Abstract
Cross contamination of β-lactams is one of the highest risks for patients using pharmaceutical products. Penicillin and some non-penicillin β-lactams may cause potentially life-threatening allergic reactions. The trace detection of β-lactam antibiotics in cleaning rinse solutions of common reactors and manufacturing aids in pharmaceutical facilities is very crucial. Therefore, the common facilities adopt sophisticated cleaning procedures and develop analytical methods to assess traces of these compounds in rinsed solutions. For this, a highly sensitive and reproducible ultra-performance liquid chromatography with triple quadrupole mass spectrometry (UHPLC-MS/MS) method was developed for the analysis of Cephapirin and Ceftiofur. As per the FDA guidelines described in FDA-2011-D-0104, the contamination of these β-lactam antibiotics must be regulated. The analysis was performed on an XBridge C18 column with 100 mm length, 4.6 mm diameter, and 3.5 µm particle size at an oven temperature of about 40 °C. The mobile phase was composed of 0.15% formic acid in water and acetonitrile as mobile phases A and B, and a flow rate was set to 0.6 mL/min. The method was validated for Cephapirin and Ceftiofur. The quantification precision and accuracy were determined to be the lowest limit of detection 0.15 parts per billion (ppb) and the lowest limit of quantification 0.4 ppb. This method was linear in the range of 0.4 to 1.5 ppb with the determination of coefficient (R2 > 0.99). This sensitive and fast method was fit-for-purpose for detecting and quantifying trace amounts of β-lactam contamination, monitoring cross contamination in facility surface cleaning, and determining the acceptable level of limits for regulatory purposes.
1. Introduction
Cross-contamination among production lines is a critical concern in drug manufacturing, as it can subject both patients and workers at the risk of adverse health effects [1]. Various regulatory bodies in the U.S., Europe, and others are tightening regulations to increase safety and control exposure as the global pharmaceutical markets grow and drugs become increasingly more potent. Tablets or a complex combination of drugs are generally manufactured in large production plants to keep costs low and manufacturing efficient, production lines for a range of different active pharmaceutical ingredients (APIs) are often run in parallel. However, it increases the risk of cross contamination, where active ingredients from one line can be carried across to the other—through the contaminated equipment, air, or via workers’ clothing. If certain sensitizing compounds, such as penicillin and β-lactam antibiotics, make their way into drug production, they can trigger allergic reactions, even at low levels [2]. The risks range from small infections like a non-pruritic, non-urticarial skin rash, or itchy eyes to dangerous immune responses including fatal anaphylactic reactions [3,4,5,6,7]. In fact, penicillin allergy is the most common cause of drug-induced anaphylaxis, and the allergy accounts for up to 1000 deaths per year [8]. In addition to humans, β-lactams antibiotics’ residues are also found most frequently in milk as these compounds are commonly applied as antibiotics in the management of dairy cattle [9]. These residual antibiotics in the milk can also potentially affect human health in the form of allergies and the development of resistance to bacteria [10,11].
β-lactam antibiotics include the following five classes: penicillins, cephalosporins, penems, carbacephems, and monobactams [12,13]. A β-lactam moiety is typically present in penicillin or other non-penicillin drugs including cephalosporins, carbacephems, and monobactams of antibiotics with a long history in the treatment of a broad range of infectious diseases in humans [14,15]. Excessive misuse of β-lactam antibiotics led to β-lactam resistance; additionally, these substances have several side effects like allergy and toxicity [16,17]. Intake of these drugs causes a potential risk in humans who are hypersensitive to them, an important group of patients that are allergic to penicillin, making up around 10% of the adult population [4]. β -lactam antibiotics contain different molecules with diverse molecular structures which consist of a variety of beta-lactam rings; these can be recognized by the immune system leading to hypersensitivity in some patients [18]. For example, cephalosporin induced hypersensitivity reaction and anaphylaxis in patients with IgE-mediated allergy are reported [19]. Apart from this, the toxic effect of β-lactams on the central nervous system is also well-known; for example, the reports of penicillin mediated disorientation, twitching, somnolence, and myoclonus are available in the literature [20]. In addition to their toxic effects, the compounds of cephalosporins including cephapirin, ceftiofur, and many more are potential contaminants present in the production reactors, which are carried forward into the cleaning solution during the cleaning process. The β-lactam of the cleaning solution is present completely unchanged or as an uncyclized form. Moreover, disposal of β-lactam cleaning solution reaches the river, ocean, agricultural lands, landfills and fish farms via municipal sewage [21,22,23]. Many cases were reported with allergic reactions after the consumption of foods and drugs containing antibiotic residues in the literature [24]. Thus, the monitoring of β-lactam compounds in a cleaning solution of drug production manufacturing facilities is essential. The current FDA’s guidance, non-penicillin beta-lactam drugs [25], directed a test method of 1965 that was not sufficiently sensitive [26].
