Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies
Abstract
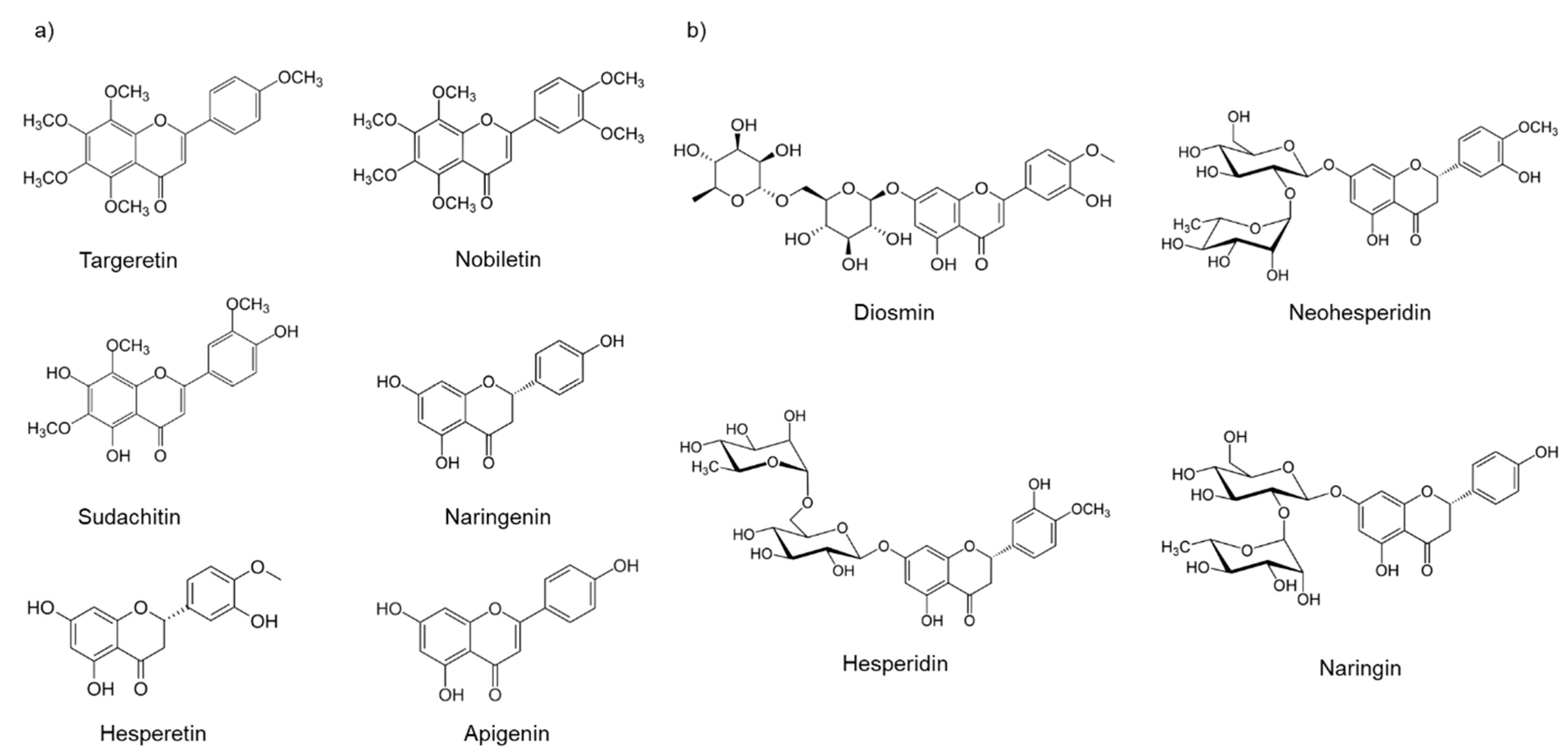
1. Introduction
2. Results
| No | Compound Name | Dosing | Follow Up Period | Animal Model | Study Outcomes | Reference | |
|---|---|---|---|---|---|---|---|
| Type of Animal | Age/Weight | ||||||
| 1 | Naringin | 100 mg/kg | 4 weeks | STZ-NA Wistar rat | 130–150 g | Blood biomarkers, OGTT, liver-specific enzyme activity, gene expression, and biomarkers content | [12] |
| Naringenin | |||||||
| 2 | Hesperidin | 10 mg/kg (1%) * | 4 weeks | Goto-Kakizaki rat | 3 weeks old | Blood biomarkers, liver-specific enzyme activity and gene expression | [13] |
| 3 | Hesperidin | 10 g/kg (1%) * | 4 weeks | STZ Wistar rat | 3 weeks old | Blood biomarkers, tissue-specific biomarkers content, and enzyme activity, serum flavonoid content, bone density | [14] |
| 4 | Naringenin | 30 mg/kg (3%) * | 4 weeks | Lep ob/ob mouse | 8–12 weeks old | Blood biomarkers, OGTT, ITT, tissue-specific biomarkers content, and gene expression, IHC analysis, anthropometric micro-CT imaging | [15] |
| 5 | Naringenin | 30 mg/kg (3%) * | 12 weeks | C57BL/6J Ldlr−/− mouse | 10–12 weeks old | Blood biomarkers, tissue-specific biomarkers content and gene expression, OGTT, ITT, IHC analysis, flow cytometry analysis for cell identification | [16] |
| 6 | Hesperetin | 40 mg/kg | 6 weeks | Wistar rat | 170–200 g | Blood biomarkers, tissue-specific biomarkers content, IHC analysis, oxidative stress assessment | [17] |
| 7 | Neohesperidin | 50 mg/kg | 6 weeks | KK-Ay mice | 8 weeks old | OGTT, ITT, blood biomarkers, liver-specific parameters, tissue-specific gene expression and protein quantification | [8] |
| 8 | Hesperidin | 0.2 g/kg * | 5 weeks | db/db mice | 5 weeks old | Blood biomarkers, liver-specific parameters, IHC analysis | [18] |
| Naringin | (23 g) | ||||||
| 9 | Hesperidin | 0.2 g/kg * | 5 weeks | db/db mice | 5 weeks old | Blood biomarkers, tissue-specific gene expression, protein quantification, and enzyme activity | [19] |
| Naringin | |||||||
| 10 | Hesperidin | 50 mg/kg | 4 weeks | HFD/STZ-Wistar rat | 180–200 g | OGTT, blood biomarkers, liver-specific enzyme activity, in situ intestinal glucose absorption, in vitro insulin secretion test | [20] |
| Naringin | |||||||
| 11 | Naringin | 100 mg/kg | 4 weeks | High fructose diet Sprague- Dawley rat | 180–200 g | Vascular reactivity, blood biomarkers, artery-specific protein quantification | [21] |
