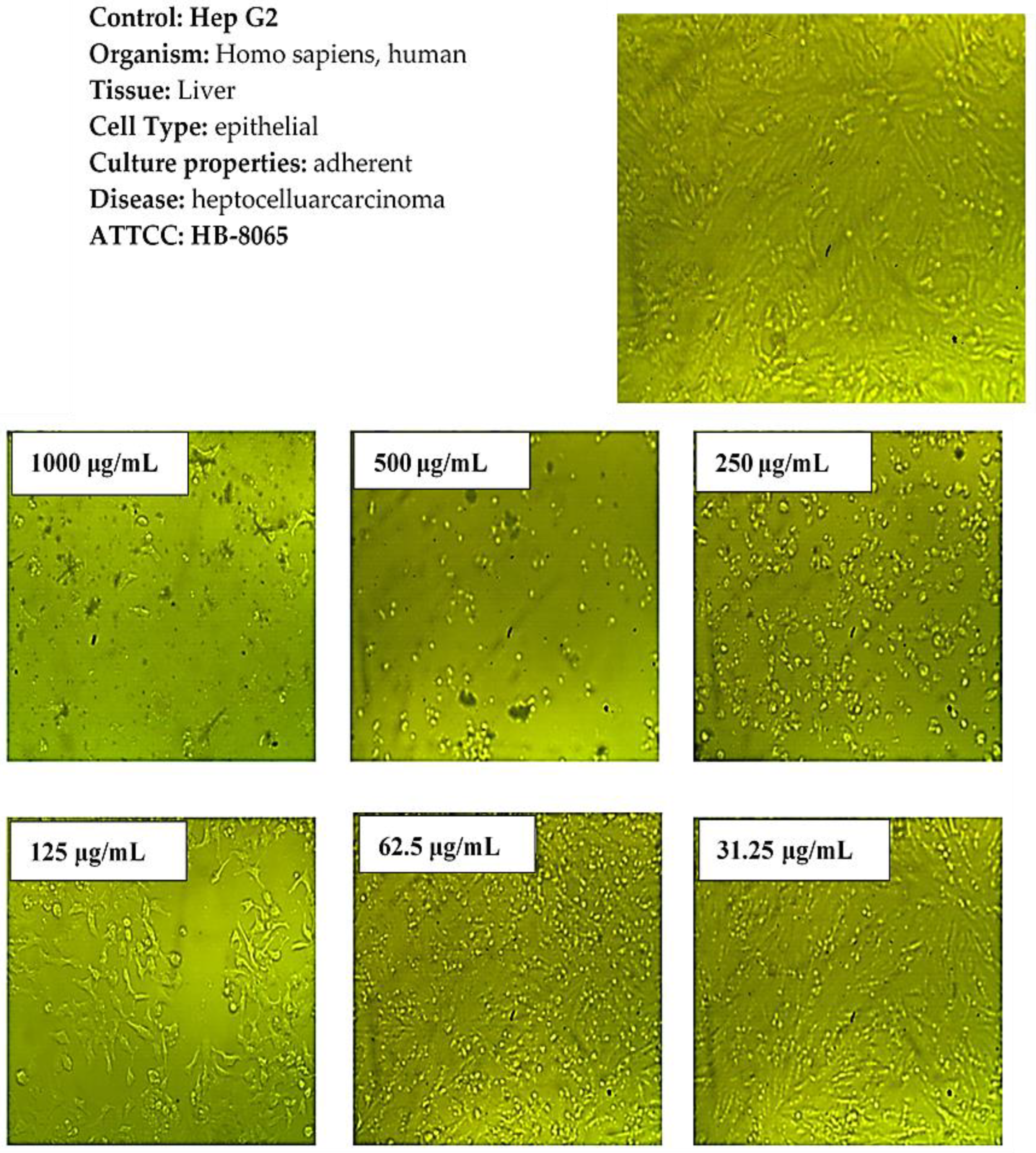
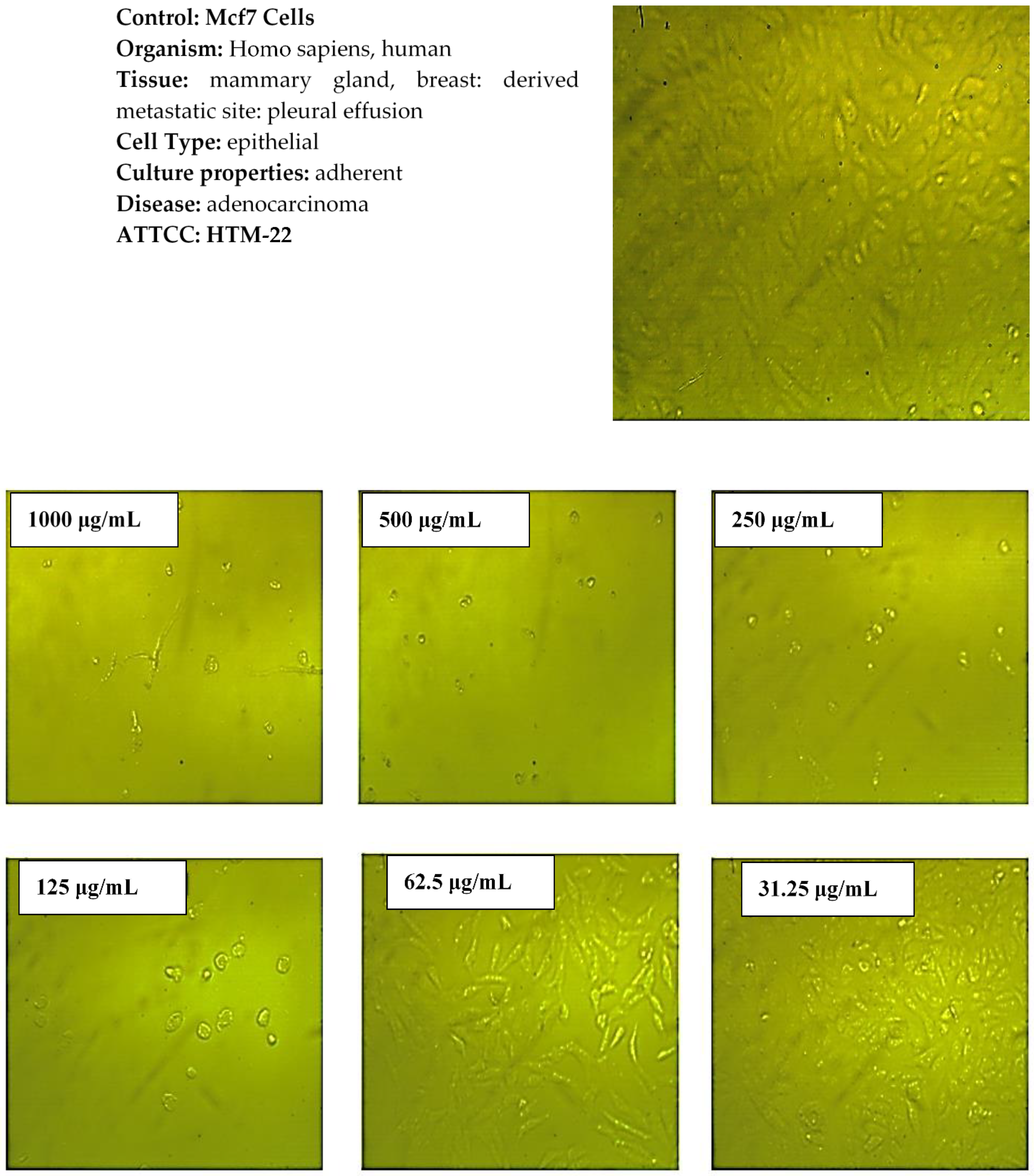
2.7. Cytotoxicity Activity
3. Experiments
3.1. Materials
3.2. Preparation of the Rosemary Leaf Extract
3.3. Biosynthesis of the Co3O4 NPs
3.4. Characterization of the Co3O4 NPs
3.5. In Vitro Cytotoxicity Evaluation
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manigandan, R.; Giribabu, K.; Suresh, R.; Vijayalakshm, L.; Stephen, A.; Narayanan, V. Cobalt oxide nanoparticles: Characterization and its electrocatalytic activity towards nitrobenzene. Chem. Sci. Trans. 2013, 2, S47–S50. [Google Scholar]
- Waris, A.; Misbahud, D.; Asmat, A.; Shakeeb, A.; Abdul, B.; Atta Ullah, K.; Muhammad, A. Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review. Open Life Sci. 2021, 16, 14–30. [Google Scholar] [CrossRef]
- Anele, A.; Obare, S.; Wei, J. Recent Trends and Advances of Co3O4 Nanoparticles in Environmental Remediation of Bacteria in Wastewater. Nanomaterials 2022, 12, 1129. [Google Scholar] [CrossRef]
- Sharma, J.; Pratibha, S.; Gurdip, M.; Shaheer, S.; Ameen, S. Green synthesis of Co3O4 nanoparticles and their applications in thermal decomposition of ammonium perchlorate and dye-sensitized solar cells. Mater. Sci. Eng. B 2015, 193, 181–188. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Sustainable synthesis of cobalt and cobalt oxide nanoparticles and their catalytic and biomedical applications. Green Chem. 2020, 22, 2643–2661. [Google Scholar] [CrossRef]
- Hafeez, M.; Shaheen, R.; Akram, B.; Haq, S. Green synthesis of cobalt oxide nanoparticles for potential biological applications. Mater. Res. Express 2020, 7, 025019. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, Y.; Liu, Y.; Li, D.; Zhu, X.; Ji, Q.; Quan, F. Synthesis of Co/Co3O4 nanoparticles embedded in porous carbon nanofibers for high performance lithium-ion battery anodes. J. Porous Mater. 2017, 24, 551–557. [Google Scholar] [CrossRef]
- Hsueh, T.; Wu, S.-S. Highly sensitive Co3O4 nanoparticles/MEMS NO2 gas sensor with the adsorption of the Au nanoparticles. Sens. Actuators B Chem. 2021, 329, 129201. [Google Scholar] [CrossRef]
