Biological Assessment of the NO-Dependent Endothelial Function
Abstract
1. Introduction
2. Vascular Sources of NO
2.1. Oxygen-Dependent Nitric Oxide Synthesis
2.2. Nitrite-Dependent Nitric Oxide Synthesis
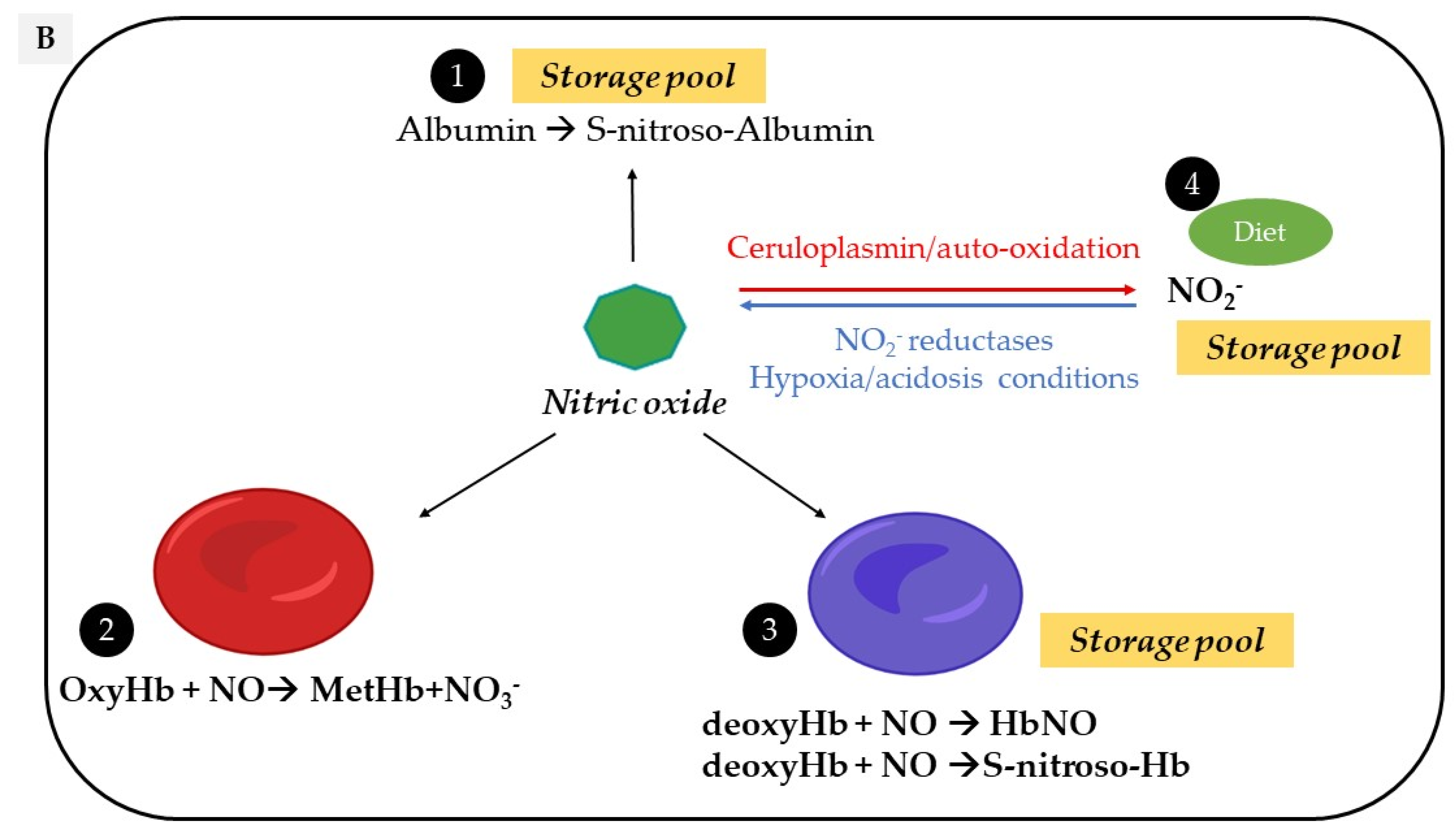
2.3. Nitroso/Nitrosyl Species
2.4. Red Blood Cells
3. Endothelial Dysfunction and the Vascular Redox Status
4. NO Bioavailability Biomarkers
4.1. The Nitrate/Nitrite
4.2. The Cyclic Guanosine Monophosphate
4.3. The Nitrosyl-Hemoglobin and Others NO Species
5. Electron Paramagnetic Resonance Technique for Measurement of Fresh Frozen Erythrocytes HbNO
5.1. There Are Several Paramagnetic Forms of Nitrosyl-Hemoglobin
5.2. EPR Signal Subtraction Procedure to Unmask the Hyper Fine (hf) Complex
5.3. Optimal Conditions of Fresh Frozen Erythrocytes HbNO Preservation for Diagnostic Use
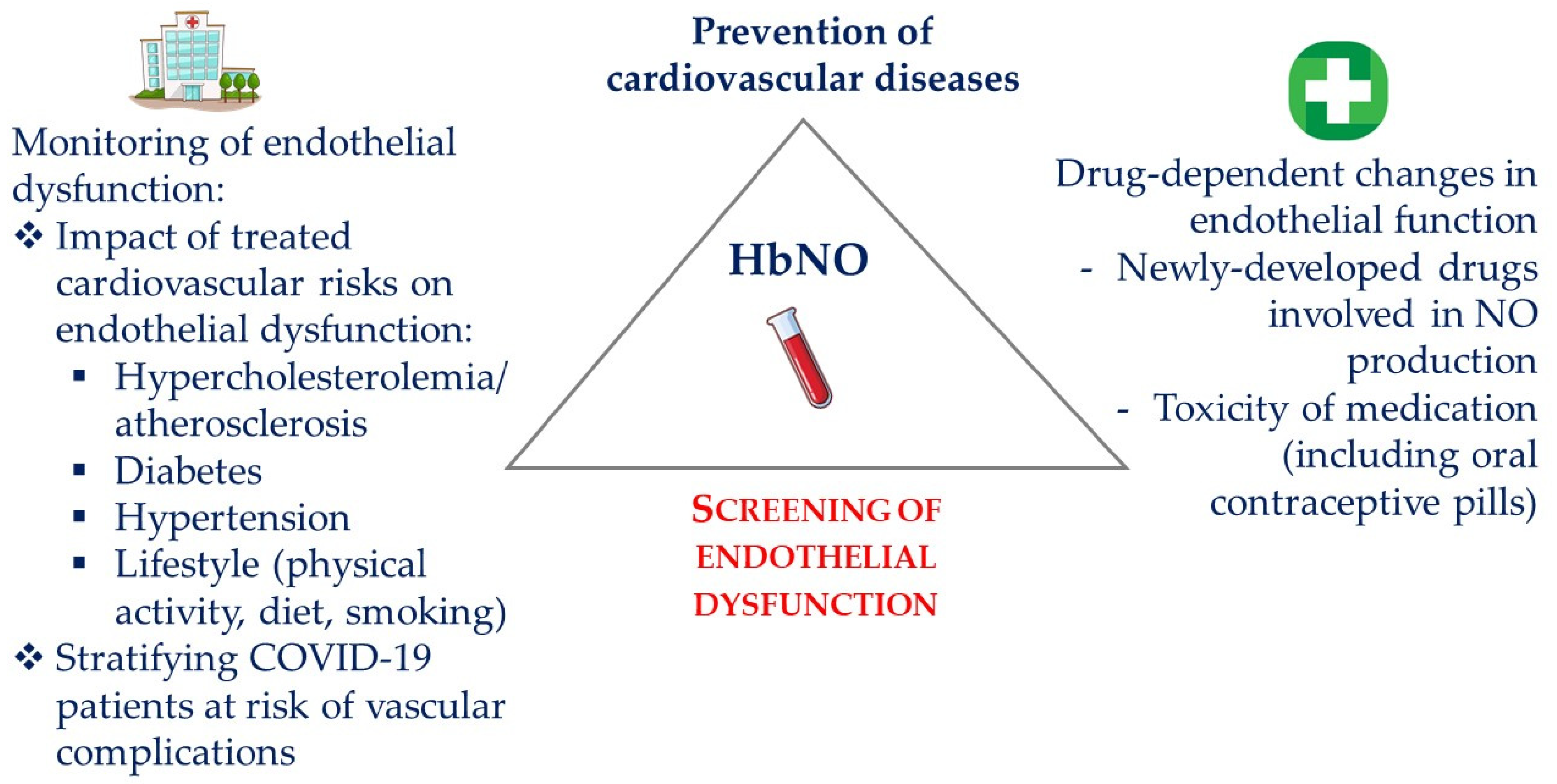
6. HbNO in Clinical Research
6.1. HbNO Is Correlated with Endothelial Function in Healthy Volunteers
6.2. HbNO Reflects NO Formation from the Vasculature In Vivo
6.3. HbNO Is Influenced by the Vascular Redox Balance
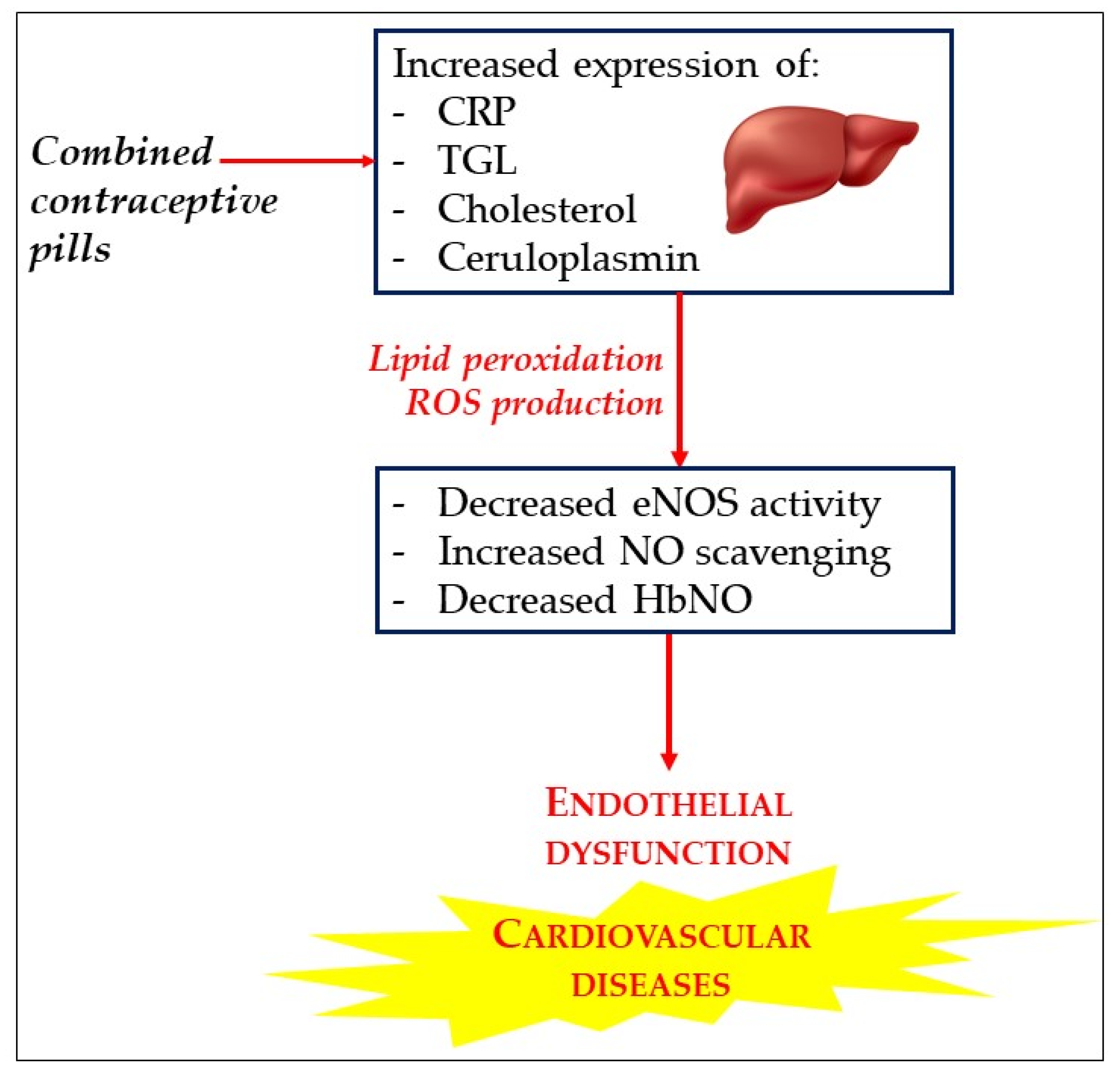
6.4. HbNO Is a Biomarker of Oxidant Stress and Endothelial Dysfunction under Oral Contraceptive Pill
6.5. HbNO Is a Biomarker of Vascular and Respiratory Degradation in COVID-19 Patients
6.6. HbNO as Biomarker for Detecting the Development of a Cardiovascular Complications during or after Surgery
6.7. Limitations of HbNO
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- West, J.B. Joseph Priestley, Oxygen, and the Enlightenment. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L111–L119. [Google Scholar] [CrossRef] [PubMed]
- Gruetter, C.A.; Barry, B.K.; McNamara, D.B.; Gruetter, D.Y.; Kadowitz, P.J.; Ignarro, L. Relaxation of Bovine Coronary Artery and Activation of Coronary Arterial Guanylate Cyclase by Nitric Oxide, Nitroprusside and a Carcinogenic Nitrosoamine. J. Cyclic Nucleotide Res. 1979, 5, 211–224. [Google Scholar] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The Obligatory Role of Endothelial Cells in the Relaxation of Arterial Smooth Muscle by Acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.M.; Ferrige, A.G.; Moncada, S. Nitric Oxide Release Accounts for the Biological Activity of Endothelium-Derived Relaxing Factor. Nature 1987, 327, 524–526. [Google Scholar] [CrossRef]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric Oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- SoRelle, R. Nobel Prize Awarded to Scientists for Nitric Oxide Discoveries. Circulation 1998, 98, 2365–2366. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Kim, H.; Kim, S.; Han, S.; Rane, P.P.; Fox, K.M.; Qian, Y.; Suh, H.S. Prevalence and Incidence of Atherosclerotic Cardiovascular Disease and Its Risk Factors in Korea: A Nationwide Population-Based Study. BMC Public Health 2019, 19, 1112. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.C.; Feletou, M. Endothelial Dysfunction and Vascular Disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Signal Transduction of ENOS Activation. Cardiovasc. Res. 1999, 43, 532–541. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Schwartz, M.A. Vascular Mechanobiology: Homeostasis, Adaptation, and Disease. Annu. Rev. Biomed. Eng. 2021, 23, 1–27. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.-L. Nitric Oxide Signalling in Cardiovascular Health and Disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Mount, P.F.; Kemp, B.E.; Power, D.A. Regulation of Endothelial and Myocardial NO Synthesis by Multi-Site ENOS Phosphorylation. J. Mol. Cell Cardiol. 2007, 42, 271–279. [Google Scholar] [CrossRef]
- Kraehling, J.R.; Sessa, W.C. Contemporary Approaches to Modulating the Nitric Oxide–CGMP Pathway in Cardiovascular Disease. Circ. Res. 2017, 120, 1174–1182. [Google Scholar] [CrossRef]
- Lima, B.; Forrester, M.T.; Hess, D.T.; Stamler, J.S. S-Nitrosylation in Cardiovascular Signaling. Circ. Res. 2010, 106, 633–646. [Google Scholar] [CrossRef]
- Wallerath, T.; Gath, I.; Aulitzky, W.E.; Pollock, J.S.; Kleinert, H.; Förstermann, U. Identification of the NO Synthase Isoforms Expressed in Human Neutrophil Granulocytes, Megakaryocytes and Platelets. Thromb. Haemost. 1997, 77, 163–167. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Rassaf, T.; Lauer, T.; Dejam, A.; Jax, T.; Kumara, I.; Gharini, P.; Kabanova, S.; Ozüyaman, B.; et al. Red Blood Cells Express a Functional Endothelial Nitric Oxide Synthase. Blood 2006, 107, 2943–2951. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Heymes, C.; Benessiano, J.; Geske, R.S.; Lévy, B.I.; Vanhoutte, P.M. Neuronal Nitric Oxide Synthase Is Expressed in Rat Vascular Smooth Muscle Cells: Activation by Angiotensin II in Hypertension. Circ. Res. 1998, 83, 1271–1278. [Google Scholar] [CrossRef]
- Singel, D.J.; Stamler, J.S. Chemical Physiology of Blood Flow Regulation by Red Blood Cells: The Role of Nitric Oxide and S-Nitrosohemoglobin. Annu. Rev. Physiol. 2005, 67, 99–145. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, J.L. Evidence for the Presence of L-Arginine-Nitric Oxide Pathway in Human Red Blood Cells: Relevance in the Effects of Red Blood Cells on Platelet Function. J. Cardiovasc. Pharmacol. 1998, 32, 57–61. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Kelm, M. Endothelial Nitric Oxide Synthase in Red Blood Cells: Key to a New Erythrocrine Function? Redox Biol. 2014, 2, 251–258. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Lobysheva, I.I.; Balligand, J.-L. Nitrosyl-Hemoglobin Formation in Rodent and Human Venous Erythrocytes Reflects NO Formation from the Vasculature in Vivo. PLoS ONE 2018, 13, e0200352. [Google Scholar] [CrossRef]
- Ulker, P.; Sati, L.; Celik-Ozenci, C.; Meiselman, H.J.; Baskurt, O.K. Mechanical Stimulation of Nitric Oxide Synthesizing Mechanisms in Erythrocytes. Biorheology 2009, 46, 121–132. [Google Scholar] [CrossRef]