Many sophisticated and laborious analytical techniques have been utilized for the determination of β-lactam, involving screening methods which include microbial inhibition, radioimmunoassay, enzyme-linked immunosorbent, bioluminescent immunoassay and biosensors [27,28,29,30]. For instance, enzyme immunoassay is conventionally used for the screening of antibiotic residue including aflatoxin M1 or melamine [31]. Apart from this, molecularly imprinted polymer nanoparticles and other metal and metal oxide nanoparticles have been used for the detection and removal of hazardous antibiotic pollutants by enhancing surface area, fast binding kinetics, binding capacity and stability [32,33]. In particular, electrochemical sensing of antibiotics based on various nanomaterials has widely been reported in the current literature [34,35,36]. Unfortunately, using MIP nanoparticles still has a major obstacle, namely inefficient recovery via filtration (significant loss of materials) and centrifugation (time-consuming and laborious). However, they lack specificity and are suitable only when qualitative information is desired. Spectroscopic methods are another alternative method, but the absence of chromophores in β-lactam antibiotics makes them insignificant to UV absorbance; hence, this method needs a suitable derivatization to obtain color [37,38,39]. In addition, many of these methods were developed using conventional high-performance liquid chromatography (HPLC) with UV detection and suffer from a long analysis time [40,41,42]. LC-MS/MS methods were also reported to determine the antibiotics in drug products [43,44] and drug manufacturing surfaces [44]. However, these methods were not developed to determine β-lactam compounds of Cephapirin and Ceftiofur for trace level. Therefore, the development of an easy, rapid, and accurate multi-residue analytical method involving ultra-high performance liquid chromatographic technique is highly required. For example, the eco-friendly QuEChERS technique is broadly applied for the identification of antibiotic residues in various materials including food and other agricultural products [45]. For example, Li et al. have developed a multi-residue analytical technique of QuECHERS in combination with UHPLC-MS/MS to detect several β-lactam antibiotics in aquaculture products [46]. In this perspective, there is a need to develop a sensitive and reproducible method to assess the content of the Cephapirin and Ceftiofur in the solution obtained from the cleaning of reactors used for manufacturing of Ceftiofur free acid, Ceftiofur Hydrochloride, Ceftiofur Sodium, Cephapirin sodium, and Cephapirin Benzathine (Figure 1).
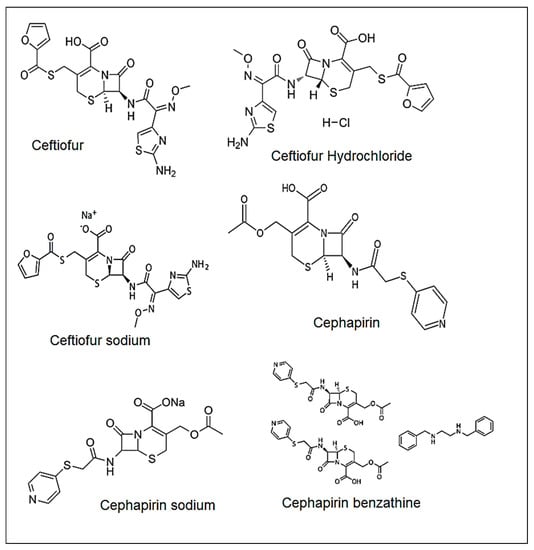
Figure 1.
Structures of Ceftiofur, Ceftiofur Hydrochloride, Ceftiofur Sodium, Cephapirin, Cephapirin sodium, and Cephapirin Benzathine.
In this present work, we developed a precise, reproducible, and rapid ultrahigh-performance liquid chromatography (UHPLC-MS/MS) method to determine traces of β-lactams in cleaning solutions of the production reactors to prevent the occurrence of cross contaminations in the reactor (Scheme 1).
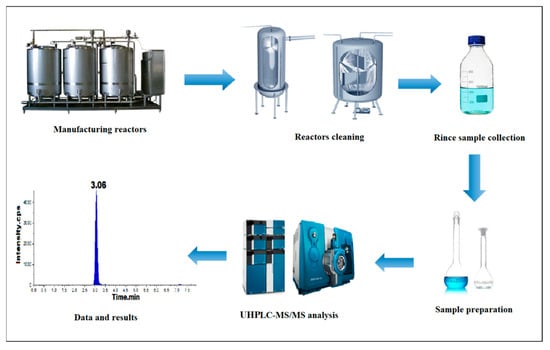
Scheme 1.
Process flow of cleaning and analysis.
