Simultaneous Determination of Moxifloxacin Hydrochloride and Dexamethasone Sodium Phosphate in Rabbit Ocular Tissues and Plasma by LC-MS/MS: Application for Pharmacokinetics Studies
Abstract
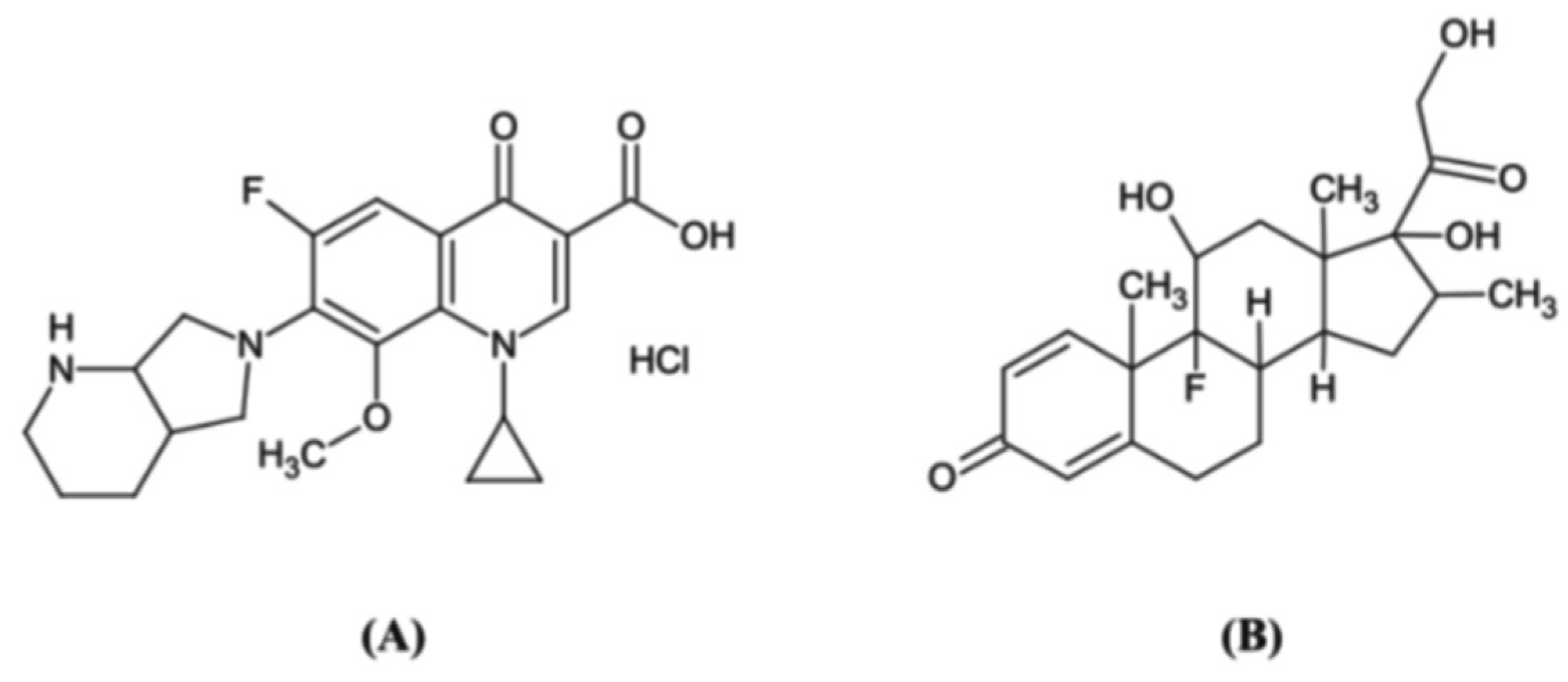
1. Introduction
2. Results and Discussion
2.1. Method Development
2.2. Method Validation
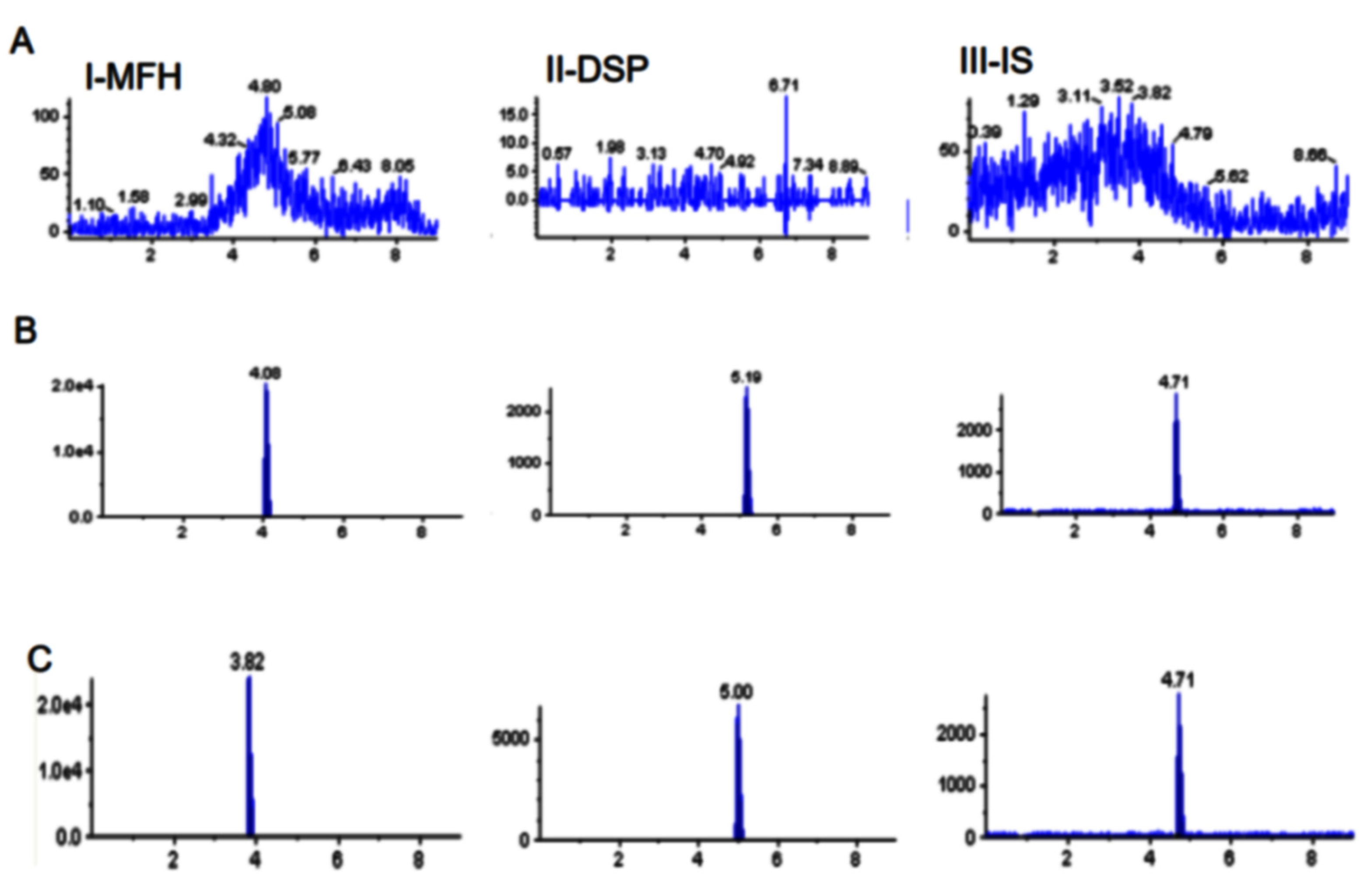
2.2.1. Selectivity and Sensitivity
2.2.2. Linearity and Lower Limits of Quantitative Detection
2.2.3. Accuracy and Precision
2.2.4. Recovery and Matrix Effect
2.2.5. Stability
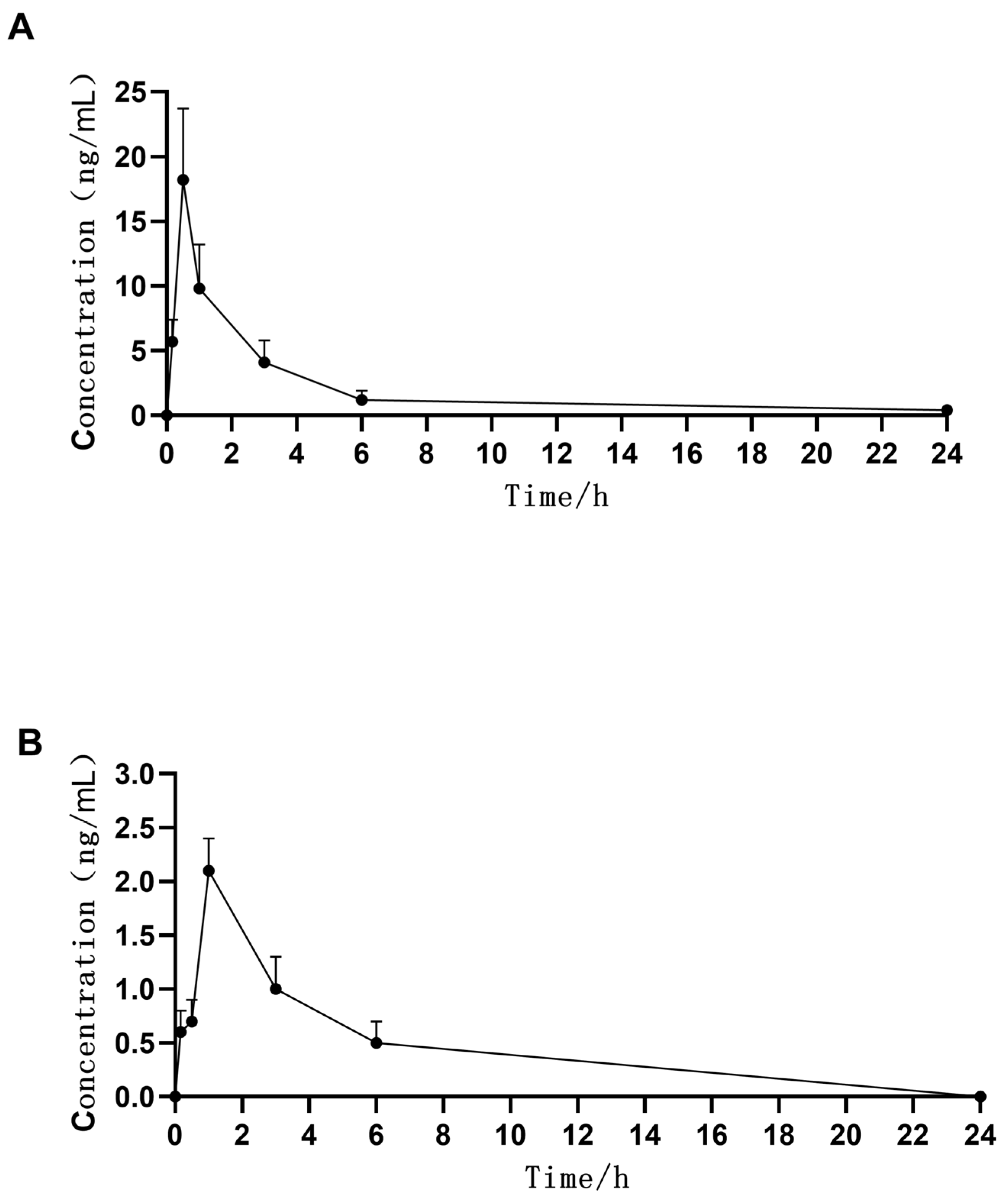
2.3. Pharmacokinetics in Different Ocular Tissues
2.4. Pharmacokinetics in Rabbit Plasma
3. Materials and Methods
3.1. Materials and Reagents
3.2. LC-MS Conditions
3.2.1. Liquid Chromatography
3.2.2. Mass Spectrometry
3.3. Preparation of Standard Solutions, Calibration, and Quality Control Samples
3.4. Sample Preparation
3.5. Bioanalytical Method Validation
3.5.1. Selectivity
3.5.2. Sensitivity
3.5.3. Linearity
3.5.4. Accuracy and Precision
3.5.5. Recovery and Matrix Effect
3.5.6. Stability
3.6. Pharmacokinetic Applications
3.6.1. Animals
3.6.2. Animal Grouping
3.6.3. Tissue Extraction
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Leal, S.M., Jr.; Rodino, K.G.; Fowler, W.C.; Gilligan, P.H. Practical guidance for clinical microbiology laboratories: Diagnosis of ocular infections. Clin. Microbiol. Rev. 2021, 34, e0007019. [Google Scholar] [CrossRef]