- Khalid, N.; Gull, A.; Ali, F.; Tahir, M.; Iqbal, T. Bi-functional green-synthesized of Co3O4 NPs for photocatalytic and electrochemical applications. Ceram. Int. 2022, 48, 32009–32021. [Google Scholar] [CrossRef]
- Jarestan, M.; Khalatbari, K.; Sadat Shandiz, S.; Beigi, S. Preparation, characterization, and anticancer efficacy of novel cobalt oxide nanoparticles conjugated with thiosemicarbazide. Biotech 2020, 10, 230. [Google Scholar] [CrossRef]
- Bhargava, R.; Khan, S.; Ahmad, N. Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar]
- Farhadi, S.; Sepahdar, A.; Jahanara, K. Spinel-type cobalt oxide (Co3O4) nanoparticles from the mer-Co (NH3)3(NO2)3 complex: Preparation, characterization, and study of optical and magnetic properties. J. Nanostruct. 2013, 3, 199–207. [Google Scholar]
- Khan, M.; Ali, F.; Faisal, S.; Rizwan, M.; Hussain, Z. Exploring the therapeutic potential of Hibiscus rosa sinensis synthesized cobalt oxide (Co3O4-NPs) and magnesium oxide nanoparticles (MgO-NPs). Saudi J. Biol. Sci. 2021, 28, 5157–5167. [Google Scholar]
- Anuradha, C.; Raji, P. Citrus limon fruit juice-assisted biomimetic synthesis, characterization and antimicrobial activity of cobalt oxide (Co3O4) nanoparticles. Appl. Phys. A 2021, 127, 55. [Google Scholar] [CrossRef]
- Ngnintedem Yonti, C.; Patrice, K.; Roussin, L.; Francois, D.; John Lambi, N. Green Synthesis of Iron-Doped Cobalt Oxide Nanoparticles from Palm Kernel Oil via Co-Precipitation and Structural Characterization. Nanomaterials 2021, 11, 2833. [Google Scholar] [CrossRef]
- Niu, M.; Wang, Y.; Cheng, Y.; Chen, G.; Cui, L. Fabrication of Co3O4 cubic nanoframes: Facet-preferential chemical etching of Fe3+ ions to Co3O4 nanocubes. Mater. Lett. 2009, 63, 837–839. [Google Scholar] [CrossRef]
- Shaalan, N.; Rashad, M.; Moharram, A. Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Matinise, N.; Mayedwa, N.; Fuku, X. Green synthesis of cobalt (II, III) oxide nanoparticles using Moringa Oleifera natural extract as high electrochemical electrode for supercapacitors. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar]