2. Results and Discussion
2.1. Optimization of Mass Spectrometric Parameters
Mass parameters optimization can play a critical role in method development. Interpretation and selection of mass fragments play a key role in the identification and analysis in ppb level impurities analysis. Mass tuning was performed for Cephapirin and Ceftiofur to identify Q1 and Q3 values. Mass tuning was performed by using different ion sources such as atmospheric pressure chemical ionization (APCI) positive, APCI negative and ESI positive, and ESI negative. The m/z 424.0 > 320.0 (for qualification), m/z 424.0 > 292.0 (for quantification) of Cephapirin, and transition ion pairs of m/z 523.8 > 285.0 (for qualification), m/z 523.8 > 241.1 (for quantification) of Ceftiofur MRM mode with ESI ion source and positive ion polarity were finalized; other mass parameters are DP 40, EP 10, CE 22, 25, and CAD medium; GS1 and GS2 are the nebulizer gas 45 and MS temperature 400 °C. The solubility of analytes is checked by using mass-compatible solvents like water, methanol, and acetonitrile. Cephapirin and Ceftiofur are soluble in water, and mass fragments were identified.
2.2. Optimization of Chromatographic Conditions
Chromatographic conditions are to be established by using different mass compatible solvents and buffers. Different volatile acidic and basic buffers were used—for example, using ammonia and formic acid with the combination of different solvents like methanol and acetonitrile as mobile phase, different HPLC column chemistries (C8, C18, phenyl), and different column lengths (250 mm, 150 mm, 100 mm, and 50 mm) and different particle size (5 µm and 3.5 µm). Finally, the method was optimized by using 0.15% formic acid in water and acetonitrile as a mobile phase-A and mobile phase-B, with a gradient program and flow rate of 0.6 mL/min by using an XBridge C18 column with 100 mm length, 4.6 mm diameter and 3.5 µm particle size; column temperature is about 40 °C. Cephapirin and Ceftiofur response and ionizations are very good by using the above chromatographic and mass conditions, and retention time was found to be about 3 and 4.4 min.
2.3. Method Validation Study
To prove that the method is capable of its intended use, the developed method for the quantification of β-lactam antibiotic traces was validated. The final method was validated in line with ICH guidelines [47]. The validation parameters are system suitability and specificity, limit of detection (LOD), limit of quantification (LOQ), LOQ precision, linearity, method precision, intermediate precision, accuracy, solution stability, and robustness.
2.4. Specificity and System Suitability
As a validation process to ensure the identity of each analyte and analyte retention time, system suitability is performed to check the analyte reproducible response and system efficiency. Specificity followed by system stability was performed, a blank sample was injected, the sample was spiked, an individual standard was prepared in the diluent, and 1 ppb concentration of standards was prepared in each dilution. The peak area percentage relative standard deviation (RSD (%)) of the standard is within the limit, and no interference was observed at the retention time (RT) of Cephapirin and Ceftiofur. The retention times of both the analyte peaks in a spiked sample, standard and individual analytes are eluted at the same retention times. Therefore, this method is specific (Table 1) (Figure 2).

Table 1.
Summary of method validation results.
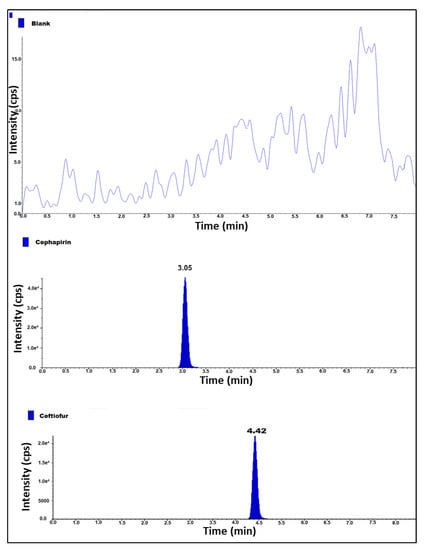
Figure 2.
MS/MS chromatogram of blank, cephapirin and ceftiofur standard solution.
2.5. LOD, LOQ and LOQ Precision
Establish a limit of detection (LOD) and limit of quantification (LOQ) by injecting diluted standard solutions, while taking the known concentration of the Cephapirin and Ceftiofur in triplicate, the final concentrations of LOD and LOQ with respect to sample concentration are 0.15 ppb and 0.4 ppb, and the signal-to-noise ratio (S/N) is equal to or greater than 3 for LOD solutions and is equal to or greater than 10 for LOQ solutions. LOQ precision was performed by injecting six replicate injections of LOQ solution. Based on the results, the s/n ratio was greater than 3 for LOD and 10 for LOQ solutions. The area RSD (%) for six replicate injections of LOQ precision is 1.7 and 2.1 for Cephapirin and Ceftiofur (Table 1) (Figure 3).
