Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta domesticus)
Abstract
1. Introduction
2. Results and Discussion
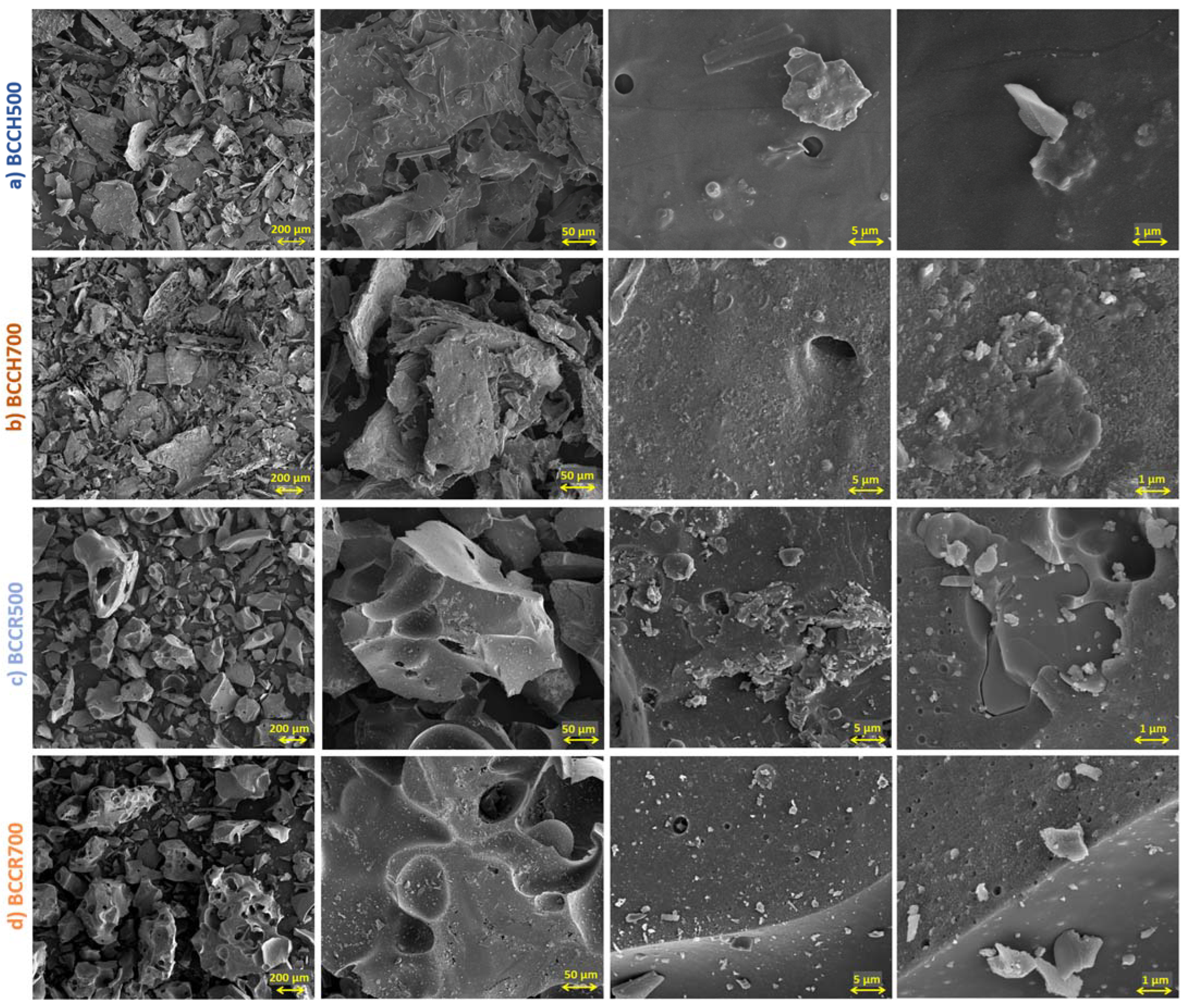
2.1. Physiochemical Characterization
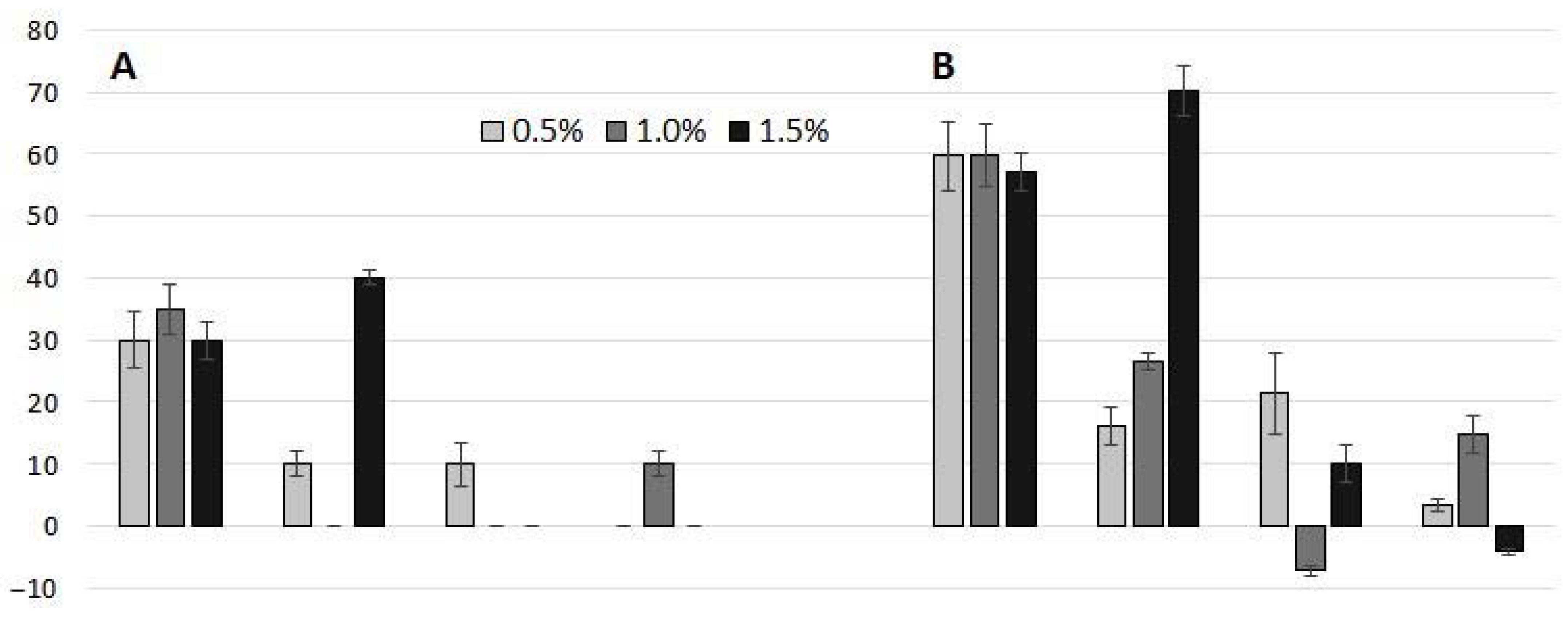
2.2. Phytoxicity of Biochars
3. Materials and Methods
3.1. Fractionation of Cricket Powder
3.2. Chemicals
3.3. Biochar Preparation
3.4. Physiochemical Characterization
3.5. Phytotoxicity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghinoi, S.; Silvestri, F.; Steiner, B. The role of local stakeholders in disseminating knowledge for supporting the Circular Economy: A network analysis approach. Ecol. Econ. 2020, 169, 106446. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Hawkey, K.J.; Lopez-Viso, C.; Brameld, J.M.; Parr, T.; Salter, A.M. Insects: A potential source of protein and other nutrients for feed and food. Annu. Rev. Anim. Biosci. 2021, 9, 333–354. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing food’s environmental impacts through producers and consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.A.; Worth, D.E.; Verg´e, X.P.C.; Desjardins, R.L. Impact of recommended red meat consumption in Canada on the carbon footprint of Canadian livestock production. J. Clean Prod. 2020, 266, 121785. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Toviho, O.; Bársony, P. Insect base-protein: A new opportunity in animal nutrition. Acta Agrar. Debr. 2020, 1, 129–138. [Google Scholar] [CrossRef]
- Poveda, J. Insect frass in the development of sustainable agriculture. A review Agron. Sustain. Dev. 2021, 41, 1–10. [Google Scholar] [CrossRef]
- Vauterin, A.; Steiner, B.; Sillman, J.; Kahiluoto, H. The potential of insect protein to reduce food-based carbon footprints in Europe: The case of broiler meat production. J. Clean Prod. 2021, 320, 128799. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed (IPIFF) The European Insect Sector Today: Challenges, Opportunities and Regulatory Landscape. IPIFF Vision Paper on the Future of the Insect Sector towards 2030. IPIFF 2018. Available online: https://ipiff.org/wp-content/uploads/2021/04/Apr-27-2021-IPIFF_The-European-market-of-insects-as-feed.pdf (accessed on 1 October 2022).