| 12 | Naringenin | 10, 30 mg/kg (1% or 3%) * | 4 weeks | C57BL/6J Ldlr−/− mouse | 12 weeks old | OGTT, ITT, blood biomarkers, tissue-specific parameters, gene expression, and enzyme activity, in situ intestinal lipid absorption, energy expenditure | [22] |
| 13 | Nobiletin | 10, 30 mg/kg (1% or 3%) * | 8–26 weeks | C57BL/6J Ldlr−/− mouse | 12 weeks old | Tissue- and cell-specific gene expression, blood biomarkers, hyperinsulinemic—euglycemic clamp and pyruvate tolerance test energy expenditure | [23] |
| 14 | Naringenin | 6, 12.5, 25 mg/kg | 6 weeks | HFD/STZ Wistar rat | 12–13 weeks old (75–100 g) | Blood biomarkers, lipid peroxidation determination, tissue-specific enzyme activity, gene expression, and protein quantification, IHC analysis | [24] |
| 15 | Naringin | 50, 100, 200 mg/kg * | 3 weeks | HFD/STZ Wistar rat | 150–200 g | Blood biomarkers, tissue-specific lipid determination, enzyme activity, and gene expression | [25] |
| 16 | Diosmin | 100 mg/kg | 6 weeks | STZ Wistar rat | 200–220 g | Blood biomarkers, tissue-specific enzyme activity, IHC analysis | [26] |
| 17 | Diosmin | 100 mg/kg | 6 weeks | STZ Wistar rat | 180–220 g | Blood biomarkers, tissue-specific lipid determination and enzyme activity | [27] |
| 18 | Targeretin | 100 mg/kg | 4 weeks | STZ Wistar rat | 180–200 g | OGTT, blood biomarkers, liver-specific enzyme activity and glycogen content, IHC analysis | [28] |
| 19 | Hesperidin | 25, 50, 100 mg/kg | 4 weeks | STZ Wistar rat | 200–220 g | OGTT, blood biomarkers, liver-specific enzyme activity and glycogen content, IHC analysis | [29] |
| 20 | Hesperidin | 20 ppm ** | 2 weeks, 3 days | STZ albino mouse † | ~30 g | Blood biomarkers, malformations rate, number of diabetic fetuses, whole body staining analysis | [30] |
| 21 | Sudachitin | 5 mg/kg | 12 weeks | HFD and db/db mouse | 4 weeks old | Blood biomarkers, anthropometric computed tomography analysis, OGTT, ITT, tissue-specific gene expression, energy expenditure | [31] |
| No | Compound Name | Dosing | Treatment Duration | Animal Model | Efficacy Outcome | Reference | |
|---|---|---|---|---|---|---|---|
| Type of Animal | Age/Weight | ||||||
| 1 | Hesperidin | 50 mg/kg | 4 weeks | SHR rat | 15 weeks | Blood pressure changes, vascular reactivity | [32] |
| 2 | Nobiletin | 20, 40 mg/kg * | 4 weeks | SHR (stroke prone) rat | 7 weeks | Blood pressure changes, cerebral vessels thrombogenesis test | [33] |