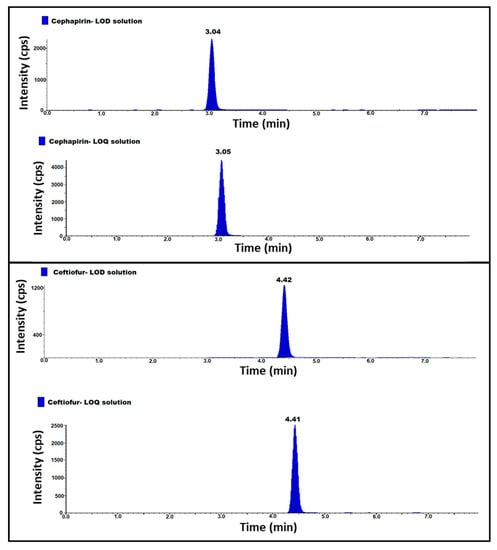
Figure 3.
MS/MS LOD and LOQ chromatogram of cephapirin and ceftiofur standard solutions.
2.6. Linearity and Range
The linearity was established from LOQ to a 150% concentration of cephapirin and ceftiofur (0.4 ppb, 0.5 ppb, 1 ppb, 1.2 ppb, and 1.5 ppb) with respect to sample concentration. Five different known concentrations of LOQ, 50%, 100%, 120%, and 150% are injected in duplicate. The linearity graph peak responses plotted against peak concentrations of cephapirin and ceftiofur evaluated the square of the correlation coefficient (r2) and found 0.999 for both of the analytes. Hence, the method was linear (Table 1).
2.7. Method Precision
The method precision (MP) was established by using a sample. Six samples were prepared as such, and six samples were prepared by spiking each 1.0 ppb of cephapirin and ceftiofur at the specification level and injecting all the solutions. For each preparation, one injection was given to determine the presence of analytes in as such sample’s reproducibility, spiked sample analytes content reproducibility and RSD (%) for the content of cephapirin and ceftiofur.
As such, the samples do not have any content and the reproducibility of spiked solutions’ content results are repeatable, the obtained content RSD (%) of the spiked solution is 1.0 and 0.6 for cephapirin and ceftiofur peaks. Hence, this method was precise and repeatable (Table 2).

Table 2.
Method validation results summary.
2.8. Intermediate Precision
The intermediate precision (IP) was established by repeating MP parameters with different analysts, different days and different lots of columns. The content and RSD (%) of the impurity were determined in sample and spike solutions. As such, the sample solutions do not have the impurity content. The spiked sample solutions (n = 6) having RSD (%) were 1.1 and 1.0 for cephapirin and ceftiofur peaks. RSD (%) for preparations (n = 12) of MP and IP spiked sample at specification levels less than 20.0. From the results, the method was rugged. (Table 2).
2.9. Accuracy
The accuracy was established by spiking cephapirin and ceftiofur into the sample in the range of LOQ to 150% level concentration. The solutions were prepared by spiking cephapirin and ceftiofur into the sample at LOQ, 50%, 100% and 150% (0.4 ppb, 0.5 ppb, 1.0 ppb, and 1.5 ppb concentrations). Each level was prepared in triplicate, and each level was given a single injection. Determine the %recovery of analytes content from spiked sample solutions. The % recovery was observed between 80% to 120% for all the recovery levels. Hence, the method was accurate (Table 2).
2.10. Robustness
The robustness parameter is used to confirm the ability of the method when slight changes are applied to the final method. By changing the column flow rate plus (+) flow 0.7 mL/min, minus (−) flow 0.5 mL/min and column oven temperature changes to plus (+) column oven temperature at 42 °C and minus (−) column oven temperature at 38 °C, results are compared with the standard and 1.0 ppb spike solution at specification levels of method precision (MP) for retention time (RT) and concentration of cephapirin and ceftiofur. The % difference of cephapirin and ceftiofur content between the results obtained in the method precision and robustness study is less than 5%, and the retention time variation of the analyte ≤ 0.5 min (Table 2).
2.11. Solution Stability
Stability studies were performed using a secondary intermediate stock solution of cephapirin, ceftiofur and spiked samples with cephapirin and ceftiofur at 100% concentration levels up to 48 h at ambient laboratory temperature (25 ± 5 °C) and refrigerated condition (2–8 °C). The percent recoveries of primary standard solutions of cephapirin and ceftiofur and spiked samples subjected to stability studies were calculated by comparing them against the freshly prepared primary standard solutions of cephapirin and ceftiofur (Table 2).
