Role of AuNPs in Active Food Packaging Improvement: A Review
Abstract
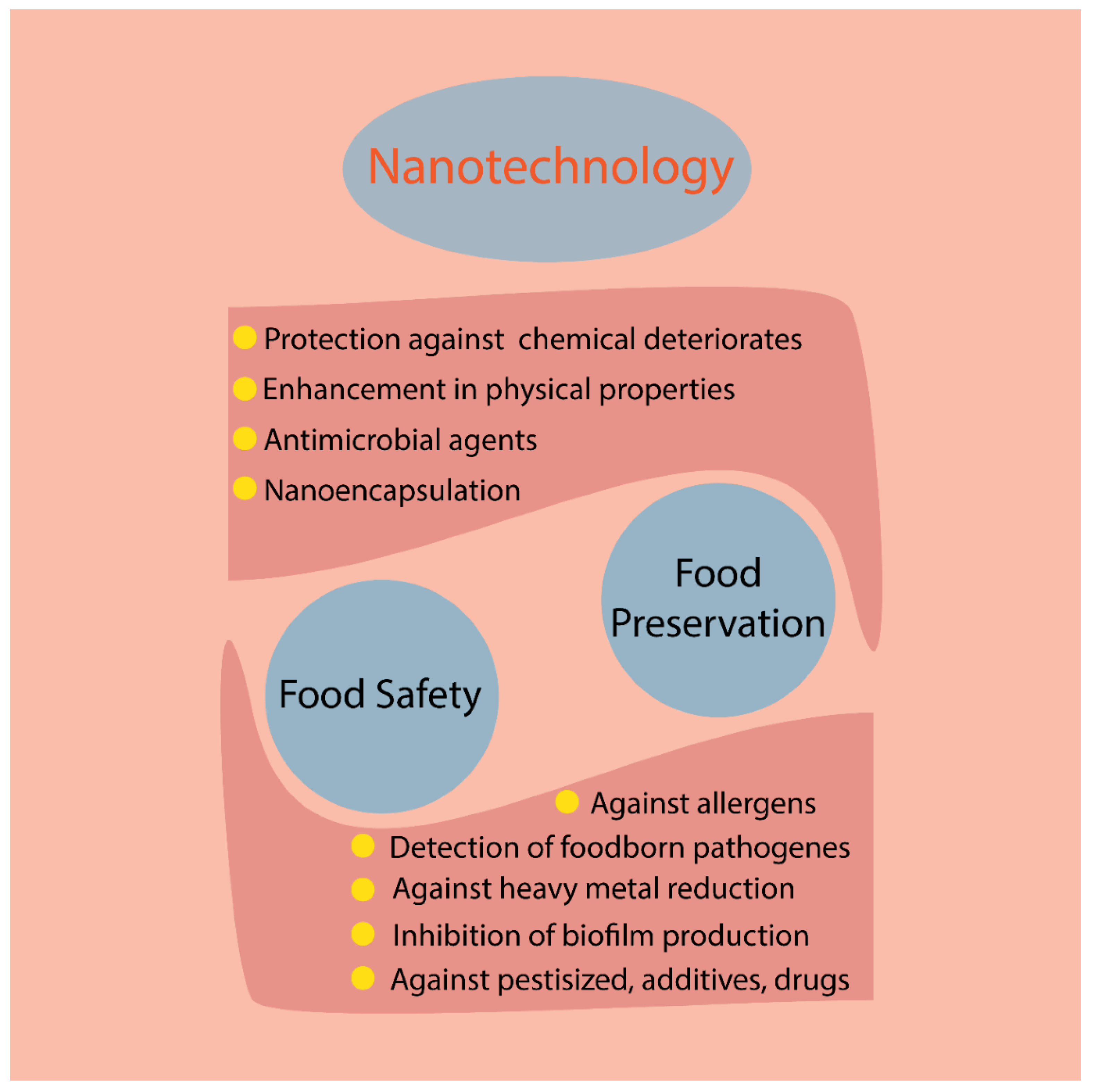
1. Introduction
2. Search Method
3. Synthesis of AuNPs
3.1. Biosynthesis of AuNPs Using Microbial Strains
3.2. Biosynthesis of AuNPs Using Leaf Extract
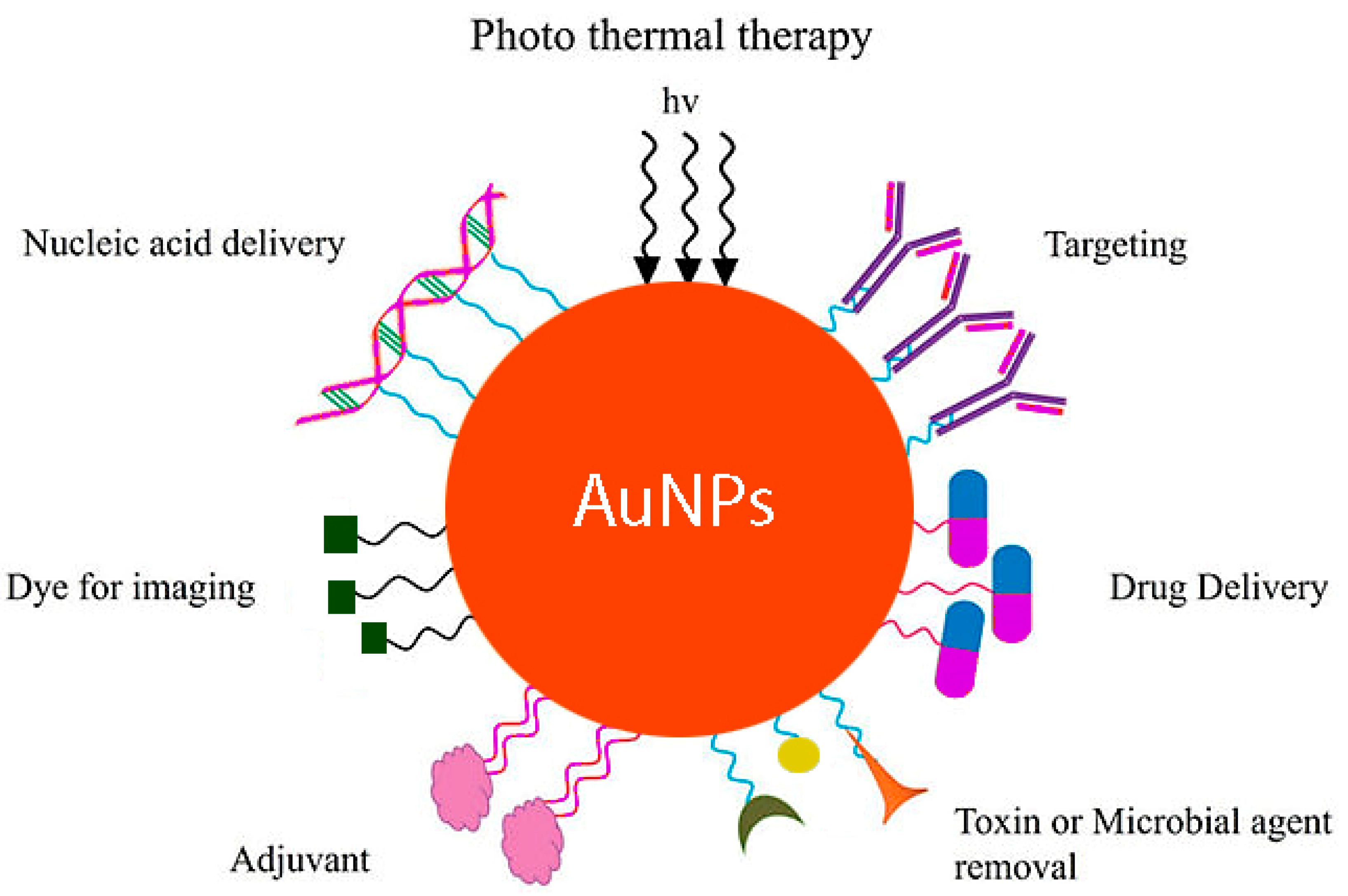
4. Potential Application of AuNPs in Food Packaging
4.1. Antibacterial Activities
4.2. Barrier Properties
4.3. Antioxidant Properties
4.4. Biosensing
5. Hazard
6. Future Prospective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amani, P.; Gadde, L.-E. Shelf life extension and food waste reduction. Proc. Food Syst. Dyn. 2015, 7–14. [Google Scholar]
- Jedermann, R.; Nicometo, M.; Uysal, I.; Lang, W. Reducing food losses by intelligent food logistics. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130302. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E. Nanotechnology in Food Systems: A Review. Acta Aliment. 2020, 49, 460–474. [Google Scholar] [CrossRef]
- Sahraei, F.; Ahari, H.; Kakoolaki, S. Effect of Bacillus subtilis as a probiotic on protein, lipid content, and trypsin and chymotrypsin enzymes in rainbow trout biometry (Oncorhynchus mykiss). Aquac. Int. 2018, 27, 141–153. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Dekkers, S.; Noordam, M.; Hagens, W.; Bulder, A.; De Heer, C.; ten Voorde, S.; Wijnhoven, S.; Sips, A. Health Impact of Nanotechnologies in Food Production; RIKILT Report; National Institute for Public Health and the Environment Ministry of Health, Welfare and Sport: Wageningen, The Netherlands, 2007. [Google Scholar]
- Sandarani, M.; Dasanayaka, D.; Jayasinghe, C. Strategies used to prolong the shelf life of fresh commodities. J. Agric. Sci. Food Res. 2018, 9, 1–6. [Google Scholar]
- Esmaeili, Y.; Zamindar, N.; Paidari, S.; Ibrahim, S.A.; Nafchi, A.M. The synergistic effects of aloe vera gel and modified atmosphere packaging on the quality of strawberry fruit. J. Food Process. Preserv. 2021, 45, e16003. [Google Scholar] [CrossRef]
- Pessu, P.; Agoda, S.; Isong, I.; Ikotun, I. The concepts and problems of post–harvest food losses in perishable crops. Afr. J. Food Sci. 2011, 5, 603–613. [Google Scholar]
- Shafiq, M.; Anjum, S.; Hano, C.; Anjum, I.; Abbasi, B.H. An Overview of the Applications of Nanomaterials and Nanodevices in the Food Industry. Foods 2020, 9, 148. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Hwang, H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Gómez-Guillén, M.C. A state-of-the-art review on the elaboration of fish gelatin as bioactive packaging: Special emphasis on nanotechnology-based approaches. Trends Food Sci. Technol. 2018, 79, 125–135. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. npj Sci. Food 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Anvar, A.; Kajavi, S.H.; Ahari, H.; Sharifan, A.; Motallebi, A.; Kakoolaki, S.; Paidari, S. Evaluation of the antibacterial effects of Ag-Tio2 nanoparticles and optimization of its migration to sturgeon caviar (Beluga). Iran. J. Fish. Sci. 2019, 18, 954–967. [Google Scholar] [CrossRef]
- Dey, A.; Neogi, S. Oxygen scavengers for food packaging applications: A review. Trends Food Sci. Technol. 2019, 90, 26–34. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yuan, L.; Yong, H.; Liu, J. Preparation and characterization of antioxidant, antimicrobial and pH-sensitive films based on chitosan, silver nanoparticles and purple corn extract. Food Hydrocoll. 2019, 96, 102–111. [Google Scholar] [CrossRef]
- Esmaeili, Y.; Zamindar, N.; Mohammadi, R. The effect of polypropylene film containing nano-hydroxyapatite on Physicochemical and microbiological properties of button mushrooms (Agaricus bisporus) under Modified atmosphere packaging. J. Food Meas. Charact. 2022, 1–14. [Google Scholar] [CrossRef]