| 3 | Hesperidin | 30 mg/kg * | 25 weeks | SHR rat | 3 weeks | Blood pressure and heart rate changes | [34] |
| Glucosyl hesperidin | |||||||
| 4 | Apigenin | 1.44 mg/kg ** | 6 weeks | L-NAME Sprague–Dawley | 300–325 g | Blood pressure and heart rate changes, vascular reactivity, IHC analysis | [35] |
| Diosmin | 7.16 mg/kg ** | ||||||
| 5 | Nobiletin | 20, 40 mg/kg | 2 weeks | L-NAME Sprague–Dawley | 220–250 g | Conscious and unconscious blood pressure changes, vascular reactivity, artery-specific protein quantification, blood nitrate/nitrite quantification, IHC analysis | [36] |
| 6 | Hesperidin-naringenin mixture | 150 mg/kg | 4 weeks | SHR rat | 250–300 g | Blood pressure and heart rate changes, vascular reactivity | [37] |
| 7 | Hesperidin | 1 mg/kg * | 12 weeks | HFaD Apo-E KO mouse | 9 weeks old | Blood biomarkers, vascular reactivity, IHC analysis | [38] |
| Glucosyl hesperidin | 5 mg/kg * | ||||||
| 8 | Hesperidin | 20, 40 mg/kg | 4 weeks | One-clipped kidney Sprague-Dawley rat | 150–180 g | Conscious and unconscious blood pressure changes, blood biomarkers, vascular reactivity, artery-specific enzyme activity and protein content, blood nitrate/nitrite quantification | [39] |
3. Discussion
4. Materials and Methods
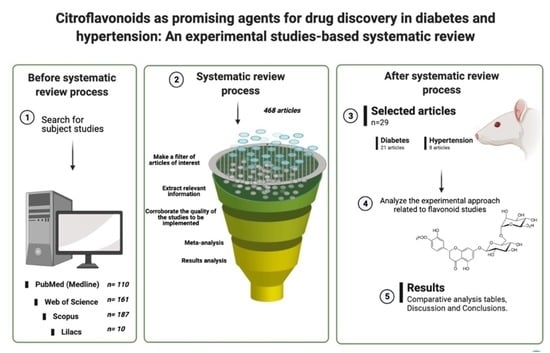
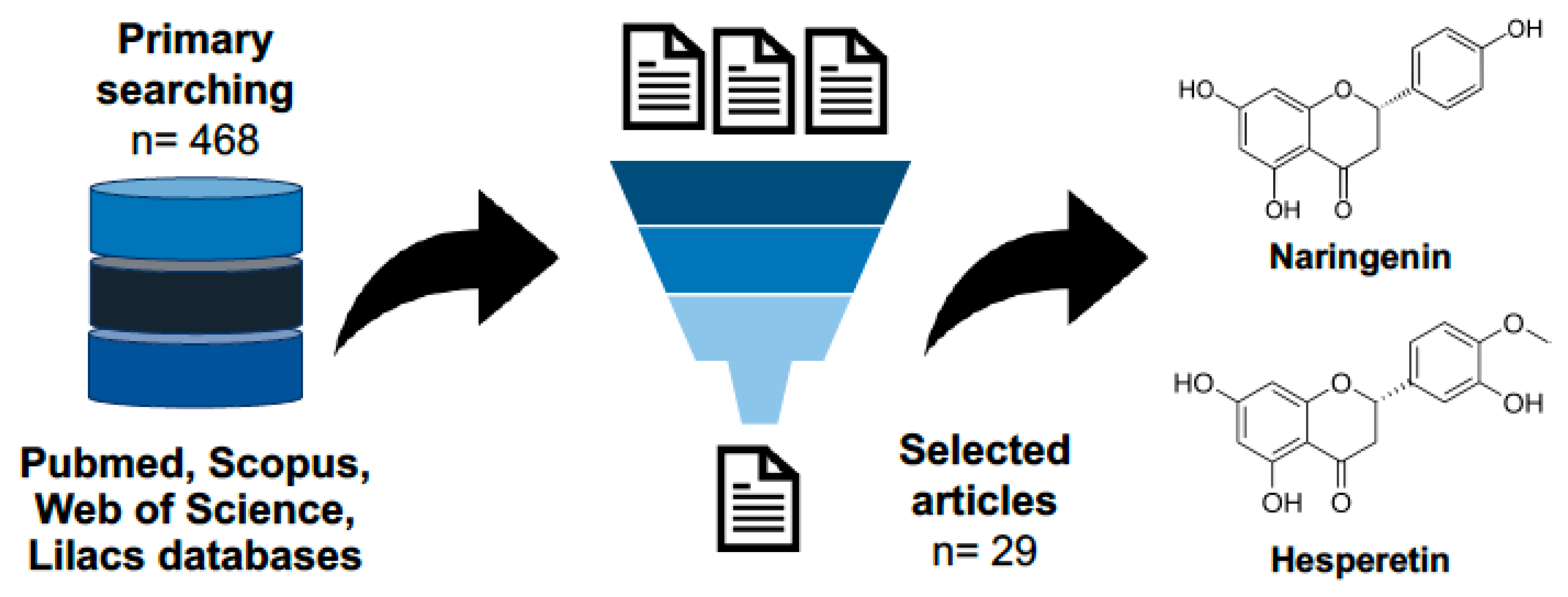
4.1. Literature Sources
4.2. Eligibility Criteria
4.3. Studies Selection
4.4. Meta-Analysis
4.5. Risk of Bias Assessment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreca, D.; Trombetta, D. Flavonoids as Promising Therapeutics of the Future: A Hub for Cells Survival or Death. Curr. Med. Chem. 2019, 26, 5092–5093. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.J.; Li, Y.; Cao, Q.H.; Wu, H.X.; Tang, X.Y.; Gao, X.H.; Yu, J.Q.; Chen, Z.; Yang, Y. In vitro and in vivo evidence that quercetin protects against diabetes and its complications: A systematic review of the literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Ellwood, L.; Torun, G.; Bahar, Z.; Fernandez, R. Effects of flavonoid-rich fruits on hypertension in adults: A systematic review. Libr. Syst. Rev. 2019, 17, 2075–2105. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, L.; Song, Y.; Hu, Y.; Wang, G.; Sun, S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim. Care Diabetes 2016, 10, 272–280. [Google Scholar] [CrossRef]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study. Phytother. Res. PTR 2019, 33, 1921–1933. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Nicolaides, A.N. Efficacy of micronized purified flavonoid fraction (Daflon®) on improving individual symptoms, signs and quality of life in patients with chronic venous disease: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2018, 37, 143–154. [Google Scholar] [CrossRef]
- Sánchez-Salgado, J.C.; Estrada-Soto, S.; García-Jiménez, S.; Montes, S.; Gómez-Zamudio, J.; Villalobos-Molina, R. Analysis of Flavonoids Bioactivity for Cholestatic Liver Disease: Systematic Literature Search and Experimental Approaches. Biomolecules 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Nakaya, Y.; Ishimi, Y.; Uehara, M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci. Biotechnol. Biochem. 2009, 73, 2779–2782. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef]
- Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Morrow, M.R.; Sawyez, C.G.; Edwards, J.Y.; Huff, M.W. Naringenin enhances the regression of atherosclerosis induced by a chow diet in Ldlr(-/-) mice. Atherosclerosis 2019, 286, 60–70. [Google Scholar] [CrossRef]
- Assini, J.M.; Mulvihill, E.E.; Burke, A.C.; Sutherland, B.G.; Telford, D.E.; Chhoker, S.S.; Sawyez, C.G.; Drangova, M.; Adams, A.C.; Kharitonenkov, A.; et al. Naringenin prevents obesity, hepatic steatosis, and glucose intolerance in male mice independent of fibroblast growth factor 21. Endocrinology 2015, 156, 2087–2102. [Google Scholar] [CrossRef]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Jeong, K.S.; Choi, M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Park, Y.B.; Kang, M.A.; Choi, M.S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006, 38, 1134–1145. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ahmed, O.M.; Ashour, M.B.; Abdel-Moneim, A. In vivo and in vitro antidiabetic effects of citrus flavonoids; a study on the mechanism of action. Int. J. Diabetes Dev. Ctries 2015, 35, 250–263. [Google Scholar] [CrossRef]
- Jayaraman, R.; Subramani, S.; Sheik Abdullah, S.H.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Assini, J.M.; Lee, J.K.; Allister, E.M.; Sutherland, B.G.; Koppes, J.B.; Sawyez, C.G.; Edwards, J.Y.; Telford, D.E.; Charbonneau, A.; et al. Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 2011, 60, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Jayakumar, M.; Thirumurugan, K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J. Funct. Foods 2015, 14, 363–373. [Google Scholar] [CrossRef]