Ultra-performance liquid chromatography with triple quadrupole mass spectrometry is a powerful analytical technique for highly specific and quantitative measurements of very low levels of analytes and impurities determination in the pharmaceutical industry. An optimized UHPLC–MS/MS method was developed to determine the cephapirin and ceftiofur content in the cleaning rinse solution of the common reactor in pharmaceutical manufacturing facilities. Since molecular mass is more specific for each compound, no interferences were observed at the retention time of the analyte due to other drug substances or blank. An advantage of this method is the detection of cephapirin and ceftiofur in ppb levels, whereas the reported methods [27,28,29,30,37,38,39,40,41,42,43,44,47] like HPLC, UV Spectrophotometric and LC-MS methods are silent about the content and determination of the cephapirin and ceftiofur. The developed method is simple and direct and lacks any other derivatization process required. The method has the following advantages over the other methods reported. Detection using UHPLC–MS/MS would be a more sensitive and reproducible approach; the proposed method has indicated high accuracy and precision results found during the validation study. The sensitivity was evaluated by the limit of quantification. The LOQ was determined to be 0.4 ppb. This method is as good or superior to that reported in the other papers. Apart from pharmaceuticals, this type of high-performance analytical technique can be used to detect other toxic secondary metabolites generated by the plant and other microbial sources [48,49].
3. Material and Methods
3.1. Materials and Reagents
Reactor rinsing solutions, Cephapirin, and Ceftiofur have been procured from Jisai Pharma Pvt Ltd. (Hyderabad, India). All chemicals and solvents were of analytical grade, Formic acid and acetonitrile and methanol have been procured from Fischer Chemicals and J.T Baker (Mumbai, India). Water for HPLC grade has been procured from Rankem® (Tiruppur, India) and used for the preparation of all buffers and standard solutions.
3.2. Equipment
The traces of β-lactams were determined using a UHPLC system connected with triple quadrupole QTRAP MS/MS equipped with electrospray ionization (ESI) probe make; ABsciex QTRAP 4500 has been used for method development and validation. Analyst software was used to collect and analyze data. For standards and sample weighing, a Mettler Toledo analytical balance was used.
3.3. Chromatographic Conditions
Chromatographic conditions were finalized by considering both analytes, based on method development data. The quantification of the compound was achieved with a C18 column (100 × 4.6 mm, 3.5 µm particle size) at an oven temperature of 40 °C. The mobile phase was composed of 0.15% formic acid in water, and acetonitrile as a mobile phase A and B, and a flow rate was set to 0.6 mL/min and deployed the ‘gradient elution program’, which gave the best response within the shortest and acceptable analysis time and column back pressure. The injection volume was 50 µL.
3.4. Mass Spectrometer Conditions
The MS/MS detector is highly sensitive and reproducible. The MS detector was operated with electrospray ionization (ESI), which is selected as a positive ion source, and multiple reaction monitoring (MRM) selected transition ion pairs of m/z 424.0 > 320.0 (for qualification) and m/z 424.0 > 292.0 (for quantification) of Cephapirin (Figure 4). It also selected transition ion pairs of m/z 523.8 > 285.0 (for qualification) and m/z 523.8 > 241.1 (for quantification) of Ceftiofur (Figure 5). De-clustering potential (DP 40), entrance potential (EP 10) and MS temperature of 400 °C were used as MS/MS detector conditions. Final liquid chromatographic and mass spectrometric method conditions were tabulated. (Table 3).
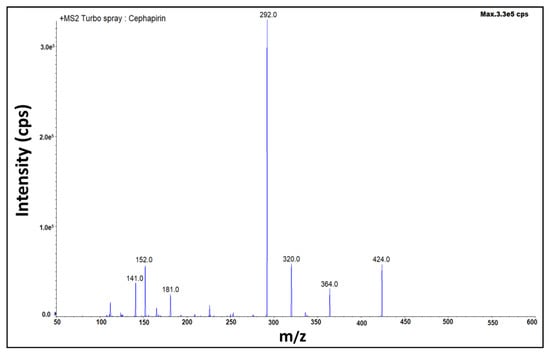
Figure 4.
Fragmentation pattern of Cephapirin.

Figure 5.
Fragmentation pattern of Ceftiofur.

Table 3.
Liquid chromatographic and mass spectrometric method conditions.
3.5. Preparation of Standard and Test Sample Solutions
Standard and sample concentrations were finalized by using required dilutions, based on the response of impurities during the study by using water as a diluent. Preparation of 1 ppb concentration of Cephapirin and Ceftiofur standards in water used the required dilutions. Directly inject reactor rinse solutions without any dilution. The sample solution was filtered by using a 0.45 µm nylon filter and injected water as a blank. Supplementary chromatograms of the method validation study are provided in Supplementary Files (Figures S1–S14).
