Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms †
Abstract
1. Introduction
1.1. SMCs and Atherosclerosis
1.2. Nitric Oxide (NO) and Atherosclerosis
1.3. NO and SMC Vasodilation and Proliferation
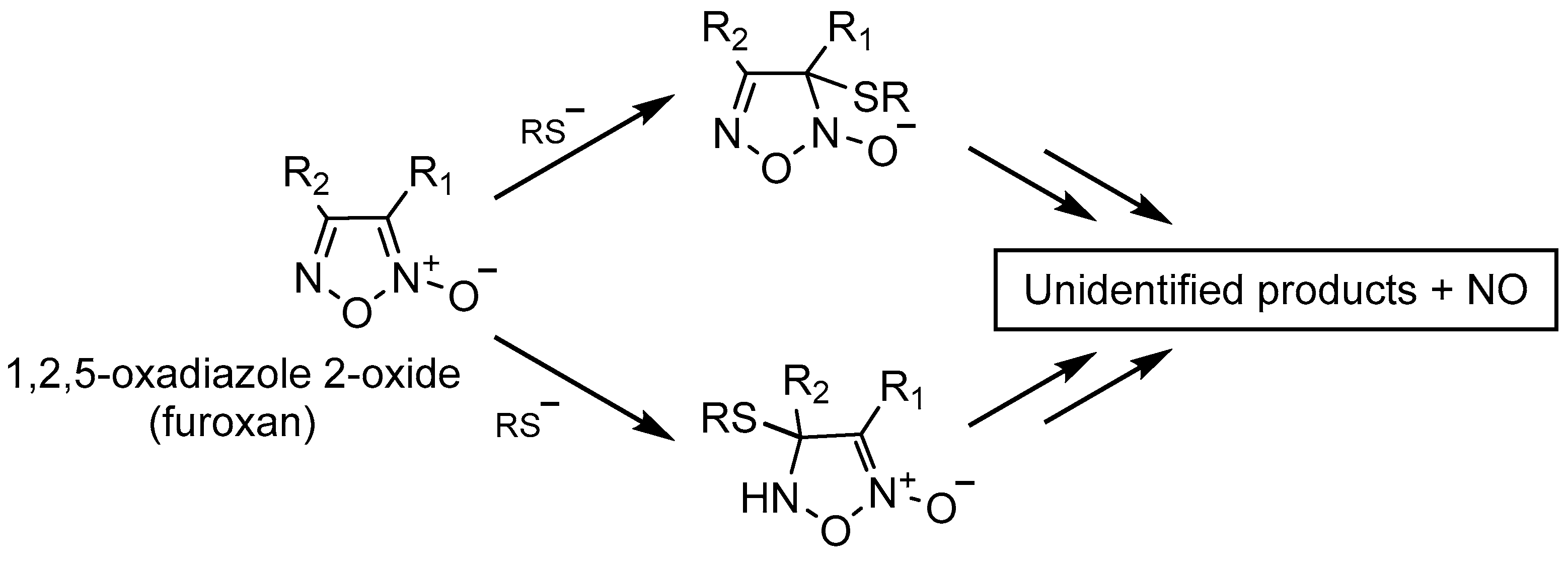
1.4. NO Donors
- (a)
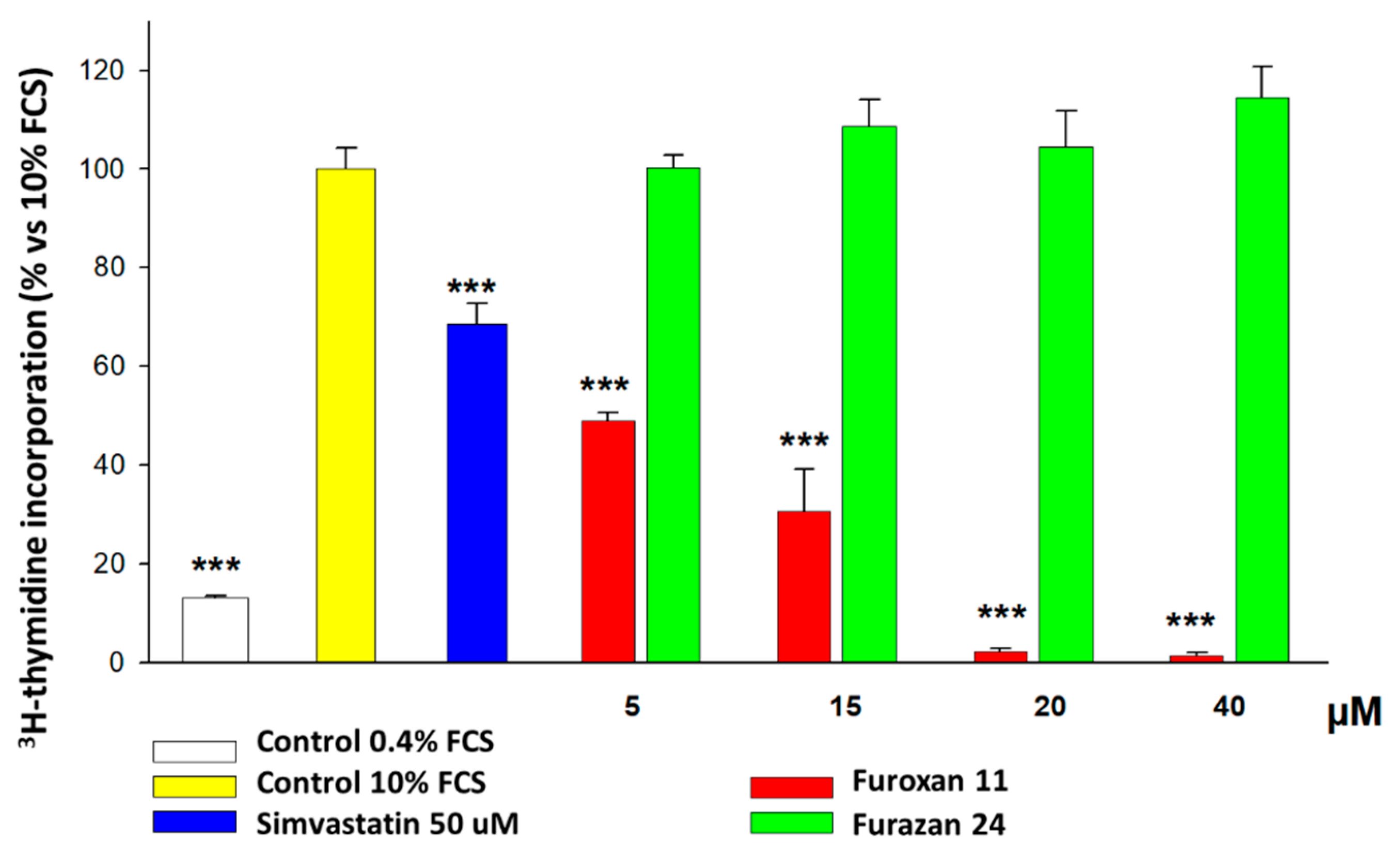
- We ran preliminary screening on the possible antiproliferative effects exerted by different NO donors and, among these furoxans, on human and rat vascular SMCs. After determining that furoxans are really effective, to understand the mechanism of action of this effect, we tested whether their ring-opening abilities and the nature of the substituent in position 3 may affect these properties. To address this point, we also tested furazans (compounds structurally and chemically related to furoxans devoid of NO-releasing properties).
- (b)
- We also aimed at comprehending whether these molecules may possess a phase-specific effect on the cell cycle, since it is known that NO causes a block in the G1-S phase [83]. For this purpose, we utilized the incorporation assay of radiolabeled thymidine in the DNA of rat aorta SMCs.
- (c)
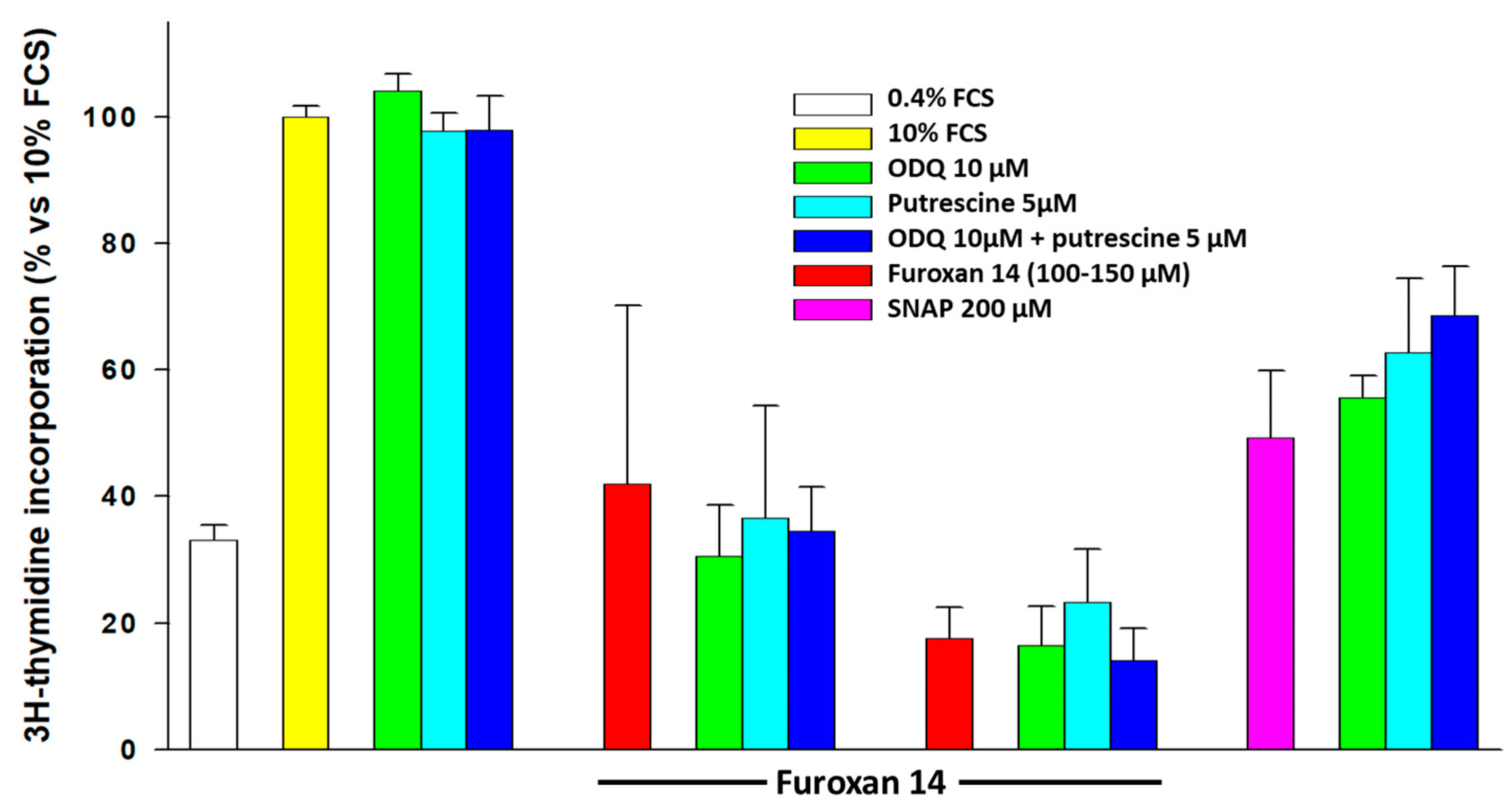
- In order to understand whether NO mediates the antiproliferative effect, we organized a series of experiments in which we co-incubated furoxans with 1H-[1,2,4]oxadiazole[4,3-α]quinoxalin-1-one (ODQ) and/or putrescine, thanks to their effect on the two known pathways by which NO exerts its antiproliferative effects on SMCs.
- (d)
- Finally, we used a proteomic approach (SILAC, Western blot, and MetaCore) to find proteins targeted by furoxans and to unravel the pathways and molecular mechanism(s) underlying their pharmacological effect on SMC proliferation.