- Wood, K.C.; Cortese-Krott, M.M.; Kovacic, J.C.; Noguchi, A.; Liu, V.B.; Wang, X.; Raghavachari, N.; Boehm, M.; Kato, G.J.; Kelm, M.; et al. Circulating Blood Endothelial Nitric Oxide Synthase Contributes to the Regulation of Systemic Blood Pressure and Nitrite Homeostasis. ATVB 2013, 33, 1861–1871. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Rodriguez-Mateos, A.; Sansone, R.; Kuhnle, G.G.C.; Thasian-Sivarajah, S.; Krenz, T.; Horn, P.; Krisp, C.; Wolters, D.; Heiß, C.; et al. Human Red Blood Cells at Work: Identification and Visualization of Erythrocytic ENOS Activity in Health and Disease. Blood 2012, 120, 4229–4237. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Shiva, S. Nitrite: A Physiological Store of Nitric Oxide and Modulator of Mitochondrial Function. Redox Biol. 2013, 1, 40–44. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-Arginine-Nitric Oxide Pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Ford, P.C.; Wink, D.A.; Stanbury, D.M. Autoxidation Kinetics of Aqueous Nitric Oxide. FEBS Lett. 1993, 326, 1–3. [Google Scholar] [CrossRef]
- Shiva, S.; Wang, X.; Ringwood, L.A.; Xu, X.; Yuditskaya, S.; Annavajjhala, V.; Miyajima, H.; Hogg, N.; Harris, Z.L.; Gladwin, M.T. Ceruloplasmin Is a NO Oxidase and Nitrite Synthase That Determines Endocrine NO Homeostasis. Nat. Chem. Biol. 2006, 2, 486–493. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric Oxide Signaling in Health and Disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological Nitric Oxide Signalling: Chemistry and Terminology: NO Chemical Biology and Terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef]
- Maron, B.A.; Tang, S.-S.; Loscalzo, J. S-Nitrosothiols and the S-Nitrosoproteome of the Cardiovascular System. Antioxid. Redox Signal. 2013, 18, 270–287. [Google Scholar] [CrossRef]
- Hess, D.T.; Matsumoto, A.; Kim, S.-O.; Marshall, H.E.; Stamler, J.S. Protein S-Nitrosylation: Purview and Parameters. Nat. Rev. Mol. Cell Biol. 2005, 6, 150–166. [Google Scholar] [CrossRef]
- Belcastro, E.; Wu, W.; Fries-Raeth, I.; Corti, A.; Pompella, A.; Leroy, P.; Lartaud, I.; Gaucher, C. Oxidative Stress Enhances and Modulates Protein S-Nitrosation in Smooth Muscle Cells Exposed to S-Nitrosoglutathione. Nitric Oxide 2017, 69, 10–21. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Ghatge, M.S.; Safo, M.K. Hemoglobin: Structure, Function and Allostery. In Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins; Hoeger, U., Harris, J.R., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2020; Volume 94, pp. 345–382. ISBN 978-3-030-41768-0. [Google Scholar]
- Kim-Shapiro, D.B.; Schechter, A.N.; Gladwin, M.T. Unraveling the Reactions of Nitric Oxide, Nitrite, and Hemoglobin in Physiology and Therapeutics. Arter. Thromb. Vasc. Biol. 2006, 26, 697–705. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Schechter, A.N. NO Contest: Nitrite Versus S -Nitroso-Hemoglobin. Circ. Res. 2004, 94, 851–855. [Google Scholar] [CrossRef]
