The Effects of Different Doses of ROCK Inhibitor, Antifreeze Protein III, and Boron Added to Semen Extender on Semen Freezeability of Ankara Bucks
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals, Animals, and Sperm Freezing
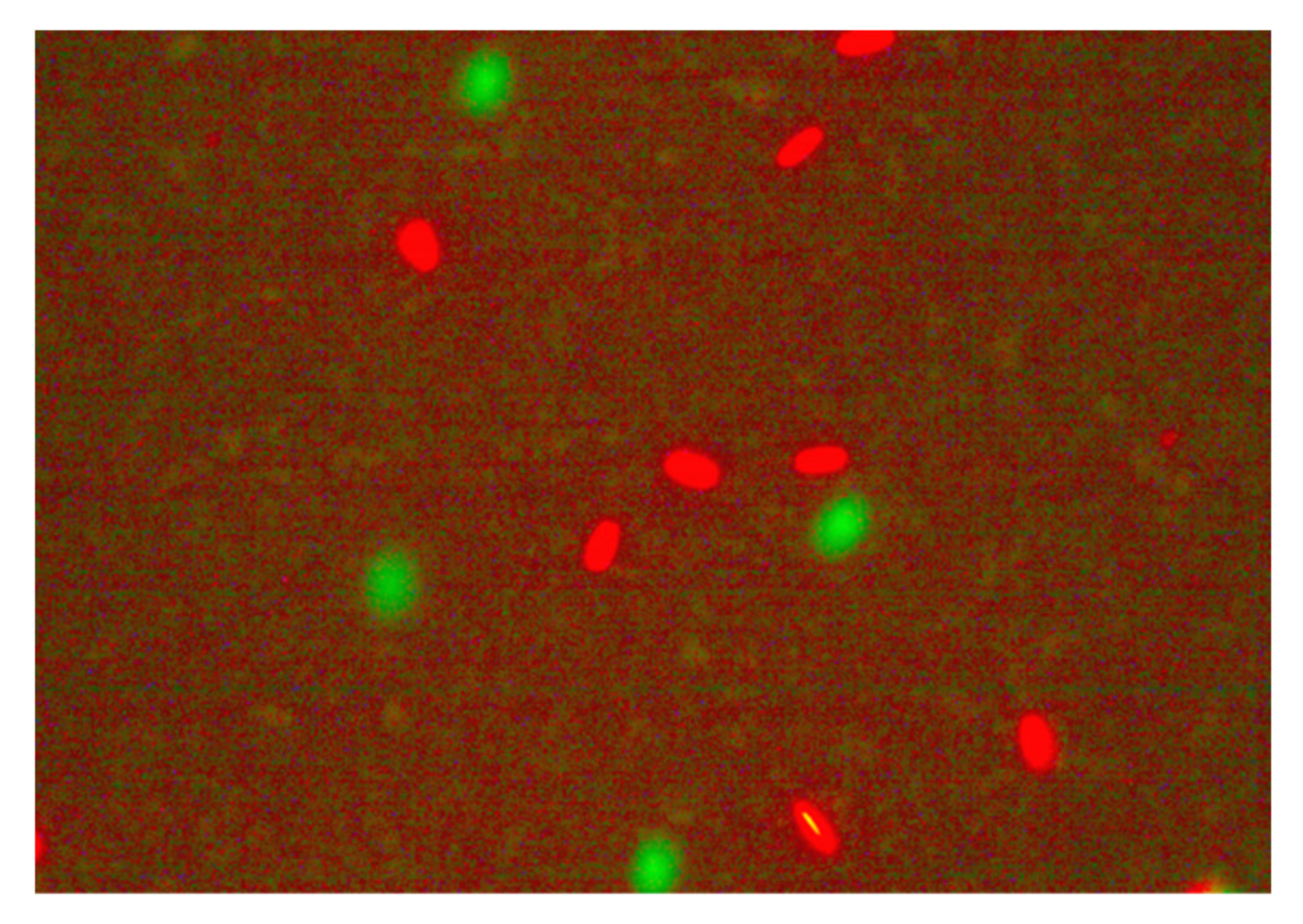
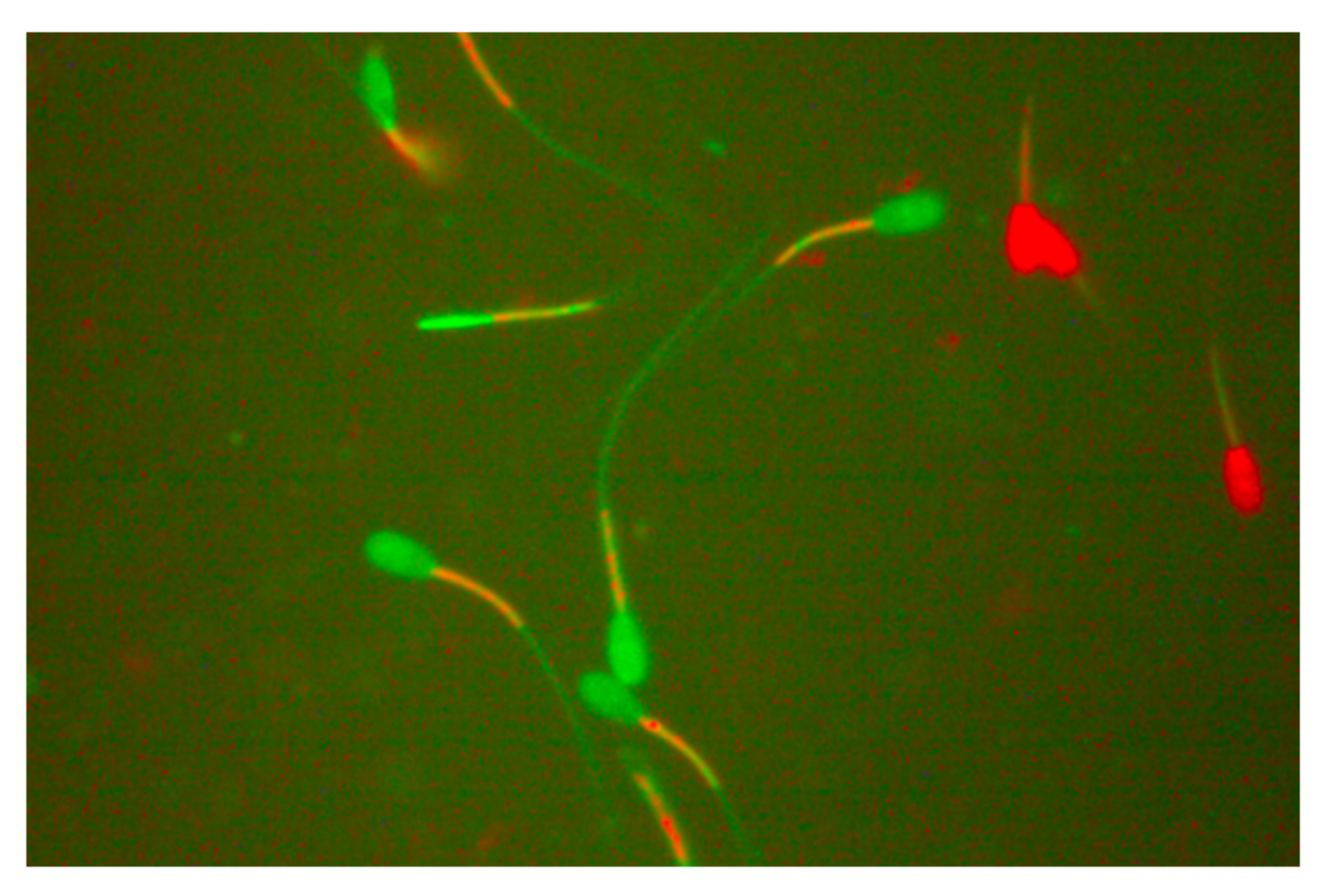
2.2. Post-Thaw Microscopic Sperm Parameters
