Novel Ascorbic Acid Co-Crystal Formulations for Improved Stability
Abstract
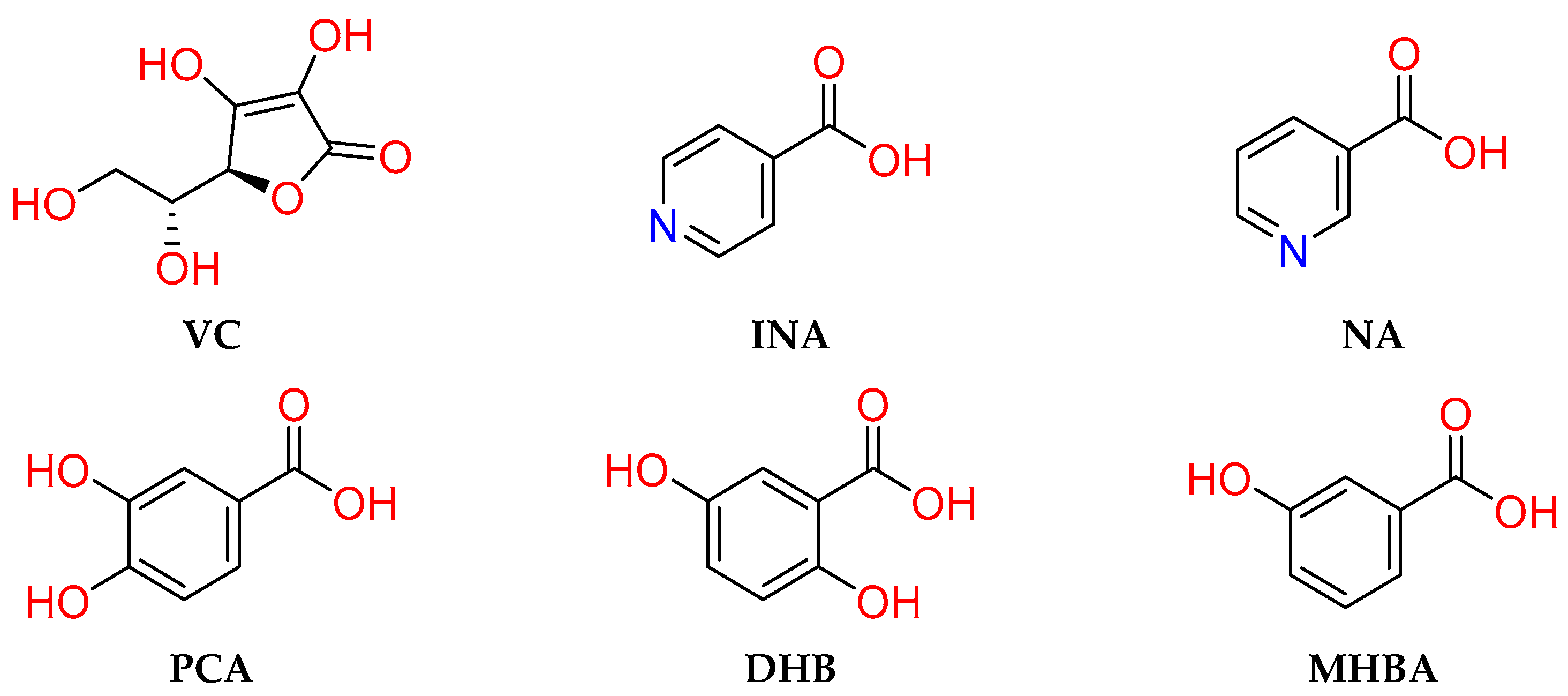
1. Introduction
2. Results and Discussion
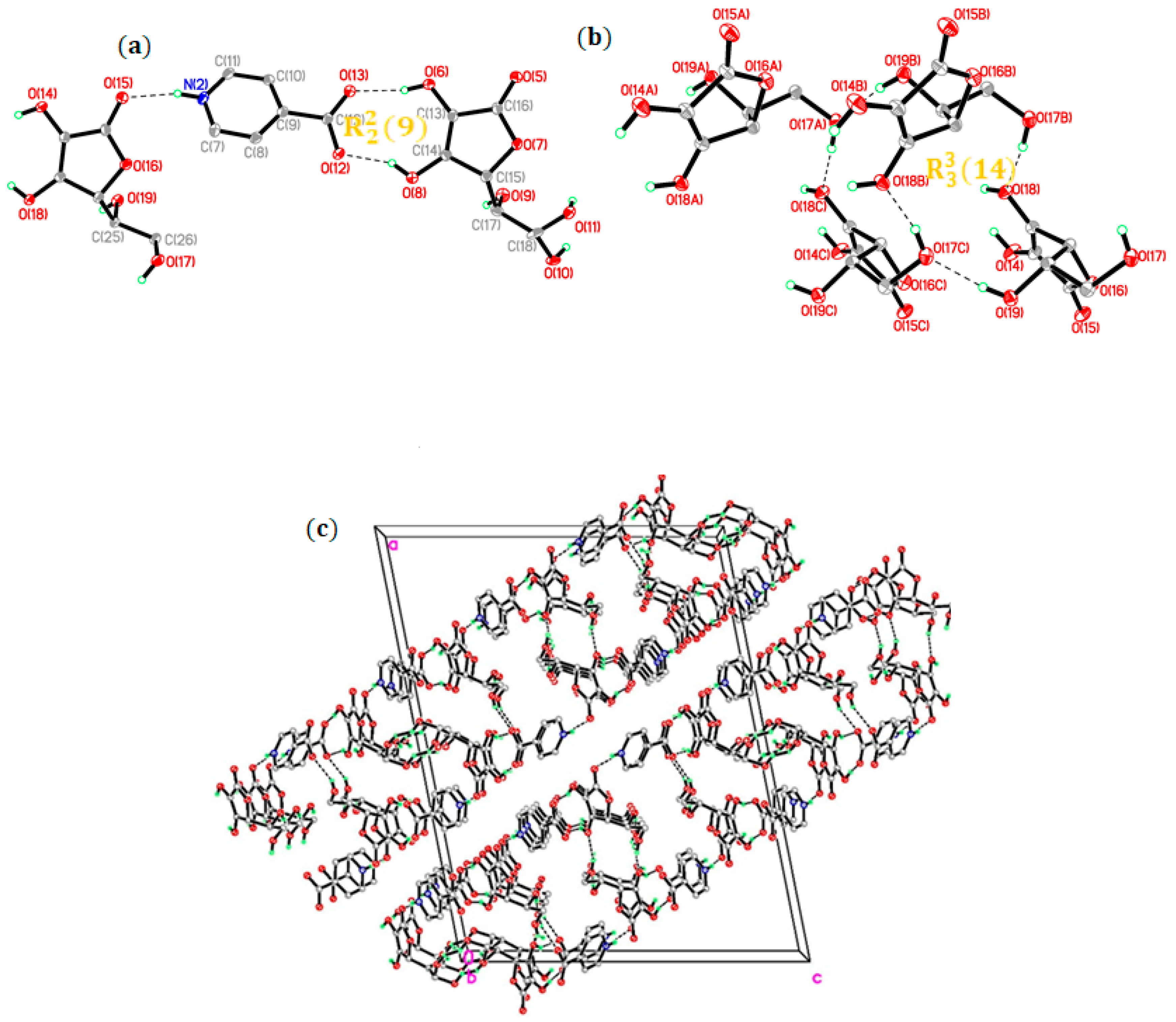
2.1. Crystal Structure Analysis
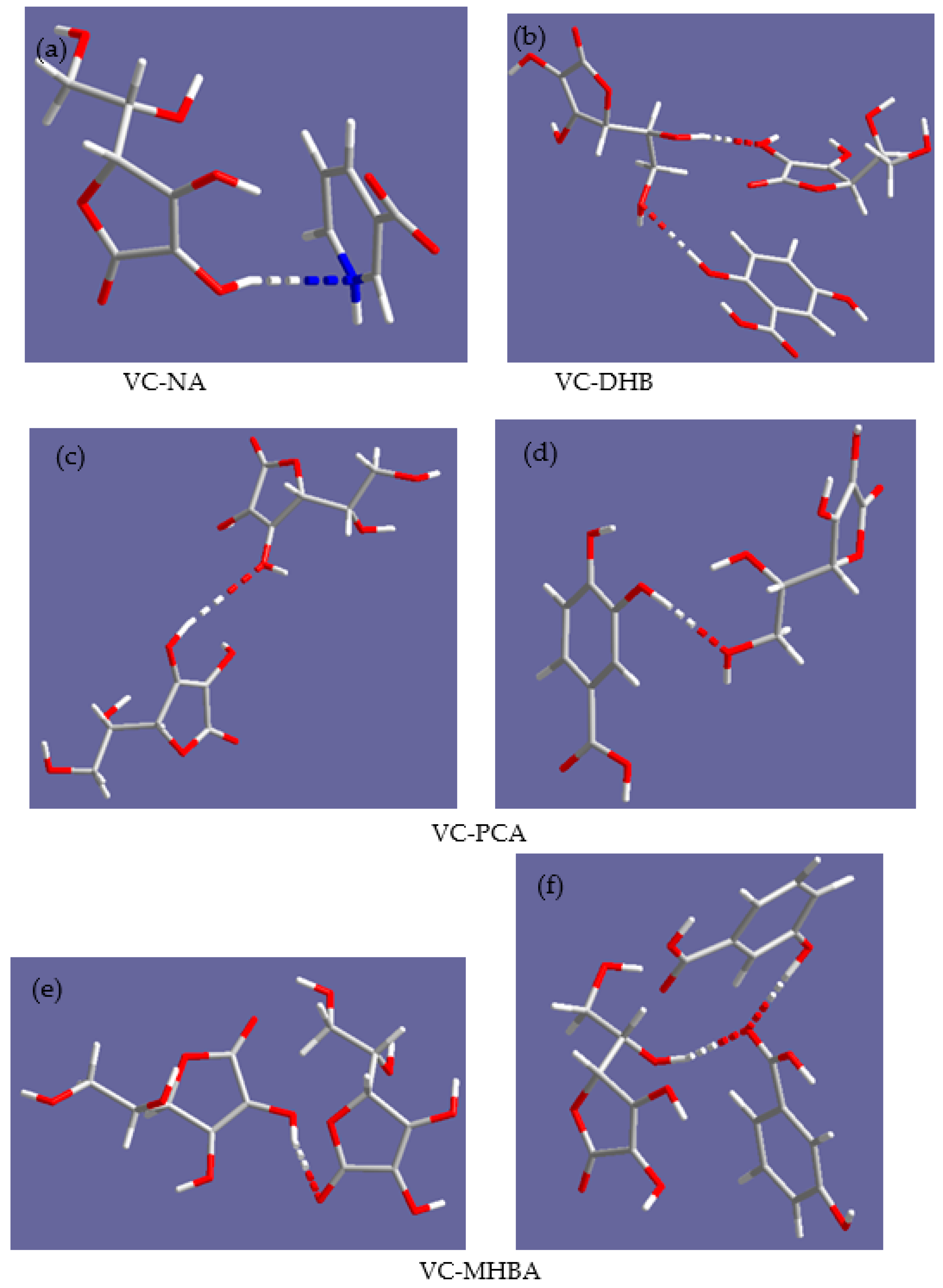
2.2. MD Simulations
2.3. Thermal Analysis
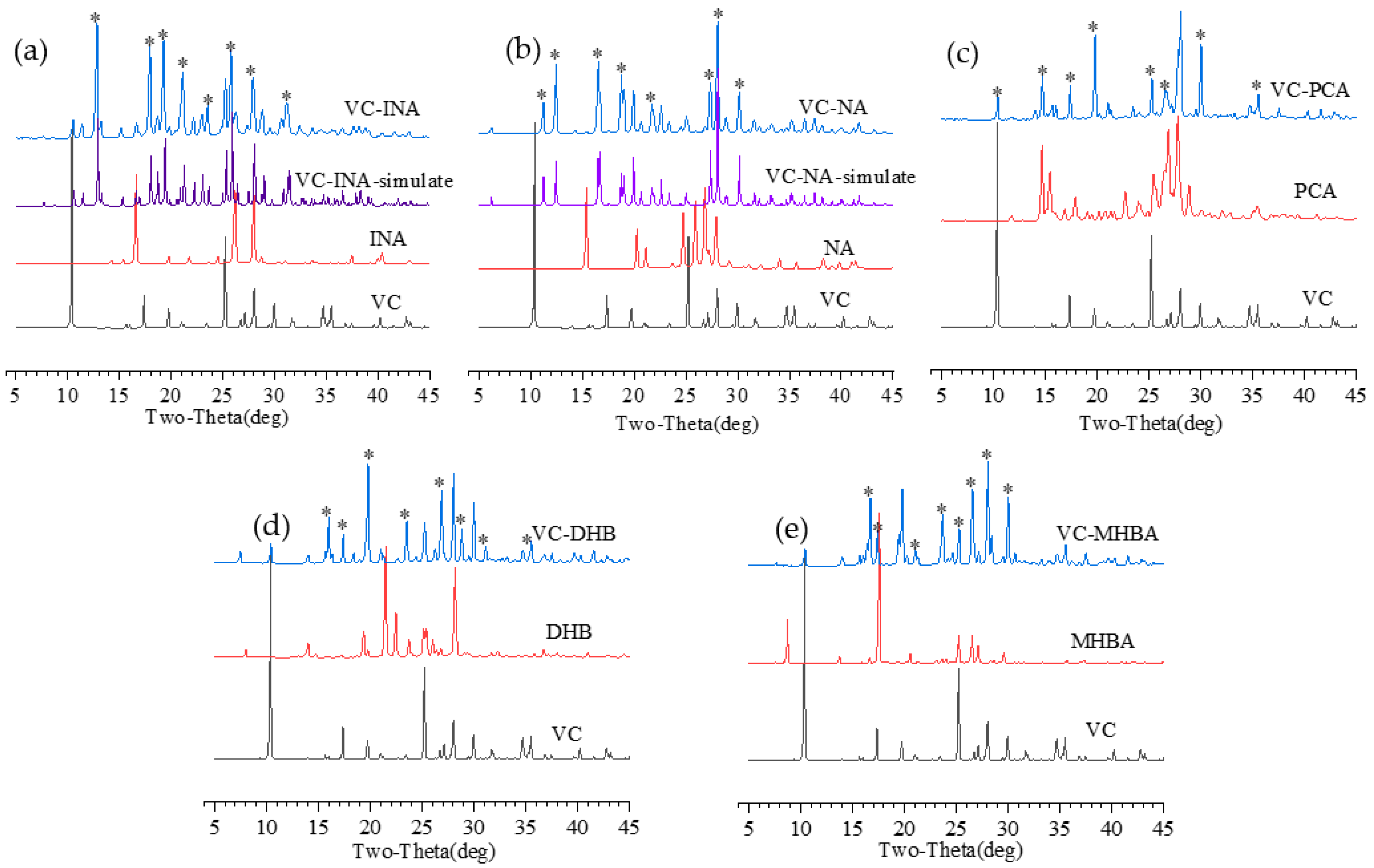
2.4. X-ray Powder Diffraction
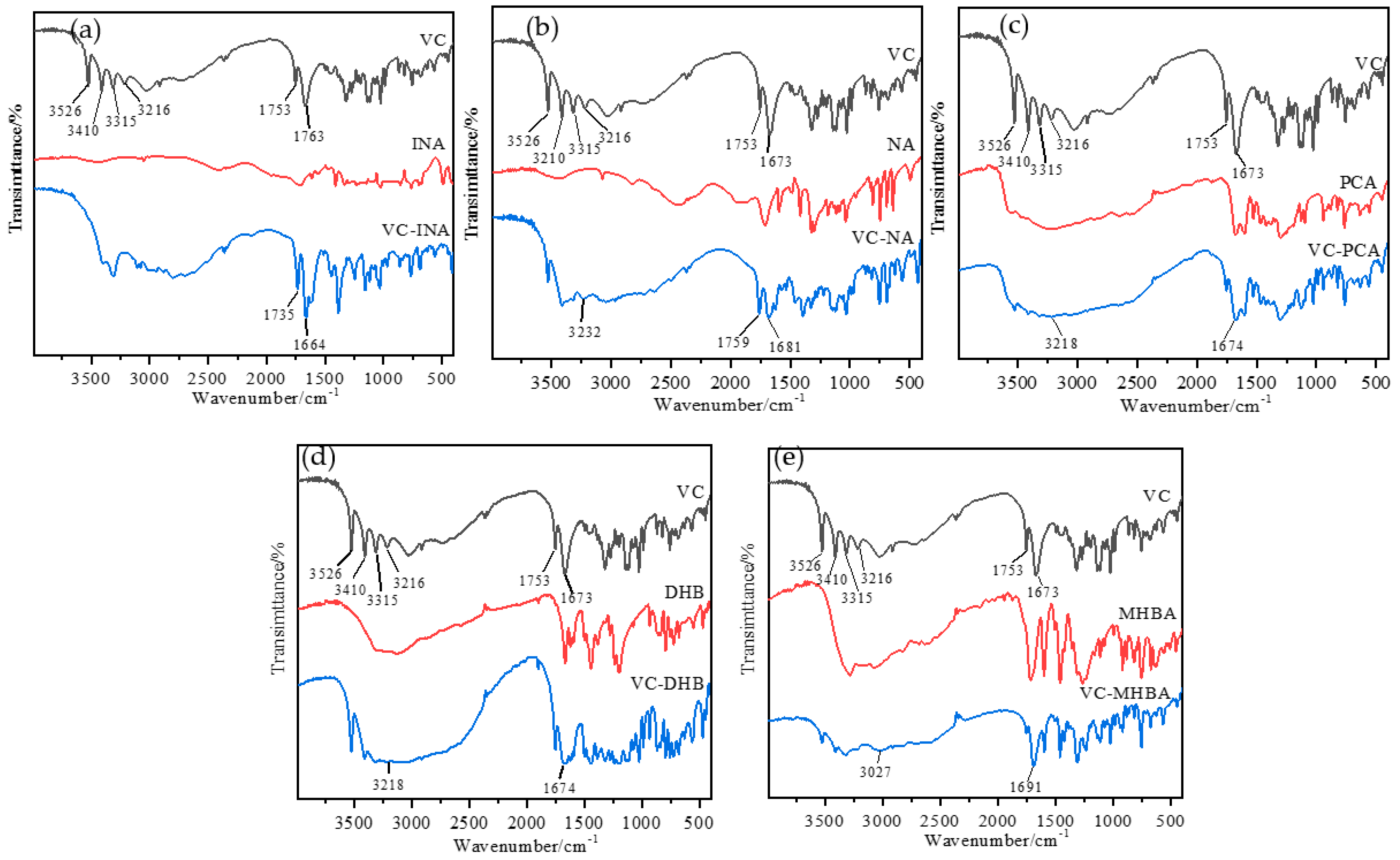
2.5. Fourier Transform Infrared Analysis
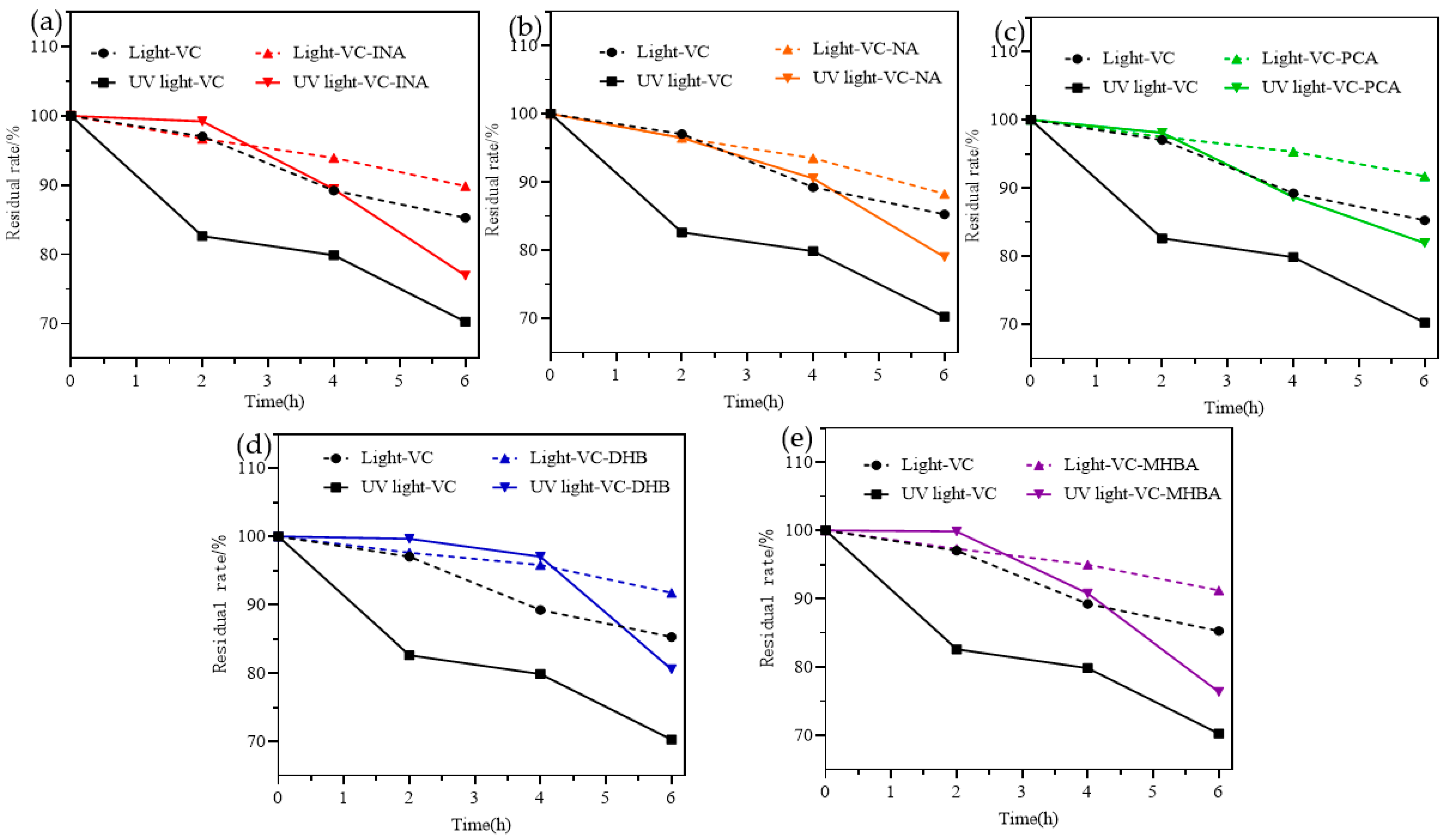
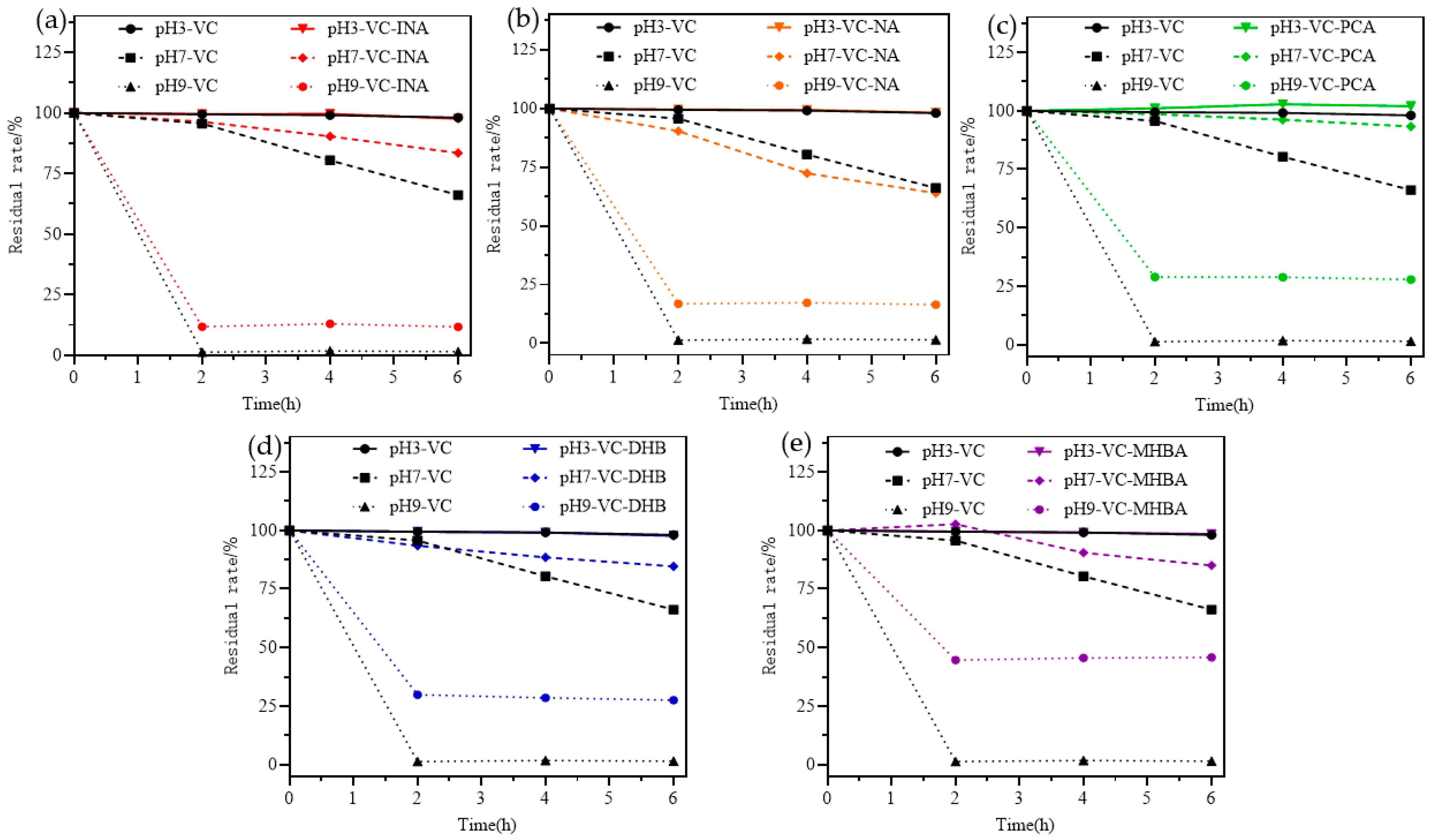
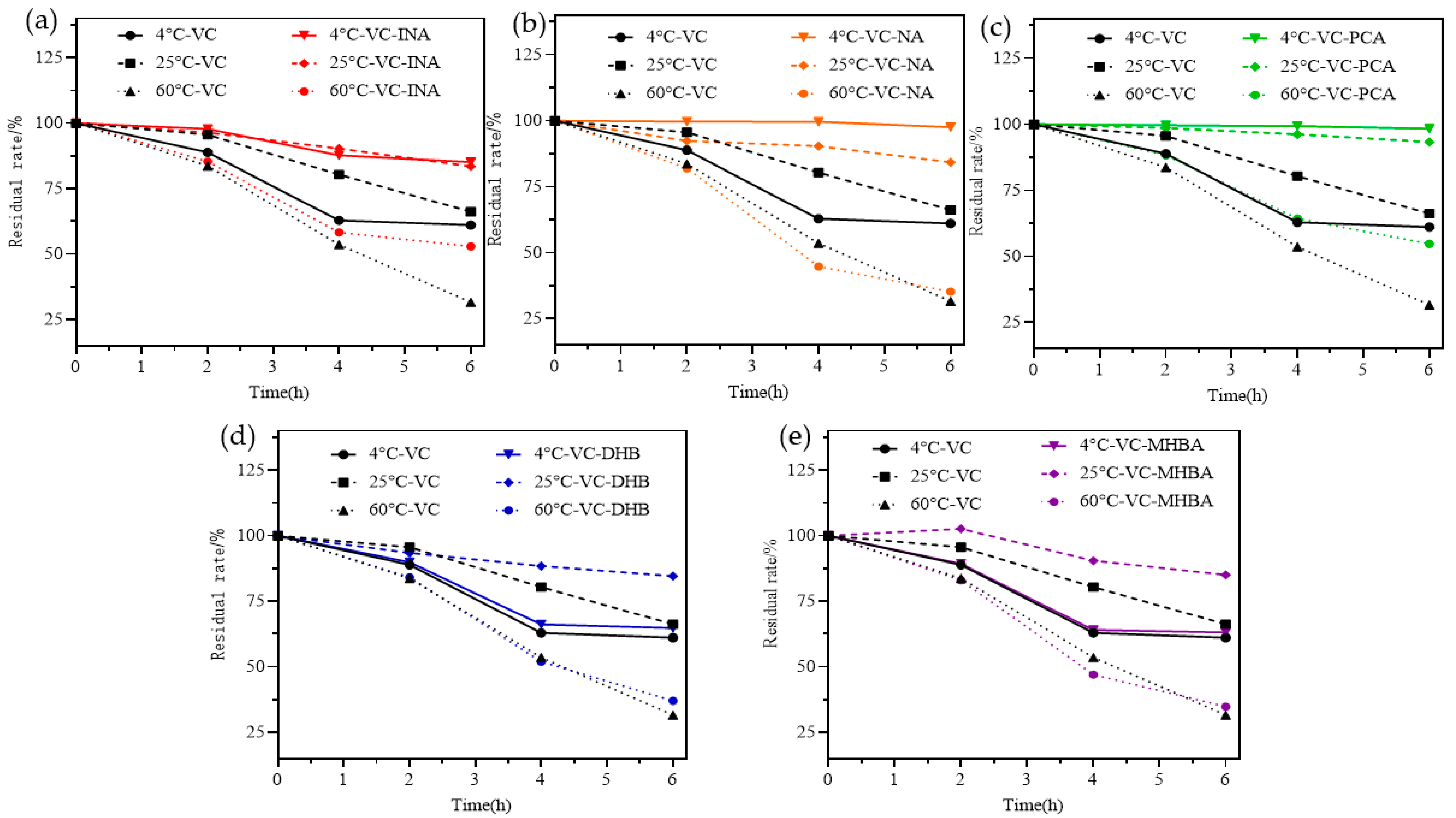
2.6. Stability Analysis
3. Materials and Methods
3.1. Materials
3.2. Dynamic Simulation
3.3. Co-Crystal Preparation
3.4. Single-Crystal X-ray Diffraction Analysis
3.5. Differential Scanning Calorimetry
3.6. X-ray Powder Diffraction
3.7. FT-IR Microscopy System
3.8. Photostability Analysis
3.9. Thermostability Analysis
3.10. pH Stability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.-Y.; Chen, J.-M.; Lu, T.-B. Improving the Solubility and Bioavailability of Apixaban via Apixaban–Oxalic Acid Cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical Cocrystals: A Review of Preparations, Physicochemical Properties and Applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, S.M.; Smilgies, D.-M.; Giri, G. Controlling Polymorphism in Pharmaceutical Compounds Using Solution Shearing. Cryst. Growth Des. 2018, 18, 602–606. [Google Scholar] [CrossRef]
- Ma, X.; Huang, S.; Lowinger, M.B.; Liu, X.; Lu, X.; Su, Y.; Williams, R.O. Influence of Mechanical and Thermal Energy on Nifedipine Amorphous Solid Dispersions Prepared by Hot Melt Extrusion: Preparation and Physical Stability. Int. J. Pharm. 2019, 561, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical Solvates, Hydrates and Amorphous Forms: A Special Emphasis on Cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Devi, V.K.; Clara, D.; Malviya, N.; Ganguly, S.; Desiraju, G.R. Cocrystals of Hydrochlorothiazide: Solubility and Diffusion/Permeability Enhancements through Drug–Coformer Interactions. Mol. Pharm. 2015, 12, 1615–1622. [Google Scholar] [CrossRef]
- Braun, D.E.; Kahlenberg, V.; Gelbrich, T.; Ludescher, J.; Griesser, U.J. Solid State Characterisation of Four Solvates of R-Cinacalcet Hydrochloride. CrystEngComm 2008, 10, 1617. [Google Scholar] [CrossRef]
- Zencirci, N.; Griesser, U.J.; Gelbrich, T.; Kahlenberg, V.; Jetti, R.K.R.; Apperley, D.C.; Harris, R.K. New Solvates of an Old Drug Compound (Phenobarbital): Structure and Stability. J. Phys. Chem. B 2014, 118, 3267–3280. [Google Scholar] [CrossRef]
- Panzade, P.S.; Shendarkar, G.R. Pharmaceutical Cocrystal: A Game Changing Approach for the Administration of Old Drugs in New Crystalline Form. Drug Dev. Ind. Pharm. 2020, 46, 1559–1568. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Qiao, N.; Chen, Y.; Gao, L. Preparation and Characterization of Carbamazepine Cocrystal in Polymer Solution. Pharmaceutics 2017, 9, 54. [Google Scholar] [CrossRef]
- Bofill, L.; de Sande, D.; Barbas, R.; Prohens, R. New Cocrystal of Ubiquinol with High Stability to Oxidation. Cryst. Growth Des. 2020, 20, 5583–5588. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Smith, A.J.; Wojtas, Ł.; Shytle, R.D.; Zaworotko, M.J. Physical Stability Enhancement and Pharmacokinetics of a Lithium Ionic Cocrystal with Glucose. Cryst. Growth Des. 2014, 14, 6135–6142. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Gao, Y.; Zhang, J.; Shi, L. Baicalein–Nicotinamide Cocrystal with Enhanced Solubility, Dissolution, and Oral Bioavailability. J. Pharm. Sci. 2014, 103, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Maginn, E.J. From Discovery to Data: What Must Happen for Molecular Simulation to Become a Mainstream Chemical Engineering Tool. AIChE J. 2009, 55, 1304–1310. [Google Scholar] [CrossRef]
- Cadden, J.; Gupta, K.M.; Kanaujia, P.; Coles, S.J.; Aitipamula, S. Cocrystal Formulations: Evaluation of the Impact of Excipients on Dissolution by Molecular Simulation and Experimental Approaches. Cryst. Growth Des. 2021, 21, 1006–1018. [Google Scholar] [CrossRef]
- Yani, Y.; Kanaujia, P.; Chow, P.S.; Tan, R.B.H. Effect of API-Polymer Miscibility and Interaction on the Stabilization of Amorphous Solid Dispersion: A Molecular Simulation Study. Ind. Eng. Chem. Res. 2017, 56, 12698–12707. [Google Scholar] [CrossRef]
- El Shinnawy, H.; Khedr, A.; Alghitany, A.; Ramzy, M.; Baki, A. Role of Intravenous Ascorbic Acid in the Management of Anemia in Hemodialysis Patients. Indian J. Nephrol. 2021, 31, 230. [Google Scholar]
- Evtushenko, D.N.; Arkhipov, S.G.; Fateev, A.V.; Izaak, T.I.; Egorova, L.A.; Skorik, N.A.; Vodyankina, O.V.; Boldyreva, E.V. A Cocrystal of L -Ascorbic Acid with Picolinic Acid: The Role of O—H...O, N—H...O and C—H...O Hydrogen Bonds and L -Ascorbic Acid Conformation in Structure Stabilization. Acta Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 2020, 76, 967–978. [Google Scholar] [CrossRef]
- Lapidus, S.H.; Stephens, P.W.; Arora, K.K.; Shattock, T.R.; Zaworotko, M.J. A Comparison of Cocrystal Structure Solutions from Powder and Single Crystal Techniques. Cryst. Growth Des. 2010, 10, 4630–4637. [Google Scholar] [CrossRef]
- Kavuru, P.; Aboarayes, D.; Arora, K.K.; Clarke, H.D.; Kennedy, A.; Marshall, L.; Ong, T.T.; Perman, J.; Pujari, T.; Wojtas, Ł.; et al. Hierarchy of Supramolecular Synthons: Persistent Hydrogen Bonds Between Carboxylates and Weakly Acidic Hydroxyl Moieties in Cocrystals of Zwitterions. Cryst. Growth Des. 2010, 10, 3568–3584. [Google Scholar] [CrossRef]
- Wang, J.-R.; Bao, J.; Fan, X.; Dai, W.; Mei, X. PH-Switchable Vitamin B9 Gels for Stoichiometry-Controlled Spherical Co-Crystallization. Chem. Commun. 2016, 52, 13452–13455. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, V.; Vijayan, M. X-Ray Studies on Crystalline Complexes Involving Amino Acids. IV. The Structure of L-Arginine L-Ascorbate. Acta C34ryst. 1980, 36, 120–125. [Google Scholar] [CrossRef]
- Sudhakar, V.; Bhat, T.N.; Vijayan, M. Studies on Crystalline Complexes Involving Amino Acids. V. The Structure of L-Serine-L-Ascorbic Acid. Acta Cryst. 1980, 36, 125–128. [Google Scholar] [CrossRef]
- Goher, M.A.S.; Mautner, F.A.; Mak, T.C.W.; Abu-Youssef, M.A.M. Synthesis and Crystal Structures of [(Nicotinic Acid)2H]+I−, [(2-Amino-6-Methylpyridine)H]+(NO3)−, And the 1:1 Complex Between 1-Isoquinoline Carboxylic Acid (Zwitter Ion) and L-Ascorbic Acid Assembled via Hydrogen Bonds. Mon. Chem./Chem. Mon. 2003, 134, 1519–1527. [Google Scholar] [CrossRef]
- Sylvester, E.; McGovern, M.; Young Lee, A.; Nguyen, P.; Park, J.; Benedict, J.B. Partial Charge Transfer in the Salt Co-Crystal of l-Ascorbic Acid and 4,4′-Bi-Pyridine. Acta Crystallogr. E Crystallogr. Commun. 2019, 75, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fan, X.; Ding, Q.; Mei, X. Determination of absolute configuration using heavy atom based co-crystallization method: Halogen atom effects. J. Mol. Sruct. 2016, 1119, 269–275. [Google Scholar] [CrossRef]
- GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 17 September 2022).
- Stilinović, V.; Kaitner, B. Salts and Co-Crystals of Gentisic Acid with Pyridine Derivatives: The Effect of Proton Transfer on the Crystal Packing (and Vice Versa). Cryst. Growth Des. 2012, 12, 5763–5772. [Google Scholar] [CrossRef]
- ChemicalBook—Chemical Information Search. Available online: https://www.chemicalbook.com/ProductIndex.aspx (accessed on 20 September 2022).
- Wang, J.; Dai, X.-L.; Lu, T.-B.; Chen, J.-M. Temozolomide–Hesperetin Drug–Drug Cocrystal with Optimized Performance in Stability, Dissolution, and Tabletability. Cryst. Growth Des. 2021, 21, 838–846. [Google Scholar] [CrossRef]
- Nicolov, M.; Ghiulai, R.M.; Voicu, M.; Mioc, M.; Duse, A.O.; Roman, R.; Ambrus, R.; Zupko, I.; Moaca, E.A.; Coricovac, D.E.; et al. Cocrystal Formation of Betulinic Acid and Ascorbic Acid: Synthesis, Physico-Chemical Assessment, Antioxidant, and Antiproliferative Activity. Front. Chem. 2019, 7, 92. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Chem. Phys. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]


| Compound | VC-INA |
|---|---|
| Empirical formula | C12H13NO8 |
| Formula weight | 299.23 |
| Temperature/K | 150.00(10) |
| Crystal system | monoclinic |
| Space group | C2 (no. 5) |
| a/Å | 30.073(2) |
| b/Å | 5.4222(4) |
| c/Å | 23.6087(12) |
| α/° | 90 |
| β/° | 101.250(5) |
| γ/° | 90 |
| Volume/Å3 | 3775.7(4) |
| Z | 12 |
| ρcalcg/cm3 | 1.579 |
| μ/mm−1 | 1.173 |
| F(000) | 1872.0 |
| Index ranges | −35 ≤ h ≤ 36, −6 ≤ k ≤ 4, −29 ≤ l ≤ 24 |
| Data/restraints/parameters | 5980/1/597 |
| Final R indexes (I ≥ 2σ(I)) | R1 = 0.0830, wR2 = 0.2078 |
| Final R indexes (all data) | R1 = 0.0975, wR2 = 0.2227 |
| Goodness-of-fit on F2 | 1.014 |
| Flack parameter | −0.1(4) |
| System | Ecoul | ELJ | Etotal | ΔE * |
|---|---|---|---|---|
| VC-INA | ||||
| VC | −3088.88 | −306.384 | −3395.26 | |
| INA | −241.67 | −222.065 | −463.735 | |
| VC-INA | −3369.94 | −921.246 | −4291.19 | −432.195 |
| VC-NA | ||||
| VC | −3088.19 | −301.88 | −3390.07 | |
| NA | 438.305 | −237.564 | 200.741 | |
| VC-NA | −2687.16 | −940.937 | −3628.1 | −438.771 |
| VC-PCA | ||||
| VC | −3093.84 | −319.397 | −3413.24 | |
| PCA | −2367.44 | −215.397 | −2582.84 | |
| VC-PCA | −5569.87 | −965.21 | −6535.08 | −539.000 |
| VC-DHB | ||||
| VC | −3098.22 | −336.478 | −3434.7 | |
| DHB | 1124.42 | −132.278 | 992.142 | |
| VC-DHB | −2084.45 | −902.098 | −2986.55 | −543.992 |
| VC-MHBA | ||||
| VC | −3090.57 | −322.092 | −3412.66 | |
| MHBA | −309.506 | −184.255 | −493.761 | |
| VC-MHBA | −3481.98 | −890.006 | −4371.99 | −465.569 |
| System | H-Bond Number |
|---|---|
| VC-INA | 20.55 |
| VC-NA | 22.07 |
| VC-PCA | 47.05 |
| VC-DHB | 48.05 |
| VC-MHBA | 32.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zeng, H.; Li, M.; Song, Y.; Tian, S.; Xiong, J.; He, L.; Liu, Y.; Wu, X. Novel Ascorbic Acid Co-Crystal Formulations for Improved Stability. Molecules 2022, 27, 7998. https://doi.org/10.3390/molecules27227998
Zhang H, Zeng H, Li M, Song Y, Tian S, Xiong J, He L, Liu Y, Wu X. Novel Ascorbic Acid Co-Crystal Formulations for Improved Stability. Molecules. 2022; 27(22):7998. https://doi.org/10.3390/molecules27227998
Chicago/Turabian StyleZhang, Hui, Huahui Zeng, Mengfei Li, Yagang Song, Shuo Tian, Jing Xiong, Lan He, Yang Liu, and Xiangxiang Wu. 2022. "Novel Ascorbic Acid Co-Crystal Formulations for Improved Stability" Molecules 27, no. 22: 7998. https://doi.org/10.3390/molecules27227998
APA StyleZhang, H., Zeng, H., Li, M., Song, Y., Tian, S., Xiong, J., He, L., Liu, Y., & Wu, X. (2022). Novel Ascorbic Acid Co-Crystal Formulations for Improved Stability. Molecules, 27(22), 7998. https://doi.org/10.3390/molecules27227998