- Helms, C.C.; Liu, X.; Kim-Shapiro, D.B. Recent Insights into Nitrite Signaling Processes in Blood. Biol. Chem. 2017, 398, 319–329. [Google Scholar] [CrossRef]
- Helms, C.C.; Gladwin, M.T.; Kim-Shapiro, D.B. Erythrocytes and Vascular Function: Oxygen and Nitric Oxide. Front. Physiol. 2018, 9, 125. [Google Scholar] [CrossRef]
- Vaughn, M.W.; Huang, K.T.; Kuo, L.; Liao, J.C. Erythrocytes Possess an Intrinsic Barrier to Nitric Oxide Consumption. J. Biol. Chem. 2000, 275, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Crawford, J.H.; Patel, R.P. The Biochemistry of Nitric Oxide, Nitrite, and Hemoglobin: Role in Blood Flow Regulation. Free Radic. Biol. Med. 2004, 36, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Jia, L.; Eu, J.P.; McMahon, T.J.; Demchenko, I.T.; Bonaventura, J.; Gernert, K.; Piantadosi, C.A. Blood Flow Regulation by S-Nitrosohemoglobin in the Physiological Oxygen Gradient. Science 1997, 276, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S.; et al. The Assessment of Endothelial Function: From Research Into Clinical Practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive Oxygen Species: Key Regulators in Vascular Health and Diseases: ROS in Vascular Diseases. Br. J. Pharmacol. 2018, 175, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.R.; Sobey, C.G. Endothelial NADPH Oxidases: Which NOX to Target in Vascular Disease? Trends Endocrinol. Metab. 2014, 25, 452–463. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M. Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. CVP 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Bouras, G.; Deftereos, S.; Tousoulis, D.; Giannopoulos, G.; Chatzis, G.; Tsounis, D.; Cleman, M.W.; Stefanadis, C. Asymmetric Dimethylarginine (ADMA): A Promising Biomarker for Cardiovascular Disease? Curr. Top. Med. Chem. 2013, 13, 180–200. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-Related Reduction of NO Availability and Oxidative Stress in Humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Rafikov, R.; Fonseca, F.V.; Kumar, S.; Pardo, D.; Darragh, C.; Elms, S.; Fulton, D.; Black, S.M. ENOS Activation and NO Function: Structural Motifs Responsible for the Posttranslational Control of Endothelial Nitric Oxide Synthase Activity. J. Endocrinol. 2011, 210, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Weydert, C.J.; Cullen, J.J. Measurement of Superoxide Dismutase, Catalase and Glutathione Peroxidase in Cultured Cells and Tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent Hydroxyl Radical Production by Peroxynitrite: Implications for Endothelial Injury from Nitric Oxide and Superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2014, 20, 3040–3077. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Holmgren, A.; Johansson, C.; Berndt, C.; Lönn, M.E.; Hudemann, C.; Lillig, C.H. Thiol Redox Control via Thioredoxin and Glutaredoxin Systems. Biochem. Soc. Trans. 2005, 33, 1375–1377. [Google Scholar] [CrossRef]