2.3. Statistical Analysis
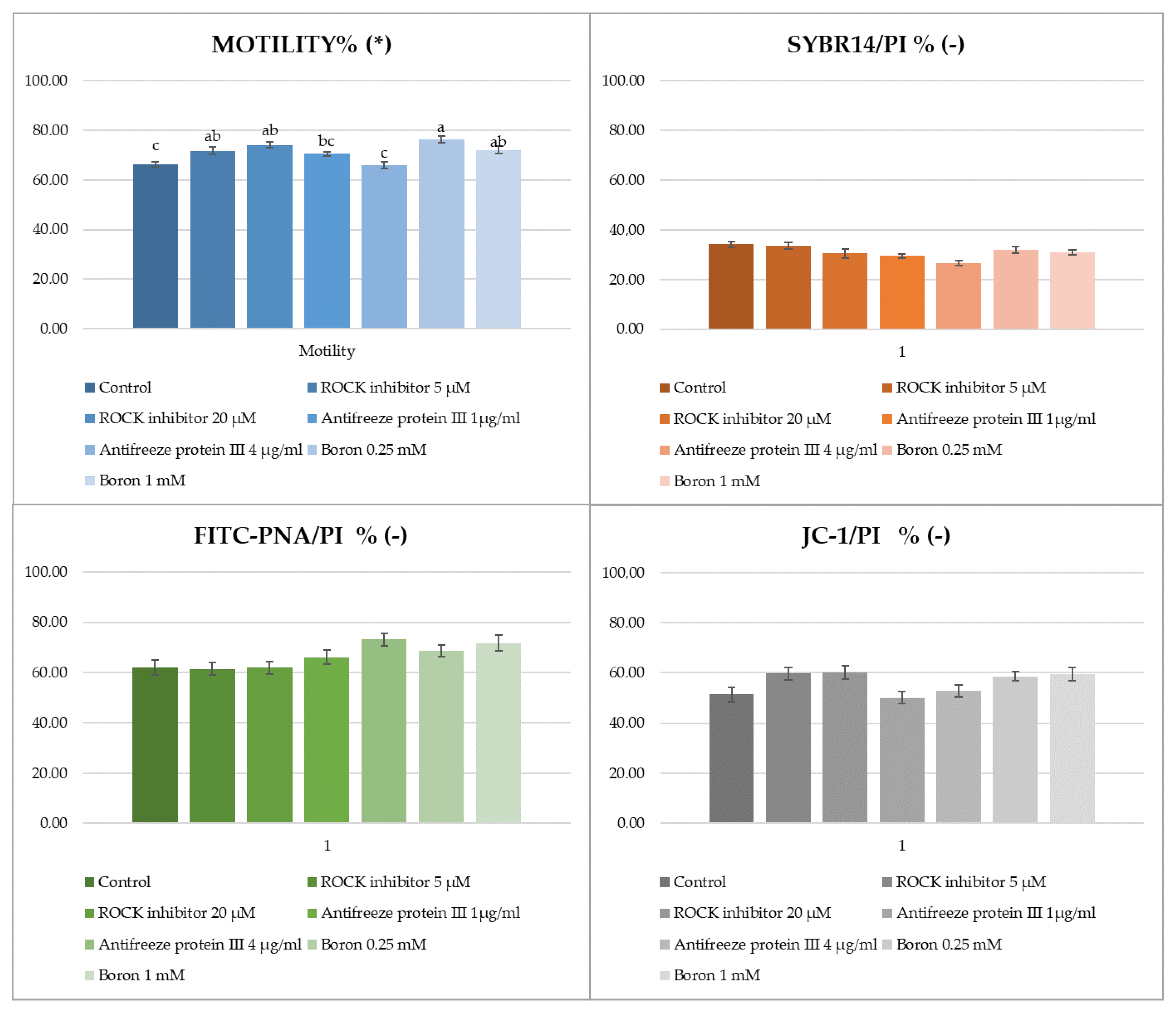
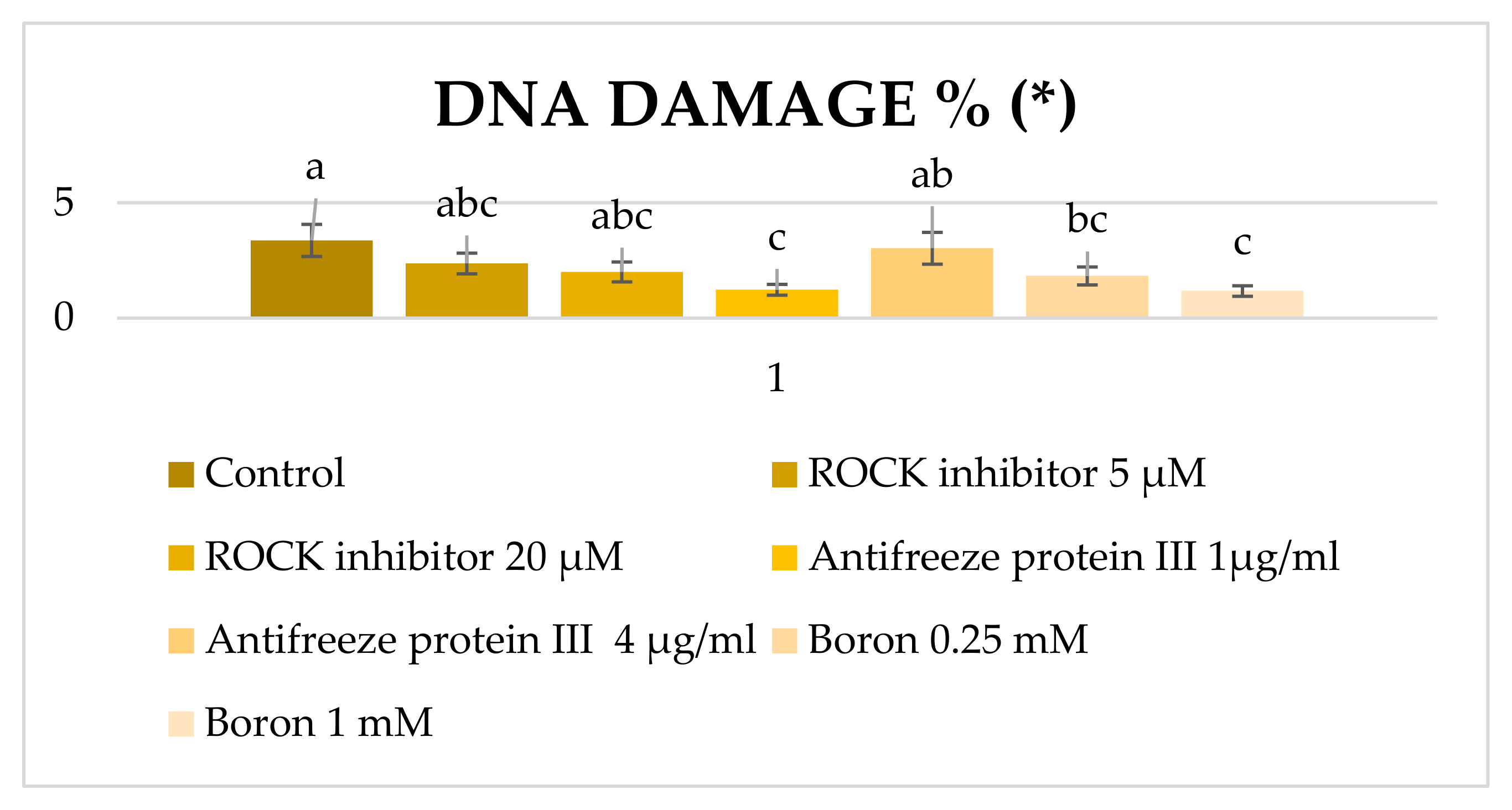
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yalcin, B.C. Sheep and Goats in Turkey. Fao Animal Production and Protection Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1986. [Google Scholar]
- Günes, H.; Horst, P.; Evrim, M.; Valle-Zárate, A. Studies on improvement of the productivity of Turkish Angora goats by crossing with South African Angora goats. Small Rumin. Res. 2002, 45, 115–122. [Google Scholar] [CrossRef]
- Ankara İli Tiftik İşleme Tesisi Ön Fizibilite Raporu; T.C. Sanayi ve Teknoloji Bakanlığı Ankara Kalkınma Ajansı: Ankara, Turkey, 2020.