- Park, S.; Jeon, Y.; Han, T.; Kim, S.; Gwon, Y.; Kim, J. Nanoscale manufacturing as an enabling strategy for the design of smart food packaging systems. Food Packag. Shelf Life 2020, 26, 100570. [Google Scholar] [CrossRef]
- Paidari, S.; Ibrahim, S.A. Potential application of gold nanoparticles in food packaging: A mini review. Gold Bull. 2021, 54, 31–36. [Google Scholar] [CrossRef]
- Hayat, M.A. Colloidal Gold: Principles, Methods, and Applications; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Sahoo, M.; Vishwakarma, S.; Panigrahi, C.; Kumar, J. Nanotechnology: Current applications and future scope in food. Food Front. 2020, 2, 3–22. [Google Scholar] [CrossRef]
- Sardar, R.; Shumaker-Parry, J.S. Spectroscopic and Microscopic Investigation of Gold Nanoparticle Formation: Ligand and Temperature Effects on Rate and Particle Size. J. Am. Chem. Soc. 2011, 133, 8179–8190. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Colloid. Synth. Plasmonic Nanometals 2020, 197–220. [Google Scholar]
- Wilton-Ely, J.D. The surface functionalisation of gold nanoparticles with metal complexes. Dalton Trans. 2008, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kumar, S.; Gandhi, K.S.; Kumar, R. Modeling of Formation of Gold Nanoparticles by Citrate Method. Ind. Eng. Chem. Res. 2006, 46, 3128–3136. [Google Scholar] [CrossRef]
- Leng, W.; Pati, P.; Vikesland, P.J. Room temperature seed mediated growth of gold nanoparticles: Mechanistic investigations and life cycle assesment. Environ. Sci. Nano 2015, 2, 440–453. [Google Scholar] [CrossRef]
- Hussain, M.H.; Abu Bakar, N.F.; Mustapa, A.N.; Low, K.-F.; Othman, N.H.; Adam, F. Synthesis of Various Size Gold Nanoparticles by Chemical Reduction Method with Different Solvent Polarity. Nanoscale Res. Lett. 2020, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cazes, J. Encyclopedia of Chromatography; Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- El Shafey, A.M. Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Kalpana, V.; Rajeswari, V.D. A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Park, Y.; Hong, Y.; Weyers, A.; Kim, Y.; Linhardt, R. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78. [Google Scholar] [CrossRef]
- Singh, S.; Dev, A.; Gupta, A.; Nigam, V.K.; Poluri, K.M. Nitrate Reductase mediated synthesis of surface passivated nanogold as broad-spectrum antibacterial agent. Gold Bull. 2019, 52, 197–216. [Google Scholar] [CrossRef]
- Ramdayal; Balasubramanian, K. Antibacterial application of polyvinylalcohol-nanogold composite membranes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 455, 174–178. [Google Scholar] [CrossRef]
- Smitha, S.; Gopchandran, K. Surface enhanced Raman scattering, antibacterial and antifungal active triangular gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 114–119. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. npj Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Mohan, C.; Guan, J.; Ravishankar, C.; Gunasekaran, S. Chitosan and gold nanoparticles-based thermal history indicators and frozen indicators for perishable and temperature-sensitive products. Food Control 2018, 85, 186–193. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lu, L.; Gunasekaran, S. Biopolymer/gold nanoparticles composite plasmonic thermal history indicator to monitor quality and safety of perishable bioproducts. Biosens. Bioelectron. 2017, 92, 109–116. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B Biointerfaces 2013, 101, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-Álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green synthesis of gold and silver nanoparticles from plant extracts and their possible applications as antimicrobial agents in the agricultural area. Nanomaterials 2020, 10, 1763. [Google Scholar] [CrossRef]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. -Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Suganya, K.U.; Govindaraju, K.; Kumar, V.G.; Dhas, T.S.; Karthick, V.; Singaravelu, G.; Elanchezhiyan, M. Blue green alga mediated synthesis of gold nanoparticles and its antibacterial efficacy against Gram positive organisms. Mater. Sci. Eng. C 2015, 47, 351–356. [Google Scholar] [CrossRef]