- Bretón-Romero, R.; Lamas, S. Hydrogen Peroxide Signaling in Vascular Endothelial Cells. Redox Biol. 2014, 2, 529–534. [Google Scholar] [CrossRef]
- Touyz, R.M.; Briones, A.M. Reactive Oxygen Species and Vascular Biology: Implications in Human Hypertension. Hypertens. Res. 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Montiel, V.; Bella, R.; Michel, L.Y.M.; Esfahani, H.; De Mulder, D.; Robinson, E.L.; Deglasse, J.-P.; Tiburcy, M.; Chow, P.H.; Jonas, J.-C.; et al. Inhibition of Aquaporin-1 Prevents Myocardial Remodeling by Blocking the Transmembrane Transport of Hydrogen Peroxide. Sci. Transl. Med. 2020, 12, eaay2176. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Verdoy, R.; Brusa, D.; Lobysheva, I.I.; Balligand, J.-L. Redox Regulation of Nitrosyl-Hemoglobin in Human Erythrocytes. Redox Biol. 2020, 34, 101399. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporin Water Channels and Endothelial Cell Function*. J. Anat. 2002, 200, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Kelm, M. Nitric Oxide Metabolism and Breakdown. Biochim. Biophys. Acta 1999, 1411, 273–289. [Google Scholar] [CrossRef]
- Rassaf, T.; Heiss, C.; Hendgen-Cotta, U.; Balzer, J.; Matern, S.; Kleinbongard, P.; Lee, A.; Lauer, T.; Kelm, M. Plasma Nitrite Reserve and Endothelial Function in the Human Forearm Circulation. Free Radic. Biol. Med. 2006, 41, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Nitrite and Nitrate Measurement by Griess Reagent in Human Plasma: Evaluation of Interferences and Standardization. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 440, pp. 361–380. ISBN 978-0-12-373967-4. [Google Scholar]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. CGMP-Dependent Protein Kinases and CGMP Phosphodiesterases in Nitric Oxide and CGMP Action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Páli, T.; Bencsik, P.; Görbe, A.; Ferdinandy, P.; Csont, T. Measurement of NO in Biological Samples: NO Detection. Br. J. Pharmacol. 2015, 172, 1620–1632. [Google Scholar] [CrossRef]
- Metzger, I.F.; Sertorio, J.T.C.; Tanus-Santos, J.E. Relationship between Systemic Nitric Oxide Metabolites and Cyclic GMP in Healthy Male Volunteers. Acta Physiol. 2006, 188, 123–127. [Google Scholar] [CrossRef]
- Bork, N.; Nikolaev, V. CGMP Signaling in the Cardiovascular System—The Role of Compartmentation and Its Live Cell Imaging. Int. J. Mol. Sci. 2018, 19, 801. [Google Scholar] [CrossRef]
- Takimoto, E.; Champion, H.C.; Belardi, D.; Moslehi, J.; Mongillo, M.; Mergia, E.; Montrose, D.C.; Isoda, T.; Aufiero, K.; Zaccolo, M.; et al. CGMP Catabolism by Phosphodiesterase 5A Regulates Cardiac Adrenergic Stimulation by NOS3-Dependent Mechanism. Circ. Res. 2005, 96, 100–109. [Google Scholar] [CrossRef]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.-S.; Hamdani, N.; Holewinski, R.; Jo, S.-H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A Controls Nitric-Oxide-Independent CGMP and Hypertrophic Heart Disease. Nature 2015, 519, 472–476. [Google Scholar] [CrossRef]
