Determination of Leuprolide–Fatty Acid Conjugate in Rat Plasma Using LC-MS/MS and Its Pharmacokinetics after Subcutaneous Administration in Rats
Abstract
1. Introduction
2. Results and Discussion
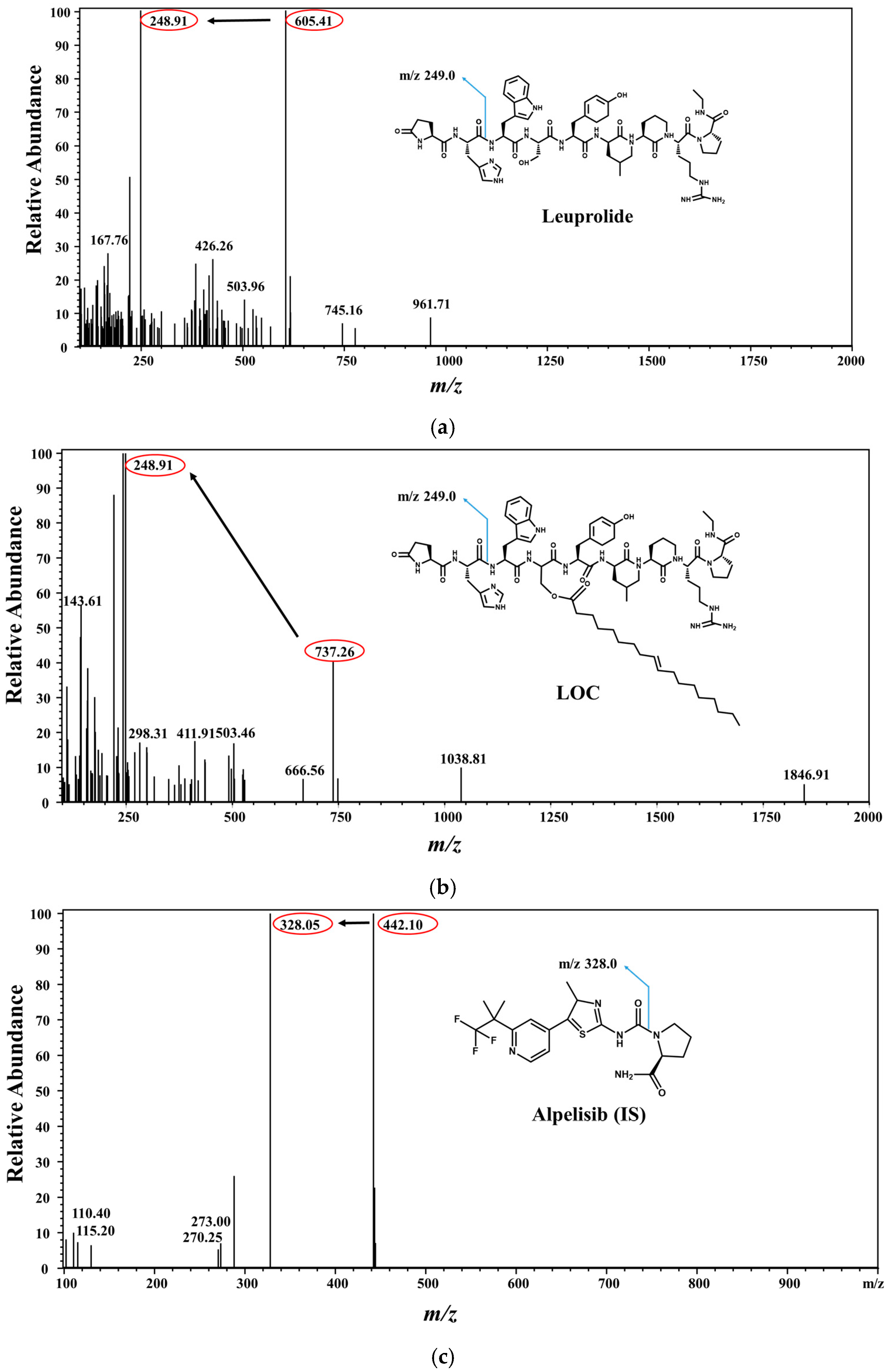
2.1. Method Development
2.2. Method Validation
2.2.1. Selectivity and Linearity
2.2.2. Precision and Accuracy
2.2.3. Extraction Recovery and Matrix Effect
2.2.4. Stability
2.3. Pharmacokinetic Application
3. Materials and Methods
3.1. Materials and Animals
3.2. Synthesis of LOC
3.3. LC-MS/MS Conditions
3.4. Calibration Standard and QC Samples
3.5. Sample Preparation
3.6. Method Validation
3.7. In Vivo Pharmacokinetic Study of Rats
3.8. Pharmacokinetic and Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Conn, P.M.; Huckle, W.R.; Andrews, W.V.; McArdle, C.A. The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Recent. Prog. Horm. Res. 1987, 43, 29–68. [Google Scholar] [PubMed]
- Teutonico, D.; Montanari, S.; Ponchel, G. Leuprolide acetate: Pharmaceutical use and delivery potentials. Expert. Opin. Drug Deliv. 2012, 9, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Comaru-Schally, A.M.; Nagy, A.; Kovacs, M.; Szepeshazi, K.; Plonowski, A.; Varga, J.L.; Halmos, G. Hypothalamic hormones and cancer. Front. Neuroendocrinol. 2001, 22, 248–291. [Google Scholar] [CrossRef] [PubMed]
- Swayzer, D.V.; Gerriets, V. Leuprolide; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Redding, T.W.; Schally, A.V. Inhibition of prostate tumor growth in two rat models by chronic administration of D-Trp6 analogue of luteinizing hormone-releasing hormone. Proc. Natl. Acad. Sci. USA 1981, 78, 6509–6512. [Google Scholar] [CrossRef] [PubMed]
- Schally, A.V.; Redding, T.W.; Comaru-Schally, A.M. Inhibition of prostate tumors by agonistic and antagonistic analogs of LH-RH. Prostate 1983, 4, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Barbieri, R.L.; Benacerraf, B.R.; Schiff, I. Treatment of leiomyomata with intranasal or subcutaneous leuprolide, a gonadotropin-releasing hormone agonist. Fertil. Steril. 1987, 48, 560–564. [Google Scholar] [CrossRef]
- Friedman, A.J.; Harrison-Atlas, D.; Barbieri, R.L.; Benacerraf, B.; Gleason, R.; Schiff, I. A randomized, placebo-controlled, double-blind study evaluating the efficacy of leuprolide acetate depot in the treatment of uterine leiomyomata. Fertil. Steril. 1989, 51, 251–256. [Google Scholar] [CrossRef]