- Barker, D.; Fitzpatrick, M.P.; Dierenfeld, E.S. Nutrient composition of selected whole invertebrates. Zoo. Biol. 1998, 17, 123–134. [Google Scholar] [CrossRef]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Ghorbel-Bellaaj, O.; Hajji, R.; Rinaudo, M.; Nasri, M.; Jellouli, K. Structural differences between chitin and chitosan extracted from three different marine sources. Int. J. Biol. Macromol. 2014, 65, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Santos, J.M.N.; Moura, J.M.; Pinto, L.A.A. Ultrasound–assisted treatment of chitin: Evaluation of physicochemical characteristics and dye removal potential. E-Polymers 2016, 16, 49–56. [Google Scholar] [CrossRef]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, characterization and environmental/electrochemical energy storage testing of low–cost biochar from natural chitin obtained via pyrolysis at mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Lee, K.Y.; Jeong, L.; Kang, Y.O.; Lee, S.J.; Park, W.H. Electrospinning of Polysaccharides for Regenerative Medicine. Adv. Drug. Deliv. Rev. 2009, 61, 1020–1032. [Google Scholar] [CrossRef]
- Ifuku, S. Chitin and Chitosan Nanofibers: Preparation and chemical modifications. Molecules 2014, 19, 18367–18380. [Google Scholar] [CrossRef]
- Su, W.; Yu, S.; Wu, D.; Xia, M.; Wen, Z.; Yao, Z.; Tang, J.; Wu, W. A critical review of cast-off crab shell recycling from the perspective of functional and versatile biomaterials. Environ. Sci. Pollut. Res. 2019, 26, 31581–31591. [Google Scholar] [CrossRef]
- Varma, R.S. Biomass-Derived Renewable Carbonaceous Materials for Sustainable Chemical and Environmental Applications. ACS Sustain. Chem. Eng. 2019, 7, 6458–6470. [Google Scholar] [CrossRef]
- Corazzari, I.; Nisticò, R.; Turci, F.; Faga, M.G.; Franzoso, F.; Tabasso, S.; Magnacca, G. Advanced physico-chemical characterization of chitosan by means of TGA coupled on-line with FTIR and GCMS: Thermal degradation and water adsorption capacity. Polym. Degrad. Stab. 2015, 112, 1–9. [Google Scholar] [CrossRef]
- Muzzarelli, R.A. Chitin; Pergamon Press Ltd.: Oxford, UK, 1977. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Hanafiah, M.A.K.M. Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr. Polym. 2011, 83, 1446–1456. [Google Scholar] [CrossRef]
- Nisticò, R.; Franzoso, F.; Cesano, F.; Scarano, D.; Magnacca, G.; Carlos, L.; Parolo, M.E. Chitosan-derived iron oxide systems for magnetically-guided and efficient water purification processes from polycyclic aromatic hydrocarbons. ACS Sustain. Chem. Eng. 2017, 5, 793–801. [Google Scholar] [CrossRef]
- Yuan, H.; Deng, L.; Cai, X.; Zhou, S.; Chen, Y.; Yuan, Y. Nitrogen–doped carbon sheets derived from chitin as non–metal bifunctional electrocatalysts for oxygen reduction and evolution. RSC Adv. 2015, 5, 56121–56129. [Google Scholar] [CrossRef]
- Zazycki, M.A.; Borba, P.A.; Silva, R.N.F.; Peres, E.C.; Perondi, D.; Collazzo, G.C.; Dotto, G.L. Chitin derived biochar as an alternative adsorbent to treat colored effluents containing methyl violet dye. Adv. Powder Technol. 2019, 30, 1494–1503. [Google Scholar] [CrossRef]
- Zhou, J.; Bao, L.; Wu, S.; Yang, W.; Wang, H. Chitin based hetero-atom doped porous carbon as electrode materials for super capacitors. Carbohydr. Polym. 2017, 173, 321–329. [Google Scholar] [CrossRef]
- Khanday, W.A.; Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. Singlestep pyrolysis of phosphoric acid-activated chitin for efficient adsorption of cephalexin antibiotic. Bioresour. Technol. 2019, 280, 255–259. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, R.N.; Tian, Y.D.; Sun, Z.; Huang, Z.H.; Wu, X.L.; Li, B. Heteroatoms-doped hierarchical porous carbon derived from chitin for flexible all-soil-state symmetric super capacitors. Chem. Eng. J. 2020, 384, 123–263. [Google Scholar] [CrossRef]
- Ji, Q.; Li, H. High surface area activated carbon derived from chitin for efficient adsorption of Crystal Violet. Diam. Relat. Mater. 2021, 118, 108516. [Google Scholar] [CrossRef]
- Gao, L.; Ma, J.; Li, S.; Liu, D.; Xu, D.; Cai, J.; Chen, L.; Xie, J.; Zhang, L. 2D Ultrathin Carbo Nanosheets with Rich N/O Content Constructed by Stripping Bulk Chitin for High-Performance Sodium Ion Batteries. Nanoscale 2019, 1, 12626–12636. [Google Scholar] [CrossRef]
- Gao, L.; Ying, D.; Shen, T.; Zheng, Y.; Cai, J.; Wang, D.; Zhang, L. Two-Dimensional Wrinkled N-Rich Carbon Nanosheets Fabricated from Chitin via Fast Pyrolysis as Optimized Electrocatalyst. ACS Sustain. Chem. Eng. 2020, 8, 10881–10891. [Google Scholar] [CrossRef]