- Priksz, D.; Bombicz, M.; Varga, B.; Kurucz, A.; Gesztelyi, R.; Balla, J.; Toth, A.; Papp, Z.; Szilvassy, Z.; Juhasz, B. Upregulation of Myocardial and Vascular Phosphodiesterase 9A in A Model of Atherosclerotic Cardiovascular Disease. Int. J. Mol. Sci. 2018, 19, 2882. [Google Scholar] [CrossRef]
- Perrin-Sarrado, C.; Zhou, Y.; Salgues, V.; Parent, M.; Giummelly, P.; Lartaud, I.; Gaucher, C. S-Nitrosothiols as Potential Therapeutics to Induce a Mobilizable Vascular Store of Nitric Oxide to Counteract Endothelial Dysfunction. Biochem. Pharmacol. 2020, 173, 113686. [Google Scholar] [CrossRef] [PubMed]
- Rassaf, T.; Feelisch, M.; Kelm, M. Circulating No Pool: Assessment of Nitrite and Nitroso Species in Blood and Tissues. Free Radic. Biol. Med. 2004, 36, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Lauer, T.; Dejam, A.; Kleinbongard, P.; Hamada, S.; Rassaf, T.; Matern, S.; Feelisch, M.; Kelm, M. Plasma Nitroso Compounds Are Decreased in Patients With Endothelial Dysfunction. J. Am. Coll. Cardiol. 2006, 47, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Gladwin, M.T.; Schechter, A.N.; Hogg, N. Electron Paramagnetic Resonance Analysis of Nitrosylhemoglobin in Humans during NO Inhalation. J. Biol. Chem. 2005, 280, 40583–40588. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, H.; Watanabe, M.; Yoshihara, H.; Harada, N.; Shiga, T. Detection of nitric oxide production in lipopolysaccbaride-treated rats by esr using carbon monoxkie hemoglobin. Biochem. Biophys. Res. Commun. 1992, 184, 6. [Google Scholar] [CrossRef]
- Hogg, N. Detection of Nitric Oxide by Electron Paramagnetic Resonance Spectroscopy. Free Radic. Biol. Med. 2010, 49, 122–129. [Google Scholar] [CrossRef]
- Kleschyov, A.L.; Wenzel, P.; Munzel, T. Electron Paramagnetic Resonance (EPR) Spin Trapping of Biological Nitric Oxide. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 12–20. [Google Scholar] [CrossRef]
- Desjardins, F.; Lobysheva, I.; Pelat, M.; Gallez, B.; Feron, O.; Dessy, C.; Balligand, J.-L. Control of Blood Pressure Variability in Caveolin-1-Deficient Mice: Role of Nitric Oxide Identified in Vivo through Spectral Analysis. Cardiovasc. Res. 2008, 79, 527–536. [Google Scholar] [CrossRef]
- Kirima, K.; Tsuchiya, K.; Sei, H.; Hasegawa, T.; Shikishima, M.; Motobayashi, Y.; Morita, K.; Yoshizumi, M.; Tamaki, T. Evaluation of Systemic Blood NO Dynamics by EPR Spectroscopy: HbNO as an Endogenous Index of NO. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H589–H596. [Google Scholar] [CrossRef]