- Diallo, A.; Beye, A.; Doyle, T.; Park, E. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Khalil, A.; Muhammad, O.; Ikram, U.; Muhammad, A.; Zabta, K.; Malik, M. Physical properties, biological applications and biocompatibility studies on biosynthesized single phase cobalt oxide (Co3O4) nanoparticles via Sageretia thea (Osbeck.). Arab. J. Chem. 2020, 13, 606–619. [Google Scholar] [CrossRef]
- Ikhuoria, E.U.; Omorogbe, S.O.; Sone, B.T.; Maaza, M. Bioinspired shape controlled antiferromagnetic Co3O4 with prism like-anchored octahedron morphology: A facile green synthesis using Manihot esculenta Crantz extract. Sci. Technol. Mater. 2018, 30, 92–98. [Google Scholar] [CrossRef]
- Edison, T.; Raji, A.; Gopalakrishnan, S.; Lee, Y. Supercapacitor performance of carbon supported Co3O4 nanoparticles synthesized using Terminalia chebula fruit. J. Taiwan Inst. Chem. Eng. 2016, 68, 489–495. [Google Scholar] [CrossRef]
- Pagar, T.; Suresh, G.; Khanderao, P.; Shreyas, P.; Rajeshwari, O. A review on bio-synthesized Co3O4 nanoparticles using plant extracts and their diverse applications. J. Chem. Rev. 2019, 1, 260–270. [Google Scholar]
- Labban, L.; Mustafa, U.E.-S.; Ibrahim, Y.M. The effects of rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid perodoxation. Int. J. Clin. Med. 2014, 2014, 44285. [Google Scholar]
- Hammer, M.; Junghanns, W. Rosmarinus officinalis L.: Rosemary. In Medicinal, Aromatic and Stimulant Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 501–521. [Google Scholar]
- Ribeiro-Santos, R.; Carvalho-Costaad, D.; Helena, C.; Gonçalves, A.T.; Conceição, C.M.; Fernando, R.; Nathália, R.; Ana, S. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Tyagi, A. The effects of rosemary (Rosemarinus officinalis) leaves powder on glucose level. World J. Pharm. Res. 2017, 6, 548–554. [Google Scholar]
- Badreah, A.A.; Ahmed, A.; Mohamed, M.; Kamel, S. Phytosynthesis of Co3O4 Nanoparticles as the High Energy Storage Material of an Activated Carbon/Co3O4 Symmetric Supercapacitor Device with Excellent Cyclic Stability Based on a Na2SO4 Aqueous Electrolyte. ACS Omega 2022, 7, 23673–23684. [Google Scholar]