- Lee, P.A.; Page, J.G. Effects of leuprolide in the treatment of central precocious puberty. J. Pediatr. 1989, 114, 321–324. [Google Scholar] [CrossRef]
- Hoffmann, G.; Spitz, J.; Behrens, R. GnRH-agonists in the therapy of endometriosis. Ther. Umsch. 1990, 47, 937–944. [Google Scholar]
- Tanaka, T.; Hibi, I.; Kato, K.; Saito, S.; Shimizu, N.; Suwa, S.; Nakajima, H.; The TAP-144-SR CPP Study Group. A dose finding study of a super long-acting luteinizing hormone-releasing hormone analog (leuprolide acetate depot, TAP-144-SR) in the treatment of central precocious puberty. Endocrinol. Jpn. 1991, 38, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Periti, P.; Mazzei, T.; Mini, E. Clinical pharmacokinetics of depot leuprorelin. Clin. Pharmacokinet. 2002, 41, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Alcala, S.; Urban-Morlan, Z.; Aguilar-Rosas, I.; Quintanar-Guerrero, D. A biodegradable polymeric system for peptide-protein delivery assembled with porous microspheres and nanoparticles, using an adsorption/infiltration process. Int. J. Nanomed. 2013, 8, 2141–2151. [Google Scholar] [CrossRef]
- Hu, Q.; van Gaal, E.V.; Brundel, P.; Ippel, H.; Hackeng, T.; Rijcken, C.J.; Storm, G.; Hennink, W.E.; Prakash, J. A novel approach for the intravenous delivery of leuprolide using core-cross-linked polymeric micelles. J. Control Release 2015, 205, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.H.; Lim, J.L.; Ko, J.Y.; Park, S.H.; Kim, J.E.; Cho, H.J.; Park, E.S.; Kim, D.D. A new injectable liquid crystal system for one month delivery of leuprolide. J. Control Release 2014, 185, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhuang, X.; Zhang, T.; Guan, Y.; Meng, Q.; Zhang, Y. PEGylated leuprolide with improved pharmacokinetic properties. Bioorg. Med. Chem. 2020, 28, 115306. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ngo, H.V.; Jin, H.E.; Lee, K.W.; Lee, B.J. Hydroxyl group-targeted conjugate and its self-assembled nanoparticle of peptide drug: Effect of degree of saturation of fatty acids and modification of physicochemical properties. Int. J. Nanomed. 2022, 17, 2243–2260. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O.; Freire, A.; Gryngarten, M.G.; Kletter, G.B.; Benson, M.; Miller, B.S.; Dajani, T.S.; Eugster, E.A.; Mauras, N. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J. Clin. Endocrinol. Metab. 2020, 105, e3660–e3671. [Google Scholar] [CrossRef] [PubMed]