4. Conclusions
The sensitive, selective, and rapid UHPLC-MS/MS method developed for the identification and quantification of cephapirin and ceftiofur in reactor rinse samples is very sensitive and detects very trace level concentrations. Thus, this new method with advanced technology is capable of the identification and detection of cephapirin and ceftiofur in ppb levels. Considering industrial and guidelines requirements, the method was validated in line with ICH and USP. The method is specific, linear, precise, accurate, rugged and robust. The results of this method demonstrated that reliable data can be obtained in further experiments such as Ceftiofur free acid, Ceftiofur Hydrochloride, Ceftiofur Sodium, Cephapirin sodium, and Cephapirin Benzathine reactor cleaning, and manufacturing aids rinse samples analysis. Therefore, when compared to instrumental analytic techniques and other immunoassays, the method developed in this study can simultaneously detect several structurally different analytes in reactor rinse samples with the advantage of rapidity, low cost and high sensitivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227920/s1, Figure S1: MS/MS chromatogram of Cephapirin system suitability standard solution; Figure S2: MS/MS chromatogram of Ceftiofur system suitability standard solution; Figure S3: MS/MS chromatogram of Cephapirin LOD solution; Figure S4: MS/MS chromatogram of Cephapirin LOQ solution; Figure S5: MS/MS chromatograms of Cephapirin LOQ Precision; Figure S6: MS/MS chromatogram of Ceftiofur LOD solution; Figure S7: MS/MS chromatogram of Ceftiofur LOQ Precision; Figure S8: MS/MS chromatogram of Ceftiofur Linearity; Figure S9: MS/MS chromatograms of Cephapirin Method precision; Figure S10: MS/MS chromatograms of Ceftiofur Method precision; Figure S11: MS/MS chromatograms of Cephapirin Intermediate precision; Figure S12: MS/MS chromatograms of Ceftiofur Intermediate precision; Figure S13: MS/MS chromatograms of Cephapirin Accuracy; Figure S14: MS/MS chromatograms of Ceftiofur Accuracy.
Author Contributions
Conceptualization, H.N.P.R.C. and J.V.S.K.; methodology, H.N.P.R.C. and J.V.S.K.; formal analysis, M.R.S., A.H.S., A.A. and B.S.; investigation, H.N.P.R.C., J.V.S.K. and M.R.S.; resources, H.N.P.R.C. and J.V.S.K.; data curation, H.N.P.R.C., J.V.S.K. and M.R.S.; writing—original draft preparation, H.N.P.R.C., J.V.S.K. and A.B.; writing—review and editing, H.N.P.R.C. and M.R.S.; supervision, J.V.S.K.; project administration, J.V.S.K.; funding acquisition, A.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Program through the Researchers Supporting Project No. (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained within the article and Supplementary File.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Program for funding this work through Researchers Supporting Project No. (RSP-2021/371), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors on reasonable request.
References
- Fabio, P.; Alessandro, C.; Stefania, S.; Iolanda, G.; Elena, M.; Aldo, C. The requirements for manufacturing highly active or sensitising drugs comparing Good Manufacturing Practices. Acta Biomed. Ateneo Parm. 2019, 90, 288. [Google Scholar]
- Bush, K.; Bradford, P.A. β-Lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Robitaille, G.; Karam, F.; Daigle, J.-M.; Bédard, F.; Biron, É.; Tardif, M.R.; Lacombe-Barrios, J.; Bégin, P. Cross-reactivity to cephalosporins and carbapenems in penicillin-allergic patients: Two systematic reviews and meta-analyses. J. Allergy Clin. Immunol. Pract. 2019, 7, 2722–2738.e2725. [Google Scholar] [CrossRef] [PubMed]
- Macy, E.; Contreras, R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef]
- Pichichero, M.E.; Zagursky, R. Penicillin and cephalosporin allergy. Ann. Allergy Asthma Immunol. 2014, 112, 404–412. [Google Scholar] [CrossRef]
- Caubet, J.-C.; Kaiser, L.; Lemaître, B.; Fellay, B.; Gervaix, A.; Eigenmann, P.A. The role of penicillin in benign skin rashes in childhood: A prospective study based on drug rechallenge. J. Allergy Clin. Immunol. 2011, 127, 218–222. [Google Scholar] [CrossRef]
- Montañez, M.I.; Mayorga, C.; Bogas, G.; Barrionuevo, E.; Fernandez-Santamaria, R.; Martin-Serrano, A.; Laguna, J.J.; Torres, M.J.; Fernandez, T.D.; Doña, I. Epidemiology, mechanisms, and diagnosis of drug-induced anaphylaxis. Front. Immunol. 2017, 8, 614. [Google Scholar] [CrossRef]
- Gruchalla, R.S.; Pirmohamed, M. Antibiotic allergy. N. Engl. J. Med. 2006, 354, 601–609. [Google Scholar] [CrossRef]