- Suman, S.; Panwar, D.S.; Gautam, S. Surface morphology properties of biochars obtained from different biomass waste. Energy Sources A Recovery Util. Environ. Eff. 2017, 39, 1007–1012. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Preparation of adsorbents from sewage sludge pyrolytic char by carbon dioxide activation. Process. Saf. Environ. Prot. 2016, 103, 76–86. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P. Characterization of biochars produced from residues from biogas production. J. Anal. Appl. Pyrolysis 2015, 115, 157–165. [Google Scholar] [CrossRef]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Gunamantha, I.M.; Widana, G.A.B. Characterization the potential of biochar from cow and pig manure for geoecology application. IOP Conf. Ser. Environ. Earth Sci. 2018, 131, 012055. [Google Scholar] [CrossRef]
- Henriques, B.S.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Determination of Chitin Content in Insects: An Alternate Method Based on Calcofluor Staining. Front. Physiol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Liu, C.H.; Chu, W.; Li, H.; Boyd, S.A.; Teppen, B.J.; Mao, J.; Lehmann, J.; Zhang, W. Quantification and characterization of dissolved organic carbon from biochars. Geoderma 2019, 335, 161–169. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Yang, L.; May, P.W.; Yin, L.; Smith, J.A.; Rosser, K.N. Ultra fine carbon nitride nanocrystals synthesized by laser ablation in liquid solution. J. Nanopart Res. 2007, 9, 1181–1185. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Bounaas, M.; Bouguettoucha, A.; Chebli, D.; Gatica, J.M.; Vidal, H. Role of the Wild Carob as Biosorbent and as Precursor of a New High-Surface-Area Activated Carbon for the Adsorption of Methylene Blue. Arab. J. Sci. Eng. 2021, 46, 325–341. [Google Scholar] [CrossRef]
- Ibrahim, I.; Tsubota, T.; Hassan, M.A.; Andou, Y. Surface Functionalization of Biochar from Oil Palm Empty Fruit Bunch through Hydrothermal Process. Processes 2021, 9, 149. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Pii, Y.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef]
- Volkov, D.S.; Rogova, O.B.; Proskurnin, M.A. Organic Matter and Mineral Composition of Silicate Soils: FTIR Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection Modalities. Agronomy 2021, 11, 1879. [Google Scholar] [CrossRef]
- Salimi, E.; Javadpour, J. Synthesis and Characterization of Nanoporous Monetite Which Can Be Applicable for Drug Carrier. J. Nanomater. 2012, 931492. [Google Scholar] [CrossRef]
- Sazali, N.E.S.; Deraman, M.; Omar, R.; Othman, M.A.R.; Suleman, M.; Shamsudin, S.A.; Tajuddin, N.S.M.; Hanappi, M.F.Y.M.; Hamdan, E.; Nor, N.S.M.; et al. Preparation and structural characterization of turbostratic-carbon/graphene derived from amylose film. AIP Conf. Proc. 2016, 1784, 040009. [Google Scholar] [CrossRef]
- Irungu, F.G.; Mutungi, C.M.; Faraj, A.K.; Affognon, H.; Tanga, C.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Minerals content of extruded fish feeds containing cricket (Acheta domesticus) and black soldier fly larvae (Hermetia illucens) fractions. Int. Aquat. Res. 2018, 10, 101–113. [Google Scholar] [CrossRef]
- Ojha, S.; Bekhit, A.E.D.; Grune, T.; Schlüter, O.K. Bioavailability of nutrients from edible insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- Gell, K.; van Groenigen, J.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard Mater. 2010, 186, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass. Bioenerg. 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; García de Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling pyrolyzed organic waste from plant nurseries, rice production and shrimp industry as peat substitute in potting substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Tzortzakis, N. Critical review of chemical properties of biochar as a component of growing media. Acta Hortic. 2021, 1317, 115–124. [Google Scholar] [CrossRef]
- AOAC-International; Latimer, G.W. Official Methods of Analysis, 19th ed.; AOAC International: Gathersburg, MD, USA, 2012. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341. [Google Scholar] [CrossRef]
- Babiker, E.E.; Hassan, A.B.; Eltayeb, M.M. Solubility and functional properties of boiled and fried sudanese tree locust flour as a function of NaCl concentration. J. Food Technol. 2007, 5, 210–214. Available online: https://medwelljournals.com/abstract/?doi=jftech.2007.210.214 (accessed on 1 October 2022).
