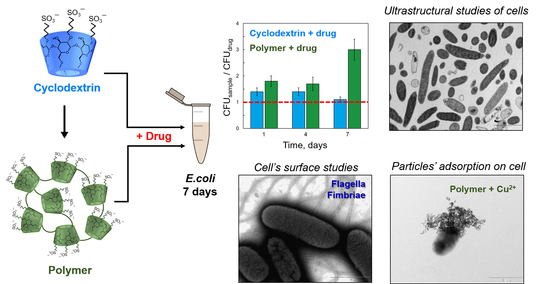
The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action
Abstract
1. Introduction
2. Results
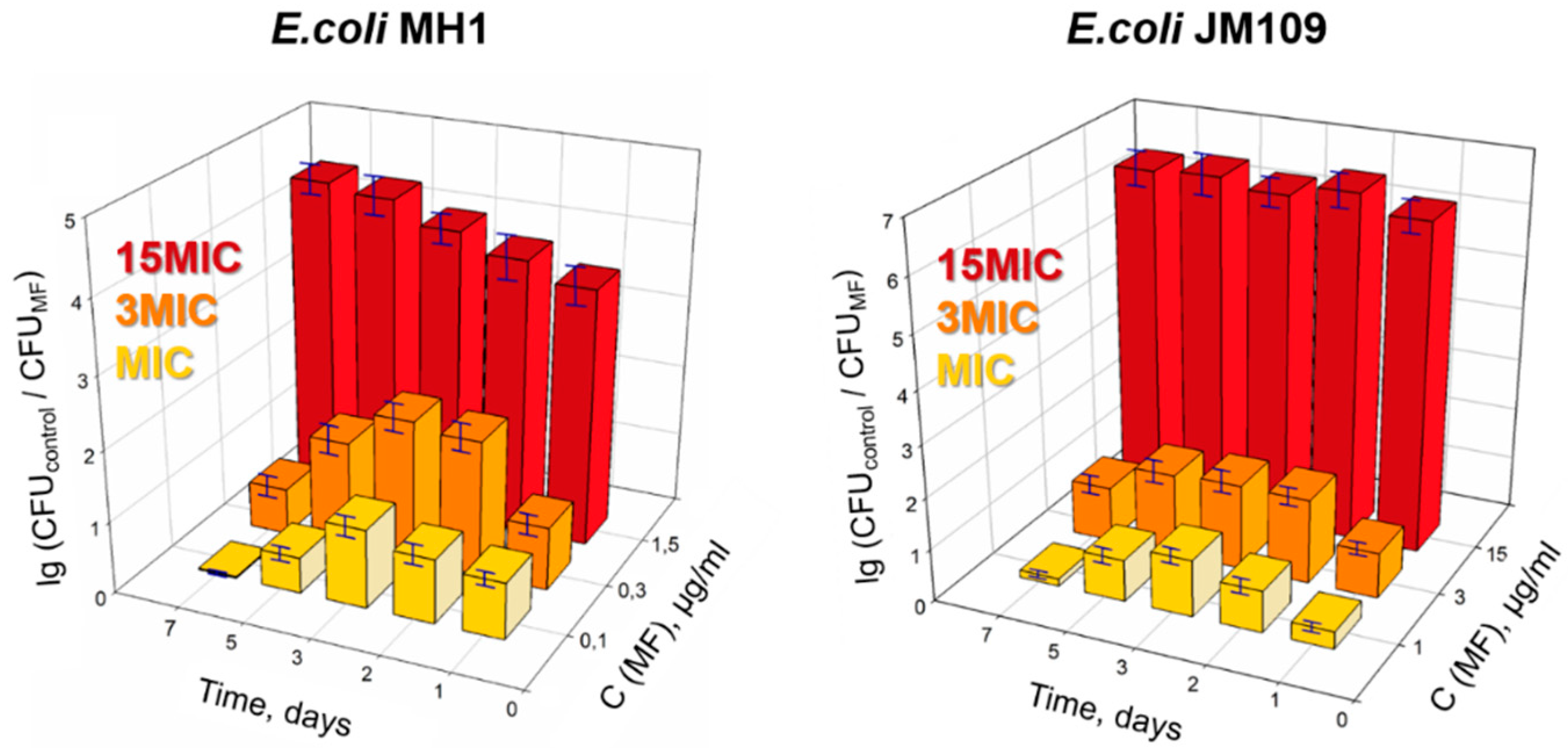
2.1. Antibacterial Action of Moxifloxacin against Two Strains of Escherichia coli
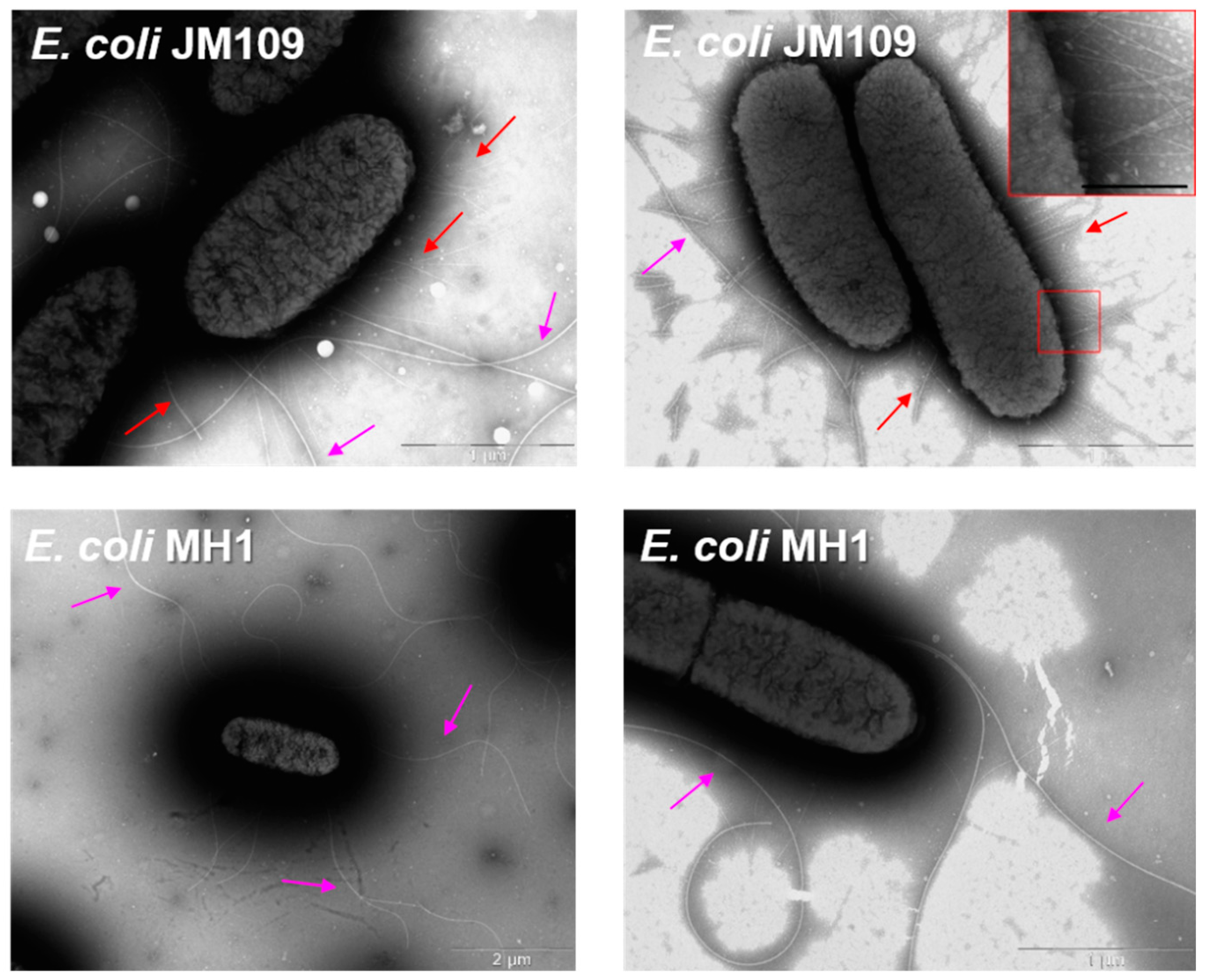
2.2. TEM Studies of Bacteria State during a 7-Day In Vitro Experiment
2.3. The Surface Morphology of E. coli MH1 and E. coli JM109
2.4. ζ-Potential of E. coli Cells in the Presence of MF-SCD Complexes
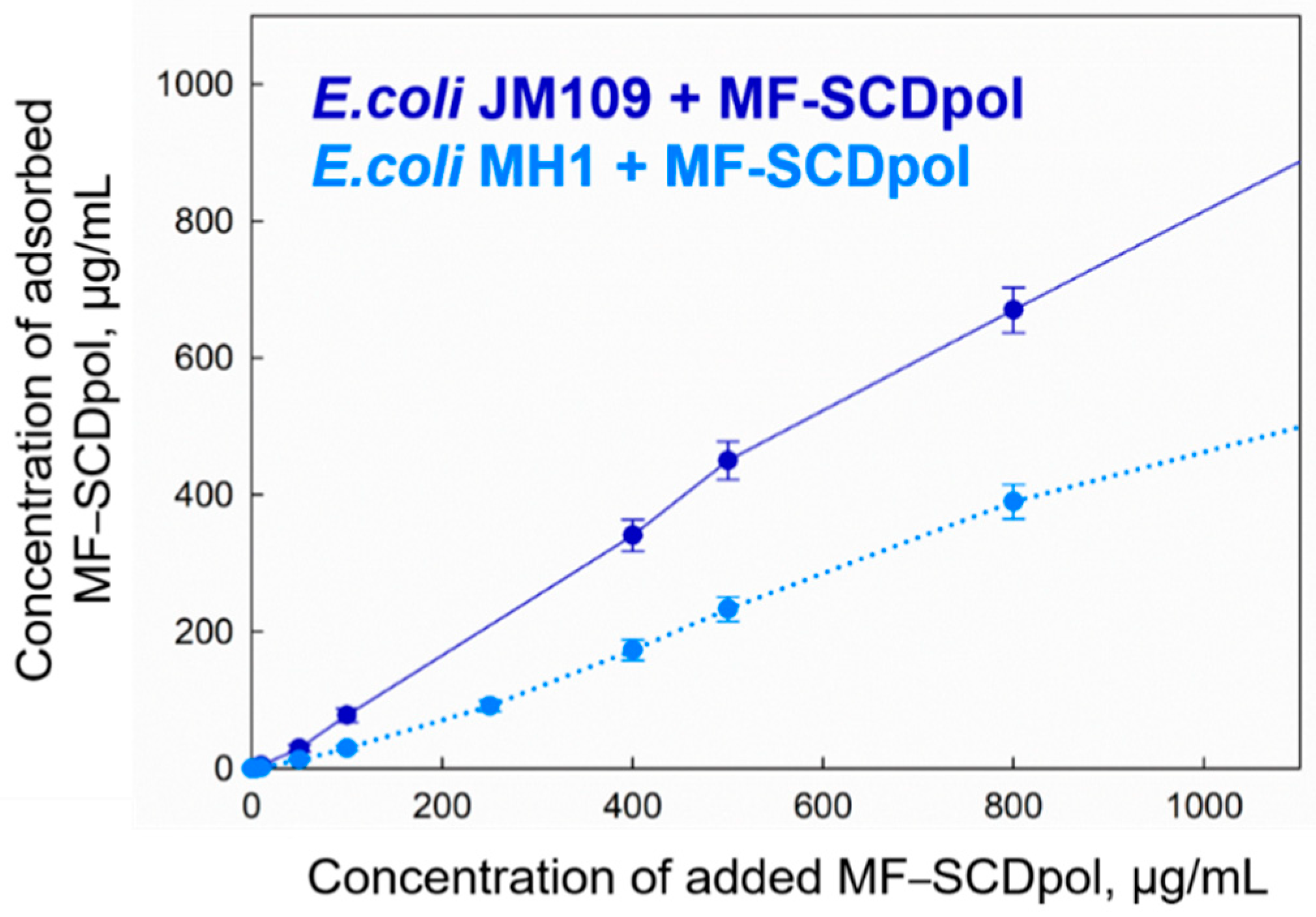
2.5. The Adsorption of MF-SCD Complexes on E. coli Cells
2.6. The Visualization of Particle Adsorption by TEM
3. Discussion
4. Materials and Methods
4.1. Reagents and Bacterial Strains
4.2. Synthesis of SCDpol
4.3. Complex Formation of MF with CD Derivatives
4.4. Dynamic Light Scattering
4.5. Nanoparticle Tracking Analysis
4.6. Fluorescence Spectroscopy
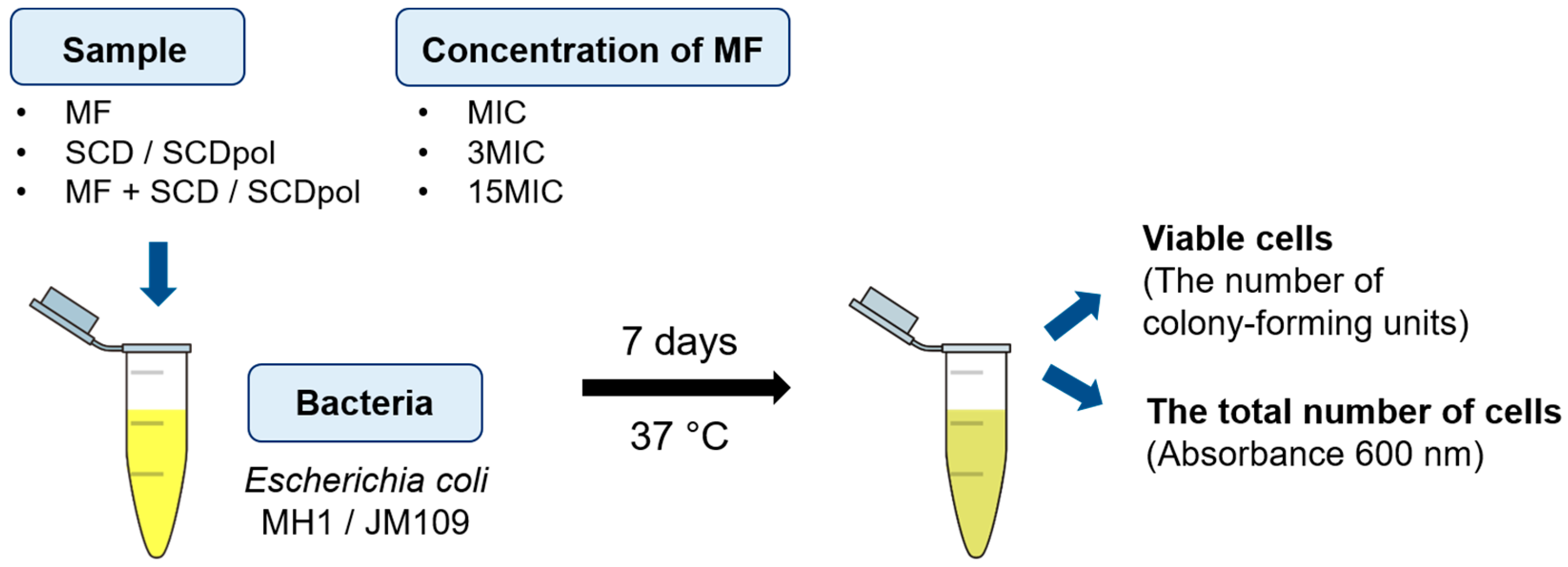
4.7. In Vitro Experiments: Growth Conditions, Antibacterial Activity Study, Surface Morphology Study, Ultrastructural Studies of Bacteria
4.8. The Particle Adsorption on Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: And Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.S.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green Fabrication, Characterization and Antibacterial Potential of Zinc Oxide Nanoparticles Using Aloe Socotrina Leaf Extract: A Novel Drug Delivery Approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101465. [Google Scholar] [CrossRef]
- Islan, G.A.; De Verti, I.P.; Marchetti, S.G.; Castro, G.R. Studies of Ciprofloxacin Encapsulation on Alginate/Pectin Matrixes and Its Relationship with Biodisponibility. Appl. Biochem. Biotechnol. 2012, 167, 1408–1420. [Google Scholar] [CrossRef]
- Le-Deygen, I.M.; Skuredina, A.A.; Uporov, I.V.; Kudryashova, E.V. Thermodynamics and Molecular Insight in Guest–Host Complexes of Fluoroquinolones with β-Cyclodextrin Derivatives, as Revealed by ATR-FTIR Spectroscopy and Molecular Modeling Experiments. Anal. Bioanal. Chem. 2017, 409, 6451–6462. [Google Scholar] [CrossRef]