- Malakul, W.; Pengnet, S.; Kumchoom, C.; Tunsophon, S. Naringin ameliorates endothelial dysfunction in fructose-fed rats. Exp. Ther. Med. 2018, 15, 3140–3146. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pari, L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem.-Biol. Interact. 2012, 195, 43–51. [Google Scholar] [CrossRef]
- Srinivasan, S.; Pari, L. Antihyperlipidemic effect of diosmin: A citrus flavonoid on lipid metabolism in experimental diabetic rats. J. Funct. Foods 2013, 5, 484–492. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of tangeretin, a polymethoxylated flavone on glucose metabolism in streptozotocin-induced diabetic rats. Phytomedicine 2014, 21, 793–799. [Google Scholar] [CrossRef]
- Sundaram, R.; Nandhakumar, E.; Haseena Banu, H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol. Mech. Methods 2019, 29, 644–653. [Google Scholar] [CrossRef]
- Toumi, M.; Merzoug, S.; Boutefnouchet, A.; Tahraoui, A.; Ouali, K.; Guellati, M. Hesperidin, a natural citrus flavanone, alleviates hyperglycaemic state and attenuates embryopathies in pregnant diabetic mice. J. Med. Plant Res. 2009, 3, 862–869. [Google Scholar]
- Tsutsumi, R.; Yoshida, T.; Nii, Y.; Okahisa, N.; Iwata, S.; Tsukayama, M.; Hashimoto, R.; Taniguchi, Y.; Sakaue, H.; Hosaka, T.; et al. Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr. Metab. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Dobiaš, L.; Petrová, M.; Vojtko, R.; Kristová, V. Long-term Treatment with Hesperidin Improves Endothelium-dependent Vasodilation in Femoral Artery of Spontaneously Hypertensive Rats: The Involvement of NO-synthase and K(v) Channels. Phytother. Res. 2016, 30, 1665–1671. [Google Scholar] [CrossRef]
- Ikemura, M.; Sasaki, Y.; Giddings, J.; Yamamoto, J. Protective Effects of Nobiletin on Hypertension and Cerebral Thrombosis in Stroke-Prone Spontaneously Hypertensive Rats (SHRSP). Food Nutr. Sci. 2012, 3, 1539–1546. [Google Scholar] [CrossRef][Green Version]
- Ohtsuki, K.; Abe, A.; Mitsuzuwi, H.; Kondo, M.; Uemura, K.; Iwasaki, Y.; Kondo, Y. Effects of long-term administration of hesperidin and glucosyl hesperidin to spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2002, 48, 420–422. [Google Scholar] [CrossRef]
