Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott
Abstract
1. Introduction
2. Experimental
2.1. Plant Collection
2.2. Extraction
2.3. Fractionation
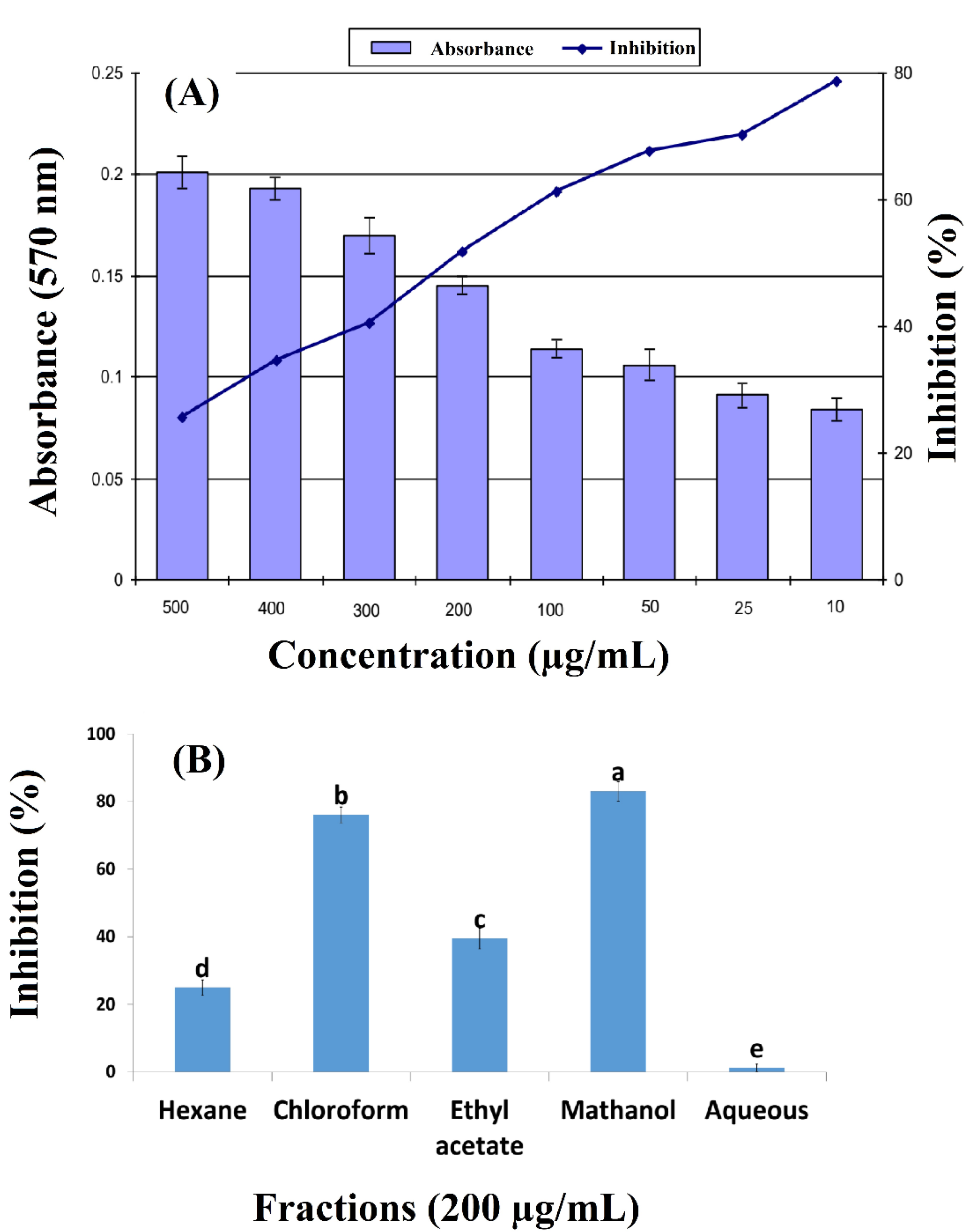
2.4. Anticancer Activity of Arisaema flavum (Forssk.) Crude Extract and Fractions
2.5. Isolation of Compounds from Chloroform Fraction
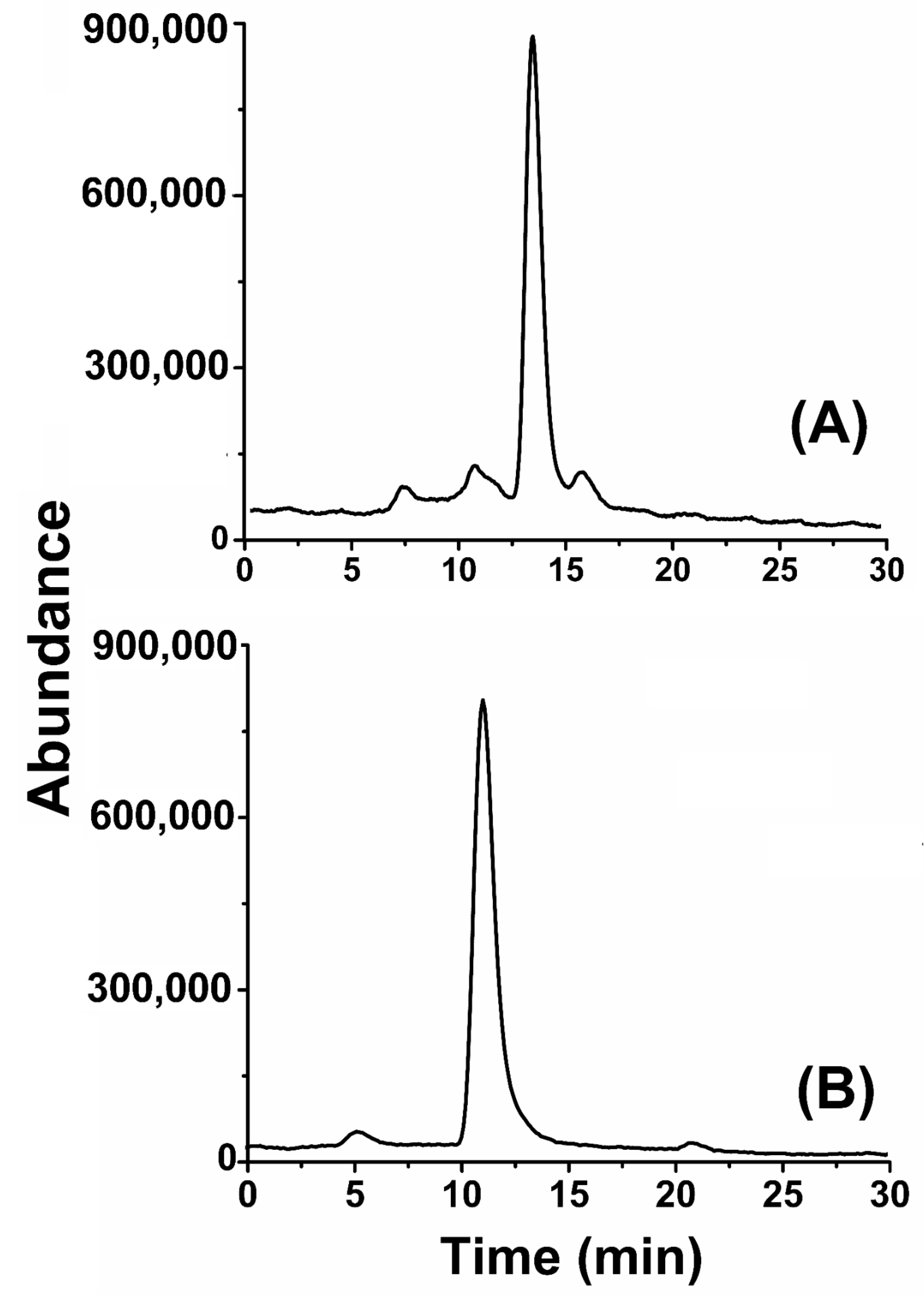
2.5.1. Chromatographic Analysis
2.5.2. GC-MS Analysis of Isolated Compounds
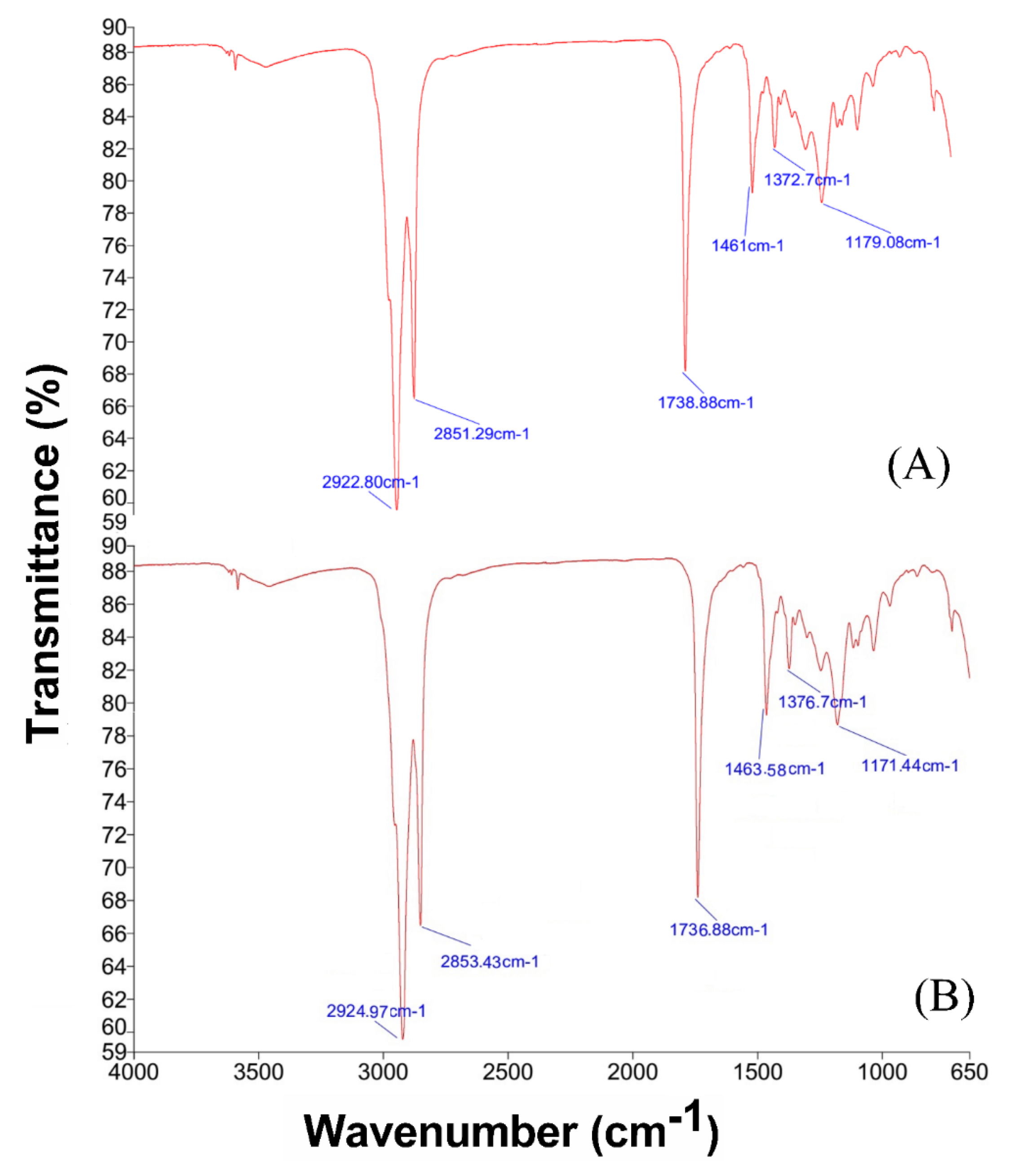
2.5.3. FTIR Analysis of Isolated Compounds
2.5.4. NMR Analysis of Isolated Compounds
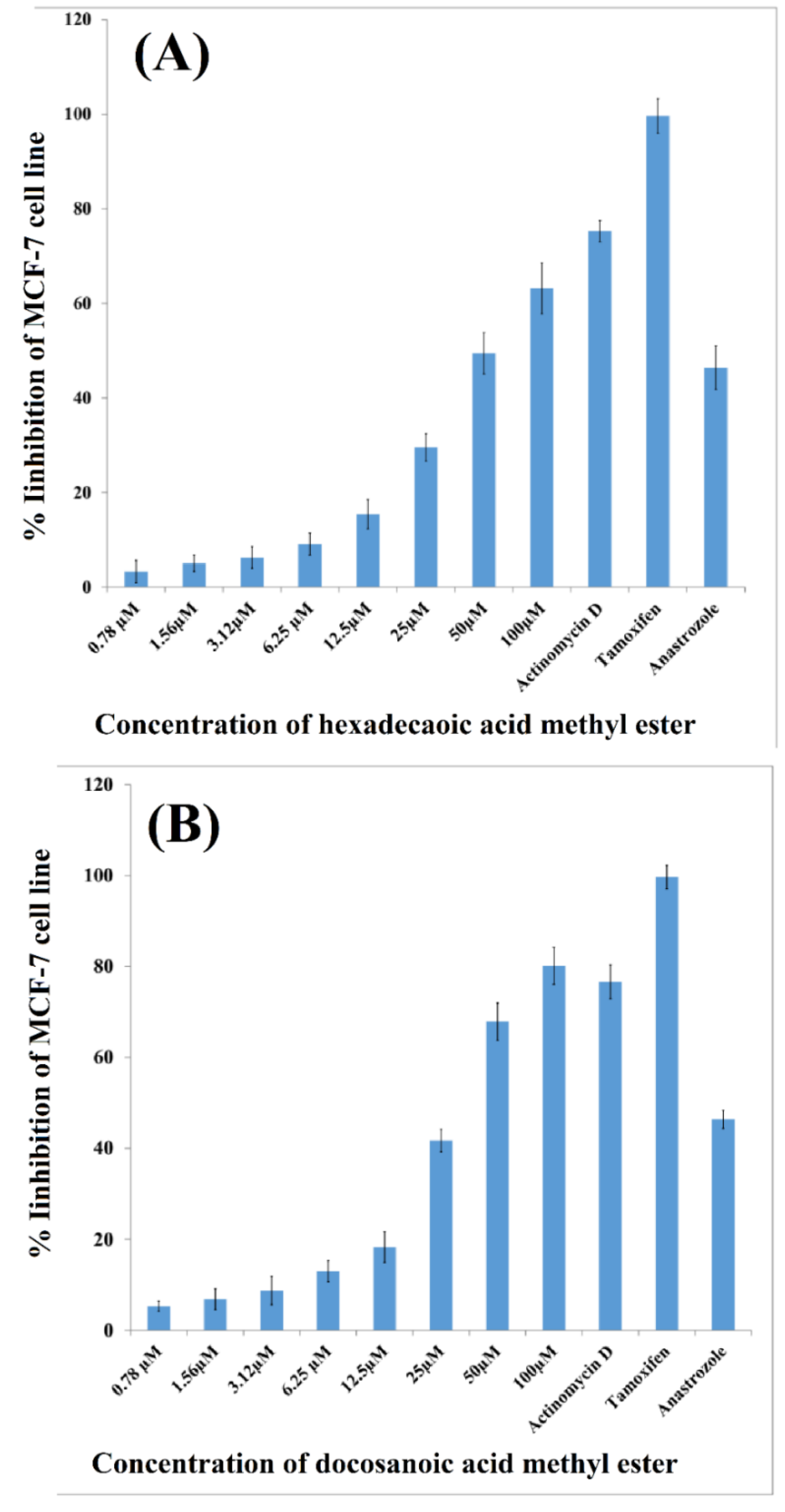
2.6. Anticancer Activity of Isolated Compounds
2.7. Statistical Analysis
3. Results
3.1. Anticancer Activity of Arisaema flavum (Forssk.)
3.2. Isolation and Characterization of Bioactive Compounds from Arisaema flavum (Forssk.) Extract
3.2.1. Spectroscopic Analysis of Isolated Compounds
3.2.2. Chromatographic Analysis of Isolated Compounds
3.2.3. FT-IR Analysis of Isolated Compounds
3.2.4. 1H-NMR of Isolated Compounds
3.3. Cytotoxic Activity of Isolated Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahaman, M.M.; Torequl Islam, M. Anticancer Activity of Plant Derived Compounds: A Literature Based Review. Clin. Oncol. 2022, 7, 1917. [Google Scholar]
- Hosseinzadeh, S.; Jafarikukhdan, A.; Hosseini, A.; Armand, R. The Application of Medicinal Plants in Traditional and Modern Medicine: A Review of Thymus vulgaris. Int. J. Clin. Med. 2015, 6, 635–642. [Google Scholar] [CrossRef]