- Dewi, N.O.M.; Yulizar, Y.; Apriandanu, D.O.B. Green synthesis of Co3O4 nanoparticles using Euphorbia heterophylla L. leaves extract: Characterization and photocatalytic activity. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Leone, P.; Antonio, G.; Rossella, F.; Antonella, A.; Eleonora, M.; Alessio, B.; Luigi, G.; Valli, D.; Nicola, S.; Vito, R. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines 2021, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Al-Qasmi, N.; Almughem, F.A.; Jarallah, S.J.; Almaabadi, A. Efficient Green Synthesis of (Fe3O4) and (NiFe2O4) Nanoparticles Using Star Anise (Illicium verum) Extract and Their Biomedical Activity against Some Cancer Cells. Materials 2022, 15, 4832. [Google Scholar] [CrossRef]
- Al-Qasmi, N. Facial eco-friendly synthesis of copper oxide nanoparticles using chia seeds extract and evaluation of its electrochemical activity. Processes 2021, 9, 2027. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qasmi, N. Sustainable and Efficacy Approach of Green Synthesized Cobalt Oxide (Co3O4) Nanoparticles and Evaluation of Their Cytotoxicity Activity on Cancerous Cells. Molecules 2022, 27, 8163. https://doi.org/10.3390/molecules27238163
Al-Qasmi N. Sustainable and Efficacy Approach of Green Synthesized Cobalt Oxide (Co3O4) Nanoparticles and Evaluation of Their Cytotoxicity Activity on Cancerous Cells. Molecules. 2022; 27(23):8163. https://doi.org/10.3390/molecules27238163
Chicago/Turabian StyleAl-Qasmi, Noha. 2022. "Sustainable and Efficacy Approach of Green Synthesized Cobalt Oxide (Co3O4) Nanoparticles and Evaluation of Their Cytotoxicity Activity on Cancerous Cells" Molecules 27, no. 23: 8163. https://doi.org/10.3390/molecules27238163
APA StyleAl-Qasmi, N. (2022). Sustainable and Efficacy Approach of Green Synthesized Cobalt Oxide (Co3O4) Nanoparticles and Evaluation of Their Cytotoxicity Activity on Cancerous Cells. Molecules, 27(23), 8163. https://doi.org/10.3390/molecules27238163