- McMahon, T.J.; Moon, R.E.; Luschinger, B.P.; Carraway, M.S.; Stone, A.E.; Stolp, B.W.; Gow, A.J.; Pawloski, J.R.; Watke, P.; Singel, D.J.; et al. Nitric Oxide in the Human Respiratory Cycle. Nat. Med. 2002, 8, 711–717. [Google Scholar] [CrossRef]
- Lobysheva, I.I.; Biller, P.; Gallez, B.; Beauloye, C.; Balligand, J.-L. Nitrosylated Hemoglobin Levels in Human Venous Erythrocytes Correlate with Vascular Endothelial Function Measured by Digital Reactive Hyperemia. PLoS ONE 2013, 8, e76457. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R.; Kuvin, J.T.; Lerman, A. Noninvasive Identification of Patients with Early Coronary Atherosclerosis by Assessment of Digital Reactive Hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Gerhard-Herman, M.; Creager, M.A.; Hurley, S.; Mitra, D.; Ganz, P. Role of Nitric Oxide in the Regulation of Digital Pulse Volume Amplitude in Humans. J. Appl. Physiol. 2006, 101, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in The Framingham Heart Study. Circulation 2008, 117, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gonon, A.T.; Sjöquist, P.-O.; Lundberg, J.O.; Pernow, J. Arginase Regulates Red Blood Cell Nitric Oxide Synthase and Export of Cardioprotective Nitric Oxide Bioactivity. Proc. Natl. Acad. Sci. USA 2013, 110, 15049–15054. [Google Scholar] [CrossRef] [PubMed]
- Diederich, L.; Suvorava, T.; Sansone, R.; Keller, T.C.S.; Barbarino, F.; Sutton, T.R.; Kramer, C.M.; Lückstädt, W.; Isakson, B.E.; Gohlke, H.; et al. On the Effects of Reactive Oxygen Species and Nitric Oxide on Red Blood Cell Deformability. Front. Physiol. 2018, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Rifkind, J.M.; Nagababu, E. Hemoglobin Redox Reactions and Red Blood Cell Aging. Antioxid. Redox Signal. 2013, 18, 2274–2283. [Google Scholar] [CrossRef]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Øjvind, L.; Ellen, L.; Aksel, J.; Wessel, S.C.; Niels, K. Thrombotic Stroke and Myocardial Infarction with Hormonal Contraception. N. Engl. J. Med. 2012, 366, 2257–2266. [Google Scholar]
- Lalude, O.O. Risk of Cardiovascular Events with Hormonal Contraception: Insights from the Danish Cohort Study. Curr. Cardiol. Rep. 2013, 15, 374. [Google Scholar] [CrossRef]
- Campesi, I.; Sanna, M.; Zinellu, A.; Carru, C.; Rubattu, L.; Bulzomi, P.; Seghieri, G.; Tonolo, G.; Palermo, M.; Rosano, G.; et al. Oral Contraceptives Modify DNA Methylation and Monocyte-Derived Macrophage Function. Biol. Sex Differ. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, Z.; Asadi, B.; Saadatnia, M.; Ghorbani, A.; Fatehi, F. The Effect of Low-Dose Combined Oral Contraceptive Pills on Brachial Artery Endothelial Function and Common Carotid Artery Intima-Media Thickness. J. Stroke Cerebrovasc. Dis. 2014, 23, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Lobysheva, I.I.; van Eeckhoudt, S.; Dei Zotti, F.; Rifahi, A.; Pothen, L.; Beauloye, C.; Balligand, J.-L. Heme-Nitrosylated Hemoglobin and Oxidative Stress in Women Consuming Combined Contraceptives. Clinical Application of the EPR Spectroscopy. Free. Radic. Biol. Med. 2017, 108, 524–532. [Google Scholar] [CrossRef]