- Afzal, K.; Uzair, M.; Chaudhary, B.A.; Akhtar, S.; Ahmad, A.; Afzal, S. Isolation of squarrosal and squarrosol compounds from methanol root extract of Ruelliasquarrosa(Acanthaceae). Trop. J. Pharm. Res. 2018, 17, 647–652. [Google Scholar] [CrossRef]
- Iqbal, J.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Chabner, B.A.; Amrein, P.C.; Druker, B.J.; Michaelson, M.D.; Mitsiades, C.S.; Gross, P.E.; Ryan, D.P.; Ramachandra, S.; Richardson, P.G.; Supko, J.G.; et al. Goodman and Gilman’s Pharmacological Basis of Therapeutics; Brunton, L.L., Laso, J.S., Parker, K.L., Eds.; McGraw-Hill: New York, NY, USA, 2005; pp. 1315–1403. [Google Scholar]
- Bhagat, M.; Sudan, R.; Gupta, G.; Kaul, A.; Singh, J.J. Antioxidant and Immunomodulatory Potential of Cobra lily Species of North-western Himalayan Region: A Comparative Analysis. J. Biol. Act. Prod. Nat. 2014, 4, 179–187. [Google Scholar] [CrossRef]
- Singh, G.; Kachroo, P. Forest flora of Srinagar and Plants of Neighbourhood; Bishen Singh Mehendra Pal Singh; The University of Wisconsin: Madison, WI, USA, 1976. [Google Scholar]
- Gilani, S.A.; Qureshi, R.A.; Gilani, S.J. Indigenous Uses of Some Important ethnomedicinal herbs of Ayubia National Park, Abbottabad, Pakistan. Ethnobot. Leaflets. 2006, 10, 285–293. [Google Scholar]