- Hamed, M.M.; Abdelftah, L.S. Biosynthesis of gold nanoparticles using marine Streptomyces griseus isolate (M8) and evaluating its antimicrobial and anticancer activity. Egypt. J. Aquat. Biol. Fish. 2019, 23, 173–184. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J. Biomed. Nanotechnol. 2011, 7, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine algae-mediated synthesis of gold nanoparticles using a novel Ecklonia cava. Bioprocess Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; Shen, W.; Wang, J.; Li, H.; Zhang, Z.; Li, S.; Zhou, J. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 280–285. [Google Scholar] [CrossRef]
- Dasari, T.S.; Zhang, Y.; Yu, H. Antibacterial activity and cytotoxicity of gold (I) and (III) ions and gold nanoparticles. Biochem. Pharmacol. Open Access 2015, 4. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef]
- Beveridge, T.; Murray, R. Sites of metal deposition in the cell wall of Bacillus subtilis. J. Bacteriol. 1980, 141, 876–887. [Google Scholar] [CrossRef]
- Satisha, S.; Syed, B.; Prasad, N. Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity. J. Microsc. Ultrastruct. 2016, 4, 162–166. [Google Scholar] [CrossRef]
- Pourali, P.; Badiee, S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Singh, P.K.; Kundu, S. Biosynthesis of Gold Nanoparticles Using Bacteria. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2013, 84, 331–336. [Google Scholar] [CrossRef]
- Konishi, Y.; Tsukiyama, T.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S. Intracellular recovery of gold by microbial reduction of AuCl4− ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 2006, 81, 24–29. [Google Scholar] [CrossRef]
- Xu, W.; Mulhern, P.J.; Blackford, B.L.; Jericho, M.H.; Firtel, M.; Beveridge, T.J. Modeling and measuring the elastic properties of an archaeal surface, the sheath of Methanospirillum hungatei, and the implication of methane production. J. Bacteriol. 1996, 178, 3106–3112. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Biosynthesis of Monodisperse Gold Nanoparticles by a Novel Extremophilic Actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Ravichandran, R. Nanotechnology Applications in Food and Food Processing: Innovative Green Approaches, Opportunities and Uncertainties for Global Market. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, P72–P96. [Google Scholar] [CrossRef]
- Sahoo, G.; Sarkar, N.; Swain, S.K. Antimicrobial Properties of Nanogold-Imprinted Starch Bionanocomposites. Polym. Technol. Eng. 2016, 56, 334–345. [Google Scholar] [CrossRef]
- Rapa, M.; Vinci, G.; Ciano, S.; Cerra, S.; Fratoddi, I. Gold nanoparticles-based extraction of phenolic compounds from olive mill wastewater: A rapid and sustainable method. AIP Conf. Proc. 2020, 2257, 020010. [Google Scholar] [CrossRef]
- Can, M. Green gold nanoparticles from plant-derived materials: An overview of the reaction synthesis types, conditions, and applications. Rev. Chem. Eng. 2019, 36, 859–877. [Google Scholar] [CrossRef]
- Rajathi, F.A.A.; Parthiban, C.; Kumar, V.G.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Ramkumar, R.; Rahuman, A.A.; Perumal, P. Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschus esculentus and its antifungal activity. Ind. Crop. Prod. 2013, 45, 423–429. [Google Scholar] [CrossRef]