- Grandi, G.; Bianco, G.; Boattini, M.; Scalabrin, S.; Iannaccone, M.; Fea, A.; Cavallo, R.; Costa, C. Bacterial etiology and antimicrobial resistance trends in ocular infections: A 30-year study, Turin area, Italy. Eur. J. Ophthalmol. 2021, 31, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, Q.; Tan, Y.; Lin, L.; Huang, Q.; Wu, K. Bacterial spectrum and antibiotic resistance patterns of ocular infection: Differences between external and intraocular diseases. J. Ophthalmol. 2015, 2015, 813979. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the management of infectious keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Kang, E.Y.; Yeh, L.K.; Ma, D.H.K.; Tan, H.Y.; Chen, H.C.; Hung, K.H.; Huang, Y.C.; Hsiao, C.H. Clinical features and molecular characteristics of methicillin-susceptible Staphylococcus aureus ocular infection in Taiwan. Antibiotics 2021, 10, 1445. [Google Scholar] [CrossRef]
- Chen, R.; Xue, H.; Xu, Y.; Ma, T.; Liu, Y.; Zhang, J.; Shi, X.; Guo, D. Moxifloxacin derivatives with potential antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Curr. Top. Med. Chem. 2021, 21, 2474–2482. [Google Scholar] [CrossRef]
- Melega, M.V.; Alves, M.; Cavalcanti Lira, R.P.; Cardoso da Silva, I.; Ferreira, B.G.; Assis Filho, H.L.; Pedreira Chaves, F.R.; Martini, A.A.F.; Dias Freire, L.M.; Reis, R.D.; et al. Safety and efficacy of intracameral moxifloxacin for prevention of post-cataract endophthalmitis: Randomized controlled clinical trial. J. Cataract Refract. Surg. 2019, 45, 343–350. [Google Scholar] [CrossRef]