- Shah, G.M.; Khan, M.A. Common medicinal folk recipes of Siran Valley, Mansehra, Pakistan. Ethnobotany. Leaflets. 2006, 10, 49–62. [Google Scholar]
- Kunwar, R.M.; Adhikari, N. Ethnomedicine of Dolpa district, Nepal: The plants, their vernacular names and uses. Lyonia. J. Ecol. Application. 2005, 8, 43–49. [Google Scholar]
- Bibi, Y.; Nisa, S.; Zia, M.; Chaudhary, M.F. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement. Altern. Med. 2011, 11, 52. [Google Scholar] [CrossRef]
- Singh, J.; Singh, J.; Kamboj, S.S. A novel mitogenic and antiproliferativelectin from a wild cobra lily, Arisaema flavum. Biochem. Biophys. Res. Commun. 2004, 318, 1057–1065. [Google Scholar] [CrossRef]
- Kakrani, P.H.; Kakrani, H.; Raval, M. Isolation of cytotoxic constituent from bioactivity guided fraction of alysicarpus monilifer l. (dc.). Int. J. Pharm. Pharm. Sci. 2019, 11, 69–77. [Google Scholar] [CrossRef]
- Vijayarathna, S.; Sasidharan, S. Cytotoxicity of methanol extracts of Elaeisguineensis on MCF-7 and Vero cell lines. Asian Pac. J. Trop. Biomed. 2012, 2, 826–829. [Google Scholar] [CrossRef]
- Kowalczuk, D.; Pitucha, M. Application of FTIR Method for the Assessment of Immobilization of Active Substances in the Matrix of Biomedical Materials. Materials 2019, 12, 2972. [Google Scholar] [CrossRef]