- Kim, H.W.; Setyabrata, D.; Lee, Y.; Jones, O.G.; Kim, Y.H.B. Effect of house cricket (Acheta domesticus) flour addition on physicochemical and textural properties of meat emulsion under various formulations. J. Food Sci. 2017, 82, 2787–2793. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]

| Element wt% | BCCH500 | BCCH700 | BCCR500 | BCCR700 |
|---|---|---|---|---|
| C | 69.49 ± 0.57 | 67.03 ± 1.86 | 71.58 ± 0.90 | 70.65 ± 2.11 |
| N | 9.84 ± 0.62 | 8.00 ± 0.37 | 11.80 ± 2.18 | 11.15 ± 0.70 |
| O | 13.28 ± 0.57 | 15.69 ± 1.81 | 9.23 ± 0.93 | 9.33 ± 2.45 |
| Na | 4.10 ± 0.13 | 4.83 ± 0.61 | 1.19 ± 0.36 | 1.24 ± 0.18 |
| Ca | 1.28 ± 0.42 | 1.80 ± 0.45 | ||
| K | 0.84 ± 0.29 | 2.87 ± 0.67 | 4.17 ± 1.48 | |
| P | 2.04 ± 0.55 | 2.06 ± 0.46 |
| Value | Ash | pH | EC | TOC | DOC | C | H | N | O | O/C | (O+N)/C | H/C | SBET | Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (µS/cm) | (%) | (mg/L) | (%) | (%) | (%) | (%) | (m2/g) | (%) | |||||
| Chitin | 9.4 | 10.13 | 11060 | 47.3 | nd | 46.2 | 6.3 | 10.2 | 27.9 | 0.45 | 0.64 | 1.62 | nd | - |
| BCCH500 | 15.2 | 12.2 | 8.8 | 60.1 | 63.4 | 65.7 | 2.3 | 9.8 | 7.0 | 0.08 | 0.21 | 0.41 | 0.05 | 25.1 |
| BCCH700 | 16.4 | 12.4 | 12.1 | 53.4 | 9.6 | 70.3 | 1.2 | 9.4 | 2.7 | 0.03 | 0.14 | 0.21 | 0.17 | 24.5 |
| Cricket | 3.8 | 5.5 | 5560 | 47.6 | nd | 52.7 | 7.9 | 10.6 | 24.9 | 0.35 | 0.53 | 1.79 | nd | - |
| BCCR500 | 17.9 | 8.7 | 4.2 | 57.6 | 4.0 | 63.6 | 2.2 | 11.2 | 5.1 | 0.06 | 0.21 | 0.42 | 0.22 | 22.9 |
| BCCR700 | 16.2 | 10.8 | 2076.7 | 52.2 | 3.3 | 69.1 | 1.0 | 10.6 | 3.1 | 0.03 | 0.17 | 0.17 | 0.04 | 22.3 |
| Metal [mg/kg] | BCCH500 | BCCH700 | BCCR500 | BCCR700 |
|---|---|---|---|---|
| Na | 41,792.36 | 33,476.92 | 14,177.70 | 9614.68 |
| Ca | 16,028.90 | 17,858.92 | 5475.49 | 4301.13 |
| Mg | 3298.81 | 3644.61 | 3419.12 | 2120.66 |
| Zn | 888.74 | 936.21 | 892.77 | 630.40 |
| Fe | 476.31 | 379.71 | 534.31 | 289.26 |
| Mn | 274.75 | 294.43 | 159.31 | 117.17 |
| B | 50.86 | 62.87 | 21.45 | 100.08 |
| Cu | 37.83 | 40.46 | 88.85 | 26.24 |
| Pb | <LOD | <LOD | <LOD | 3.66 |
| Ni | <LOD | <LOD | <LOD | <LOD |
| Co | <LOD | <LOD | <LOD | <LOD |
| Cd | <LOD | <LOD | <LOD | <LOD |
| Cr | <LOD | <LOD | <LOD | <LOD |
| Inhibition of Seed Germination | Root Growth Inhibition | |||||
|---|---|---|---|---|---|---|
| 0.5% | 1.0% | 1.5% | 0.5% | 1.0% | 1.5% | |