- Tufail, A.; Lightman, S.; Kamal, A.; Pleyer, U.; Paniagua, N.M.G.; Dot, C.; Li, X.Y.; Jiao, J.; Lou, J.; Hashad, Y.; et al. Post-marketing surveillance study of the safety of dexamethasone intravitreal implant in patients with retinal vein occlusion or noninfectious posterior segment uveitis. Clin. Ophthalmol. 2018, 12, 2519–2534. [Google Scholar] [CrossRef]
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef]
- Li, A.F.Y.; Wang, C.L.; Tai, H.Y.; Fu, Y.J.; Tsai, F.T.; Tsai, Y.C.; Ko, Y.L.; Li, M.J.; Lin, C.C.; Chang, T.J. Pandemic aspect of dexamethasone: Molecular mechanisms and clinical application. J. Chin. Med. Assoc. 2021, 84, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Mohammadinejad, R.; Uzieliene, I.; Nabavi, N.; Dehshahri, A.; García-Couce, J.; Tavakol, S.; Moghassemi, S.; Dadashzadeh, A.; Makvandi, P.; et al. Dexamethasone: Insights into pharmacological aspects, therapeutic mechanisms, and delivery systems. ACS Biomater. Sci. Eng. 2022, 8, 1763–1790. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Gu, X.; Wang, X.; Liu, Y.; Di, X. Pharmacokinetic studies of three alkaloids in rats after intragastrical administration of Lycopodii Herba extract by LC-MS/MS. Molecules 2019, 24, 1930. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Duong, V.A.; Vo, D.K.; Jo, J.; Maeng, H.J. Development and validation of a bioanalytical LC-MS/MS method for simultaneous determination of sirolimus in porcine whole blood and lung tissue and pharmacokinetic application with coronary stents. Molecules 2021, 26, 425. [Google Scholar] [CrossRef]