- Tiftik Raporu, 2020. T.C. Ticaret Bakanlığı Esnaf, Sanatkârlar ve Kooperatifçilik Genel Müdürlüğü. Available online: https://esnafkoop.ticaret.gov.tr/data/5d44168e13b876433065544f/2019%20Tiftik%20Raporu.pdf (accessed on 2 May 2022).
- TÜİK, 2022. İstatistiksel Tablolar, Küçükbaş Hayvan Sayıları. Available online: https://data.tuik.gov.tr/Kategori/GetKategori?p=tarim-111&dil=1 (accessed on 9 February 2022).
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Watson, P. Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Purdy, P.H. A review on goat sperm cryopreservation. Small Rumin. Res. 2006, 63, 215–225. [Google Scholar] [CrossRef]
- Öztürk, A.E.; Bucak, M.N.; Bodu, M.; Başpınar, N.; Çelik, İ.; Shu, Z.; Keskin, N.; Gao, D. Cryobiology and cryopreservation of sperm. In Cryopreservation-Current Advances and Evaluations; Quain, M., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Bucak, M.; Tekin, N. Effect of some cryoprotectans in cryopreservation of Angora Buck semen. J. Lalahan Livest. Res. Inst. 2008, 48, 59–66. [Google Scholar]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Ulutaş, P.A.; Akçadağ, H.İ. Effect of antioxidants on microscopic semen parameters, lipid peroxidation and antioxidant activities in Angora goat semen following cryopreservation. Small Rumin. Res. 2009, 81, 90–95. [Google Scholar] [CrossRef]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Bucak, M.N.; Tuncer, P.B.; Taşdemir, U.; Kinet, H.; Ulutaş, P.A. Effects of different extenders and centrifugation/washing on postthaw microscopic-oxidative stress parameters and fertilizing ability of Angora buck sperm. Theriogenology 2010, 73, 316–323. [Google Scholar] [CrossRef]
- Edmondson, M.A.; Shipley, C.F. Theriogenology of sheep, goats, and cervids. In Sheep, Goat, and Cervid Medicine; Pugh, D.G., Baird, N.N., Edmondson, M., Passler, T., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Redden, R.; Thorne, J.W. Reproductive management of sheep and goats. In Animal Agriculture; Bazer, F.W., Lamb, G.C., Wu, G., Eds.; Elsevier: Cambridge, MA, USA, 2020; pp. 211–230. [Google Scholar]
- Holt, W.V.; North, R.D. Cryopreservation, actin localization and thermotropic phase transitions in ram spermatozoa. Reproduction 1991, 91, 451–461. [Google Scholar] [CrossRef][Green Version]
- Bucak, M.N.; Keskin, N.; Ili, P.; Bodu, M.; Akalın, P.P.; Öztürk, A.E.; Özkan, H.; Topraggaleh, T.R.; Sari, F.; Başpınar, N. Decreasing glycerol content by co-supplementation of trehalose and taxifolin hydrate in ram semen extender: Microscopic, oxidative stress, and gene expression analyses. Cryobiology 2020, 96, 19–29. [Google Scholar] [CrossRef]
- Keskin, N.; Erdogan, C.; Bucak, M.N.; Ozturk, A.E.; Bodu, M.; Ili, P.; Baspinar, N.; Dursun, S. Cryopreservation effects on ram sperm ultrastructure. Biopreserv. Biobank. 2020, 18, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.E.; Bodu, M.; Bucak, M.N.; Ağır, V.; Özcan, A.; Keskin, N.; İli, P.; Topraggaleh, T.R.; Sidal, H.; Başpınar, N. The synergistic effect of trehalose and low concentrations of cryoprotectants can improve post-thaw ram sperm parameters. Cryobiology 2020, 95, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Akalın, P.P.; Keskin, N.; Bodu, M.; Öztürk, A.E.; İli, P.; Özkan, H.; Topraggaleh, T.R.; Arslan, H.O.; Başpınar, N. Combination of fetuin and trehalose in presence of low glycerol has beneficial effects on freeze-thawed ram spermatozoa. Andrology 2021, 9, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Bucak, M.N.; Keskin, N.; Bodu, M.; Bülbül, B.; Kırbaş, M.; Öztürk, A.E.; Frootan, F.; İli, P.; Özkan, H.; Başpınar, N. Combination of trehalose and low boron in presence of decreased glycerol improves post-thawed ram sperm parameters: A model study in boron research. Andrology 2022, 10, 585–594. [Google Scholar] [CrossRef]
- Holt, W.V. Basic aspects of frozen storage of semen. Anim. Reprod. Sci. 2000, 62, 3–22. [Google Scholar] [CrossRef]