2. Results
2.1. Effect of Different NO Donors on NO Release, SMC Proliferation, and Possible Mechanisms
2.2. Cell-Phase-Specific Antiproliferative Effect of Furoxans
2.3. Effect of Intermediates of the Known NO-Dependent Pathways Regulating SMC Proliferation
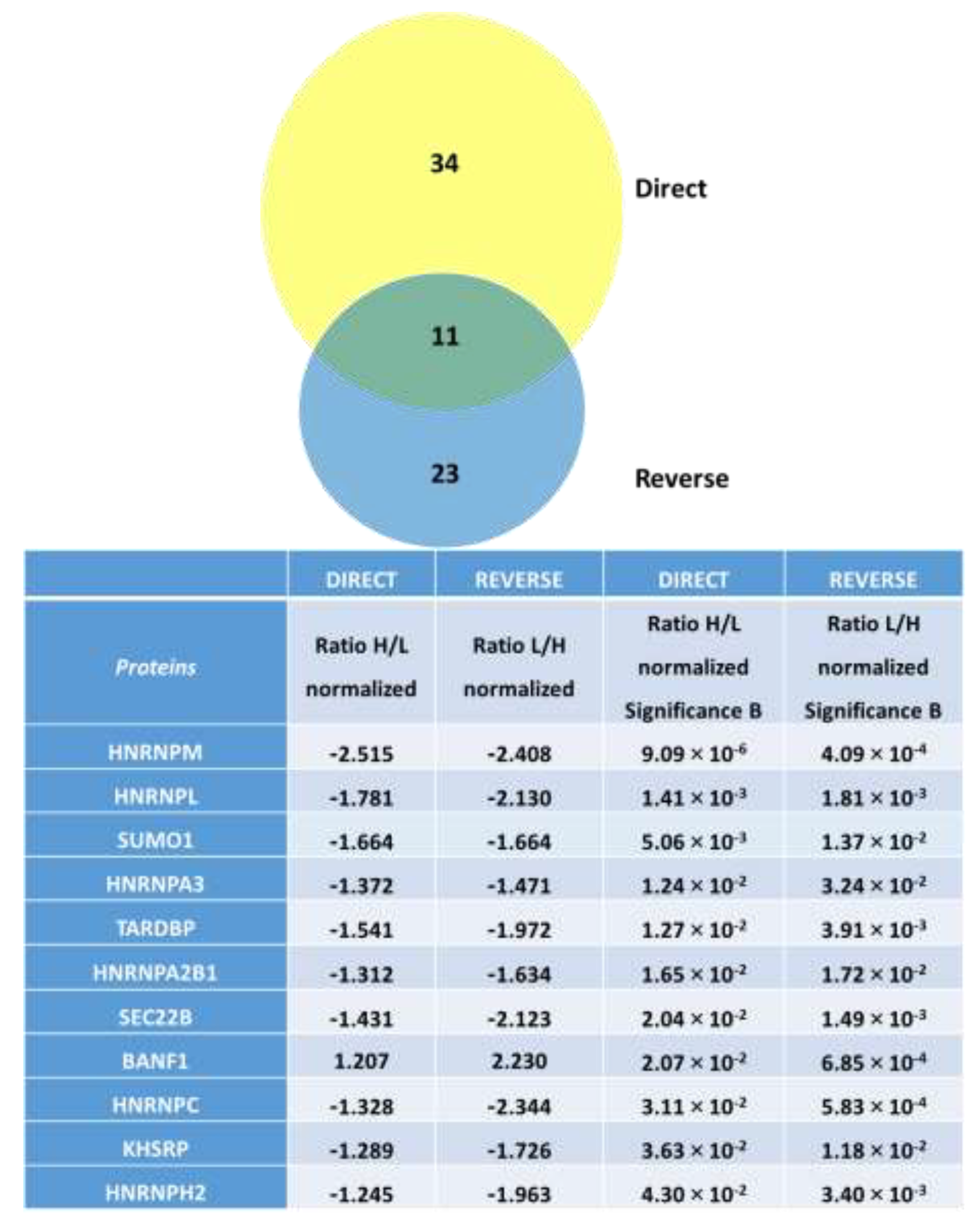
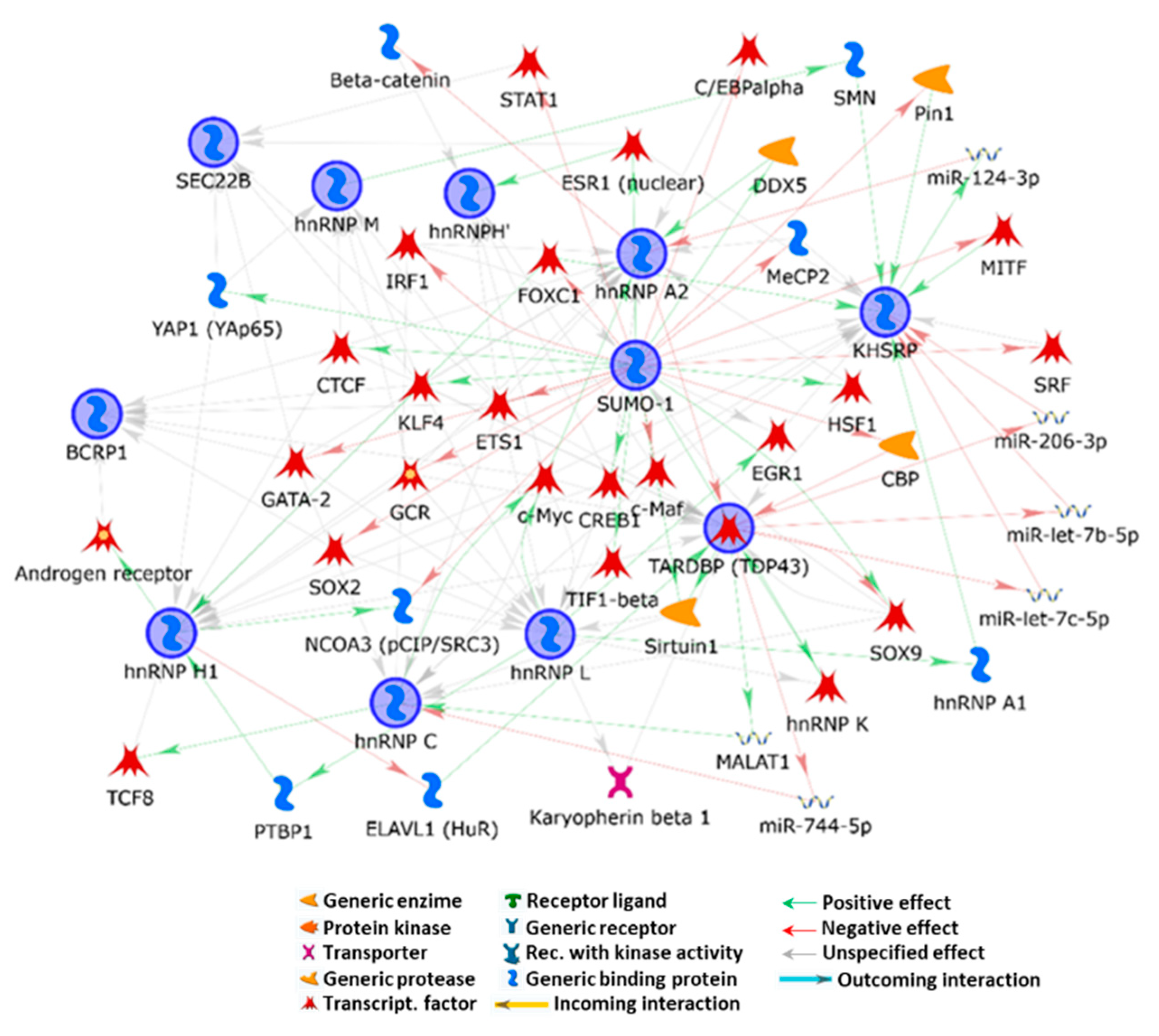
2.4. Effect of Furoxans on Cellular Protein Expression by SILAC and MetaCore
3. Discussion
- (a)
- That upon thiol-mediated activation, furoxans time- and dose-dependently generate NO (Figures S1 and S2);
- (b)
- The ability of all furoxans, despite different potencies, but not of furazans, to inhibit SMC proliferation;
- (c)
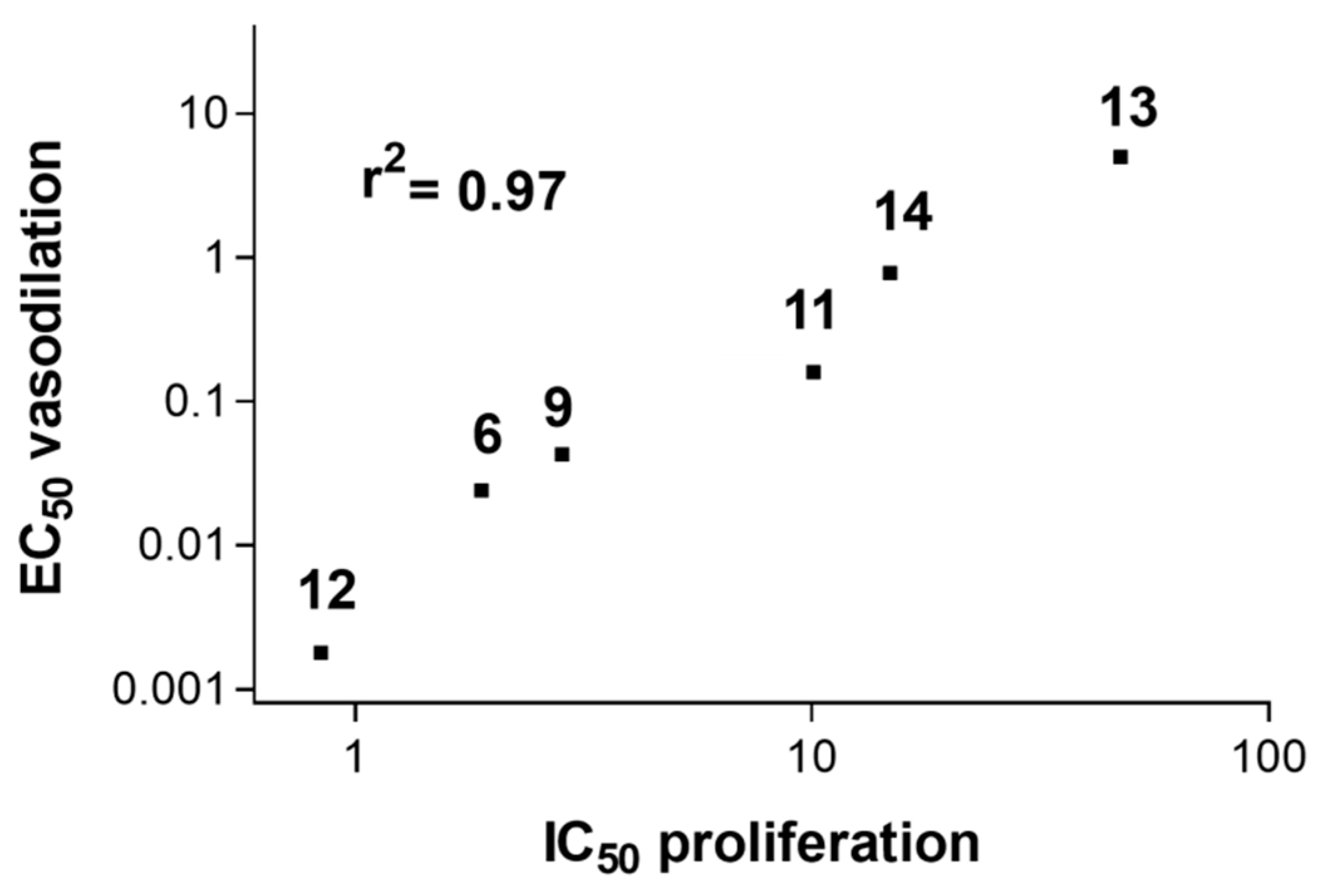
- A significant strong (r2 = 0.97) direct correlation (Figure 2) between the reduction in SMC growth and a NO-dependent vasodilating effect on isolated rat aorta stripes exerted by furoxans;
- (d)
- (a)
- The inhibitor of soluble guanylyl cyclase, ODQ, to deplete cells from cGMP;
- (b)
- Putrescine, a product of ornithine decarboxylase, the enzyme inhibited by NO eventually released by furoxans;
- (c)
- Their association.
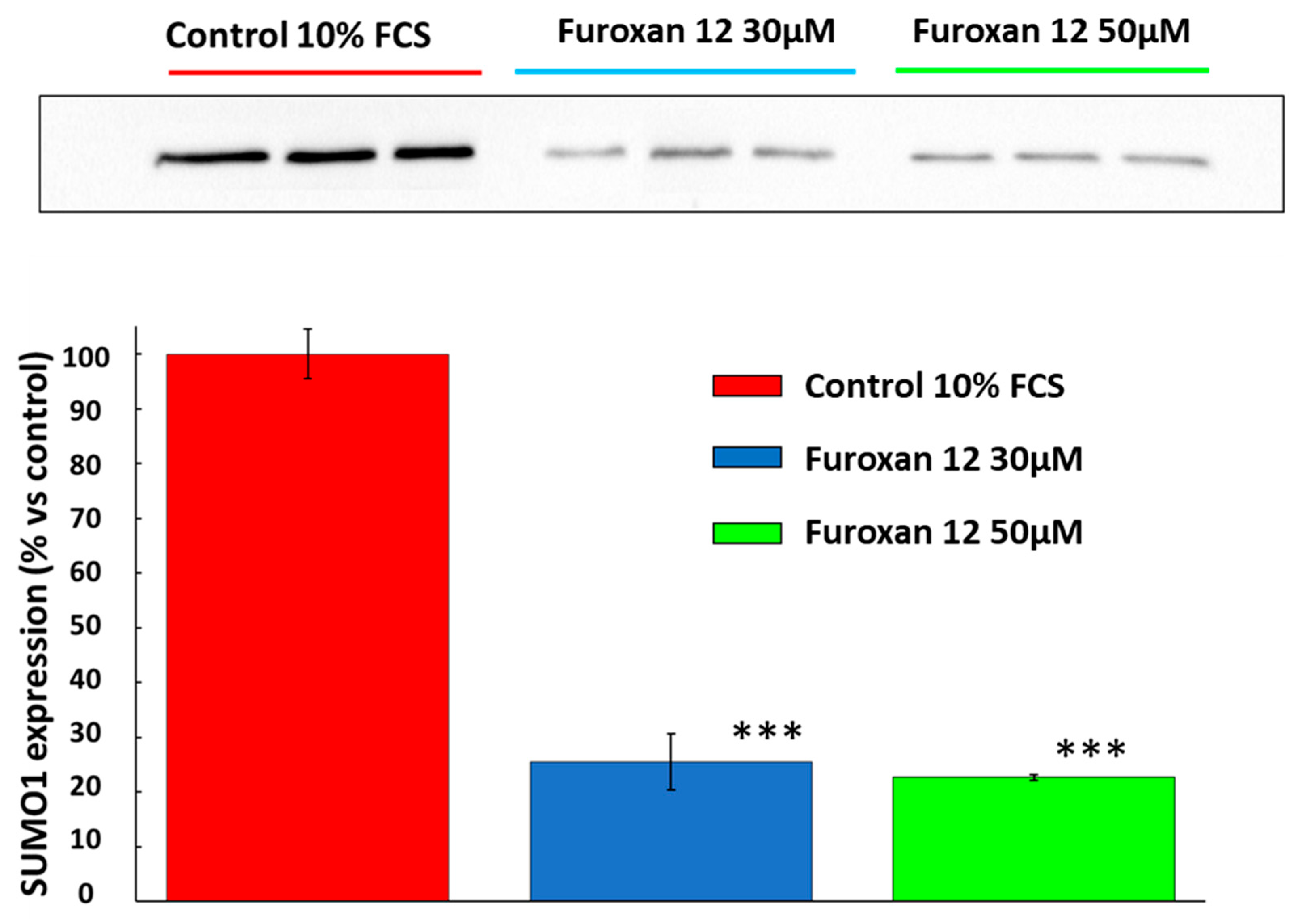
3.1. Small Ubiquitin-Related Modifier 1 (SUMO-1)
3.2. BANF1 and Vascular SMC Mechanical Stress
3.3. Furoxan Modulation of Coding and Non-Coding RNA-Binding Proteins and VSMC Proliferation
3.4. Furoxan Treatment Implications in Vesicle Trafficking
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of the Study Compounds
4.3. Reactivity and NO Release of 3-cyano-4-phenyl Furoxan (Compound 12) and 3-phenylsulfonyl-4-ethoxy Furoxan (Compound 5)
4.4. Cells and Cellular Protocols
4.5. Electrophoresis and Western Blotting
4.6. Protein Evaluation by SILAC and Mass Spectrometry
4.7. Proteomics
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Raines, E.W. The Extracellular Matrix Can Regulate Vascular Cell Migration, Proliferation, and Survival: Relationships to Vascular Disease. Int. J. Exp. Pathol. 2000, 81, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Tabas, I. The Response-to-Retention Hypothesis of Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease: Pathophysiological, Genetic, and Therapeutic Insights: A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Dzau, V.J.; Braun-Dullaeus, R.C.; Sedding, D.G. Vascular Proliferation and Atherosclerosis: New Perspectives and Therapeutic Strategies. Nat. Med. 2002, 8, 1249–1256. [Google Scholar] [CrossRef]
- Iyemere, V.P.; Proudfoot, D.; Weissberg, P.L.; Shanahan, C.M. Vascular Smooth Muscle Cell Phenotypic Plasticity and the Regulation of Vascular Calcification. J. Intern. Med. 2006, 260, 192–210. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting Inflammation in Atherosclerosis—From Experimental Insights to the Clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Luo, X.; Lv, Y.; Bai, X.; Qi, J.; Weng, X.; Liu, S.; Bao, X.; Jia, H.; Yu, B. Plaque Erosion: A Distinctive Pathological Mechanism of Acute Coronary Syndrome. Front. Cardiovasc. Med. 2021, 8, 711453. [Google Scholar] [CrossRef]
- Campbell, G.R.; Campbell, J.H.; Manderson, J.A.; Horrigan, S.; Rennick, R.E. Arterial Smooth Muscle. A Multifunctional Mesenchymal Cell. Arch. Pathol. Lab. Med. 1988, 112, 977–986. [Google Scholar]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, X.; Xia, Y.; Mao, L. An Update on the Phenotypic Switching of Vascular Smooth Muscle Cells in the Pathogenesis of Atherosclerosis. Cell. Mol. Life Sci. 2022, 79, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Ikeda, U. Matrix Metalloproteinases and Atherosclerosis. Curr. Atheroscler. Rep. 2004, 6, 112–120. [Google Scholar] [CrossRef]
- Johnson, J.L. Metalloproteinases in Atherosclerosis. Eur. J. Pharmacol. 2017, 816, 93–106. [Google Scholar] [CrossRef]
- Allahverdian, S.; Chaabane, C.; Boukais, K.; Francis, G.A.; Bochaton-Piallat, M.L. Smooth Muscle Cell Fate and Plasticity in Atherosclerosis. Cardiovasc. Res. 2018, 114, 540–550. [Google Scholar] [CrossRef]
- Dubland, J.A.; Francis, G.A. So Much Cholesterol: The Unrecognized Importance of Smooth Muscle Cells in Atherosclerotic Foam Cell Formation. Curr. Opin. Lipidol. 2016, 27, 155–161. [Google Scholar] [CrossRef]
- Xiang, P.; Blanchard, V.; Francis, G.A. Smooth Muscle Cell-Macrophage Interactions Leading to Foam Cell Formation in Atherosclerosis: Location, Location, Location. Front. Physiol. 2022, 13, 921597. [Google Scholar] [CrossRef]
- Alencar, G.F.; Owsiany, K.M.; Karnewar, S.; Sukhavasi, K.; Mocci, G.; Nguyen, A.T.; Williams, C.M.; Shamsuzzaman, S.; Mokry, M.; Henderson, C.A.; et al. Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation 2020, 142, 2045–2059. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Pan, H.; Xue, C.; Auerbach, B.J.; Fan, J.; Bashore, A.C.; Cui, J.; Yang, D.Y.; Trignano, S.B.; Liu, W.; Shi, J.; et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation 2020, 142, 2060–2075. [Google Scholar] [CrossRef]
- Newman, A.A.C.; Serbulea, V.; Baylis, R.A.; Shankman, L.S.; Bradley, X.; Alencar, G.F.; Owsiany, K.; Deaton, R.A.; Karnewar, S.; Shamsuzzaman, S.; et al. Multiple Cell Types Contribute to the Atherosclerotic Lesion Fibrous Cap by PDGFRβ and Bioenergetic Mechanisms. Nat. Metab. 2021, 3, 166–181. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Finigan, A.; Figg, N.L.; Uryga, A.K.; Bennett, M.R. SIRT6 Protects Smooth Muscle Cells From Senescence and Reduces Atherosclerosis. Circ. Res. 2021, 128, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Zhang, C.; Shuja, F.; Sturmlechner, I.; Trewartha, S.; Velasco, R.F.; Baker, D.J.; Li, H.; van Deursen, J.M. Senescent Cells Suppress Innate Smooth Muscle Cell Repair Functions in Atherosclerosis. Nat. Aging 2021, 1, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the Road Ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.M.; da Silva, M.C.; Nascimento, D.V.G.; Lima Silva, E.M.; Gouvêa, F.F.F.; de França Lopes, L.G.; Araújo, A.V.; Ferraz Pereira, K.N.; de Queiroz, T.M. Nitric Oxide as a Central Molecule in Hypertension: Focus on the Vasorelaxant Activity of New Nitric Oxide Donors. Biology 2021, 10, 1041. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The Chemical Biology of Nitric Oxide: Implications in Cellular Signaling. Free Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef]
- Horton, A.; Schiefer, I.T. Pharmacokinetics and Pharmacodynamics of Nitric Oxide Mimetic Agents. Nitric Oxide 2019, 84, 69–78. [Google Scholar] [CrossRef]
- Pautz, A.; Li, H.; Kleinert, H. Regulation of NOS Expression in Vascular Diseases. Front. Biosci. 2021, 26, 85–101. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric Oxide Synthase in Innate and Adaptive Immunity: An Update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Walford, G.; Loscalzo, J. Nitric Oxide in Vascular Biology. J. Thromb. Haemost. 2003, 1, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Costa, D.; Napoli, C. Novel Insights Regarding Nitric Oxide and Cardiovascular Diseases. Angiology 2021, 72, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Heymes, C.; Benessiano, J.; Geske, R.S.; Lévy, B.I.; Vanhoutte, P.M. Neuronal Nitric Oxide Synthase Is Expressed in Rat Vascular Smooth Muscle Cells: Activation by Angiotensin II in Hypertension. Circ. Res. 1998, 83, 1271–1278. [Google Scholar] [CrossRef]
- Bachetti, T.; Comini, L.; Curello, S.; Bastianon, D.; Palmieri, M.; Bresciani, G.; Callea, F.; Ferrari, R. Co-Expression and Modulation of Neuronal and Endothelial Nitric Oxide Synthase in Human Endothelial Cells. J. Mol. Cell. Cardiol. 2004, 37, 939–945. [Google Scholar] [CrossRef]
- Brophy, C.M.; Knoepp, L.; Xin, J.; Pollock, J.S. Functional Expression of NOS 1 in Vascular Smooth Muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H991–H997. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Tsutsui, M.; Shimokawa, H.; Horiuchi, M.; Tanimoto, A.; Suda, O.; Tasaki, H.; Huang, P.L.; Sasaguri, Y.; Yanagihara, N.; et al. Vasculoprotective Roles of Neuronal Nitric Oxide Synthase. FASEB J. 2002, 16, 1994–1996. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, M.; Shimokawa, H.; Otsuji, Y.; Yanagihara, N. Pathophysiological Relevance of NO Signaling in the Cardiovascular System: Novel Insight from Mice Lacking All NO Synthases. Pharmacol. Ther. 2010, 128, 499–508. [Google Scholar] [CrossRef]
- Buttery, L.D.K.; Springall, D.R.; Chester, A.H.; Evans, T.J.; Standfield, N.; Parums, D.V.; Yacoub, M.H.; Polak, J.M. Inducible Nitric Oxide Synthase Is Present within Human Atherosclerotic Lesions and Promotes the Formation and Activity of Peroxynitrite. Lab. Investig. 1996, 75, 77–85. [Google Scholar]
- Hevel, J.M.; White, K.A.; Marletta, M.A. Purification of the Inducible Murine Macrophage Nitric Oxide Synthase: Identification as a Flavoprotein. J. Biol. Chem. 1991, 266, 22789–22791. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Fukai, T.; Siegfried, M.R.; Ushio-Fukai, M.; Cheng, Y.; Kojda, G.; Harrison, D.G. Regulation of the Vascular Extracellular Superoxide Dismutase by Nitric Oxide and Exercise Training. J. Clin. Invest. 2000, 105, 631–1639. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Balla, J.; Rosenberg, M.; Nath, K.; Apple, F.; Eaton, J.W.; Vercellotti, G.M. Ferritin: A Cytoprotective Antioxidant Strategem of Endothelium. J. Biol. Chem. 1992, 267, 18148–18153. [Google Scholar] [CrossRef] [PubMed]
- Groves, P.; Kurz, S.; Just, H.; Drexler, H. Role of Endogenous Bradykinin in Human Coronary Vasomotor Control. Circulation 1995, 92, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Joannides, R.; Haefeli, W.E.; Linder, L.; Richard, V.; Bakkali, E.H.; Thuillez, C.; Lüscher, T.F. Nitric Oxide Is Responsible for Flow-Dependent Dilatation of Human Peripheral Conduit Arteries in Vivo. Circulation 1995, 91, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Marui, N.; Offermann, M.K.; Swerlick, R.; Kunsch, C.; Rosen, C.A.; Ahmad, M.; Wayne Alexander, R.; Medford, R.M. Vascular Cell Adhesion Molecule-1 (VCAM-1) Gene Transcription and Expression Are Regulated through an Antioxidant-Sensitive Mechanism in Human Vascular Endothelial Cells. J. Clin. Invest. 1993, 92, 1866–1874. [Google Scholar] [CrossRef]
- Ziche, M.; Parenti, A.; Ledda, F.; DelL’Era, P.; Granger, H.J.; Maggi, C.A.; Presta, M. Nitric Oxide Promotes Proliferation and Plasminogen Activator Production by Coronary Venular Endothelium through Endogenous BFGF. Circ. Res. 1997, 80, 845–852. [Google Scholar] [CrossRef]
- Dulak, J.; Józkowicz, A.; Dembinska-Kiec, A.; Guevara, I.; Zdzienicka, A.; Zmudzinska-Grochot, D.; Florek, I.; Wójtowicz, A.; Szuba, A.; Cooke, J.P. Nitric Oxide Induces the Synthesis of Vascular Endothelial Growth Factor by Rat Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 659–666. [Google Scholar] [CrossRef]
- Shen, Y.H.; Wang, X.L.; Wilcken, D.E.L. Nitric Oxide Induces and Inhibits Apoptosis through Different Pathways. FEBS Lett. 1998, 433, 125–131. [Google Scholar] [CrossRef]
- Keaney, J.F.; Vita, J.A. Atherosclerosis, Oxidative Stress, and Antioxidant Protection in Endothelium-Derived Relaxing Factor Action. Prog. Cardiovasc. Dis. 1995, 38, 129–154. [Google Scholar] [CrossRef]
- Naseem, K.M. The Role of Nitric Oxide in Cardiovascular Diseases. Mol. Aspects Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Gresele, P.; Momi, S.; Guglielmini, G. Nitric Oxide-Enhancing or -Releasing Agents as Antithrombotic Drugs. Biochem. Pharmacol. 2019, 166, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Ihrig, M.; Dangler, C.A.; Fox, J.G. Mice Lacking Inducible Nitric Oxide Synthase Develop Spontaneous Hypercholesterolaemia and Aortic Atheromas. Atherosclerosis 2001, 156, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H. The Basic Chemistry of Nitrogen Monoxide and Peroxynitrite. Free Radic. Biol. Med. 1998, 25, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-Induced Membrane Lipid Peroxidation: The Cytotoxic Potential of Superoxide and Nitric Oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Bonini, M.G.; Augusto, O. Carbon Dioxide Stimulates the Production of Thiyl, Sulfinyl, and Disulfide Radical Anion from Thiol Oxidation by Peroxynitrite. J. Biol. Chem. 2001, 276, 9749–9754. [Google Scholar] [CrossRef]
- Horowitz, A.; Menice, C.B.; Laporte, R.; Morgan, K.G. Mechanisms of Smooth Muscle Contraction. Physiol. Rev. 1996, 76, 967–1003. [Google Scholar] [CrossRef]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric Oxide Directly Activates Calcium-Dependent Potassium Channels in Vascular Smooth Muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef]
- Pfeifer, A.; Klatt, P.; Massberg, S.; Ny, L.; Sausbier, M.; Hirneiß, C.; Wang, G.X.; Korth, M.; Aszódi, A.; Andersson, K.E.; et al. Defective Smooth Muscle Regulation in CGMP Kinase I-Deficient Mice. EMBO J. 1998, 17, 3045–3051. [Google Scholar] [CrossRef]
- Cornwell, T.L.; Arnold, E.; Boerth, N.J.; Lincoln, T.M. Inhibition of Smooth Muscle Cell Growth by Nitric Oxide and Activation of CAMP-Dependent Protein Kinase by CGMP. Am. J. Physiol. 1994, 267, C1405–C1413. [Google Scholar] [CrossRef]
- Schlossmann, J.; Ammendola, A.; Ashman, K.; Zong, X.; Huber, A.; Neubauer, G.; Wang, G.X.; Allescher, H.D.; Korth, M.; Wilm, M.; et al. Regulation of Intracellular Calcium by a Signalling Complex of IRAG, IP3 Receptor and CGMP Kinase Iβ. Nature 2000, 404, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.; Nolte, C.; Walter, U. Regulation of Calcium Mobilization and Entry in Human Platelets by Endothelium-Derived Factors. Am. J. Physiol.–Cell Physiol. 1994, 267, C236–C244. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Uhrin, P.; Mocan, A.; Waltenberger, B.; Breuss, J.M.; Tewari, D.; Mihaly-Bison, J.; Huminiecki, Ł.; Starzyński, R.R.; Tzvetkov, N.T.; et al. Vascular Smooth Muscle Cell Proliferation as a Therapeutic Target. Part 1: Molecular Targets and Pathways. Biotechnol. Adv. 2018, 36, 1586–1607. [Google Scholar] [CrossRef]
- Bennett, M.R.; Anglin, S.; McEwan, J.R.; Jagoe, R.; Newby, A.C.; Evan, G.I. Inhibition of Vascular Smooth Muscle Cell Proliferation in Vitro and in Vivo by C-Myc Antisense Oligodeoxynucleotides. J. Clin. Invest. 1994, 93, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Sasaguri, T.; Kosaka, C.; Nojima, H.; Ogata, J. Induction of the Cyclin-Dependent Kinase Inhibitor P21(Sdi1/Cip1/Waf1) by Nitric Oxide-Generating Vasodilator in Vascular Smooth Muscle Cells. J. Biol. Chem. 1997, 272, 10050–10057. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wei, L.H.; Bauer, P.M.; Wu, G.; Del Soldato, P. Role of the Arginine-Nitric Oxide Pathway in the Regulation of Vascular Smooth Muscle Cell Proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 4202–4208. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; He, Q. Strategies for Engineering Advanced Nanomedicines for Gas Therapy of Cancer. Natl. Sci. Rev. 2020, 7, 1485–1512. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Z.; Li, L.L. Advanced Nitric Oxide Donors: Chemical Structure of NO Drugs, NO Nanomedicines and Biomedical Applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef]
- Stamler, J.S.; Singel, D.J.; Loscalzo, J. Biochemistry of Nitric Oxide and Its Redox-Activated Forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Rao, C.V.; Reddy, B.S.; Steele, V.E.; Wang, C.X.; Liu, X.; Ouyang, N.; Patlolla, J.M.R.; Simi, B.; Kopelovich, L.; Rigas, B. Nitric Oxide-Releasing Aspirin and Indomethacin Are Potent Inhibitors against Colon Cancer in Azoxymethane-Treated Rats: Effects on Molecular Targets. Mol. Cancer Ther. 2006, 5, 1530–1538. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric Oxide Donors: Chemical Activities and Biological Applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Kim, J.; Saravanakumar, G.; Choi, H.W.; Park, D.; Kim, W.J. A Platform for Nitric Oxide Delivery. J. Mater. Chem. B 2014, 2, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Ermondi, G.; Fruttero, R.; Di Stilo, A.; Ferretti, C.; Alberto, G. Furoxans as Nitric Oxide Donors. 4-Phenyl-3-Furoxancarbonitrile: Thiol-Mediated Nitric Oxide Release and Biological Evaluation. J. Med. Chem. 1994, 37, 4412–4416. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, R.; Fazzini, A.; Folco, G.C.; Fruttero, R.; Calvino, R.; Gasco, A.; Bongrani, S.; Civelli, M. No-Mimetic Furoxans: Arylsulphonylfuroxans and Related Compounds. Pharmacol. Res. 1993, 28, 203–212. [Google Scholar] [CrossRef]
- Ferioli, R.; Folco, G.C.; Ferretti, C.; Gasco, A.M.; Medana, C.; Fruttero, R.; Civelli, M.; Gasco, A. A New Class of Furoxan Derivatives as NO Donors: Mechanism of Action and Biological Activity. Br. J. Pharmacol. 1995, 114, 816–820. [Google Scholar] [CrossRef]
- Chegaev, K.; Lazzarato, L.; Marcarino, P.; Di Stilo, A.; Fruttero, R.; Vanthuyne, N.; Roussel, C.; Gasco, A. Synthesis of Some Novel Organic Nitrates and Comparative in Vitro Study of Their Vasodilator Profile. J. Med. Chem. 2009, 52, 4020–4025. [Google Scholar] [CrossRef]
- Abu Yousef, M.; Matsubara, R. Recent Progress in Synthesis and Application of Furoxan. RSC Adv. 2023, 13, 5228–5248. [Google Scholar] [CrossRef]
- Blangetti, M.; Rolando, B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Durante, M.; Masini, E.; Almirante, N.; Bastia, E.; Impagnatiello, F.; et al. New Furoxan Derivatives for the Treatment of Ocular Hypertension. Bioorg. Med. Chem. Lett. 2017, 27, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Bohn, H.; Brendel, J.; Martorana, P.A.; Schönafinger, K. Cardiovascular Actions of the Furoxan CAS 1609, a Novel Nitric Oxide Donor. Br. J. Pharmacol. 1995, 114, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Burilov, A. Novel Structural Hybrids on the Base of Benzofuroxans and Furoxans. Mini-Review. Curr. Top. Med. Chem. 2016, 17, 986–1005. [Google Scholar] [CrossRef]
- Feelisch, M.; Schönafingeri, K.; Noack, H. Thiol-Mediated Generation of Nitric Oxide Accounts for the Vasodilator Action of Furoxans. Biochem. Pharmacol. 1992, 44, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Sodano, F.; Gazzano, E.; Rolando, B.; Marini, E.; Lazzarato, L.; Fruttero, R.; Riganti, C.; Gasco, A. Tuning NO Release of Organelle-Targeted Furoxan Derivatives and Their Cytotoxicity against Lung Cancer Cells. Bioorg. Chem. 2021, 111, 104911. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Andres, V.; Walsh, K. Nitric Oxide-Induced Downregulation of Cdk2 Activity and Cyclin A Gene Transcription in Vascular Smooth Muscle Cells. Circulation 1998, 97, 2066–2072. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.M.V.; Rangrez, A.Y.; Frey, N. SUMO Proteins in the Cardiovascular System: Friend or Foe? J. Biomed. Sci. 2020, 27, 98. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, I.A.; Vertegaal, A.C.O. SUMO in the DNA Damage Response. Oncotarget 2015, 6, 15734–15735. [Google Scholar] [CrossRef]
- Zheng, B.; Bernier, M.; Zhang, X.H.; Suzuki, T.; Nie, C.-Q.; Li, Y.H.; Zhang, Y.; Song, L.L.; Shi, H.J.; Liu, Y.; et al. MiR-200c-SUMOylated KLF4 Feedback Loop Acts as a Switch in Transcriptional Programs That Control VSMC Proliferation. J. Mol. Cell. Cardiol. 2015, 82, 201–212. [Google Scholar] [CrossRef]
- Psakhye, I.; Castellucci, F.; Branzei, D. SUMO-Chain-Regulated Proteasomal Degradation Timing Exemplified in DNA Replication Initiation. Mol. Cell 2019, 76, 632–645.e6. [Google Scholar] [CrossRef]
- Verger, A.; Perdomo, J.; Crossley, M. Modification with SUMO. A Role in Transcriptional Regulation. EMBO Rep. 2003, 4, 137–142. [Google Scholar] [CrossRef]
- Augstein, A.; Mierke, J.; Poitz, D.M.; Strasser, R.H. Sox9 Is Increased in Arterial Plaque and Stenosis, Associated with Synthetic Phenotype of Vascular Smooth Muscle Cells and Causes Alterations in Extracellular Matrix and Calcification. Biochim. Biophys. Acta–Mol. Basis Dis. 2018, 1864, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Pateras, I.; Giaginis, C.; Tsigris, C.; Patsouris, E.; Theocharis, S. NF-ΚB Signaling at the Crossroads of Inflammation and Atherogenesis: Searching for New Therapeutic Links. Expert Opin. Ther. Targets 2014, 18, 1089–1101. [Google Scholar] [CrossRef]
- Rai, V.; Jadhav, G.P.; Chandra, C.B. Role of Transcription Factors in Regulating Development and Progression of Atherosclerosis. Ann. Vasc. Med. 2019, 2, 1007. [Google Scholar]
- Shen, Q.; Chen, Q.; Liu, Y.; Xue, X.; Shen, X.; He, Q.; Wang, G.; Han, F. Aspirin Relieves the Calcification of Aortic Smooth Muscle Cells by Enhancing the Heat Shock Response. Pharm. Biol. 2022, 60, 17–24. [Google Scholar] [CrossRef]
- Khachigian, L.M. Early Growth Response-1, an Integrative Sensor in Cardiovascular and Inflammatory Disease. J. Am. Heart Assoc. 2021, 10, e023539. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.W.; Hu, Y.; Liu, J.; Yang, H.; Huang, P. Interleukin-22: A Potential Therapeutic Target in Atherosclerosis. Mol. Med. 2021, 27, 88. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, Z.; Mao, S.; Zhang, Y.; Jiang, W.; Wang, H. IRF-1 Contributes to the Pathological Phenotype of VSMCs during Atherogenesis by Increasing CCL19 Transcription. Aging 2020, 13, 933–943. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, F.; Wei, F.; Yang, L.; Kuang, C.; Zhang, H.; Deng, J.; Wu, Q. Silencing of Long Non-Coding RNA H19 Downregulates CTCF to Protect against Atherosclerosis by Upregulating PKD1 Expression in ApoE Knockout Mice. Aging 2019, 11, 10016–10030. [Google Scholar] [CrossRef]
- Sikorski, K.; Czerwoniec, A.; Bujnicki, J.M.; Wesoly, J.; Bluyssen, H.A.R. STAT1 as a Novel Therapeutical Target in Pro-Atherogenic Signal Integration of IFNγ, TLR4 and IL-6 in Vascular Disease. Cytokine Growth Factor Rev. 2011, 22, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Haemmig, S.; Deng, Y.; Chen, J.; Simion, V.; Yang, D.; Sukhova, G.; Shvartz, E.; Khyrul Wara, A.K.M.; