Antimicrobial Properties of Plant Fibers
Abstract
1. Introduction
2. Pathogens in Biomedical Devices
2.1. Escherichia coli
2.2. Pseudomonas aeruginosa
2.3. Staphylococcus aureus
2.4. Staphylococcus epidermidis
2.5. Viruses
2.6. Fungi
2.7. Biofilms
3. Comparison between Natural and Synthetic Fibers
4. Antimicrobial Mechanism in the Vegetable Fibers
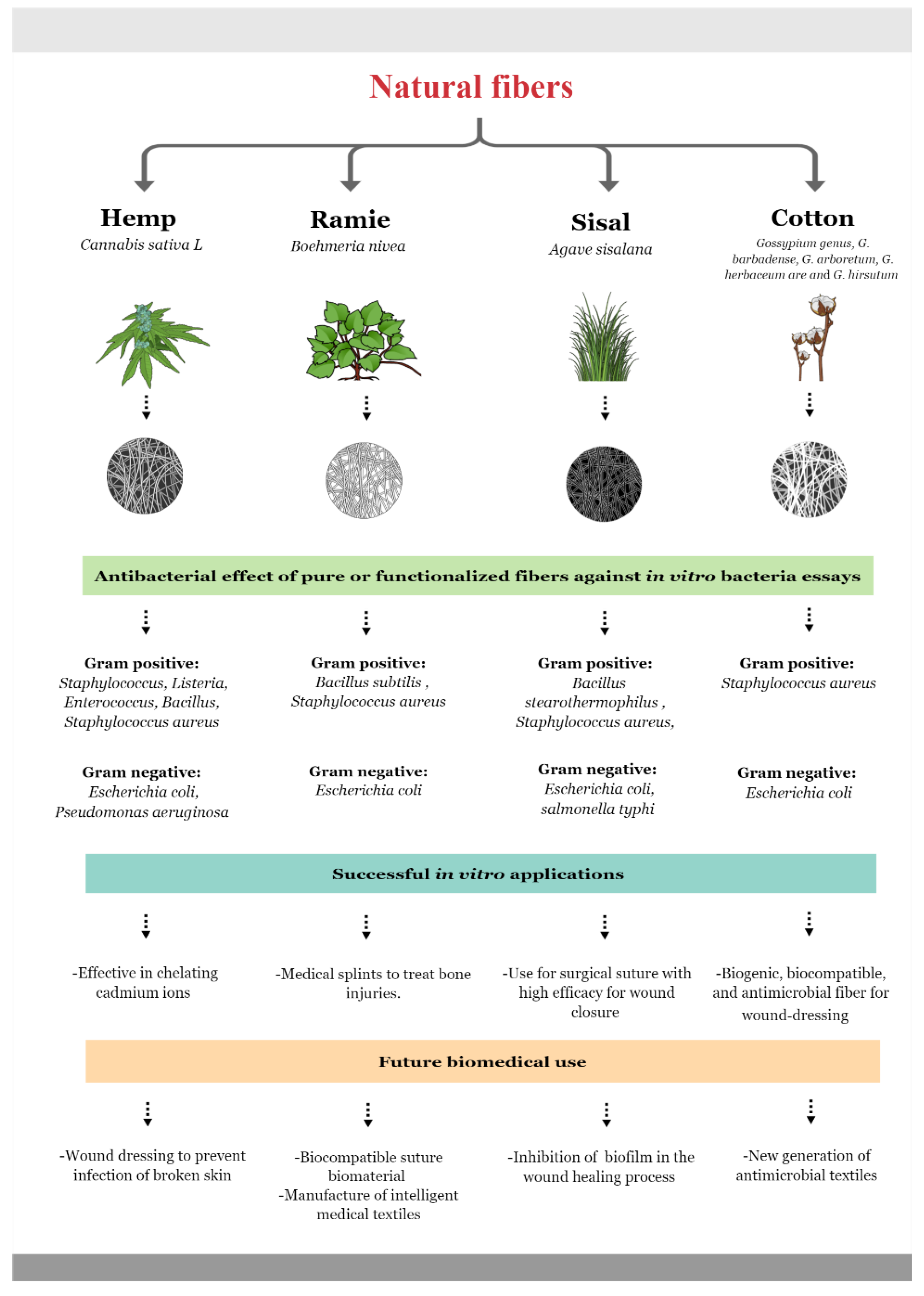
5. Plant Fiber Composition and Antimicrobial Properties
5.1. Hemp Fiber
5.2. Ramie Fiber
5.3. Sisal Fiber
5.4. Cotton Fiber
5.5. Linen or Flax Fiber
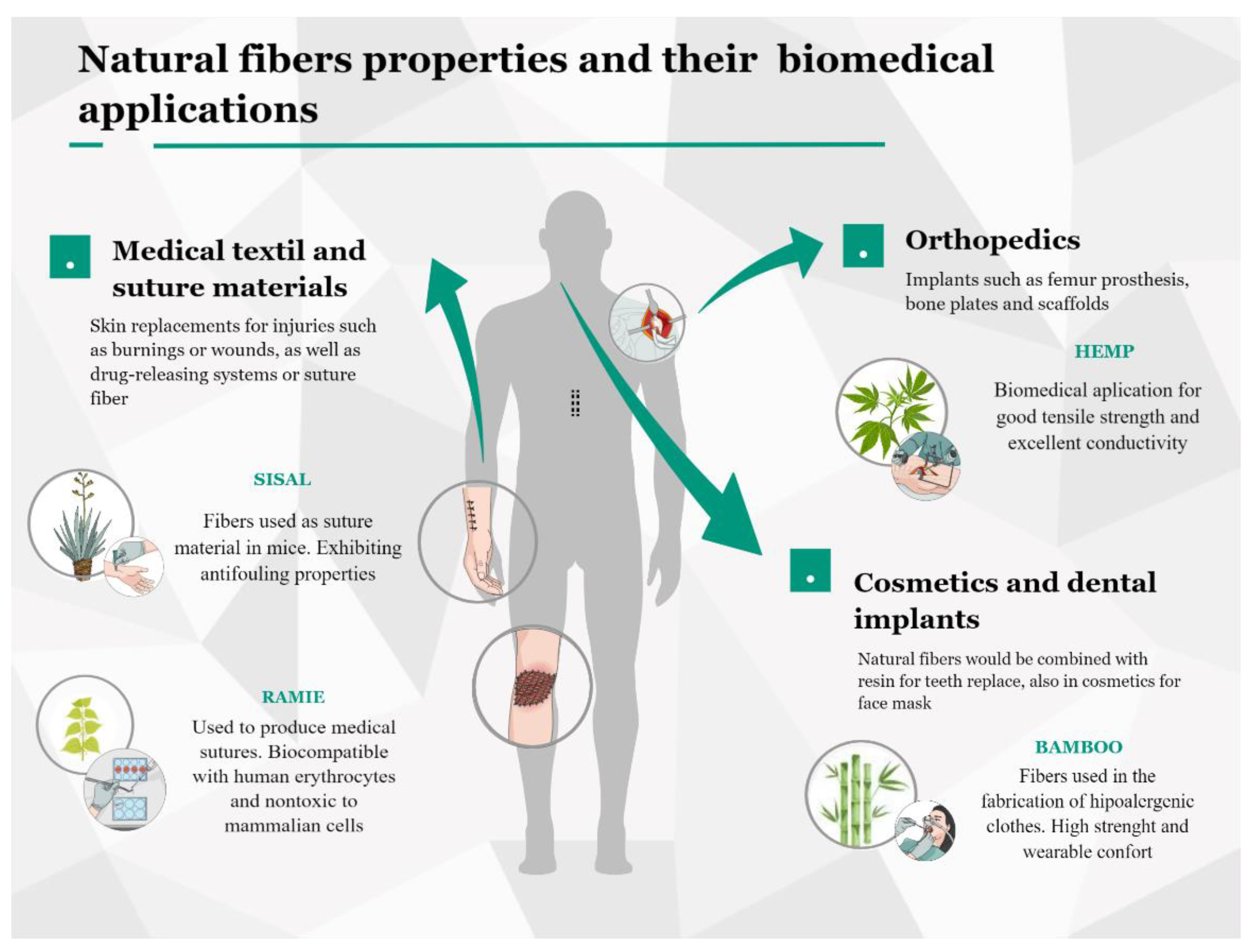
6. Biomedical Applications of Antimicrobial Fibers
6.1. Natural Fibers on Medical Textiles
6.2. Natural Fibers on Orthopedics, Dental Implants, and Cosmetics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices—An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Government of India. Annual Report 2016–2017; National Centre for Disease Control, National Surveillance Programme for Communicable Diseases, Directorate General of Health Services, Ministry of Health, and Family Welfare: Delhi, India, 2017. Available online: https://www.ncdc.gov.in (accessed on 15 October 2022).
- Khan, I.D.; Basu, A.; Kiran, S.; Trivedi, S.; Pandit, P.; Chattoraj, A. Device-Associated Healthcare-Associated Infections (DA-HAI) and the caveat of multiresistance in a multidisciplinary intensive care unit. Med J. Armed Forces India 2017, 73, 222–231. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Annual Epidemiological Report on Communicable Diseases in Europe. Healthcare-associated infections: Surgical site infections. In Annual Epidemiological Report for 2017; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu (accessed on 22 October 2022).
- Maki, G.; Zervos, M. Health Care–Acquired Infections in Low- and Middle-Income Countries and the Role of Infection Prevention and Control. Infect. Dis. Clin. N. Am. 2021, 35, 827–839. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Wei, N.; Zhang, J.; Ma, W.; Zhao, H.; Han, X. Epidemiological and clinical characteristics of healthcare-associated infection in elderly patients in a large Chinese tertiary hospital: A 3-year surveillance study. BMC Infect. Dis. 2020, 20, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Masia, M.D.; Dettori, M. Antimicrobial Resistance, Healthcare-Associated Infections, and Environmental Microbial Contamination. Healthcare 2022, 10, 242. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Making Health Care Safer. In Protect Patients from Antibiotic Resistance; U.S. Department of Health & Human Services: Atlanta, GA, USA, 2016. Available online: https://www.cdc.gov (accessed on 25 September 2022).
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef]
- Dhingra, S.; Joshi, A.; Singh, N.; Saha, S. Infection resistant polymer brush coating on the surface of biodegradable polyester. Mater. Sci. Eng. C 2021, 118, 111465. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Mikulskis, P.; Hook, A.L.; Dundas, A.A.; Irvine, D.J.; Sanni, O.; Anderson, D.G.; Langer, R.; Alexander, M.R.; Williams, P.; Winkler, D.A. Prediction of Broad-Spectrum Pathogen Attachment to Coating Materials for Biomedical Devices. ACS Appl. Mater. Interfaces 2018, 10, 139–149. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Wallace, M.J.; Fishbein, S.R.S.; Dantas, G. Antimicrobial resistance in enteric bacteria: Current state and next-generation solutions. Gut Microbes 2020, 12, 1799654. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yu, Q.; Sun, H. Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef] [PubMed]
- Sifri, Z.; Chokshi, A.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Nkansa-Gyamfi, N.A.; Kazibwe, J.; Traore, D.A.K.; Nji, E. Prevalence of multidrug-, extensive drug-, and pandrug-resistant commensal Escherichia coli isolated from healthy humans in community settings in low- and middle-income countries: A systematic review and meta-analysis. Glob. Health Action 2019, 12, 1815272. [Google Scholar] [CrossRef]
- Puca, V.; Traini, T.; Guarnieri, S.; Carradori, S.; Sisto, F.; Macchione, N.; Muraro, R.; Mincione, G.; Grande, R. The Antibiofilm Effect of a Medical Device Containing TIAB on Microorganisms Associated with Surgical Site Infection. Molecules 2019, 24, 2280. [Google Scholar] [CrossRef]
- Edmiston, C.E.; Seabrook, G.R.; Goheen, M.P.; Krepel, C.J.; Johnson, C.P.; Lewis, B.D.; Brown, K.R.; Towne, J.B. Bacterial Adherence to Surgical Sutures: Can Antibacterial-Coated Sutures Reduce the Risk of Microbial Contamination? J. Am. Coll. Surg. 2006, 203, 481–489. [Google Scholar] [CrossRef]
- Gomes, L.C.; Silva, L.N.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Escherichia coli adhesion, biofilm development and antibiotic susceptibility on biomedical materials. J. Biomed. Mater. Res. Part A 2014, 103, 1414–1423. [Google Scholar] [CrossRef]
- Mueller, M.; Tainter, C.R. Escherichia Coli. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Martinez-Medina, M. Special Issue: Pathogenic Escherichia coli: Infections and Therapies. Antibiotics 2021, 10, 112. [Google Scholar] [CrossRef]
- Reisner, A.; Maierl, M.; Jörger, M.; Krause, R.; Berger, D.; Haid, A.; Tesic, D.; Zechner, E.L. Type 1 Fimbriae Contribute to Catheter-Associated Urinary Tract Infections Caused by Escherichia coli. J. Bacteriol. 2013, 196, 931–939. [Google Scholar] [CrossRef]
- Hoque, E.; Rayhan, A.M.; Shaily, S.I. Natural Fiber-based Green Composites: Processing, Properties and Biomedical Applications. Appl. Sci. Eng. Prog. 2021, 14, 689–718. [Google Scholar] [CrossRef]
- Wilson, M.G.; Pandey, S. Pseudomonas Aeruginosa. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa Biofilms in Disease. Microb. Ecol. 2013, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Angeletti, S.; Cella, E.; Prosperi, M.; Spoto, S.; Fogolari, M.; De Florio, L.; Antonelli, F.; Dedej, E.; De Flora, C.; Ferraro, E.; et al. Multi-drug resistant Pseudomonas aeruginosa nosocomial strains: Molecular epidemiology and evolution. Microb. Pathog. 2018, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Troeman, D.P.R.; Van Hout, D.; Kluytmans, J.A.J.W. Antimicrobial approaches in the prevention of Staphylococcus aureus infections: A review. J. Antimicrob. Chemother. 2018, 74, 281–294. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2019, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.; Unakal, C. Staphylococcus Aureus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.G.; Diep, B.A. Colonization, Fomites, and Virulence: Rethinking the Pathogenesis of Community-Associated Methicillin-Resistant Staphylococcus aureus Infection. Clin. Infect. Dis. 2008, 46, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Yang, S.; Zhang, C.; Li, Z.; Li, X.; Shen, Z.; Zhu, W. Facile and Versatile Modification of Cotton Fibers for Persistent Antibacterial Activity and Enhanced Hygroscopicity. ACS Appl. Mater. Interfaces 2018, 10, 38506–38516. [Google Scholar] [CrossRef] [PubMed]
- Mahé, P.; Tournoud, M. Predicting bacterial resistance from whole-genome sequences using k-mers and stability selection. BMC Bioinform. 2018, 19, 383. [Google Scholar] [CrossRef]
- Méric, G.; Mageiros, L.; Pensar, J.; Laabei, M.; Yahara, K.; Pascoe, B.; Kittiwan, N.; Tadee, P.; Post, V.; Lamble, S.; et al. Disease-associated genotypes of the commensal skin bacterium Staphylococcus epidermidis. Nat. Commun. 2018, 9, 5034. [Google Scholar] [CrossRef]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis—Skin friend or foe? PLOS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef]
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G. Clinical characteristics of Staphylococcus epidermidis: A systematic review. GMS Hyg. Infect. Control 2014, 9, Doc23. [Google Scholar] [CrossRef]
- Mack, D.; Davies, A.P.; Harris, L.G.; Jeeves, R.; Pascoe, B.; Knobloch, J.K.-M.; Rohde, H.; Wilkinson, T.S. Staphylococcus epidermidis in Biomaterial-Associated Infections. In Biomaterials Associated Infection; Springer: New York, NY, USA, 2013; pp. 25–56. [Google Scholar] [CrossRef]
- França, A.; Carvalhais, V.; Vilanova, M.; Pier, G.B.; Cerca, N. Characterization of an in vitro fed-batch model to obtain cells released from S. epidermidis biofilms. AMB Express 2016, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Saporito, P.; Mouritzen, M.V.; Løbner-Olesen, A.; Jenssen, H. LL-37 fragments have antimicrobial activity against Staphylococcus epidermidis biofilms and wound healing potential in HaCaT cell line. J. Pept. Sci. 2018, 24, e3080. [Google Scholar] [CrossRef] [PubMed]
- Afzal, F.; Ashraf, M.; Manzoor, S.; Aziz, H.; Nosheen, A.; Riaz, S. Development of novel antiviral nanofinishes for bioactive textiles. Polym. Bull. 2022. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, W.; Sun, Y.; Chen, W.; Zhang, Y. Application of antiviral materials in textiles: A review. Nanotechnol. Rev. 2021, 10, 1092–1115. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Shymborska, Y.; Stetsyshyn, Y.; Raczkowska, J.; Skirtach, A.G.; Peretiatko, T.; Budkowski, A. Passive antifouling and active self-disinfecting antiviral surfaces. Chem. Eng. J. 2022, 446, 137048. [Google Scholar] [CrossRef]
- Galante, A.J.; Pilsbury, B.C.; Yates, K.A.; LeMieux, M.; Bain, D.J.; Shanks, R.M.Q.; Romanowski, E.G.; Leu, P.W. Reactive silver inks for antiviral, repellent medical textiles with ultrasonic bleach washing durability compared to silver nanoparticles. PLoS ONE 2022, 17, e0270718. [Google Scholar] [CrossRef]
- Iyigundogdu, Z.U.; Demir, O.; Asutay, A.B.; Sahin, F. Developing Novel Antimicrobial and Antiviral Textile Products. Appl. Biochem. Biotechnol. 2016, 181, 1155–1166. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front. Cell. Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Tejera, E.; Machado, A. Prevalence of biofilms in Candida spp. bloodstream infections: A meta-analysis. PLoS ONE 2022, 17, e0263522. [Google Scholar] [CrossRef] [PubMed]
- Filippovich, S.Y.; Bachurina, G.P. Antifungal Surfaces. Appl. Biochem. Microbiol. 2022, 58, 507–517. [Google Scholar] [CrossRef]
- Firacative, C. Invasive fungal disease in humans: Are we aware of the real impact? Memórias Do Inst. Oswaldo Cruz 2020, 115, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alkan, R.; Torgan, E.; Karadag, R. The Investigation of Antifungal Activity and Durability of Natural Silk Fabrics Dyed with Madder and Gallnut. J. Nat. Fibers 2017, 14, 769–780. [Google Scholar] [CrossRef]
- Arenas-Chávez, C.A.; de Hollanda, L.M.; Arce-Esquivel, A.A.; Alvarez-Risco, A.; Del-Aguila-Arcentales, S.; Yáñez, J.A.; Vera-Gonzales, C. Antibacterial and Antifungal Activity of Functionalized Cotton Fabric with Nanocomposite Based on Silver Nanoparticles and Carboxymethyl Chitosan. Processes 2022, 10, 1088. [Google Scholar] [CrossRef]
- Okla, M.; Alatar, A.; Al-Amri, S.; Soufan, W.; Ahmad, A.; Abdel-Maksoud, M. Antibacterial and Antifungal Activity of the Extracts of Different Parts of Avicennia marina (Forssk.) Vierh. Plants 2021, 10, 252. [Google Scholar] [CrossRef]
- Coenye, T.; Bjarnsholt, T. Editorial: The complexity of microbial biofilm research—An introduction to the third thematic issue on biofilms. Pathog. Dis. 2016, 74, ftw053. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Alhede, M.; Eickhardt-Sørensen, S.R.; Moser, C.; Kühl, M.; Jensen, P.; Høiby, N. The in vivo biofilm. Trends Microbiol. 2013, 21, 466–474. [Google Scholar] [CrossRef]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Saheb, D.N.; Jog, J.P. Natural fiber polymer composites: A review. Adv. Polym. Technol. 1999, 18, 351–363. [Google Scholar] [CrossRef]
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Adi, D.S.; Ismadi; Damayanti, R.; Subiyanto, B.; Fatriasari, W.; Fudholi, A. A review on natural fibers for development of eco-friendly bio-composite: Characteristics, and utilizations. J. Mater. Res. Technol. 2021, 13, 2442–2458. [Google Scholar] [CrossRef]
- Girijappa, Y.G.T.; Rangappa, S.M.; Parameswaranpillai, J.; Siengchin, S. Natural Fibers as Sustainable and Renewable Resource for Development of Eco-Friendly Composites: A Comprehensive Review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Kandemir, A.; Pozegic, T.R.; Hamerton, I.; Eichhorn, S.J.; Longana, M.L. Characterisation of Natural Fibres for Sustainable Discontinuous Fibre Composite Materials. Materials 2020, 13, 2129. [Google Scholar] [CrossRef]
- Gulgunje, P.V.; Newcomb, B.A.; Gupta, K.; Chae, H.G.; Tsotsis, T.K.; Kumar, S. Low-density and high-modulus carbon fibers from polyacrylonitrile with honeycomb structure. Carbon 2015, 95, 710–714. [Google Scholar] [CrossRef]
- Radzi, A.; Sapuan, S.; Huzaifah, M.; Azammi, A.N.; Ilyas, R.; Nadlene, R. A Review of the Mechanical Properties of Roselle Fiber-Reinforced Polymer Hybrid Composites. In Roselle; Elsevier: Amsterdam, The Netherlands, 2021; pp. 259–269. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L.; Gopalakrishna, K.; Yogesha, B. Applications of Natural Fibers and Its Composites: An Overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef]
- Njoku, C.E.; Alaneme, K.K.; Omotoyinbo, J.A.; Daramola, M.O. Natural Fibers as Viable Sources for the Development of Structural, Semi-Structural, and Technological Materials—A Review. Adv. Mater. Lett. 2019, 10, 682–694. [Google Scholar] [CrossRef]
- Vasilev, K.; Cook, J.; Griesser, H. Antibacterial surfaces for biomedical devices. Expert Rev. Med Devices 2009, 6, 553–567. [Google Scholar] [CrossRef]
- Bshena, O.; Heunis, T.D.; Dicks, L.M.; Klumperman, B. Antimicrobial fibers: Therapeutic possibilities and recent advances. Futur. Med. Chem. 2011, 3, 1821–1847. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J.; Chatterjee, K. Recent advances in engineering topography mediated antibacterial surfaces. Nanoscale 2015, 7, 15568–15575. [Google Scholar] [CrossRef] [PubMed]
- Berni, R.; Cai, G.; Hausman, J.-F.; Guerriero, G. Plant Fibers and Phenolics: A Review on Their Synthesis, Analysis and Combined Use for Biomaterials with New Properties. Fibers 2019, 7, 80. [Google Scholar] [CrossRef]
- Lobo, F.C.M.; Franco, A.R.; Fernandes, E.M.; Reis, R.L. An Overview of the Antimicrobial Properties of Lignocellulosic Materials. Molecules 2021, 26, 1749. [Google Scholar] [CrossRef]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Júnior, A.F. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Mwitari, P.G.; Ayeka, P.A.; Ondicho, J.; Matu, E.N.; Bii, C.C. Antimicrobial Activity and Probable Mechanisms of Action of Medicinal Plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. PLoS ONE 2013, 8, e65619. [Google Scholar] [CrossRef]
- Sham, S.; Hansi, P.; Kavitha, T. Antimicrobial activity and phytochemical analysis of selected Indian folk medicinal plants. Int. J. Pharma Sci. Res. 2010, 1, 430–434. [Google Scholar]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical Characterization and Evaluation of the Antibacterial Activity of Essential Oils from Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Khan, B.; Warner, P.; Wang, H. Antibacterial Properties of Hemp and Other Natural Fibre Plants: A Review. Bioresources 2014, 9, 3642–3659. [Google Scholar] [CrossRef]
- Jeff, K.; Williams, D.W. Hemp Agronomy-Grain and Fiber Production. In ASA, CSSA and SSSA Books; Wiley: Madison, WI, USA, 2019; pp. 58–72. [Google Scholar] [CrossRef]
- Zimniewska, M.; Rozańska, W.; Gryszczynska, A.; Romanowska, B.; Kicinska-Jakubowska, A. Antioxidant Potential of Hemp and Flax Fibers Depending on Their Chemical Composition. Molecules 2018, 23, 1993. [Google Scholar] [CrossRef] [PubMed]
- Jankauskienė, Z.; Butkutė, B.; Gruzdevienė, E.; Cesevičienė, J.; Fernando, A.L. Chemical composition and physical properties of dew- and water-retted hemp fibers. Ind. Crop. Prod. 2015, 75, 206–211. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of Natural Fiber-Reinforced Biocomposites for Biomedical Applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of Phenolic Compounds and Terpenes from Cannabis sativa L. By-Products: From Conventional to Intensified Processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Chaudhary, V.; Bajpai, P.K.; Maheshwari, S. An Investigation on Wear and Dynamic Mechanical behavior of Jute/Hemp/Flax Reinforced Composites and Its Hybrids for Tribological Applications. Fibers Polym. 2018, 19, 403–415. [Google Scholar] [CrossRef]
- Chang, L.; Duan, W.; Huang, S.; Chen, A.; Li, J.; Tang, H.; Pan, G.; Deng, Y.; Zhao, L.; Li, D. Improved antibacterial activity of hemp fibre by covalent grafting of quaternary ammonium groups. R. Soc. Open Sci. 2021, 8, rsos.201904. [Google Scholar] [CrossRef]
- Cassano, R.; Trombino, S.; Ferrarelli, T.; Nicoletta, F.P.; Mauro, M.V.; Giraldi, C.; Picci, N. Hemp fiber (Cannabis sativa L.) derivatives with antibacterial and chelating properties. Cellulose 2013, 20, 547–557. [Google Scholar] [CrossRef]
- Li, F.; Zeng, Z.; Huang, R.; Wang, Y.; Liu, T. Identification of proteins associated with bast fiber growth of ramie by differential proteomic analysis. BMC Genom. 2021, 22, 865. [Google Scholar] [CrossRef]
- Rehman, M.; Gang, D.; Liu, Q.; Chen, Y.; Wang, B.; Peng, D.; Liu, L. Ramie, a multipurpose crop: Potential applications, constraints and improvement strategies. Ind. Crop. Prod. 2019, 137, 300–307. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Chen, L.; Abbasi, A.M.; Guo, X.; Liu, R.H. Comparative Study of Phenolic Profiles, Antioxidant and Antiproliferative Activities in Different Vegetative Parts of Ramie (Boehmeria nivea L.). Molecules 2019, 24, 1551. [Google Scholar] [CrossRef] [PubMed]
- Bin Tang, B.; Yao, Y.; Li, J.; Qin, S.; Zhu, H.; Kaur, J.; Chen, W.; Sun, L.; Wang, X. Functional Application of Noble Metal Nanoparticles In Situ Synthesized on Ramie Fibers. Nanoscale Res. Lett. 2015, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Kalita, S.; Choudhury, B.; Devi, D.; Kalita, D.; Kalita, K.; Dash, S.; Kotoky, J. Fiber from ramie plant (Boehmeria nivea): A novel suture biomaterial. Mater. Sci. Eng. C 2016, 62, 816–822. [Google Scholar] [CrossRef]
- Bunshell, A. Plant fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Elsevier: Oxford, UK, 2018; pp. 59–103. [Google Scholar]
- Romanzini, D.; Júnior, H.L.O.; Amico, S.C.; Zattera, A.J. Preparation and characterization of ramie-glass fiber reinforced polymer matrix hybrid composites. Mater. Res. 2012, 15, 415–420. [Google Scholar] [CrossRef]
- Dang, C.-Y.; Shen, X.-J.; Nie, H.-J.; Yang, S.; Shen, J.-X.; Yang, X.-H.; Fu, S.-Y. Enhanced interlaminar shear strength of ramie fiber/polypropylene composites by optimal combination of graphene oxide size and content. Compos. Part B Eng. 2019, 168, 488–495. [Google Scholar] [CrossRef]
- Zheng, G.H.; Ji, F.L.; Ren, J.H. Multifunctional Finishing of Ramie Fabric Using Titanium Dioxide Nanoparticles. Adv. Mater. Res. 2012, 586, 185–190. [Google Scholar] [CrossRef]
- Fink, J.K. Compatibilization. In Reactive Polymers Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 373–409. [Google Scholar] [CrossRef]
- Debnath, M.; Padney, M.; Sharma, R.; Thankur, G.; Lal, P. Biotechnological intervention of Agave Sisalana: A unique fiber yielding plant with medicinal property. J. Med. Plant Res. 2010, 4, 177–187. [Google Scholar]
- Benítez-Guerrero, M.; Pérez-Maqueda, L.A.; Artiaga, R.; Sánchez-Jiménez, P.E.; Pascual-Cosp, J. Structural and Chemical Characteris-677 tics of Sisal Fiber and Its Components: Effect of Washing and Grinding. J. Nat. Fibers 2017, 14, 26–39. [Google Scholar] [CrossRef]
- Naveen, J.; Jawaid, M.; Amuthakkannan, P.; Chandrasekar, M. Mechanical and physical properties of sisal and hybrid sisal fiber-reinforced polymer composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 427–440. [Google Scholar] [CrossRef]
- Guambo, M.P.R.; Spencer, L.; Vispo, N.S.; Vizuete, K.; Debut, A.; Whitehead, D.C.; Santos-Oliveira, R.; Alexis, F. Natural Cellulose Fibers for Surgical Suture Applications. Polymers 2020, 12, 3042. [Google Scholar] [CrossRef]
- Shegute, T.; Wasihun, Y. Antibacterial Activity and Phytochemical Components of Leaf Extracts of Agave americana. J. Exp. Pharmacol. 2020, 12, 447–454. [Google Scholar] [CrossRef]
- Zwane, P.; Dlamini, A.; Nkambule, N. Antimicrobial Properties of Sisal (Agave sisalana) Used as an Ingredient in Petroleum 686 Jelly Production in Swaziland. Curr. Res. J. Biol. Sci. 2010, 2, 370–374. [Google Scholar]
- Hammuel, C.; Yebpella, G.G.; A Shallangwa, G.; Magomya, A.M.; Agbajp, A.S. Phytochemical and antimicrobial screening of methanol and aqueous extracts of Agave sisalana. Acta Pol. Pharm. 2011, 68, 535–539. [Google Scholar] [PubMed]
- Chen, S.; Liu, J.; Zeng, H. Structure and antibacterial activity of silver-supporting activated carbon fibers. J. Mater. Sci. 2005, 40, 6223–6231. [Google Scholar] [CrossRef]
- Günaydin, G.K.; Avinc, O.; Palamutcu, S.; Yavas, A.; Soydan, A.S. Naturally Colored Organic Cotton and Naturally Colored Cotton Fiber Production; Springer: Singapure, 2019; pp. 81–99. [Google Scholar] [CrossRef]
- Aripov, K.; Ioelovich, M. Comparative study of supramolecular structure of cellulose in cotton fibers pf gosspium hisutum and gossypium barbadense. Cellul. Chem. Technol. 2020, 54, 635–641. [Google Scholar] [CrossRef]
- Serra, A.; Tarrés, Q.; Chamorro, M.À.; Soler, J.; Mutjé, P.; Espinach, F.X.; Vilaseca, F. Modeling the Stiffness of Coupled and Uncoupled Recycled Cotton Fibers Reinforced Polypropylene Composites. Polymers 2019, 11, 1725. [Google Scholar] [CrossRef]
- Badr, A.A. Anti-microbial and durability characteristics of socks made of cotton and regenerated cellulosic fibers. Alex. Eng. J. 2018, 57, 3367–3373. [Google Scholar] [CrossRef]
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules 2020, 25, 5802. [Google Scholar] [CrossRef]
- Xu, A.Y.; McGillivray, D.J.; Dingley, A.J. Active antibacterial coating of cotton fabrics with antimicrobial proteins. Cellulose 2021, 28, 8077–8094. [Google Scholar] [CrossRef]
- Wang, J.; Ma, M.; Zhou, W. Antibacterial Properties of White Cotton/Naturally Brown-Colored Cotton Blended Fabrics. AATCC J. Res. 2019, 6, 10–14. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO–cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coatings Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Gao, S.; Su, J.; Wang, W.; Fu, J.; Wang, H. Highly efficient and durable antibacterial cotton fabrics finished with zwitterionic polysulfobetaine by one-step eco-friendly strategy. Cellulose 2020, 28, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, D.; Yan, J.; Xiao, Y.; Gu, W.; Zang, C. Study on the Photocatalytic and Antibacterial Properties of TiO2 Nanoparticles-Coated Cotton Fabrics. Materials 2019, 12, 2010. [Google Scholar] [CrossRef]
- Akin, D.E. Linen most useful: Perspectives on structure, chemistry, and enzymes for retting flax. ISRN Biotechnol. 2013, 2013, 186534. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Abo, W.; Salem, M.; Ali, H.; Fares, Y.; Elshikh, M. Impact of some plant source additives on enhancing the properties and antifungal activities of pulp made from linen fibers. Bioresources 2019, 14, 6025–6046. [Google Scholar]
- Foksowicz-Flaczyk, J.; Walentowska, J. Antifungal activity of ionic liquid applied to linen fabric. Int. Biodeterior. Biodegradation 2013, 84, 412–415. [Google Scholar] [CrossRef]
- Namvar, F.; Jawaid, M.; Tanir, P.; Mohamad, R.; Azizi, S.; Khodavandi, A.; Rahman, H.S.; Nayeri, M.D. Potential use of plant fibres and their composites for biomedical applications. BioResources 2014, 9, 5688–5706. [Google Scholar] [CrossRef]
- Witika, B.; Makoni, P.; Matafwali, S.; Chabalenge, B.; Mwila, C.; Kalungia, A.; Nkanga, C.; Bapolisi, A.; Walker, R. Biocompatibility of Biomaterials for Nanoencapsulation: Current Approaches. Nanomaterials 2020, 10, 1649. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Torre, L. Natural fiber biodegradable composites and nanocomposites. In Biomass, Biopolymer-Based Materials, and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–201. [Google Scholar] [CrossRef]
- Tudu, B.K.; Kumar, A.; Bhushan, B. Fabrication of superoleophobic cotton fabric for multi-purpose applications. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2019, 377, 20190129. [Google Scholar] [CrossRef]
- Ahmed, F.; Shaikh, I.A.; Hussain, T.; Ahmad, I.; Munir, S.; Zameer, M. Developments in Health Care and Medical Textiles—A Mini Review-1. Pak. J. Nutr. 2014, 13, 780–783. [Google Scholar] [CrossRef]
- Cesarelli, G.; Donisi, L.; Coccia, A.; Amitrano, F.; D’Addio, G.; Ricciardi, C. The E-Textile for Biomedical Applications: A Systematic Review of Literature. Diagnostics 2021, 11, 2263. [Google Scholar] [CrossRef] [PubMed]
- Saber, D.; El-Aziz, K.A. Advanced materials used in wearable health care devices and medical textiles in the battle against coronavirus (COVID-19): A review. J. Ind. Text. 2021, 51, 246S–271S. [Google Scholar] [CrossRef]
- Al-Mubarak, L.; Al-Haddab, M. Cutaneous wound closure materials: An overview and update. J. Cutan. Aesthetic Surg. 2013, 6, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Sneha, K.R.; Steny, P.S.; Sailaja, G.S. Intrinsically radiopaque and antimicrobial cellulose based surgical sutures from mechanically powerful Agave sisalana plant leaf fibers. Biomater. Sci. 2021, 9, 7944–7961. [Google Scholar] [CrossRef]
- Guna, V.; Ilangovan, M.; Hu, C.; Nagananda, G.S.; Ananthaprasad, M.G.; Venkatesh, K.; Reddy, N. Antimicrobial Natural Cellulose Fibers from Hyptis suaveolens for Potential Biomedical and Textiles Applications. J. Nat. Fibers 2019, 18, 867–876. [Google Scholar] [CrossRef]
- Zhu, L.M.; Yu, D.G. Drug delivery systems using biotextiles. Biotext. Med. Implant. 2013, 2013, 213–231. [Google Scholar] [CrossRef]
- Rostamitabar, M.; Abdelgawad, A.M.; Jockenhoevel, S.; Ghazanfari, S. Drug-Eluting Medical Textiles: From Fiber Production and Textile Fabrication to Drug Loading and Delivery. Macromol. Biosci. 2021, 21, 2100021. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Arumugam, S.; Kandasamy, J.; Shah, A.M.; Sultan, M.H.; Safri, S.; Majid, M.A.; Basri, A.; Mustapha, F. Investigations on the Mechanical Properties of Glass Fiber/Sisal Fiber/Chitosan Reinforced Hybrid Polymer Sandwich Composite Scaffolds for Bone Fracture Fixation Applications. Polymers 2020, 12, 1501. [Google Scholar] [CrossRef]
- Gouda, D.; Bellary, I.; Dinesh, D.; Gouda, H.; Prashanth, D.N. Characterization and Investigation of Mechanical Properties of Hybrid Natural Fiber Polymer Composite Materials for Femur Bone Prosthesis. J. Mech. Civ. Eng. 2014, 11, 40–52. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, W.; Zhang, Z.; Zhou, X.; Ye, Y.; Lin, P.; Liu, A.; Wu, Y.; Li, B.; Zhang, C.; et al. A trilogy antimicrobial strategy for multiple infections of orthopedic implants throughout their life cycle. Bioact. Mater. 2021, 6, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Y.; Xiong, C.; Su, S.; Ding, H. Preparation and Properties of Bamboo Fiber/Nano-hydroxyapatite/Poly(lactic-co-glycolic) Composite Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2017, 9, 4890–4897. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, Z.; Li, S.; Yi, X. Effect of Ramie Fabric Chemical Treatments on the Physical Properties of Thermoset Polylactic Acid (PLA) Composites. Aerospace 2018, 5, 93. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Thomas, V.M.; Yoo, C.G.; Ozcan, S.; Deng, Y.; Nelson, K.; Ragauskas, A.J. Recent advancements of plant-based natural fiber–reinforced composites and their applications. Compos. Part B Eng. 2020, 200, 108254. [Google Scholar] [CrossRef]
- Oleiwi, J.; Salih, S.I.; Fadhil, H. Study Compression and Impact Properties of PMMA Reinforced by Natural Fibers Used in Denture. Eng. Technol. J. 2018, 36, 652–655. [Google Scholar] [CrossRef]
- Varoni, E.M.; Iriti, M.; Rimondini, L. Plant Products for Innovative Biomaterials in Dentistry. Coatings 2012, 2, 179–194. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Lefkelidou, A.; Papadopoulou, L.; Pavlidou, E.; Paraskevopoulos, K.M.; Fenno, J.C.; Flannagan, S.; González-Cabezas, C.; Kotsanos, N.; Papagerakis, P. Bactericidal and Bioactive Dental Composites. Front. Physiol. 2018, 9, 103. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, B. Development and performance study of a natural silk fiber facial mask paper. J. Eng. Fibers Fabr. 2020, 15, 155892502097575. [Google Scholar] [CrossRef]
- Allafi, F.; Hossain, S.; Lalung, J.; Shaah, M.; Salehabadi, A.; Ahmad, M.I.; Shadi, A. Advancements in Applications of Natural Wool Fiber: Review. J. Nat. Fibers 2020, 19, 497–512. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Zhao, F. Study on the Design of Avocado-fiber Skincare Knitted Underwear. E3S Web Conf. 2021, 237, 01038. [Google Scholar] [CrossRef]
- Dhaliwal, J.S. Natural Fibers: Applications. In Generation, Development and Modifications of Natural Fibers; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
| Characteristic | Natural Fibers | Synthetic Fibers | Refs |
|---|---|---|---|
| Source | It is produced from plants, animals, and minerals | It is manufactured from petroleum-based chemicals. | [60] |
| Density | It makes the composites lighter because the density is between 1.2 and 1.6 g/cm3 | It has limited application for composites application by their density (glass fiber = 2.4 g/cm3, carbon fiber = 1.9/cm3) | [62,63] |
| Production | Relatively aligned, long and discontinuous fibers | Well-aligned continuous fibers | [62] |
| Principal compounds | The presence of cellulose, lignin, hemicellulose, and pectin | Formed by joining chemical monomers into polymers | [64] |
| Mechanical properties | High specific properties related to elastic modulus and strength, but drawbacks such as hydrophilic character and low thermal stability | High thermal stability, high elasticity and durability | [61] |
| Nature | Hydrophilic | Hydrophobic | [65] |
| Environmental | It is renewable and recyclable | High durability and cost | [66] |
| Hemp Modification | Bacteria | Type | Method | Result | Ref |
|---|---|---|---|---|---|
| Hemp Fiber Extracts | |||||
| Essential oils from hemp fiber-type (CBD, α-Pinene, β-Pinene β-Myrcene α-Terpinolene β-Caryophyllene) | Staphylococcus, Listeria, Enterococcus, Bacillus | Gram- positive | Agar disk diffusion (ADD) assay: plates with Tryptic Soy Agar, controls were ampicillin and ciprofloxacin Minimum inhibitory concentration (MIC): microwell dilution method was used | ADD: Showed good inhibition of bacterial growth compared with antibiotics. MIC: showed non or lower antibacterial activity on Staphylococcus, samples showed anti-Listeria activity, good antibacterial activity against Enterococcus, and excellent antibacterial activity against Bacillus | [78] |
| Modified Hemp Fibers | |||||
| Hemp fiber grafted with quaternary ammonium groups | Escherichia coli | Gram- negative | Antibacterial activity is based on the change of electrochemical potential by electrostatic reaction reducing the cell membrane releasing cytoplasm substances and cells dissolution | The antibacterial activity was 95.41% and after washing 89.78% | [86] |
| Staphylococcus aureus | Gram- positive | The antibacterial activity was 99.64% and after washing 91.12% | |||
| Hemp fiber with 2-benzyl-4-chlorophenol | Staphylococcus aureus | Gram- positive | Minimum inhibitory concentration (MIC) and Minimum Bactericidal Concentration (MCB) test | This resulted in the death of 99.9 % of bacteria | [87] |
| Pseudomonas aeruginosa | Gram- negative | This resulted in the death of 99.9% of bacteria | |||
| Ramie Modification | Bacteria | Type | Method | Result | Ref |
|---|---|---|---|---|---|
| Non-modified Ramie Fibers | |||||
| Ramie plant fiber as surgical suture material | Escherichia coli (MTCC40) | Gram- negative | Agar plate method: agar plates are prepared with nutrients and a layer of bacteria was added to the plate as well as sterile suture fiber. | Good antibacterial activity with a zone of inhibition of 16 mm | [92] |
| Bacillus subtilis (MTCC441) and Staphylococcus aureus (MTCC3160) | Gram- positive | B. subtilis showed a zone of inhibition of 14 mm and the S. aureus strain showed a zone of inhibition of 11 mm | |||
| Modified Ramie Fibers | |||||
| Ramie Fabric Using Titanium Dioxide Nanoparticles | Escherichia coli | Gram- negative | Antibacterial effect by the percentage of bacteria reduction (R%) by the equation: W is bacteria colonies of control and Q bacteria colonies of treated | Decreased cellular growth with the increasing content of nano-TiO2. With 0.8 g/L of nano-TiO2 there was a 98.5% of bacteria reduction | [96] |
| Staphylococcus aureus | Gram- positive | Decreased cellular growth with the increasing content of nano-TiO2. With 0.8 g/L of nano-TiO2 there was a 99.0% of bacteria reduction | |||
| Silver and Gold Nanoparticles on Ramie Fibers | Escherichia coli | Gram- negative | AATCC 100-2004 test: after exposing the fibers to the bacteria for 18 h at 120 rpm and 37 °C, the fibers are set aside and the cells are diluted, counted, and compared to the initial count. | Silver nanoparticles in ramie fiber showed 100% antibacterial activity because there was no growth of bacterial colonies on the culture plate. | [91] |
| Sisal Modification | Bacteria | Type | Method | Result | Ref |
|---|---|---|---|---|---|
| Sisal Extracts | |||||
| Sisal extract | Escherichia coli | Gram- negative | Disc diffusion—Qualitative method, absence or presence of a zone of inhibition in different concentrations | Significant inhibition, with zones or rings of inhibition of 10.69–12.17 mm. In addition, if the bacterial population is lower, the growth-inhibitory properties of the sisal extract could be improved | [103] |
| Bacillus stearothermophilus | Gram- positive | Delvo test SP-NT kit—Broad spectrum microbial inhibition and antibiotic residues test—Colorimetric method: Purple color means to contain an inhibitor and Yellow color does not contain an inhibitor of microbial growth | 100% inhibition—All samples were purple | ||
| Aqueous Sisal extract | Staphylococcus aureus | Gram- positive | Two methods: Agar well diffusion method in vitro, absence or presence of a zone of inhibition in different concentrations. The test tube dilution method determines levels of resistance to an antimicrobial substance and measures minimum inhibitory concentration (MIC) | Significant inhibition, with zones or rings of inhibition of 29 mm. Its MIC of 10 mg/mL demonstrated significant biological activity against the test pathogenic organisms | [104] |
| Salmonella typhi | Gram- negative | Significant inhibition, with zones or rings of inhibition of 27 mm. Its MIC of 20 mg/mL demonstrated a medium biological activity against the test pathogenic organisms | |||
| Non-Modified Sisal Fibers | |||||
| Sisal fibers | Escherichia coli | Gram- negative | Microtiter plate biofilm assay semiquantitative assessment of the biofilm. Measure the optical density (OD) and classify it into strong, moderate, or weak biofilm creators | Microtiter plate biofilm assay semiquantitative assessment of the biofilm. Measure the optical density (OD) and classify it into strong, moderate, or weak biofilm creators | [101] |
| Modified Sisal Fibers | |||||
| Sisal-based activated carbon fibers | Escherichia coli | Gram- negative | The flasks with fibers and bacteria were shaken at 37°C for different periods. Surviving bacteria in the solution after contact with samples were counted by the spread plate culture method. The survival amount of bacteria (CFU/mL) was counted from the colony formed on the medium. | Completely kill E. coli at the concentration of 5 × 108 CFU/mL in 8 h | [105] |
| Cotton Modification | Bacteria | Type | Method | Result | Ref |
|---|---|---|---|---|---|
| Non-Modified Cotton Fibers | |||||
| Naturally brown-colored cotton | Escherichia coli | Gram- negative | Antibacterial activity evaluated by AATCCTM100-2004 with the use of ethylene oxide in the sterilization process | Fibers showed antibacterial activity of 86.9% | [112] |
| Staphylococcus aureus | Gram- positive | Fibers showed antibacterial activity of 91.7% | |||
| Modified Cotton Fibers | |||||
| CuO-Cotton Fiber | Escherichia coli | Gram- negative | The viable bacteria were monitored by counting the number of colony-forming units from the appropriate dilution on nutrient agar plates | Treatment for 1 h with the coated cotton leads to the complete inhibition of E. coli and S. aureus growth. | [113] |
| Staphylococcus aureus | Gram- positive | ||||
| Poly (sulfobetaine-acrylamide-allyl glycidyl ether) onto cotton | Escherichia coli (ATCC8739) | Gram- negative | Antibacterial properties were quantitatively evaluated by the viable cell count method | Functionalized cotton fibers exhibited a high level of antibacterial rate effect of 95.18% | [114] |
| Staphylococcus aureus (ATCC6538) | Gram- positive | The modified fibers have efficient antibacterial properties of 98.74% | |||
| TiO2 Nanoparticles-Coated Cotton Fabrics | Escherichia coli | Gram- negative | Antibacterial activity was qualitatively evaluated by the shake-flask method under visible light | Exhibited excellent antibacterial results because nearly no bacteria grew, then the antibacterial reduction rate reaches more than 99% | [115] |
| Staphylococcus aureus | Gram- positive | ||||
| Linen Modification | Fungi | Method | Result | Ref |
|---|---|---|---|---|
| Modified Linen Fibers | ||||
| Linen fiber with didecyldimethylammonium nitrate—[DDA][NO3] | Aspergillus niger van Tieghem, Chaetomium globosum Kunze, Gliocladium virens Miller, Paecilomyces variotii Bainier, Penicillium ochrochloron Biourge | Evaluation of fungal growth measured according to the EN 14119, 2003 Standard | Showed antifungal activity. [DDA][NO3] applied in amount of 0.5% per 1 g of the dry fabric caused visible inhibition of mold growth on the fabric surface. | [118] |
| Pulp made from Linen fibers | Aspergillus terreus Ate456, Aspergillus niger Ani245, Fusarium culmorum Fcu761 | Antifungal evaluation against the growth of fungi. The inhibition zones were reported. | Samples had positive effects against the growth of A. terreus and A. niger | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamora-Mendoza, L.; Guamba, E.; Miño, K.; Romero, M.P.; Levoyer, A.; Alvarez-Barreto, J.F.; Machado, A.; Alexis, F. Antimicrobial Properties of Plant Fibers. Molecules 2022, 27, 7999. https://doi.org/10.3390/molecules27227999
Zamora-Mendoza L, Guamba E, Miño K, Romero MP, Levoyer A, Alvarez-Barreto JF, Machado A, Alexis F. Antimicrobial Properties of Plant Fibers. Molecules. 2022; 27(22):7999. https://doi.org/10.3390/molecules27227999
Chicago/Turabian StyleZamora-Mendoza, Lizbeth, Esteban Guamba, Karla Miño, Maria Paula Romero, Anghy Levoyer, José F. Alvarez-Barreto, António Machado, and Frank Alexis. 2022. "Antimicrobial Properties of Plant Fibers" Molecules 27, no. 22: 7999. https://doi.org/10.3390/molecules27227999
APA StyleZamora-Mendoza, L., Guamba, E., Miño, K., Romero, M. P., Levoyer, A., Alvarez-Barreto, J. F., Machado, A., & Alexis, F. (2022). Antimicrobial Properties of Plant Fibers. Molecules, 27(22), 7999. https://doi.org/10.3390/molecules27227999