- Bucak, M.N.; Tekin, N. Kryoprotektanlar ve gamet hücrelerinin dondurulmasında kryoprotektif etki. Ank. Üniv. Vet. Fak. Derg. 2007, 54, 67–72. [Google Scholar]
- Duncan, A.E.; Watson, P.F. Predictive water loss curves for ram spermatozoa during cryopreservation: Comparison with experimental observations. Cryobiology 1992, 29, 95–105. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Bank, H.; Mazur, P. Visualization of freezing damage. J. Cell Biol. 1973, 57, 729–742. [Google Scholar] [CrossRef]
- Raabe, D. A texture-component Avrami model for predicting recrystallization textures, kinetics and grain size. Model Simul. Mater. Sci. Eng. 2006, 15, 39. [Google Scholar] [CrossRef]
- Farrant, J.; Walter, C.; Lee, H.; McGann, L. Use of two-step cooling procedures to examine factors influencing cell survival following freezing and thawing. Cryobiology 1977, 14, 273–286. [Google Scholar] [CrossRef]
- Zavos, P.M.; Graham, E.F. Effects of various degrees of supercooling and nucleation temperatures on fertility of frozen turkey spermatozoa. Cryobiology 1983, 20, 553–559. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; El-Euch, H.; Gargouri, J.; Bahloul, A.; Keskes, L.A. Effects of cryopreservation on human sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 93, 159–166. [Google Scholar] [CrossRef]
- Martin, G.; Sabido, O.; Durand, P.; Levy, R. Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 2004, 71, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, R.Z.; Sharma, R.K.; Poenicke, K.; Jha, R.; Paasch, U.; Grunewald, S.; Agarwal, A. Evaluation of poly (ADP-ribose) polymerase cleavage (cPARP) in ejaculated human sperm fractions after induction of apoptosis. Fertil. Steril. 2009, 91, 2210–2220. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ng, S.C. Fertilizing ability of DNA damaged spermatozoa. J. Exp. Zool. 1999, 284, 696–704. [Google Scholar] [CrossRef]
- Rath, D.; Bathgate, R.; Rodriguez-Martinez, H.; Roca, J.; Strzezek, J.; Waberski, D. Recent advances in boar semen cryopreservation. In Control of Pig Reproduction VIII; Rodriguez-Martinez, H., Vallet, J.L., Ziecik, A.J., Eds.; Nottingham University Press: Nottingham, UK, 2009; pp. 51–66. [Google Scholar]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Ulutaş, P.A.; Çoyan, K.; Başpınar, N.; Özkalp, B. Effects of hypotaurine, cysteamine and aminoacids solution on post-thaw microscopic and oxidative stress parameters of Angora goat semen. Res. Vet. Sci. 2009, 87, 468–472. [Google Scholar] [CrossRef]
- Tuncer, P.B.; Bucak, M.N.; Sarıözkan, S.; Sakin, F.; Yeni, D.; Çiğerci, İ.H.; Ateşşahin, A.; Avdatek, F.; Gündoğan, M.; Büyükleblebici, O. The effect of raffinose and methionine on frozen/thawed Angora buck (Capra hircus ancryrensis) semen quality, lipid peroxidation and antioxidant enzyme activities. Cryobiology 2010, 61, 89–93. [Google Scholar] [CrossRef]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic Fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef]
- Strom, C.; Liu, X.; Jia, Z. Ice surface reconstruction as antifreeze protein-induced morphological modification mechanism. J. Am. Chem. Soc. 2005, 127, 428–440. [Google Scholar] [CrossRef]
- Wathen, B.; Jia, Z. Controlling the freezing process with antifreeze proteins. In Emerging Technologies for Food Processing; Sun, D.W., Ed.; Elsevier Academic Press: Cambridge, MA, USA, 2005; pp. 653–674. [Google Scholar]
- Scotter, A.; Marshall, C.; Graham, L.; Gilbert, J.; Garnham, C.; Davies, P. The basis for hyperactivity of antifreeze proteins. Cryobiology 2006, 53, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Robles, V.; Barbosa, V.; Herráez, M.; Martínez-Páramo, S.; Cancela, M. The antifreeze protein type I (AFP I) increases seabream (Sparus aurata) embryos tolerance to low temperatures. Theriogenology 2007, 68, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, H.; Wang, L.; Guo, X. Effect of carrot (Daucus carota) antifreeze proteins on texture properties of frozen dough and volatile compounds of crumb. LWT-Food Sci. Technol. 2008, 41, 1029–1036. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Vieira, M.C.; Silva, C.L.M. The response of watercress (Nasturtium officinale) to vacuum impregnation: Effect of an antifreeze protein type I. J. Food Eng. 2009, 95, 339–345. [Google Scholar] [CrossRef]