- Paredes, M.D.; Romecín, P.; Atucha, N.M.; O’Valle, F.; Castillo, J.; Ortiz, M.C.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Potue, P.; Wunpathe, C.; Maneesai, P.; Kukongviriyapan, U.; Prachaney, P.; Pakdeechote, P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019, 10, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Recillas, A.; Gonzalez, N.; Barrera, V.; Ibarra-Barajas, M.; Estrada-Soto, S.; Ortiz-Andrade, R. Vasorelaxant and Antihypertensive Activities of Citroflavonoids (Hesperidin/Naringenin Mixture): Potential Prophylactic of Cardiovascular Endothelial Dysfunction. Pharmacogn. Mag. 2019, 15, S84–S91. [Google Scholar]
- Sugasawa, N.; Katagi, A.; Kurobe, H.; Nakayama, T.; Nishio, C.; Takumi, H.; Higashiguchi, F.; Aihara, K.I.; Shimabukuro, M.; Sata, M.; et al. Inhibition of Atherosclerotic Plaque Development by Oral Administration of α-Glucosyl Hesperidin and Water-Dispersible Hesperetin in Apolipoprotein E Knockout Mice. J. Am. Coll. Nutr. 2019, 38, 15–22. [Google Scholar] [CrossRef]
- Wunpathe, C.; Potue, P.; Maneesai, P.; Bunbupha, S.; Prachaney, P.; Kukongviriyapan, U.; Kukongviriyapan, V.; Pakdeechote, P. Hesperidin Suppresses Renin-Angiotensin System Mediated NOX2 Over-Expression and Sympathoexcitation in 2K-1C Hypertensive Rats. Am. J. Chin. Med. 2018, 46, 751–767. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Andrade, R.; Araujo León, J.A.; Sánchez-Salgado, J.C.; Sánchez-Recillas, A.; Vazquez-Garcia, P.; Hernández-Núñez, E. Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies. Molecules 2022, 27, 7933. https://doi.org/10.3390/molecules27227933
Ortiz-Andrade R, Araujo León JA, Sánchez-Salgado JC, Sánchez-Recillas A, Vazquez-Garcia P, Hernández-Núñez E. Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies. Molecules. 2022; 27(22):7933. https://doi.org/10.3390/molecules27227933
Chicago/Turabian StyleOrtiz-Andrade, Rolffy, Jesús Alfredo Araujo León, Juan Carlos Sánchez-Salgado, Amanda Sánchez-Recillas, Priscila Vazquez-Garcia, and Emanuel Hernández-Núñez. 2022. "Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies" Molecules 27, no. 22: 7933. https://doi.org/10.3390/molecules27227933
APA StyleOrtiz-Andrade, R., Araujo León, J. A., Sánchez-Salgado, J. C., Sánchez-Recillas, A., Vazquez-Garcia, P., & Hernández-Núñez, E. (2022). Citroflavonoids as Promising Agents for Drug Discovery in Diabetes and Hypertension: A Systematic Review of Experimental Studies. Molecules, 27(22), 7933. https://doi.org/10.3390/molecules27227933