- Shahriari, M.; Hemmati, S.; Zangeneh, A.; Zangeneh, M.M. Biosynthesis of gold nanoparticles using Allium noeanum Reut. ex Regel leaves aqueous extract; characterization and analysis of their cytotoxicity, antioxidant, and antibacterial properties. Appl. Organomet. Chem. 2019, 33. [Google Scholar] [CrossRef]
- Patra, J.K.; Kwon, Y.; Baek, K.-H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv. Powder Technol. 2016, 27, 2204–2213. [Google Scholar] [CrossRef]
- Annamalai, A.; Christina, V.; Sudha, D.; Kalpana, M.; Lakshmi, P. Green synthesis, characterization and antimicrobial activity of Au NPs using Euphorbia hirta L. leaf extract. Colloids Surf. B Biointerfaces 2013, 108, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; Farh, M.E.-A.; Aceituno, V.C.; Kim, Y.J. In vitro anti-inflammatory activity of spherical silver nanoparticles and monodisperse hexagonal gold nanoparticles by fruit extract of Prunus serrulata: A green synthetic approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar]
- Geethalakshmi, R.; Sarada, D. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375–5384. [Google Scholar] [CrossRef]
- Lokina, S.; Suresh, R.; Giribabu, K.; Stephen, A.; Sundaram, R.L.; Narayanan, V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 129, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Hatipoğlu, A. Green synthesis of gold nanoparticles from Prunus cerasifera pissardii nigra leaf and their antimicrobial activities on some food pathogens. Prog. Nutr. 2021, 23, e2021241. [Google Scholar]
- Al-Radadi, N.S. Facile one-step green synthesis of gold nanoparticles (AuNp) using licorice root extract: Antimicrobial and anticancer study against HepG2 cell line. Arab. J. Chem. 2020, 14, 102956. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ruvalcaba, F.; Sanchez, V.; López, M.G.; Silva-Jara, J.; Hernandez-Adame, L.; Angulo, C. Green synthesis of gold nanoparticles using Turnera diffusa Willd enhanced antimicrobial properties and immune response in Longfin yellowtail leukocytes. Aquac. Res. 2021, 52, 3391–3402. [Google Scholar] [CrossRef]
- Nayak, S.; Sajankila, S.P.; Rao, C.V. Green synthesis of gold nanoparticles from banana pith extract and its evaluation of antibacterial activity and catalytic reduction of malachite green dye. J. Microbiol. Biotechnol. Food Sci. 2018, 7, 641–645. [Google Scholar] [CrossRef]
- Rahman, T.U.; Khan, H.; Liaqat, W.; Zeb, M.A. Phytochemical screening, green synthesis of gold nanoparticles, and antibacterial activity using seeds extract of Ricinus communis L. Microsc. Res. Tech. 2021, 85, 202–208. [Google Scholar] [CrossRef]
- Li, S.; Al-Misned, F.A.; El-Serehy, H.A.; Yang, L. Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab. J. Chem. 2020, 14, 102931. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Fadaka, A.; Aluko, O.; Awawu, S.; Theledi, K. Green Synthesis of Gold Nanoparticles using Pimenta dioica Leaves Aqueous Extract and Their Application as Photocatalyst, Antioxidant, and Antibacterial Agents. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 78–88. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Yao, B.; Folorunso, A.S. Green Synthesis, Characterization, and Antibacterial Investigation of Synthesized Gold Nanoparticles (AuNPs) from Garcinia kola Pulp Extract. Plasmonics 2020, 16, 157–165. [Google Scholar] [CrossRef]
- Varghese, B.A.; Nair, R.V.R.; Jude, S.; Varma, K.; Amalraj, A.; Kuttappan, S. Green synthesis of gold nanoparticles using Kaempferia parviflora rhizome extract and their characterization and application as an antimicrobial, antioxidant and catalytic degradation agent. J. Taiwan Inst. Chem. Eng. 2021, 126, 166–172. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Lee, C.-S. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 8489094. [Google Scholar] [CrossRef]
- León, E.R.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chan, C.; Huang, S.; Lin, Y. Green biosynthesis of gold nanoparticles using Chenopodium formosanum shell extract and analysis of the particles’ antibacterial properties. J. Sci. Food Agric. 2019, 99, 3693–3702. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Akkam, Y.; Al Zoubi, M.S.; Al-Batayneh, K.M.; Al-Trad, B.; Alrob, O.A.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Al Saqr, A.; Khafagy, E.-S.; Alalaiwe, A.; Aldawsari, M.; Alshahrani, S.; Anwer, K.; Khan, S.; Lila, A.; Arab, H.; Hegazy, W. Synthesis of Gold Nanoparticles by Using Green Machinery: Characterization and In Vitro Toxicity. Nanomaterials 2021, 11, 808. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Jayanthi, S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater. Nanotechnol. 2020, 10, 1847980420961697. [Google Scholar] [CrossRef]
- Yasmin, A.; Ramesh, K.; Rajeshkumar, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 1–7. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Arunachalam, K.; Annamalai, S.K.; Hari, S. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomed. 2013, 8, 1307–1315. [Google Scholar] [CrossRef]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Chaudhry, Q.; Scotter, M.; Blackburn, J.; Ross, B.; Boxall, A.; Castle, L.; Aitken, R.; Watkins, R. Applications and implications of nanotechnologies for the food sector. Food Addit. Contam. Part A 2008, 25, 241–258. [Google Scholar] [CrossRef]
- Chhipa, H. Applications of Nanotechnology in Agriculture. In Methods in Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–142. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Tzegkas, S.G.; Danezis, G.P. Nanomaterials in food packaging: State of the art and analysis. J. Food Sci. Technol. 2018, 55, 2862–2870. [Google Scholar] [CrossRef]
- Sekhon, B. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, S.; Ojha, N.; Shrivastava, A.; Barla, A.; Rai, V.; Bose, S. Facets of Nanotechnology as Seen in Food Processing, Packaging, and Preservation Industry. BioMed Res. Int. 2015, 2015, 365672. [Google Scholar] [CrossRef]
- Banjare, J. Application of nanotechnology in food technology and targeted drug therapy for prevention of obesity: An overview. J. Crit. Rev. 2016, 4, 7. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Han, S.G.; Chin, K.B. Nanotechnology in Meat Processing and Packaging: Potential Applications—A Review. Asian-Australas. J. Anim. Sci. 2015, 28, 290–302. [Google Scholar] [CrossRef]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Song, M.Y.; Song, K.S.; Ryu, H.R.; Yoon, J.U.; Jeon, K.S.; Jeong, J.; Han, B.S.; et al. Subchronic inhalation toxicity of gold nanoparticles. Part. Fibre Toxicol. 2011, 8, 16. [Google Scholar] [CrossRef]
- Bansal, S.A.; Kumar, V.; Karimi, J.; Singh, A.P.; Kumar, S. Role of gold nanoparticles in advanced biomedical applications. Nanoscale Adv. 2020, 2, 3764–3787. [Google Scholar] [CrossRef]
- Virgili, A.H.; Laranja, D.C.; Malheiros, P.S.; Pereira, M.B.; Costa, T.M.; de Menezes, E.W. Nanocomposite film with antimicrobial activity based on gold nanoparticles, chitosan and aminopropylsilane. Surf. Coatings Technol. 2021, 415, 127086. [Google Scholar] [CrossRef]
- Glišić, B.; DjuraN, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Castillo-Henríquez, L. Gold/Silver Nanoparticles Antimicrobial Applications. Scholarly Community Encyclopedia. 2021. Available online: https://encyclopedia.pub/entry/history/show/4817 (accessed on 18 September 2022).
- Elmetwalli, A. Impact of Antibiotic Interactions with Essential Oils on Bacterial growth. J. Complement. Med. Res. 2022, 13, 114. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Manzoor, N.; Ahmed, J.; Asiri, A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B Biointerfaces 2013, 107, 227–234. [Google Scholar] [CrossRef] [PubMed]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 2017, 12, 2813. [Google Scholar] [CrossRef] [PubMed]
- M, N.; D, S.; Hans, S.; Varghese, A.; Fatima, Z.; Hameed, S. Studies on the antifungal activity of biotemplated gold nanoparticles over Candida albicans. Mater. Res. Bull. 2019, 119, 110563. [Google Scholar] [CrossRef]
- Jia, X.; Yao, Y.; Yu, G.; Qu, L.; Li, T.; Li, Z.; Xu, C. Synthesis of gold-silver nanoalloys under microwave-assisted irradiation by deposition of silver on gold nanoclusters/triple helix glucan and antifungal activity. Carbohydr. Polym. 2020, 238, 116169. [Google Scholar] [CrossRef]
- Tirumale, S.; Wani, N.A.; Khanday, W.I. Phytochemical analysis and evaluation of antibacterial activity of different extracts of soil-isolated fungus chaetomium cupreum. J. Nat. Sci. Biol. Med. 2020, 11, 72. [Google Scholar] [CrossRef]
- Beurton, J.; Clarot, I.; Stein, J.; Creusot, B.; Marcic, C.; Marchioni, E.; Boudier, A. Long-lasting and controlled antioxidant property of immobilized gold nanoparticles for intelligent packaging. Colloids Surf. B Biointerfaces 2019, 176, 439–448. [Google Scholar] [CrossRef]
- Jha, P.; Saraf, A.; Sohal, J.K. Antimicrobial Activity of Biologically Synthesized Gold Nanoparticles from Wild Mushroom Cantharellus Species. J. Sci. Res. 2021, 65, 78–83. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Suvorov, D. Antibacterial nanocomposite of functionalized nanogold and gallium-doped hydroxyapatite. Mater. Lett. 2017, 193, 126–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, H.; Huang, W.; Zhou, Y.; Yan, D. Facile preparation and characterization of highly antimicrobial colloid Ag or Au nanoparticles. J. Colloid Interface Sci. 2008, 325, 371–376. [Google Scholar] [CrossRef]
- Tan, P.-L.; Soo, K.Q.-S.; Khor, Y.-P.; Abas, F.; Tan, C.-P. Enzyme-Assisted Water Extraction Optimization, Antioxidant Capacity and Phenolic Profiling of Extracts from Garcinia Mangostana Linn. J. Food Technol. Res. 2022, 9, 135–149. [Google Scholar] [CrossRef]
- Yu, L.; Yung, L.-Y.L.; Ong, C.N.; Tan, Y.-L.; Balasubramaniam, K.S.; Hartono, D.; Shui, G.; Wenk, M.R.; Ong, W.-Y. Translocation and effects of gold nanoparticles after inhalation exposure in rats. Nanotoxicology 2007, 1, 235–242. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Jakobi, J.; Winkel, A.; Stiesch, M.; Barcikowski, S. Alloying colloidal silver nanoparticles with gold disproportionally controls antibacterial and toxic effects. Gold Bull. 2013, 47, 83–93. [Google Scholar] [CrossRef]
- Lima, E.; Guerra, R.; Lara, V.; Guzmán, A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem. Central J. 2013, 7, 11. [Google Scholar] [CrossRef]
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater. Sci. Eng. C 2019, 108, 110338. [Google Scholar] [CrossRef]
- Datkhile, K.D.; Patil, S.R.; Patil, M.N.; Durgawale, P.P.; Jagdale, N.J.; Deshmukh, V.N. Studies on phytoconstituents, in vitro antioxidant, antibacterial, and cytotoxicity potential of Argemone mexicana Linn. (Family: Papaveraceae). J. Nat. Sci. Biol. Med. 2020, 11, 198–205. [Google Scholar]
- Thirumurugan, A.; Ramachandran, S.; Shiamala Gowri, A. Combined effect of bacteriocin with gold nanoparticles against food spoiling bacteria–An approach for food packaging material preparation. Int. Food Res. J. 2013, 20, 1909–1912. [Google Scholar]
- Pagno, C.H.; Costa, T.M.; de Menezes, E.W.; Benvenutti, E.V.; Hertz, P.F.; Matte, C.R.; Tosati, J.V.; Monteiro, A.R.; Rios, A.O.; Flôres, S.H. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015, 173, 755–762. [Google Scholar] [CrossRef]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Zaidi, N.S.R.; Mah, S.-K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and photocatalytic antibacterial Au-TiO2/sodium alginate nanocomposite films for active food packaging. Nanomaterials 2018, 8, 930. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Ananadhi, P.; Tharani, M.; Lakshmi, T. Antibacterial activity of Cinnamon and Clove oil against wound pathogens: Wound pathogens control using oil. J. Popul. Ther. Clin. Pharmacol. 2021, 28. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Li, X.; Robinson, S.M.; Gupta, A.; Saha, K.; Jiang, Z.; Moyano, D.F.; Sahar, A.; Riley, M.A.; Rotello, V.M. Functional Gold Nanoparticles as Potent Antimicrobial Agents against Multi-Drug-Resistant Bacteria. ACS Nano 2014, 8, 10682–10686. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Tavakolian, S.; Ahari, H.; Givianrad, M.H.; Hosseini, H. Improving the Barrier Properties of Food Packaging by Al2O3@TiO2 & Al2O3@SiO2 Nanoparticles. Food Bioprocess Technol. 2021, 14, 1287–1300. [Google Scholar] [CrossRef]
- Gomathy, M.; Sabarinathan, K. Microbial mechanisms of heavy metal tolerance—A review. Agric. Rev. 2010, 31, 133–138. [Google Scholar]
- Maduraiveeran, G.; Ramaraj, R. Gold nanoparticle-based sensing platform of hydrazine, sulfite, and nitrite for food safety and environmental monitoring. J. Anal. Sci. Technol. 2017, 8, 14. [Google Scholar] [CrossRef]
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green Synthesis of Gold Nanoparticles Using Sumac Aqueous Extract and Their Antioxidant Activity. Mater. Res. 2017, 20, 264–270. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Jha, P.K.; Vignesh, V.; Rajkuberan, C.; Jeyaraj, M.; Selvakumar, M.; Jha, R.; Sivaramakrishnan, S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. [Google Scholar] [CrossRef]
- Ramamurthy, C.; Padma, M.; Samadanam, I.D.M.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Ferranti, P. Food Production and Ecosystem Protection. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Bindhu, M.; Umadevi, M. Silver and gold nanoparticles for sensor and antibacterial applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.-F. Biogenic amines-and sulfides-responsive gold nanoparticles for real-time visual detection of raw meat, fish, crustaceans, and preserved meat. Food Chem. 2020, 311, 125908. [Google Scholar] [CrossRef]
- Han, C.; Zhao, A.; Varughese, E.; Sahle-Demessie, E. Evaluating weathering of food packaging polyethylene-nano-clay composites: Release of nanoparticles and their impacts. NanoImpact 2017, 9, 61–71. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Silver migration from nanosilver and a commercially available zeolite filler polyethylene composites to food simulants. Food Addit. Contam. Part A 2014, 31, 1132–1140. [Google Scholar] [CrossRef]
- Malik, A.; Erginkaya, Z.; Erten, H. Health and Safety Aspects of Food Processing Technologies; Springer: Heidelberg/Berlin, Germany, 2019. [Google Scholar]
- Koyun, O.; Sahin, Y. Voltammetric determination of nitrite with gold nanoparticles/poly(methylene blue)-modified pencil graphite electrode: Application in food and water samples. Ionics 2018, 24, 3187–3197. [Google Scholar] [CrossRef]
- García-Cambero, J.P.; García, M.N.; López, G.D.; Herranz, A.L.; Cuevas, L.; Pérez-Pastrana, E.; Cuadal, J.S.; Castelltort, M.R.; Calvo, A.C. Converging hazard assessment of gold nanoparticles to aquatic organisms. Chemosphere 2013, 93, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
| Plant Source | Reducing Agents | Biological Effects | Microorganisms | Wavelength (nm) | Shape | NP Size (nm) | Year (Ref) |
|---|---|---|---|---|---|---|---|
| prunus cerasifera pissardii nigra leaf | extract | Anti-microbial and anti-fungal properties | E. coli, S. aureus, B. subtillis, P. aeruginosa, and C. albicans. | 535 | Spherical (20 nm) | 20 | 2021 [69] |
| licorice root | extract | Antimicrobial and anticancer | Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhi | 517 | Spherical | 53.7 | 2021 [70] |
| Turnera diffusa Willd | oplopanone, γ-eudesmol, hydroquinone-β-d-glucoside (arbutin) and inositol | antimicrobial properties and immune response | Vibrio parahaemolyticus and Aeromonas hydrophila Longfin yellowtail | 540 | multiple shapes, mostly spherical | 24 | 2021 [71] |
| banana pith extract | Alkaloids, Flavonoids | antibacterial activity and catalytic reduction | Bacillus subtilis, E. coli, Pseudomonas aeruginosa | 530–560 | spherical | 470 | 2021 [72] |
| Ricinus communis L. | alkaloids, terpenoids, steroids | antibacterial activity | Bacillus cereus, Klebsiella pneumonia, Pseudomonas aeruginosa, B. cereus | 550 | spherical | 100 | 2021 [73] |
| Mentha Longifolia leaf | - | anti-human breast carcinoma | breast carcinoma (Hs 578Bst) | 512 | spherical shape particles | 36 | 2021 [74] |
| curcumin | Curcuma pseudomontana isolated curcumin | antimicrobial, antioxidant, and anti-inflammatory activities, antioxidant and radical scavenging activities. | Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis and Escherichia coli | 542 | spherical shape particles | 20 | 2021 [75] |
| sing Pimenta dioica Leaves | extract | Photocatalyst, antioxidant, and antibacterial | S. aureus and E. coli | 517 | spherical shape particles | 11 | 2021 [76] |
| Garcinia kola Pulp | extract | Antibacterial activity | Staphylococcus epidermidis, Bacillus subtilis, Staphylococcus aureus, Escherichia coli | 564 | spherical shape particles | 18–38 | 2021 [77] |
| Kaempferia parviflora rhizome | extract | antimicrobial, antioxidant, and catalytic degradation agent | Escherichia coli, Staphylococcus aureus | 540 | spherical structure with high crystal in nature | 20–60 | 2021 [78] |
| Clerodendrum inerme | extract | Antimicrobial, and antioxidant activities | B. subtilis, S. aureus, Klebsiella, and E. coli, A. niger, T. harzianum, and A. flavus | 520 | spherical | 5 | 2020 [79] |
| Nigella arvensis leaf extract | flavonoids, alkaloids, and proteins | antibacterial, antioxidant, cytotoxicity against H1299 and MCF-7 cancer cell lines, and catalytic activities | S. epidermidis, B. subtilis, S. aureus, E. coli, Serratia marcescens, and P. aeruginosa | 546 | spherical shape mostly spherical in shape and less triangle, pentagon and hexagon shapes | 3–37 | 2017 [80] |
| Aqueous Extract of Garcinia mangostana Fruit Peels | phenols, flavonoids, benzophenones, and anthocyanins | - | - | 540–550 (UV-vis) | spherical shape particles | 32 | 2016 [81] |
| Mimosa tenuiflora Bark Extract | deprotonation of hydroxyl groups present in polyphenolic molecules of extract | a moderate cytotoxic effect at 24 and 48 h was found Cytotoxicity on HUVEC cells using MTT, Cellular uptake, and catalysis 12.5 mg/L of Mt extract, we obtained a 50% inhibition (L50), | maximum in 280 nm and broad of 50 nm | multiple shapes | 20–200 | 2019 [82] | |
| Chenopodium formosanum shell extract | phenolic groups | antibacterial properties | E. coli, and S. aureus. | 533 | Most of the resultant Au NPs were spherical | 8 | 2018 [83] |
| Leaf Extract of Ziziphus zizyphus | antioxidants, enzymes, and phenolic moieties | Antimicrobial and antifungal activity | E. coli, S. marcescens or C. albicans | peak in the range of 525–540 nm with a peak maximum in the range of approximately 527–535 nm | Majorly spherical and monodisperse | 30–50 | 2018 [84] |
| Brazilian red propolis | hexane, dichloromethane and ethyl acetate, prenylated benzophenones | Antimicrobial, antifungal, and anticancer activities (dose-dependent cytotoxicity activities in bladder and prostate cancer cells) | S. aureus, E. coli, S. mutans, C. albicans | prominent peak at a range of 523–541 nm | mostly spherical shapes | 8–15 | 2021 [85] |
| fresh peel (aqueous) extracts of Benincasa hispida | reducing enzymes as well as capping agents such as secondary metabolites | In vitro toxicity (antibacterial and anticancer) | Different Gram-positive and Gram-negative bacteria. Furthermore, the biosynthesized GNPs exerted remarkable in vitro cytotoxicity against human cervical cancer cell line, while sparing normal human primary osteoblast cells | a sharp absorption peak at 520 nm, | spherical in shape | 22 | 2021 [86] |
| Platycodon grandiflorum leaf extract (Balloon flower plant) | flavonoids, saponins, alkaloids, amino acids, proteins, and carbohydrates | antipathogenic activity under optimal conditions | E. coli and B. subtilis | absorption at 545 nm | spherical in shape | 15 | 2020 [87] |
| Hibiscus rosa-sinensis extract | alkaloids and flavonoids | - | - | 520 | spherical sized nanoparticles | 16–30 | 2014 [88] |
| Galaxaura elongata (powder or free ethanolic) based extract | Anti-microbial properties | E. coli, K. pneumoniae, S. aureus, and Methicillin-Resistant S. aureus | 520 | spherical with a few rods, triangular, truncated triangular, and hexagonal AuNPs | 3–77 | 2017 [89] | |
| of Memecylon umbellatum leaf extract | Saponins, alkaloids, phytosterols, phlobatannins, phenolic compounds, phytosterols, and quinones | biocompatibility and anti-microbial activities | B. subtilis, S. pneumoniae, S. aureus, S. typhimurium, Klebsiella aerogenes, and E. coli | 540 | spherical-shaped nanoparticles | 15–20 | 2018 [90] |
| Solanum nigrum leaf extract | phenolic compounds | strong DPPH radical and hydroxyl radical scavengers and antibacterial activity | S. saprophyticus, B. subtilis, E. coli, and P. aeruginosa | 537 | spherical-shaped nanoparticles and crystaline | 50 | 2014 [91] |
| AuNPs | Average Size (nm) (Diameter) | mAuNP (10−18 g) | Volume (10−25 m3) |
|---|---|---|---|
| Citrate AuNS | 12.5 | 19.7 | 10.2 |
| Tryptophan AuNS | 8.4 | 5.9 | 3.1 |
| Tyrosine AuNS | 9.9 | 9.8 | 5.1 |
| CTAB/citrate AuNS | 8.9 | 7.1 | 3.7 |
| AuNS (CTAB) | 77.9 | 4800 | 2475.2 |
| AuNR (CTAB) | 58.8 (length) 15.3 width | 270 | 137.6 |
| AuNPr (CTAB) | 94.7 (side length) | 1900 | 970.8 |
| AuNC (CTAB) | 47.5 (side length) | 2100 | 1071.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahari, H.; Fakhrabadipour, M.; Paidari, S.; Goksen, G.; Xu, B. Role of AuNPs in Active Food Packaging Improvement: A Review. Molecules 2022, 27, 8027. https://doi.org/10.3390/molecules27228027
Ahari H, Fakhrabadipour M, Paidari S, Goksen G, Xu B. Role of AuNPs in Active Food Packaging Improvement: A Review. Molecules. 2022; 27(22):8027. https://doi.org/10.3390/molecules27228027
Chicago/Turabian StyleAhari, Hamed, Mostafa Fakhrabadipour, Saeed Paidari, Gulden Goksen, and Baojun Xu. 2022. "Role of AuNPs in Active Food Packaging Improvement: A Review" Molecules 27, no. 22: 8027. https://doi.org/10.3390/molecules27228027
APA StyleAhari, H., Fakhrabadipour, M., Paidari, S., Goksen, G., & Xu, B. (2022). Role of AuNPs in Active Food Packaging Improvement: A Review. Molecules, 27(22), 8027. https://doi.org/10.3390/molecules27228027