- Jarząbek, M.; Pukacki, P.M.; Nuc, K. Cold-regulated proteins with potent antifreeze and cryoprotective activities in spruces (Picea spp.). Cryobiology 2009, 58, 268–274. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Hashim, N.H.F.; Bharudin, I.; Nguong, D.L.S.; Higa, S.; Bakar, F.D.A.; Nathan, S.; Rabu, A.; Kawahara, H.; Illias, R.M.; Najimudin, N. Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles 2013, 17, 63–73. [Google Scholar] [CrossRef]
- Arav, A.; Rubinsky, B.; Fletcher, G.; Seren, E. Cryogenic protection of oocytes with antifreeze proteins. Mol. Reprod. Dev. 1993, 36, 488–493. [Google Scholar] [CrossRef]
- Payne, S.R.; Oliver, J.E.; Upreti, G.C. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology 1994, 31, 180–184. [Google Scholar] [CrossRef]
- Koushafar, H.; Pham, L.; Lee, C.; Rubinsky, B. Chemical adjuvant cryosurgery with antifreeze proteins. J. Surg. Oncol. 1997, 66, 114–121. [Google Scholar] [CrossRef]
- Harding, M.M.; Anderberg, P.I.; Haymet, A.D.J. ‘Antifreeze’glycoproteins from polar fish. Eur. J. Biochem. 2003, 270, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Khan, M.A.; Ansari, M.S.; Rakha, B.A.; Ejaz, R.; Iqbal, R.; Younis, M.; Ullah, N.; DeVries, A.L.; Akhter, S. Efficiency of antifreeze glycoproteins for cryopreservation of Nili-Ravi (Bubalus bubalis) buffalo bull sperm. Anim. Reprod. Sci. 2015, 157, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Ridley, A.J. Rocks: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wennerberg, K. Rho and Rac take center stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef]
- Pirone, D.M.; Liu, W.F.; Ruiz, S.A.; Gao, L.; Raghavan, S.; Lemmon, C.A.; Romer, L.H.; Chen, C.S. An inhibitory role for FAK in regulating proliferation: A link between limited adhesion and RhoA-ROCK signaling. J. Cell Biol. 2006, 174, 277–288. [Google Scholar] [CrossRef]
- Ducummon, C.C.; Berger, T. Localization of the Rho GTPases and some Rho effector proteins in the sperm of several mammalian species. Zygote 2006, 14, 249–257. [Google Scholar] [CrossRef]
- Street, C.A.; Bryan, B.A. Rho kinase proteins—Pleiotropic modulators of cell survival and apoptosis. Anticancer Res. 2011, 31, 3645–3657. [Google Scholar]
- Liu, X.; Ory, V.; Chapman, S.; Yuan, H.; Albanese, C.; Kallakury, B.; Timofeeva, O.A.; Nealon, C.; Dakic, A.; Simic, V. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am. J. Pathol. 2012, 180, 599–607. [Google Scholar] [CrossRef]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef]
- Shimokawa, H.; Rashid, M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol. Sci. 2007, 28, 296–302. [Google Scholar] [CrossRef]
- Tosello-Trampont, A.C.; Nakada-Tsukui, K.; Ravichandran, K.S. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 2003, 278, 49911–49919. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.; Versteilen, A.M.G.; Sipkema, P.; van Nieuw-Amerongen, G.P.; Musters, R.J.P.; Groeneveld, A.B. Rho-kinase-dependent F-actin rearrangement is involved in the inhibition of PI3-kinase/Akt during ischemia–reperfusion-induced endothelial cell apoptosis. Apoptosis 2008, 13, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Marroquin, B.A.; Gugliotta, W.; Tse, R.; White, S.R. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2004, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Eti Maden İşletmeleri Genel Müdürlüğü, 2022. Dünya’da Bor. Available online: https://www.etimaden.gov.tr/dunyada-bor (accessed on 1 April 2022).