- Várnaia, B.; Malangab, M.; Sohajdab, T.; Béni, S. Molecular Interactions in Remdesivir-Cyclodextrin Systems. J. Pharm. Biomed. Anal. 2022, 209, 114482. [Google Scholar] [CrossRef]
- Valizadeh, H.; Mohammadi, G.; Ehyaei, R.; Milani, M.; Azhdarzadeh, M.; Zakeri-Milani, P.; Lotfipour, F. Antibacterial Activity of Clarithromycin Loaded PLGA Nanoparticles. Pharmazie 2012, 67, 63–68. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of Cyclodextrin/Linalool-Inclusion Complex Nanofibers: Fast-Dissolving Nanofibrous Web with Prolonged Release and Antibacterial Activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef]
- Jug, M.; Kosalec, I.; Maestrelli, F.; Mura, P. Analysis of Triclosan Inclusion Complexes with β-Cyclodextrin and Its Water-Soluble Polymeric Derivative. J. Pharm. Biomed. Anal. 2011, 54, 1030–1039. [Google Scholar] [CrossRef]
- Liang, H.; Yuan, Q.; Vriesekoop, F.; Lv, F. Effects of Cyclodextrins on the Antimicrobial Activity of Plant-Derived Essential Oil Compounds. Food Chem. 2012, 135, 1020–1027. [Google Scholar] [CrossRef]
- Almekhlafi, S.; Thabit, A.A.M. Formulation and Evaluation of Lomefloxacin HCl as Semisolid Dosage Forms. J. Chem. Pharm. Res. 2014, 6, 1242–1248. [Google Scholar]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E. V Regulation of Properties of Lipid Membranes by Interaction with 2-Hydroxypropyl β-Cyclodextrin: Molecular Details. Russ. J. Bioorganic Chem. 2020, 46, 692–701. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.H.; Chai, F.; Leprêtre, S.; Blanchemain, N.; Martel, B.; Siepmann, F.; Hildebrand, H.F.; Siepmann, J.; Flament, M.P. Bone Implants Modified with Cyclodextrin: Study of Drug Release in Bulk Fluid and into Agarose Gel. Int. J. Pharm. 2010, 400, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Chai, F.; Blanchemain, N.; Neut, C.; Goube, M.; Maton, M.; Martel, B.; Hildebrand, H.F. Evaluation of Sorption Capacity of Antibiotics and Antibacterial Properties of a Cyclodextrin-Polymer Functionalized Hydroxyapatite-Coated Titanium Hip Prosthesis. Int. J. Pharm. 2014, 477, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.A.; Tychinina, A.S.; Le-Deygen, I.M.; Golyshev, S.A.; Belogurova, N.G.; Kudryashova, E.V. The Formation of Quasi-Regular Polymeric Network of Cross-Linked Sulfobutyl Ether Derivative of β-Cyclodextrin Synthesized with Moxifloxacin as a Template. React. Funct. Polym. 2021, 159, 104811. [Google Scholar] [CrossRef]
- Chuensombat, S.; Khemaleelakul, S.; Chattipakorn, S.; Srisuwan, T. Cytotoxic Effects and Antibacterial Efficacy of a 3-Antibiotic Combination: An in Vitro Study. J. Endod. 2013, 39, 813–819. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef]
- Li, L.; Feng, X.; Tang, M.; Hao, W.; Han, Y.; Zhang, G.; Wan, S. Antibacterial Activity of Lansiumamide B to Tobacco Bacterial Wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169, 522–526. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella Fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as Pharmaceutical Solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in Pharmaceutical Formulations I: Structure and Physicochemical Properties, Formation of Complexes, and Types of Complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Skuredina, A.; Le-Deygen, I.; Belogurova, N.; Kudryashova, E. Effect of Cross-Linking on the Inclusion Complex Formation of Derivatized β-Cyclodextrins with Small-Molecule Drug Moxifloxacin. Carbohydr. Res. 2020, 498, 108183. [Google Scholar] [CrossRef] [PubMed]
- Karampela, I.; Dalamaga, M. Could Respiratory Fluoroquinolones, Levofloxacin and Moxifloxacin, Prove to Be Beneficial as an Adjunct Treatment in COVID-19? Arch. Med. Res. 2020, 51, 741–742. [Google Scholar] [CrossRef]
- Damanhouri, Z.A.; Alkreathy, H.M.; Ali, A.S.; Karim, S. The Potential Role of Fluoroquinolones in the Management of COVID-19 a Rapid Review. J. Adv. Pharm. Educ. Res. 2021, 11, 125–134. [Google Scholar] [CrossRef]
- Scroggs, S.L.P.; Offerdahl, D.K.; Flather, D.P.; Morris, C.N.; Kendall, B.L.; Broeckel, R.M.; Beare, P.A.; Bloom, M.E. Fluoroquinolone Antibiotics Exhibit Low Antiviral Activity against SARS-CoV-2 and MERS-CoV. Viruses 2021, 13, 8. [Google Scholar] [CrossRef]
- Di Somma, A.; Avitabile, C.; Cirillo, A.; Moretta, A.; Merlino, A.; Paduano, L.; Duilio, A.; Romanelli, A. The Antimicrobial Peptide Temporin L Impairs E. coli Cell Division by Interacting with FtsZ and the Divisome Complex. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129606. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, Q.; Zhang, T.; Chen, X.; Wu, Z.; Zhang, N.; Shao, Y.; Cheng, Y. Antibacterial Activity and Mechanism of Green Tea Polysaccharide Conjugates against Escherichia coli. Ind. Crops Prod. 2020, 152, 112464. [Google Scholar] [CrossRef]
- Azhdarzadeh, M.; Lotfipour, F.; Zakeri-Milani, P.; Mohammadi, G.; Valizadeh, H. Anti-Bacterial Performance of Azithromycin Nanoparticles as Colloidal Drug Delivery System against Different Gram-Negative and Gram-Positive Bacteria. Adv. Pharm. Bull. 2012, 2, 17–24. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Fontaine, S.; Adam, H.; Schurek, K.; Mayer, M.; Noreddin, A.M.; Gin, A.S.; Rubinstein, E.; Hoban, D.J. A Review of New Fluoroquinolones: Focus on Their Use in Respiratory Tract Infections. Treat. Respir. Med. 2006, 5, 437–465. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.; McKenzie, E.A.; Robinson, A.M.; Gingles, N.A.; Marston, F.; Warwicker, J.; Dickson, A.J. Predictive Approaches to Guide the Expression of Recombinant Vaccine Targets in Escherichia coli: A Case Study Presentation Utilising Absynth Biologics Ltd. Proprietary Clostridium Difficile Vaccine Antigens. Appl. Microbiol. Biotechnol. 2021, 105, 5657–5674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Nie, T.; Meng, F.; Zhou, L.; Chen, M.; Sun, J.; Lu, Z.; Lu, Y. The Determination of Antibacterial Mode for Cationic Lipopeptides Brevibacillins against Salmonella Typhimurium by Quantum Chemistry Calculation. Appl. Microbiol. Biotechnol. 2021, 105, 5643–5655. [Google Scholar] [CrossRef]
- Otto, K.; Elwing, H.; Hermansson, M. The Role of Type 1 Fimbriae in Adhesion of Escherichia coli to Hydrophilic and Hydrophobic Surfaces. Colloids Surf. B Biointerfaces 1999, 15, 99–111. [Google Scholar] [CrossRef]
- Chao, Y.; Zhang, T. Probing Roles of Lipopolysaccharide, Type 1 Fimbria, and Colanic Acid in the Attachment of Escherichia coli Strains on Inert Surfaces. Langmuir 2011, 27, 11545–11553. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.; Ghosh, S.K.; Praharaj, S.; Panigrahi, S.; Basu, S.; Jana, S.; Pal, A.; Tsukuda, T.; Pal, T. Synthesis of Normal and Inverted Gold-Silver Core-Shell Architectures in β-Cyclodextrin and Their Applications in SERS. J. Phys. Chem. C 2007, 111, 10806–10813. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Xu, W.R. Enhanced Oral Bioavailability of Fluvastatin by Using Nanosuspensions Containing Cyclodextrin. Drug Des. Devel. Ther. 2018, 12, 3491–3499. [Google Scholar] [CrossRef]
- Prochowicz, D.; Kornowicz, A.; Justyniak, I.; Lewiński, J. Metal Complexes Based on Native Cyclodextrins: Synthesis and Structural Diversity. Coord. Chem. Rev. 2016, 306, 331–345. [Google Scholar] [CrossRef]
- Skuredina, A.A.; Tychinina, A.S.; Le-deygen, I.M.; Golyshev, S.A.; Kopnova, T.Y.; Le, N.T.; Belogurova, N.G.; Kudryashova, E. V Cyclodextrins and Their Polymers Affect the Lipid Membrane Permeability and Increase Levofloxacin’ s Antibacterial Activity In Vitro. Polymers 2022, 14, 4476. [Google Scholar] [CrossRef]
- Nadtochenko, V.A.; Rincon, A.G.; Stanca, S.E.; Kiwi, J. Dynamics of E. coli Membrane Cell Peroxidation during TiO2 Photocatalysis Studied by ATR-FTIR Spectroscopy and AFM Microscopy. J. Photochem. Photobiol. A Chem. 2005, 169, 131–137. [Google Scholar] [CrossRef]
- Sohrabi, S.; Haeri, A.; Mahboubi, A.; Mortazavi, A.; Dadashzadeh, S. Chitosan Gel-Embedded Moxifloxacin Niosomes: An Efficient Antimicrobial Hybrid System for Burn Infection. Int. J. Biol. Macromol. 2016, 85, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Baghdan, E.; Raschpichler, M.; Lutfi, W.; Pinnapireddy, S.R.; Pourasghar, M.; Schäfer, J.; Schneider, M.; Bakowsky, U. Nano Spray Dried Antibacterial Coatings for Dental Implants. Eur. J. Pharm. Biopharm. 2019, 139, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Pellicer, J.A.; Gómez-López, V.M.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Evaluation of Monoterpene-Cyclodextrin Complexes as Bacterial Growth Effective Hurdles. Food Control 2020, 108, 106814. [Google Scholar] [CrossRef]
- Mousa, S.A.; El-Sayed, E.S.R.; Mohamed, S.S.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Abdou, D.A.M. Novel Mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO Nanoparticles by the Endophytic Aspergillus Terreus and Evaluation of Their Antioxidant and Antimicrobial Activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T. Biodegradation and Adsorption of Antibiotics in the Activated Sludge Process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef]
- Hubicka, U.; Krzek, J.; Zuromska, B.; Walczak, M.; Zylewski, M.; Pawłowski, D. Determination of Photostability and Photodegradation Products of Moxifloxacin in the Presence of Metal Ions in Solutions and Solid Phase. Kinetics and Identification of Photoproducts. Photochem. Photobiol. Sci. 2012, 11, 351–357. [Google Scholar] [CrossRef]
- Maia, A.S.; Ribeiro, A.R.; Amorim, C.L.; Barreiro, J.C.; Cass, Q.B.; Castro, P.M.L.; Tiritan, M.E. Degradation of Fluoroquinolone Antibiotics and Identification of Metabolites/Transformation Products by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1333, 87–98. [Google Scholar] [CrossRef]
- Wasfi, R.; Elkhatib, W.F.; Khairalla, A.S. Effects of Selected Egyptian Honeys on the Cellular Ultrastructure and the Gene Expression Profile of Escherichia coli. PLoS ONE 2016, 11, e0150984. [Google Scholar] [CrossRef]
- Cramariuc, O.; Rog, T.; Javanainen, M.; Monticelli, L.; Polishchuk, A.V.; Vattulainen, I. Mechanism for Translocation of Fluoroquinolones across Lipid Membranes. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2563–2571. [Google Scholar] [CrossRef]
- Boudeau, J.; Barnich, N.; Darfeuille-Michaud, A. Type 1 Pili-Mediated Adherence of Escherichia coli Strain LF82 Isolated from Crohn’s Disease Is Involved in Bacterial Invasion of Intestinal Epithelial Cells. Mol. Microbiol. 2004, 39, 1272–1284. [Google Scholar] [CrossRef]
- Proft, T.; Baker, E.N. Pili in Gram-Negative and Gram-Positive Bacteria—Structure, Assembly and Their Role in Disease. Cell. Mol. Life Sci. 2009, 66, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Seltmann, G.; Holst, O. Cell Wall Models. In The Bacterial Cell Wall; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Halder, S.; Yadav, K.K.; Sarkar, R.; Mukherjee, S.; Saha, P.; Haldar, S.; Karmakar, S.; Sen, T. Alteration of Zeta Potential and Membrane Permeability in Bacteria: A Study with Cationic Agents. Springerplus 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Stenstrom, T.-A.; Kjelleberg, S. Fimbriae Mediated Nonspecific Adhesion of Salmonella typhimurium to Mineral Particles. Arch. Microbiol. 1985, 143, 6–10. [Google Scholar] [CrossRef]
- Eshdat, Y.; Silverblatt, F.J.; Sharon, N. Dissociation and Reassembly of Escherichia coli Type 1 Pili. J. Bacteriol. 1981, 148, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef]