- Razzaq, S.N.; Ashfaq, M.; Khan, I.U.; Mariam, I.; Razzaq, S.S.; Azeem, W. Simultaneous determination of dexamethasone and moxifloxacin in pharmaceutical formulations using stability indicating HPLC method. Arab. J. Chem. 2017, 10, 321–328. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Kiatkumjorn, T.; Jithavech, P.; Thanawattanawanich, P.; Na Bhuket, P.R.; Rojsitthisak, P. A simple and sensitive HPLC-fluorescence method for the determination of moxifloxacin in human plasma and its application in a pharmacokinetic study. Pharmazie 2018, 73, 625–629. [Google Scholar] [CrossRef]
- Gomes, R.L.; Viana, R.G.; Melo, L.A., Jr.; Cruz, A.C.; Suenaga, E.M.; Kenyon, K.R.; Campos, M. Aqueous Humor Penetration and Biological Activity of Moxifloxacin 0.5% Ophthalmic Solution Alone or with Dexamethasone 0.1. J. Ocul. Pharmacol. Ther. 2017, 33, 98–102. [Google Scholar] [CrossRef]
- Bahuon, F.; Darcos, V.; Patel, S.; Marin, Z.; Coudane, J.; Schwach, G.; Nottelet, B. Polyester-Polydopamine Copolymers for Intravitreal Drug Delivery: Role of Polydopamine Drug-Binding Properties in Extending Drug Release. Biomacromolecules 2022, 23, 4388–4400. [Google Scholar] [CrossRef]
- Le Merdy, M.; AlQaraghuli, F.; Tan, M.L.; Walenga, R.; Babiskin, A.; Zhao, L.; Lukacova, V. Clinical Ocular Exposure Extrapolation for Ophthalmic Solutions Using PBPK Modeling and Simulation. Pharm. Res. 2022. [Google Scholar] [CrossRef]
- Sanap, S.N.; Mishra, A.; Bisen, A.C.; Agrawal, S.; Biswas, A.; Verma, S.K.; Kumar, M.; Bhatta, R.S. Simultaneous determination of fluconazole and ofloxacin in rabbit tear fluid by LC-MS/MS: Application to ocular pharmacokinetic studies. J. Pharm. Biomed. Anal. 2022, 208, 114463. [Google Scholar] [CrossRef]
- Gu, X.F.; Mao, B.Y.; Xia, M.; Yang, Y.; Zhang, J.L.; Yang, D.S.; Wu, W.X.; Du, Y.X.; Di, B.; Su, M.X. Rapid, sensitive and selective HPLC-MS/MS method for the quantification of topically applied besifloxacin in rabbit plasma and ocular tissues: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2016, 117, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Shetty, K.H.; Desai, D.T.; Shah, D.O.; Willcox, M.D.P. Recent advances in ophthalmic preparations: Ocular barriers, dosage forms and routes of administration. Int. J. Pharm. 2021, 608, 121105. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kim, J.W.; Choi, H.I.; Maeng, H.J.; Koo, T.S. Development of an LC-MS/MS Method for ARV-110, a PROTAC Molecule, and Applications to Pharmacokinetic Studies. Molecules 2022, 27, 1977. [Google Scholar] [CrossRef] [PubMed]
- US-FDA. FDA Guidance for Industry: Bioanalytical Method Validation; US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research: Rockville, MD, USA, 2018. Available online: https://www.fda.gov/downloads/drugs/ (accessed on 10 June 2020).


| Ocular Tissue | Regression Equation | r | |
|---|---|---|---|
| MFH | Cornea | Y = 0.506X + 0.0503 | 0.9973 |
| Aqueous humor | Y = 0.400X + 0.0212 | 0.9964 | |
| Plasma | Y = 2.380X + 0.302 | 0.9983 | |
| DSP | Cornea | Y = 0.133X + 0.00434 | 0.9998 |
| Aqueous humor | Y = 0.151X + 0.0139 | 0.9995 | |
| Plasma | Y = 0.161X + 0.0108 | 0.9997 |
| Ocular Tissue | Concentration (ng/mL) | MFH (DSP) | |||
|---|---|---|---|---|---|
| Accuracy (%) | Precision (%) | ||||
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | ||
| Cornea | 0.5 | 102.4(107.6) | 101.7(103.3) | 6.57(4.74) | 10.12(9.8) |
| 1 | 97.8(109.7) | 104.1(111.8) | 3.03(6.82) | 11.21(7.6) | |
| 10 | 100.9(95.1) | 100.8(97.85) | 6.80(4.21) | 4.93(4.5) | |
| 150 | 99.3(91.3) | 100.4(96.9) | 2.55(3.03) | 2.69(6.6) | |
| Aqueous humor | 0.5 | 107.0(97.0) | 98.5(92.8) | 7.81(10.77) | 13.5(11.54) |
| 1 | 105.5(100.3) | 107(104.88) | 4.19(7.05) | 12.1(12.9) | |
| 10 | 103.6(102.7) | 102.9(102.5) | 6.21(3.57) | 4.9(3.23) | |
| 150 | 101.6(104) | 101.8(103.1) | 5.73(2.56) | 4.1(2.3) | |
| Plasma | 0.5 | 103.6(95.3) | 101.2(99.3) | 10.7(8.48) | 10.2(10.7) |
| 1 | 102.1(96.5) | 108.2(107.4) | 3.01(5.29) | 13.5(14.2) | |
| 10 | 98.8(97.4) | 101.6(102.0) | 6.02(4.48) | 5.7(6.23) | |
| 150 | 102.6(100.3) | 101.3(100.2) | 2.83(2.33) | 3.1(2.95) | |
| Ocular Tissue | Concentration (ng/mL) | MFH(DSP) | |||
|---|---|---|---|---|---|
| Recovery (%) | Mean Recovery (%) | Matrix Effect (%) | RSD (%) | ||
| Cornea | 1 | 107.2(100.5) | 109.0 (99.9) | 104.71(95.9) | 7.1(11.1) |
| 10 | 112.2(105.1) | 87.36(101.4) | 11.9(9.6) | ||
| 150 | 107.7(94.1) | 93.1(95.4) | 10.6(7.0) | ||
| Aqueous humor | 1 | 98.8(102.2) | 98.5 (107.8) | 101.92(105.2) | 8.4(13.7) |
| 10 | 99.6(111.3) | 90.6(94.7) | 10.4(8.6) | ||
| 150 | 97.1(109.9) | 95.59(93.8) | 7.5(5.6) | ||
| Plasma | 1 | 96.5(103.8) | 104.8 (98.7) | 114.78(102.4) | 10.9(11.8) |
| 10 | 109.4(100.1) | 90.45(94.9) | 12(8.7) | ||
| 150 | 108.5(92.3) | 98.84(109.8) | 5.8(9.3) | ||
| Ocular Tissue | Stability Condition | Nominal Conc. (ng/mL) | Mean | SD | Accuracy (%) |
|---|---|---|---|---|---|
| Cornea | Benchtop (room temperature for 10 h) | 1 | 1.0(1.0) | 0.1(0.1) | 99.4(96.9) |
| 150 | 158.3(145.5) | 10.5(4.6) | 105.6(97.0) | ||
| Autosampler (4 °C for 36 h) | 1 | 1.0(0.9) | 0.1(0.0) | 103.2(93.2) | |
| 150 | 159.3(147.7) | 10.1(3.5) | 106.2(98.4) | ||
| Freeze-thaw (3 cycles; −20 °C to room temperature) | 1 | 1.1(1.12) | 0.0(0.1) | 109.3(112.3) | |
| 150 | 158.7(149.3) | 8.4(7.8) | 105.8(99.6) | ||
| Long-term (−20 °C for 56 days) | 1 | 1.1(1.0) | 0.1(0.1) | 109.7(103.4) | |
| 150 | 139.5(142.0) | 12.5(6.5) | 93.0(94.7) | ||
| Aqueous Humor | Benchtop (room temperature for 10 h) | 1 | 1.1(1.1) | 0.4(0.3) | 107(108.7) |
| 150 | 157.5(156.7) | 16.7(15.4) | 105(104.4) | ||
| Autosampler (4 °C for 36 h) | 1 | 1(1.1) | 0.4(0.4) | 102(106.2) | |
| 150 | 150(149.2) | 21.2(19.9) | 100(99.4) | ||