- Song, E.; Yu, M.; Wang, Y.; Hu, W.; Cheng, D.; Swihart, M.T.; Song, Y. Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens. Bioelectron. 2015, 72, 320–325. [Google Scholar] [CrossRef]
- Ray, P.; Knowlton, K.F.; Shang, C.; Xia, K. Development and validation of a UPLC-MS/MS method to monitor cephapirin excretion in dairy cows following intramammary infusion. PLoS ONE 2014, 9, e112343. [Google Scholar] [CrossRef]
- Llopis, B.; Funck-Brentano, C.; Tissot, N.; Bleibtreu, A.; Jaureguiberry, S.; Fourniols, E.; Aubry, A.; Zahr, N. Development and validation of a UPLC-MS/MS method for simultaneous quantification of levofloxacin, ciprofloxacin, moxifloxacin and rifampicin in human plasma: Application to the therapeutic drug monitoring in osteoarticular infections. J. Pharm. Biomed. Anal. 2020, 183, 113137. [Google Scholar] [CrossRef] [PubMed]
- Jyrkkanen, J. Antibiotic induced changes to mitochondria result in potential contributions to carcinogenesis, heart pathologies, other medical conditions and ecosystem risks. J. Cardiol. Cardiovasc. Med. 2020, 5, 163–171. [Google Scholar]
- Trejnowska, E.; Deptuła, A.; Tarczyńska-Słomian, M.; Knapik, P.; Jankowski, M.; Misiewska-Kaczur, A.; Tamowicz, B.; Śmiechowicz, J.; Antończyk, R.; Armatowicz, P. Surveillance of antibiotic prescribing in intensive care units in Poland. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 5670238. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.B.; Veve, M.P.; Wagner, J.L. Cephalosporins: A focus on side chains and β-lactam cross-reactivity. Pharmacy 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Zagursky, R.J.; Pichichero, M.E. Cross-reactivity in β-lactam allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 72–81.e71. [Google Scholar] [CrossRef] [PubMed]
- Terico, A.T.; Gallagher, J.C. Beta-lactam hypersensitivity and cross-reactivity. J. Pharm. Pract. 2014, 27, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Blanca, M. The complex clinical picture of β-lactam hypersensitivity: Penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med. Clin. 2010, 94, 805–820. [Google Scholar] [CrossRef]
- Bozcal, E.; Dagdeviren, M. Toxicity of β-lactam antibiotics: Pathophysiology, molecular biology and possible recovery strategies. In Poisoning: From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; InTechOpen: Rijeka, Croatia, 2017; pp. 87–105. [Google Scholar]
- Dickson, S.D.; Salazar, K.C. Diagnosis and management of immediate hypersensitivity reactions to cephalosporins. Clin. Rev. Allergy Immunol. 2013, 45, 131–142. [Google Scholar] [CrossRef]
- Chow, K.; Hui, A.; Szeto, C. Neurotoxicity induced by beta-lactam antibiotics: From bench to bedside. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 649–653. [Google Scholar] [CrossRef]
- Ana, K.M.S.; Madriaga, J.; Espino, M.P. β-Lactam antibiotics and antibiotic resistance in Asian lakes and rivers: An overview of contamination, sources and detection methods. Environ. Pollut. 2021, 275, 116624. [Google Scholar] [CrossRef]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Olatoye, I.O.; Daniel, O.F.; Ishola, S.A. Screening of antibiotics and chemical analysis of penicillin residue in fresh milk and traditional dairy products in Oyo state, Nigeria. Vet. World 2016, 9, 948–954. [Google Scholar] [CrossRef]
- Food, U.; Administration, D. Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 2013.
- Carter, G.G. A Review of Procedures for the Detection of Residual Penicillins in Drugs; National Center for Antibiotics Analysis, Bureau of Drugs, FDA, BY-Lines: Silver Spring, MD, USA, 1977; Volume 8.
- Okerman, L.; De Wasch, K.; Van Hoof, J. Detection of antibiotics in muscle tissue with microbiological inhibition tests: Effects of the matrix. Analyst 1998, 123, 2361–2365. [Google Scholar] [CrossRef]
- Kricka, L.J. Selected strategies for improving sensitivity and reliability of immunoassays. Clin. Chem. 1994, 40, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hujer, A.M.; Page, M.G.; Helfand, M.S.; Yeiser, B.; Bonomo, R.A. Development of a sensitive and specific enzyme-linked immunosorbent assay for detecting and quantifying CMY-2 and SHV β-lactamases. J. Clin. Microbiol. 2002, 40, 1947–1957. [Google Scholar] [CrossRef]
- Petrişor, C.; Gherman-Ionică, N.; Bologa, R.; Sfichi, M.; Hagău, N. Basophil activation test versus radio-immunoassay in the diagnosis of β-lactam immediate-type hypersensitivity reactions. Rev. Romana Med. Lab. 2013, 21, 415–422. [Google Scholar] [CrossRef][Green Version]