- Korkmaz, M.; Şaylı, U.; Şaylı, B.S.; Bakırdere, S.; Titretir, S.; Ataman, O.Y.; Keskin, S. Estimation of human daily boron exposure in a boron-rich area. Br. J. Nutr. 2007, 98, 571–575. [Google Scholar] [CrossRef]
- Korkmaz, M.; Yenigün, M.; Bakırdere, S.; Ataman, O.Y.; Keskin, S.; Müezzinoğlu, T.; Lekili, M. Effects of chronic boron exposure on semen profile. Biol. Trace. Elem. Res. 2011, 143, 738–750. [Google Scholar] [CrossRef]
- Nielsen, F.; Mullen, L.; Nielsen, E. Dietary boron affects blood cell counts and hemoglobin concentrations in humans. J. Trace. Elem. Exp. Med. 1991, 4, 211–223. [Google Scholar]
- Blevins, D.G.; Lukaszewski, K.M. Proposed physiologic functions of boron in plants pertinent to animal and human metabolism. Environ. Health Perspect. 1994, 102, 31–33. [Google Scholar]
- Hunt, C.D. The biochemical effects of physiologic amounts of dietary boron in animal nutrition models. Environ. Health Perspect. 1994, 102, 35–43. [Google Scholar]
- WHO. Trace Elements in Human Nutrition and Health; World Health Organization: Brussels, Belgium, 1996; p. 178. [Google Scholar]
- Elkomy, A.E.; Abd El-hady, A.M.; Elghalid, O.A. Dietary boron supplementation and its impact on semen characteristics and physiological status of adult male rabbits. Asian J. Poult. Sci. 2015, 9, 85–96. [Google Scholar] [CrossRef][Green Version]
- Weir Jr, J.; Fisher, S. Toxicologic studies on borax and boric acid. Toxicol. Appl. Pharmacol. 1972, 23, 351–364. [Google Scholar] [CrossRef]
- Price, C.J.; Strong, P.L.; Marr, M.C.; Myers, C.B.; Murray, F.J. Developmental toxicity NOAEL and postnatal recovery in rats fed boric acid during gestation. Fundam. Appl. Toxicol. 1996, 32, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Chapin, R.E.; Ku, W.W. The reproductive toxicity of boric acid. Environ. Health Perspect. 1994, 102, 87–91. [Google Scholar] [PubMed]
- Evans, G.; Maxwell, W.M.C.; Salamon, S. Salamon’s Artificial Insemination of Sheep and Goats; Butterworths: Sydney, Australia, 1987; pp. 1–194. [Google Scholar]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Nagy, S.; Jansen, J.; Topper, E.K.; Gadella, B.M. A triple-stain flow cytometric method to assess plasma-and acrosome-membrane integrity of cryopreserved bovine sperm immediately after thawing in presence of egg-yolk particles. Biol. Reprod. 2003, 68, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Garner, D.L.; Thomas, C.A.; Joerg, H.W.; DeJarnette, J.M.; Marshall, C.E. Fluorometric assessments of mitochondrial function and viability in cryopreserved bovine spermatozoa. Biol. Reprod. 1997, 57, 1401–1406. [Google Scholar] [CrossRef]
- Pastuszek, E.; Kiewisz, J.; Skowronska, P.; Liss, J.; Lukaszuk, M.; Bruszczynska, A.; Jakiel, G.; Lukaszuk, K. An investigation of the potential effect of sperm nuclear vacuoles in human spermatozoa on DNA fragmentation using a neutral and alkaline Comet assay. Andrology 2017, 5, 392–398. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Delgado-Bermúdez, A.; Mateo-Otero, Y.; Viñolas, E.; Hidalgo, C.O.; Ward, W.S.; Yeste, M. Determination of double- and single-stranded DNA breaks in bovine sperm is predictive of their fertilizing capacity. J. Anim. Sci. Biotechnol. 2022, 13, 105. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes: Cell membranes are viewed as two-dimensional solutions of oriented globular proteins and lipids. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Harayama, T.; Shimizu, T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020, 61, 1150–1160. [Google Scholar] [CrossRef]
- Bharudin, I.; Abu Bakar, M.; Hashim, N.; Mat Isa, M.; Alias, H.; Firdaus-Raih, M.; Md Illias, R.; Najimudin, N.; Mahadi, N.; Abu Bakar, F.; et al. Unravelling the adaptation strategies employed by Glaciozyma antarctica PI12 on Antarctic sea ice. Mar. Environ. Res. 2018, 137, 169–176. [Google Scholar] [CrossRef]
- Hammerstedt, R.H.; Graham, J.K.; Nolan, J.P. Cryopreservation of mammalian sperm: What we ask them to survive. J. Androl. 1990, 11, 73–88. [Google Scholar] [PubMed]
- Tharasanit, T.; Tiptanavattana, N.; Oravetdilok, K.; Tuangsintanakul, T.; Sirithanyakul, P.; Tanvetthayanont, P. Optimal concentration of Rho-associated coiled-coil kinase (ROCK) inhibitor improved sperm membrane functionality and fertilizing ability of cryopreserved-thawed feline sperm. Theriogenology 2020, 144, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duan, X.; Xiong, B.; Cui, X.S.; Kim, N.H.; Rui, R.; Sun, S.C. ROCK inhibitor Y-27632 prevents porcine oocyte maturation. Theriogenology 2014, 82, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, S.H.; Ma, K.; Zhao, B.Y.; Yan, B.; Pei, C.B.; Zhou, Y.; Wang, H.H.; Wang, H.Y.; Ma, L.H. Rho/ROCK signaling pathway and anti-cryodamage ability of human sperm. Natl. J. Androl. 2019, 25, 322–328. [Google Scholar]