- Xiong, X.; Chi, J.; Gao, Q. Prevalence and Risk Factors of Thrombotic Events on Patients with COVID-19: A Systematic Review and Meta-analysis. Thromb. J. 2021, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.-H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-Associated Coagulopathy: Evidence from a Single-Centre, Cross-Sectional Study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.-R.; Fobker, M.; Kühn, J.; Braune, S.; Göbel, U.; Thölking, G.; et al. Microvascular Dysfunction in COVID-19: The MYSTIC Study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef]
- Sabatier, P.; Beusch, C.M.; Gencheva, R.; Cheng, Q.; Zubarev, R.; Arnér, E.S.J. Comprehensive Chemical Proteomics Analyses Reveal That the New TRi-1 and TRi-2 Compounds Are More Specific Thioredoxin Reductase 1 Inhibitors than Auranofin. Redox Biol. 2021, 48, 102184. [Google Scholar] [CrossRef]
- Yamasaki, H. Blood Nitrate and Nitrite Modulating Nitric Oxide Bioavailability: Potential Therapeutic Functions in COVID-19. Nitric Oxide 2020, 103, 29–30. [Google Scholar] [CrossRef]
- Green, S.J. Covid-19 Accelerates Endothelial Dysfunction and Nitric Oxide Deficiency. Microbes Infect. 2020, 22, 149–150. [Google Scholar] [CrossRef]
- Montiel, V. Oxidative Stress-Induced Endothelial Dysfunction and Decreased Vascular Nitric Oxide in COVID-19 Patients. eBioMedicine 2022, 77, 18. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.; Hu, W. An Analysis of the Risk of Perioperative Ischemic Stroke in Patients Undergoing Non-Cardiovascular and Non-Neurological Surgeries. Neurol. Res. 2020, 42, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Wei, A.N.; Chook, P.; Yin, Y.; Cheng, W.; Wu, M.J.; Celermajer, D.S.; Woo, K.S. Impact of Non-Cardiovascular Surgery on Reactive Hyperaemia and Arterial Endothelial Function. Clin. Exp. Pharmacol. Physiol. 2013, 40, 466–472. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boughaleb, H.; Lobysheva, I.; Dei Zotti, F.; Balligand, J.-L.; Montiel, V. Biological Assessment of the NO-Dependent Endothelial Function. Molecules 2022, 27, 7921. https://doi.org/10.3390/molecules27227921
Boughaleb H, Lobysheva I, Dei Zotti F, Balligand J-L, Montiel V. Biological Assessment of the NO-Dependent Endothelial Function. Molecules. 2022; 27(22):7921. https://doi.org/10.3390/molecules27227921
Chicago/Turabian StyleBoughaleb, Hasnae, Irina Lobysheva, Flavia Dei Zotti, Jean-Luc Balligand, and Virginie Montiel. 2022. "Biological Assessment of the NO-Dependent Endothelial Function" Molecules 27, no. 22: 7921. https://doi.org/10.3390/molecules27227921
APA StyleBoughaleb, H., Lobysheva, I., Dei Zotti, F., Balligand, J.-L., & Montiel, V. (2022). Biological Assessment of the NO-Dependent Endothelial Function. Molecules, 27(22), 7921. https://doi.org/10.3390/molecules27227921