- Han, M.; Gong, L.; Wang, J.; Zhang, X.; Jin, Y.; Zhao, R.; Yang, C.; He, L.; Feng, X.; Chen, Y. An octuplex lateral flow immunoassay for rapid detection of antibiotic residues, aflatoxin M1 and melamine in milk. Sens. Actuators B Chem. 2019, 292, 94–104. [Google Scholar] [CrossRef]
- Kuhn, J.; Aylaz, G.; Sari, E.; Marco, M.; Yiu, H.H.; Duman, M. Selective binding of antibiotics using magnetic molecular imprint polymer (MMIP) networks prepared from vinyl-functionalized magnetic nanoparticles. J. Hazard. Mater. 2020, 387, 121709. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Kavetskyy, T.; Khosroushahi, A.Y.; Turksoy, V.A.; Khalilov, R. Effects of quercetin loaded nanostructured lipid carriers on the paraquat-induced toxicity in human lymphocytes. Pestic. Biochem. Physiol. 2020, 167, 104586. [Google Scholar] [CrossRef]
- Joshi, A.; Kim, K.-H. Recent advances in nanomaterial-based electrochemical detection of antibiotics: Challenges and future perspectives. Biosens. Bioelectron. 2020, 153, 112046. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Arjmand, A.; Asheghvatan, A.; Švajdlenková, H.; Šauša, O.; Abiyev, H.; Ahmadian, E.; Smutok, O.; Khalilov, R.; Kavetskyy, T. The potential application of magnetic nanoparticles for liver fibrosis theranostics. Front. Chem. 2021, 9, 674786. [Google Scholar] [CrossRef]
- Ahmadian, E.; Janas, D.; Eftekhari, A.; Zare, N. Application of carbon nanotubes in sensing/monitoring of pancreas and liver cancer. Chemosphere 2022, 302, 134826. [Google Scholar] [CrossRef] [PubMed]
- Verdier, M.-C.; Tribut, O.; Tattevin, P.; Le Tulzo, Y.; Michelet, C.; Bentué-Ferrer, D. Simultaneous determination of 12 β-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: Application to therapeutic drug monitoring. Antimicrob. Agents Chemother. 2011, 55, 4873–4879. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G. Spectrophotometric determination of ampicillin, dicluxacillin, flucloxacillin and amoxicillin antibiotic drugs: Ion-pair formation with molybdenum and thiocyanate. J. Pharm. Biomed. Anal. 2001, 24, 561–567. [Google Scholar] [CrossRef]
- Salem, H.; Saleh, G.A. Selective spectrophotometric determination of phenolic β-lactam antibiotics. J. Pharm. Biomed. Anal. 2002, 28, 1205–1213. [Google Scholar] [CrossRef]
- Denooz, R.; Charlier, C. Simultaneous determination of five β-lactam antibiotics (cefepim, ceftazidim, cefuroxim, meropenem and piperacillin) in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. B 2008, 864, 161–167. [Google Scholar] [CrossRef]
- Nemutlu, E.; Kır, S.; Katlan, D.; Beksac, M.S. Simultaneous multiresponse optimization of an HPLC method to separate seven cephalosporins in plasma and amniotic fluid: Application to validation and quantification of cefepime, cefixime and cefoperazone. Talanta 2009, 80, 117–126. [Google Scholar] [CrossRef]
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B 2010, 878, 2039–2043. [Google Scholar] [CrossRef]
- Bekoe, S.O.; Bak, S.A.; Björklund, E.; Krogh, K.A.; Okine, N.N.; Adosraku, R.K.; Styrishave, B.; Hansen, M. Determination of thirteen antibiotics in drug products–A new LC-MS/MS tool for screening drug product quality. Anal. Methods 2014, 6, 5847–5855. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, H.; Ruzicka, C.; Keire, D.; Ye, H. A general LC-MS/MS method for monitoring potential β-lactam contamination in drugs and drug-manufacturing surfaces. AAPS J. 2018, 20, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Quantification of 16 β-lactams in chicken muscle by QuEChERS extraction and UPLC-Q-Orbitrap-MS with parallel reaction monitoring. J. Pharm. Biomed. Anal. 2017, 145, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Y.; Zheng, G.; Liu, S.; Zhao, C.; Ma, L.; Shan, Q.; Dai, X.; Wei, L.; Lin, J. Determining β-lactam antibiotics in aquaculture products by modified QuECHERS combined with ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Arab. J. Chem. 2022, 15, 103912. [Google Scholar] [CrossRef]
- Guideline, I.H.T. Validation of Analytical Procedures: Text and Methodology. Q2 2005, 1, 05. [Google Scholar]
- Vaclavik, L.; Krynitsky, A.J.; Rader, J.I. Targeted analysis of multiple pharmaceuticals, plant toxins and other secondary metabolites in herbal dietary supplements by ultra-high performance liquid chromatography–quadrupole-orbital ion trap mass spectrometry. Anal. Chim. Acta 2014, 810, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Jaderson, M.; Park, J.-H. Evaluation of matrix effects in quantifying microbial secondary metabolites in indoor dust using ultraperformance liquid chromatograph–tandem mass spectrometer. Saf. Health Work 2019, 10, 196–204. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).