- Solowey, E.; Lichtenstein, M.; Sallon, S.; Paavilainen, H.; Solowey, E.; Lorberboum-Galski, H. Evaluating Medicinal Plants for Anticancer Activity. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Masoko, P.; Gololo, S.S.; Mokgotho, M.P.; Eloff, J.N.; Howard, R.L.; Mampuru, L.J. Evaluation of the antioxidant, antibacterial, and antiproliferative activities of the acetone extract of the roots of Senna Italica (Fabaceae). Afr. J. Tradit. Complement Altern. Med. 2010, 7, 138–148. [Google Scholar] [CrossRef]
- Nisa, S.; Bibi, Y.; Waheed, A.; Zia, M.; Sarwar, S.; Ahmed, S.; Chaudhary, M.F. Evaluation of anticancer activity of Debregeasia Salicifolia extract against estrogen receptor positive cell line. Afr. J. Biotechnol. 2011, 10, 990–995. [Google Scholar]
- Abdolmohammadi, M.H.; Fouladdel, S.H.; Shafiee, A.; Amin, G.H.; Ghaffari, S.M.; Azizi, E. Anticancer effects and cell cycle changes by A. Persicus. DARU J. Pharm. Sci. 2008, 6, 384–388. [Google Scholar]
- Azam, A.Z.; Moni, F.; Hamiduzzaman, M.; Masud, M.M.; Hasan, C.M. Two Cinnamoyl Triterpenes and Steroids from Crotalaria incana(Fabaceae). Res. J. Phytochem. 2013, 7, 1–9. [Google Scholar] [CrossRef]
- Kumar, P.A.; Rapolu RRaju, C.K.; Sasalamari, G.; Kumar, G.S.; Awasthi, A.; Navalgund, S.G.; Surendranath, K.V. The novel acid degradation products of losartan: Isolation and characterization using Q-TOF, 2D-NMR and FTIR. J. Pharm. Biomed. Anal. 2016, 120, 65–71. [Google Scholar] [CrossRef]
- Anu, A.; Rao, J.M. Oxanthrone esters from the roots of Cassia kleinii. Photochem 2002, 59, 425–427. [Google Scholar] [CrossRef]
- Kaliyaperumal, S.; Mishra, P.K.; Gautam, G.K. Isolation And Characterization of Palmitic Acid, 2- (Tetradecyloxy) Ethyl Ester and Beta Sitosterol from Methanolic Extract of Bauhinia Purpurea Leaves. Euro. J. Biomed. Pharma. Sci. 2016, 3, 264–268. [Google Scholar]
- Chopra, N.; Alam, M.S.; Ali, M. A new derivative of benzoic acid from Pluchea lanceolata. Ind. J. Chem. 1996, 35, 1352–1353. [Google Scholar]
- Reid, R.G.; Sarker, S.D. Isolation of natural products by low-pressure column chromatography. Methods. Mol. Biol. 2012, 864, 155–187. [Google Scholar] [PubMed]
- Yang, X.D.; Zhan, L.J.; Liang, B.; Xu, L.Z.; Yang, S.L. Oligosaccharide esters isolated from plants of Polygalaceae. Chin. Tradit. Herbal. Drugs. 2002, 33, 954–958. [Google Scholar]
- Ogwuru, N.; Adamczeski, M. Bioactive natural products derived from Polygonum species of plants: Their structures and mechanisms of action. Stud. Nat. Prod. Chem. 2002, 22, 607–642. [Google Scholar]
- Kobayashi, S.; Miyase, T.; Noguchi, H. Polyphenolic glycosides and oligosaccharide multiesters from the roots of Polygala dalmaisiana. J. Nat. Prod. 2002, 65, 319–328. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Subramaniam, P. Phytochemical profiling of leaf, stem, and tuber parts of Solenaamplexicaulis (Lam.) Gandhi Using GC-MS. Int. Sch. Res. Notices. 2014, 3, 1–13. [Google Scholar]
- Ukwubile, C.A.; Ahmed, A.; Katsayal, U.A.; Yau, J.; Mejida, S. GC-MS analysis of bioactive from Melastomastrum capitatum Fern. Leaf methanol extract. An anticancer plant. Sci. Afr. 2019, 3, 900e. [Google Scholar] [CrossRef]
- Singh, S.; Nair, V.; Jain, S.; Gupta, Y.K. Evaluation of anti-inflammatory activity of plant lipids containing α-linolenic acid. Indian. J. Exp. Bio. 2008, 46, 453–456. [Google Scholar]
- Jegadeeswari, P.; Nishanthini, N.; Muthukumarasamy, S.; Mohan, V.R. GC-MS analysis of bioactive components of Aristolochiakrysagathra (Aristolochiaceae.). J. Chem. Pharm Sci. 2012, 2, 226–232. [Google Scholar]
- Ali, A.; Alharthi, S.; Al-Shalan, N.H.; Santali, E.Y. Development of Narrow-Bore C18 Column for Fast Separation of Peptides and Proteins in High-Performance Liquid Chromatography. Polymers. 2022, 14, 2576. [Google Scholar] [CrossRef]
| 1H-NMR data ofCompound-I (A) | |||
| Carbon No | 1H(δ) | 13C(δ) | Multiplicity (DEPT) |
| 1 | 173.9 | C = O | |
| 2 | 2.28(t) | 34.4 | CH2 |
| 3 | 1.60(d) | 29.65 | CH2 |
| 4–13 | 1.27(s) | 29.66 | (CH2)10 |
| 14 | 1.27(s) | 29.70 | CH2 |
| 15 | 1.29(m) | 29.60 | CH2 |
| 16 | 0.88(t) | 29.15 | CH3 |
| 17 | 4.11(dd) | 22.70 | -O-CH2 |
| 18 | 1.25(m) | 22.70 | CH3 |
| 1H-NMR data ofCompound-II (B) | |||
| 1 | 174.45 | C = O | |
| 2 | 60.23 | CH2 | |
| 3 | 1.64(dd) | 25.08 | CH2 |
| 4 | 1.59(m) | 34.48 | CH2 |
| 5 | 2.30(dd) | 188.45 | C = O |
| 6 | 34.21 | CH2 | |
| 7 | 2.30(dd) | 25.05 | CH2 |
| 8 | 1.59(m) | 22.78 | CH2 |
| 9–17 | 1.28(s) | 29.77–29.44 | (CH2)10 |
| 18 | 1.28(s) | 32.01 | CH2 |
| 19 | 1.28(s) | 29.24 | CH |
| 20,21 | 1.59(m) | 29.25 | (CH2)2 |
| 22 | 1.28(s) | 14.20 | CH3 |
| 23,24 | 0.88(m) | 29.35 | (CH2)2 |
| 25 | 1.28(s) | 14.20 | CH3 |
| 26 | 0.88(m) | 51.53 | -O-CH3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, S.; Bibi, Y.; Masood, S.; Ali, A.; Alam, S.; Sabir, M.; Qayyum, A.; Ahmed, W.; Alharthi, S.; Santali, E.Y.; et al. Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules 2022, 27, 7932. https://doi.org/10.3390/molecules27227932
Nisa S, Bibi Y, Masood S, Ali A, Alam S, Sabir M, Qayyum A, Ahmed W, Alharthi S, Santali EY, et al. Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules. 2022; 27(22):7932. https://doi.org/10.3390/molecules27227932
Chicago/Turabian StyleNisa, Sobia, Yamin Bibi, Saadia Masood, Ashraf Ali, Sadia Alam, Maimoona Sabir, Abdul Qayyum, Waqas Ahmed, Sarah Alharthi, Eman Y. Santali, and et al. 2022. "Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott" Molecules 27, no. 22: 7932. https://doi.org/10.3390/molecules27227932
APA StyleNisa, S., Bibi, Y., Masood, S., Ali, A., Alam, S., Sabir, M., Qayyum, A., Ahmed, W., Alharthi, S., Santali, E. Y., Alharthy, S. A., Bawazir, W. M., & Almashjary, M. N. (2022). Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules, 27(22), 7932. https://doi.org/10.3390/molecules27227932