- Goddard, J.M.; Caput, D.; Williams, S.R.; Martin, D.M. Cloning of Human Purine-Nucleoside Phosphorylase CDNA Sequences by Complementation in Escherichia coli. Proc. Natl. Acad. Sci. USA 1983, 80, 4281–4285. [Google Scholar] [CrossRef]
- Yanisch-Perron, C.; Vieira, J.; Messing, J. Improved Ml3 Phage Cloning Vectors and Host Strains: Nucleotide Sequences of the M13mp18 and PUC19 Vectors. Gene 1985, 33, 103–119. [Google Scholar] [CrossRef]
- Kuriki, Y. Temperature-Sensitive Amber Suppression of OmpF ‘-’ LacZ Fused Gene Expression in a SupE Mutant of Escherichia Coli K12. FEMS Microbiol. Lett. 1993, 107, 71–76. [Google Scholar] [CrossRef]





| CD | Hydrodynamic Diameter, nm | ζ-Potential, mV | M, kDa | Kdis of MF-CD, M |
|---|---|---|---|---|
| SCD | ~0.15 | −7.3 ± 0.8 | ~2.1 | 1.0 (±0.3) × 10−4 |
| SCDpol | 190 ± 15 | 18.7 ± 1.1 | 140 ± 14 | 5.2 (±0.3) × 10−6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skuredina, A.A.; Kopnova, T.Y.; Tychinina, A.S.; Golyshev, S.A.; Le-Deygen, I.M.; Belogurova, N.G.; Kudryashova, E.V. The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action. Molecules 2022, 27, 8026. https://doi.org/10.3390/molecules27228026
Skuredina AA, Kopnova TY, Tychinina AS, Golyshev SA, Le-Deygen IM, Belogurova NG, Kudryashova EV. The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action. Molecules. 2022; 27(22):8026. https://doi.org/10.3390/molecules27228026
Chicago/Turabian StyleSkuredina, Anna A., Tatiana Yu. Kopnova, Anastasia S. Tychinina, Sergey A. Golyshev, Irina M. Le-Deygen, Natalya G. Belogurova, and Elena V. Kudryashova. 2022. "The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action" Molecules 27, no. 22: 8026. https://doi.org/10.3390/molecules27228026
APA StyleSkuredina, A. A., Kopnova, T. Y., Tychinina, A. S., Golyshev, S. A., Le-Deygen, I. M., Belogurova, N. G., & Kudryashova, E. V. (2022). The New Strategy for Studying Drug-Delivery Systems with Prolonged Release: Seven-Day In Vitro Antibacterial Action. Molecules, 27(22), 8026. https://doi.org/10.3390/molecules27228026