| Freeze-thaw (3 cycles; −20 °C to room temperature) | 1 | 1.1(1.0) | 0.5(0.4) | 108.3(103.3) | |
| 150 | 150.8(147.5) | 13.9(11.7) | 100.6(98.3) | ||
| Long-term (−20 °C for 56 days) | 1 | 0.9(0.9) | 0.4(0.3) | 93.3(89.5) | |
| 150 | 145.8(165.8) | 14.3(18.3) | 97.2(110.6) | ||
| Plasma | Benchtop (room temperature for 10 h) | 1 | 0.9(1.0) | 0.4(0.2) | 92.8(96.2) |
| 150 | 170.8(136.7) | 17.2(9.3) | 113.9(91.1) | ||
| Autosampler (4 °C for 36 h) | 1 | 1.1(1.1) | 0.4(0.3) | 109.5(113.5) | |
| 150 | 146.7(156.7) | 27.1(17.8) | 97.8(104.4) | ||
| Freeze-thaw (3 cycles;−20 °C to room temperature) | 1 | 1.1(1.0) | 0.2(0.3) | 112.8(103.5) | |
| 150 | 151.7(141.7) | 23.2(13.3) | 101.1(94.4) | ||
| Long-term (−20 °C for 56 days) | 1 | 1.1(1.1) | 0.2(0.2) | 113.7(111.8) | |
| 150 | 138.3(154.0) | 7.5(15.3) | 92.2(102.7) |
| Tissue | Cmax | AUC(0–24h) | t1/2(h) | Tmax (h) | |
|---|---|---|---|---|---|
| MFH | Cornea | 6369.5 ± 1538.2 | 23,865 ± 10,777 | 2.2 ± 0.4 | 0.2 |
| Aqueous humor | 2656.7 ± 840.6 | 6311.7 ± 1627.7 | 1.3 ± 0.2 | 0.5 | |
| Plasma | 18.2 ± 5.5 | 48.1 ± 18.5 | 1.6 ± 0.3 | 0.5 | |
| DSP | Cornea | 385.8 ± 161.7 | 1266.7 ± 596.3 | 2.3 ± 0.6 | 0.2 |
| Aqueous humor | 91.4 ± 18.5 | 252.5 ± 47.8 | 1.7 ± 0.3 | 0.5 | |
| Plasma | 2.1 ± 0.3 | 13.2 ± 2.4 | 2.8 ± 1.3 | 1.0 |
| Instrumental Variable | ||||
|---|---|---|---|---|
| LC | Injection volume | 10 µL | ||
| Column temperature | 20 °C | |||
| Flow rate | 0.6 mL/min | |||
| Mobile phase | 0.1% formic acid in water (A) and methanol (B) | |||
| Gradient change | Time (min) | A% | B% | |
| 0 | 20 | 80 | ||
| 1.5 | 80 | 20 | ||
| 5.5 | 80 | 20 | ||
| 5.6 | 20 | 80 | ||
| 9.0 | 20 | 80 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Yuan, Y.; Shao, Q.; Qiao, H. Simultaneous Determination of Moxifloxacin Hydrochloride and Dexamethasone Sodium Phosphate in Rabbit Ocular Tissues and Plasma by LC-MS/MS: Application for Pharmacokinetics Studies. Molecules 2022, 27, 7934. https://doi.org/10.3390/molecules27227934
Zhao X, Yuan Y, Shao Q, Qiao H. Simultaneous Determination of Moxifloxacin Hydrochloride and Dexamethasone Sodium Phosphate in Rabbit Ocular Tissues and Plasma by LC-MS/MS: Application for Pharmacokinetics Studies. Molecules. 2022; 27(22):7934. https://doi.org/10.3390/molecules27227934
Chicago/Turabian StyleZhao, Xinxin, Yanjuan Yuan, Qing Shao, and Hongqun Qiao. 2022. "Simultaneous Determination of Moxifloxacin Hydrochloride and Dexamethasone Sodium Phosphate in Rabbit Ocular Tissues and Plasma by LC-MS/MS: Application for Pharmacokinetics Studies" Molecules 27, no. 22: 7934. https://doi.org/10.3390/molecules27227934
APA StyleZhao, X., Yuan, Y., Shao, Q., & Qiao, H. (2022). Simultaneous Determination of Moxifloxacin Hydrochloride and Dexamethasone Sodium Phosphate in Rabbit Ocular Tissues and Plasma by LC-MS/MS: Application for Pharmacokinetics Studies. Molecules, 27(22), 7934. https://doi.org/10.3390/molecules27227934