| ASH% | −0.545 | −0.821 | −0.560 | −0.541 | −0.946 * | −0.482 |
| pH | 0.410 | 0.454 | 0.868 | 0.343 | 0.839 | 0.783 |
| EC | 0.507 | 0.099 | 0.976 ** | 0.401 | 0.552 | 0.986 ** |
| TOC | 0.876 | 0.619 | 0.135 | 0.904 * | 0.401 | 0.269 |
| DOC | 0.944 * | 0.938 * | 0.491 | 0.955 ** | 0.896 | 0.528 |
| C% | −0.403 | −0.197 | 0.438 | −0.466 | 0.207 | 0.288 |
| H% | 0.749 | 0.427 | −0.006 | 0.779 | 0.174 | 0.148 |
| N% | −0.412 | −0.300 | −0.956 ** | −0.324 | −0.752 | −0.894 |
| O% | 0.857 | 0.753 | 0.037 | 0.906 * | 0.469 | 0.149 |
| Cu | 0.010 | −0.429 | −0.368 | 0.020 | −0.627 | −0.220 |
| Zn | 0.589 | −0.037 | 0.640 | 0.510 | 0.195 | 0.754 |
| Al | 0.690 | 0.379 | 0.983 ** | 0.603 | 0.762 | 0.991 ** |
| B | −0.465 | 0.083 | −0.038 | −0.452 | 0.187 | −0.204 |
| Ba | 0.687 | 0.435 | 0.981 ** | 0.604 | 0.812 | 0.976 ** |
| Bi | 0.927 * | 0.958 ** | 0.404 | 0.950 ** | 0.866 | 0.444 |
| Ca | 0.636 | 0.324 | 0.993 ** | 0.544 | 0.734 | 0.995 ** |
| Fe | 0.597 | 0.125 | 0.013 | 0.603 | −0.049 | 0.180 |
| Ga | 0.927 * | 0.958 ** | 0.404 | 0.950 ** | 0.866 | 0.444 |
| In | 0.802 | 0.598 | 0.922 | 0.739 | 0.893 | 0.928 * |
| Mg | 0.527 | −0.112 | 0.623 | 0.444 | 0.134 | 0.735 |
| Mn | 0.676 | 0.297 | 0.975 ** | 0.584 | 0.685 | 0.998 ** |
| Na | 0.849 | 0.584 | 0.905 * | 0.786 | 0.856 | 0.932 * |
| Sr | 0.595 | 0.323 | 0.998 ** | 0.503 | 0.747 | 0.985 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Różyło, K.; Jędruchniewicz, K.; Krasucka, P.; Biszczak, W.; Oleszczuk, P. Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta domesticus). Molecules 2022, 27, 8071. https://doi.org/10.3390/molecules27228071
Różyło K, Jędruchniewicz K, Krasucka P, Biszczak W, Oleszczuk P. Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta domesticus). Molecules. 2022; 27(22):8071. https://doi.org/10.3390/molecules27228071
Chicago/Turabian StyleRóżyło, Krzysztof, Katarzyna Jędruchniewicz, Patrycja Krasucka, Wojciech Biszczak, and Patryk Oleszczuk. 2022. "Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta domesticus)" Molecules 27, no. 22: 8071. https://doi.org/10.3390/molecules27228071
APA StyleRóżyło, K., Jędruchniewicz, K., Krasucka, P., Biszczak, W., & Oleszczuk, P. (2022). Physicochemical Characteristics of Biochar from Waste Cricket Chitin (Acheta domesticus). Molecules, 27(22), 8071. https://doi.org/10.3390/molecules27228071