- Di, L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Dhimitruka, I.; Santalucia, J., Jr. Investigation of the Yamaguchi esterification mechanism. Synthesis of a lux-s enzyme inhibitor using an improved esterification method. Org. Lett. 2006, 8, 47–50. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Bioanalytical Method Validation Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry (accessed on 22 May 2018).
- Seo, S.W.; Han, D.G.; Choi, E.; Park, T.; Byun, J.H.; Cho, H.J.; Jung, I.H.; Yoon, I.S. Development and application of a physiologically based pharmacokinetic model for entrectinib in rats and scale-up to humans: Route-dependent gut wall metabolism. Biomed. Pharmacother. 2022, 146, 112520. [Google Scholar] [CrossRef]
- Seo, S.W.; Kim, J.M.; Han, D.G.; Geum, D.; Yun, H.; Yoon, I.S. A sensitive HPLC-FLD method for the quantification of alpelisib, a novel phosphatidylinositol 3-kinase inhibitor, in rat plasma: Drug metabolism and pharmacokinetic evaluation in vitro and in vivo. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1163, 122508. [Google Scholar] [CrossRef] [PubMed]



| Nominal Concentration (ng/mL) | Precision (%) | Accuracy (%) | Recovery (%) | Matrix Effect (%) | ||
|---|---|---|---|---|---|---|
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |||
| Leuprolide | ||||||
| Lower limit of quantification (LLOQ) (1) | ≤12.4 | ≤6.92 | 104 ± 13 | 98.1 ± 6.8 | ||
| Low quality control (LQC) (3) | ≤4.04 | ≤8.33 | 109 ± 4 | 99.8 ± 8.3 | 105 ± 2 | 108 ± 4 |
| Medium quality control (MQC) (75) | ≤3.21 | ≤7.44 | 107 ± 3 | 97.0 ± 7.2 | 105 ± 5 | 104 ± 7 |
| High quality control (HQC) (800) | ≤6.10 | ≤4.90 | 99.3 ± 6.0 | 93.0 ± 4.6 | 103 ± 4 | 106 ± 9 |
| LOC | ||||||
| LLOQ (1) | ≤10.1 | ≤4.74 | 110 ± 11 | 103 ± 4.9 | ||
| LQC (3) | ≤1.36 | ≤4.35 | 100 ± 1 | 102 ± 4.4 | 99.2 ± 3.9 | 96.7 ± 3.4 |
| MQC (75) | ≤5.15 | ≤5.78 | 97.4 ± 5.0 | 97.5 ± 5.6 | 95.8 ± 6.1 | 95.0 ± 5.6 |
| HQC (800) | ≤5.96 | ≤7.04 | 94.7 ± 5.6 | 97.7 ± 6.9 | 98.8 ± 3.8 | 90.6 ± 4.1 |
| Nominal Concentration (ng/mL) | Bench-Top 1 | Autosampler 2 | Freeze–Thaw 3 | Long-Term 4 |
|---|---|---|---|---|
| Leuprolide | ||||
| LQC (3) | 96.5 ± 2.4 | 103 ± 6 | 92.6 ± 4.5 | 91.1 ± 4.4 |