- Crevel, R.W.R.; Fedyk, J.K.; Spurgeon, M.J. Antifreeze proteins: Characteristics, occurrence and human exposure. Food Chem. Toxicol. 2002, 40, 899–903. [Google Scholar] [CrossRef]
- Bektaş, G.I.; Altıntaş, A. Antifiriz proteinler. Etlik. Vet. Mikrobiyol. Derg. 2007, 18, 27–32. [Google Scholar]
- Ustun, N.S.; Turhan, S. Antifreeze proteins: Characteristics, function, mechanism of action, sources and application to foods. J. Food Process. Preserv. 2015, 39, 3189–3197. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G. Hypothermic protection-a fundamental property of “antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef]
- Correia, L.F.L.; Espírito-Santo, C.G.; Braga, F.; Carvalho-de-Paula, J.; da Silva, A.; Brandão, F.Z.; Freitas, V.J.F.; Ungerfeld, R.; Souza-Fabjan, J.M.G. Addition of antifreeze protein type I or III to extenders for ram sperm cryopreservation. Cryobiology 2021, 98, 194–200. [Google Scholar] [CrossRef]
- Lv, C.; Larbi, A.; Memon, S.; Liang, J.; Fu, X.; Wu, G.; Quan, G. The Effects of Antifreeze Protein III Supplementation on the Cryosurvival of Goat Spermatozoa During Cryopreservation. Biopreserv. Biobank. 2021, 19, 298–305. [Google Scholar] [CrossRef]
- Suarez, S.S.; Marquez, B.; Harris, T.P.; Schimenti, J.C. Different regulatory systems operate in the midpiece and principal piece of the mammalian sperm flagellum. Soc. Reprod. Fertil. Suppl. 2007, 65, 331–334. [Google Scholar] [PubMed]
- Moreira, P.I.; Harris, P.L.R.; Zhu, X.; Santos, M.S.; Oliveira, C.R.; Smith, M.A.; Perry, G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J. Alzheimer’s Dis. 2007, 12, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Kazachenko, A.V.; Vyssokikh, M.Y.; Vasileva, A.K.; Tcvirkun, D.V.; Isaev, N.K.; Kirpatovsky, V.I.; Zorov, D.B. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007, 72, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, K.; Tanaka, M.; Sakai, Y.; Koshimoto, C.; Morimoto, M.; Watanabe, T.; Fan, J.; Kitajima, S. Effects of type III antifreeze protein on sperm and embryo cryopreservation in rabbit. Cryobiology 2014, 69, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, B.B.; Selvaraju, S.; Gowda, N.K.S.; Subramanya, K.B.; Pal, D.; Archana, S.S.; Bhatta, R. Dietary boron supplementation enhances sperm quality and immunity through influencing the associated biochemical parameters and modulating the genes expression at testicular tissue. J. Trace Elem. Med. Biol. 2019, 55, 6–14. [Google Scholar] [CrossRef]
- Yeni, D.; Avdatek, F.; Gündoğan, M. The effect of Boron addition on spermatological parameters, oxidative stress and DNA damage after frozen-thawed process in ramlic ram semen. Fırat. Üniv. Sağlık. Bilim. Vet. Derg. 2018, 32, 53–57. [Google Scholar]
- Twigg, J.; Fulton, N.; Gomez, E.; Irvine, D.S.; Aitken, R.J. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: Lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum. Reprod. 1998, 13, 1429–1436. [Google Scholar] [CrossRef]
- Kumar, T.R.; Doreswamy, K.; Shrilatha, B. Oxidative stress associated DNA damage in testis of mice: Induction of abnormal sperms and effects on fertility. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2002, 513, 103–111. [Google Scholar] [CrossRef]
- De Lamirande, E.; Gagnon, C. Reactive oxygen species and human spermatozoa: I. Effects on the motility of intact spermatozoa and on sperm axonemes. J. Androl. 1992, 13, 368–378. [Google Scholar]
- Garner, D.L.; Hafez, E.S.E. Spermatozoa and seminal plasma. In Reproduction in Farm Animals; Hafez, E.S.E., Ed.; Lea&Febiger: Philadelphia, PA, USA, 1993; pp. 167–182. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaşör, Ö.F.; Bucak, M.N.; Cenariu, M.; Bodu, M.; Taşpınar, M.; Taşpınar, F. The Effects of Different Doses of ROCK Inhibitor, Antifreeze Protein III, and Boron Added to Semen Extender on Semen Freezeability of Ankara Bucks. Molecules 2022, 27, 8070. https://doi.org/10.3390/molecules27228070
Karaşör ÖF, Bucak MN, Cenariu M, Bodu M, Taşpınar M, Taşpınar F. The Effects of Different Doses of ROCK Inhibitor, Antifreeze Protein III, and Boron Added to Semen Extender on Semen Freezeability of Ankara Bucks. Molecules. 2022; 27(22):8070. https://doi.org/10.3390/molecules27228070
Chicago/Turabian StyleKaraşör, Ömer Faruk, Mustafa Numan Bucak, Mihai Cenariu, Mustafa Bodu, Mehmet Taşpınar, and Filiz Taşpınar. 2022. "The Effects of Different Doses of ROCK Inhibitor, Antifreeze Protein III, and Boron Added to Semen Extender on Semen Freezeability of Ankara Bucks" Molecules 27, no. 22: 8070. https://doi.org/10.3390/molecules27228070
APA StyleKaraşör, Ö. F., Bucak, M. N., Cenariu, M., Bodu, M., Taşpınar, M., & Taşpınar, F. (2022). The Effects of Different Doses of ROCK Inhibitor, Antifreeze Protein III, and Boron Added to Semen Extender on Semen Freezeability of Ankara Bucks. Molecules, 27(22), 8070. https://doi.org/10.3390/molecules27228070