| HQC (800) | 97.4 ± 3.7 | 101 ± 4 | 90.8 ±2.5 | 96.3 ± 2.5 |
| LOC | ||||
| LQC (3) | 105 ± 7 | 102 ± 9 | 107 ± 4 | 91.2 ± 4.0 |
| HQC (800) | 97.7± 2.0 | 92.7 ±1.2 | 107 ± 4 | 93.4 ± 2.6 |
| Parameter | Leuprolide Group | LOC Group |
|---|---|---|
| Leuprolide | LOC | |
| Total area under the plasma concentration versus time curve from time zero to time infinity (AUCinf) (ng·min/mL) | 5484 ± 364 | 23,581 ± 4068 |
| The area under the plasma concentration–time curve from time zero to the last sampling time (AUClast) (ng·min/mL) | 5396 ± 322 | 19,545 ± 2483 |
| Half-life of drug elimination at the terminal phase (t1/2) (min) | 38.2 ± 4.3 | 172 ± 66 |
| Peak plasma concentration (Cmax) (ng/mL) | 70.0 ± 3.7 | 52.9 ± 7.1 |
| Time to reach Cmax (Tmax) (min) | 15 | 300 (240–360) |
| Mean residence time (MRTinf) (min) | 64.9 ± 4.6 | 405 ± 64 |
| Leuprolide after administration of LOC | ||
| AUCinf (ng·min/mL) | 1634 ± 328 | |
| AUClast (ng·min/mL) | 1265 ± 317 | |
| t1/2 (min) | 166 ± 38 | |
| Cmax (ng/mL) | 4.9 ± 1.3 | |
| Tmax (min) | 120 | |
| MRTinf (min) | 283 ± 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.-S.; Seo, S.-W.; Cho, J.Y.; Lee, K.W.; Lee, B.-J.; Yoon, I.-S.; Jin, H.-E. Determination of Leuprolide–Fatty Acid Conjugate in Rat Plasma Using LC-MS/MS and Its Pharmacokinetics after Subcutaneous Administration in Rats. Molecules 2022, 27, 8716. https://doi.org/10.3390/molecules27248716
Seong G-S, Seo S-W, Cho JY, Lee KW, Lee B-J, Yoon I-S, Jin H-E. Determination of Leuprolide–Fatty Acid Conjugate in Rat Plasma Using LC-MS/MS and Its Pharmacokinetics after Subcutaneous Administration in Rats. Molecules. 2022; 27(24):8716. https://doi.org/10.3390/molecules27248716
Chicago/Turabian StyleSeong, Gi-Sang, Seong-Wook Seo, Ji Young Cho, Kye Wan Lee, Beom-Jin Lee, In-Soo Yoon, and Hyo-Eon Jin. 2022. "Determination of Leuprolide–Fatty Acid Conjugate in Rat Plasma Using LC-MS/MS and Its Pharmacokinetics after Subcutaneous Administration in Rats" Molecules 27, no. 24: 8716. https://doi.org/10.3390/molecules27248716
APA StyleSeong, G.-S., Seo, S.-W., Cho, J. Y., Lee, K. W., Lee, B.-J., Yoon, I.-S., & Jin, H.-E. (2022). Determination of Leuprolide–Fatty Acid Conjugate in Rat Plasma Using LC-MS/MS and Its Pharmacokinetics after Subcutaneous Administration in Rats. Molecules, 27(24), 8716. https://doi.org/10.3390/molecules27248716






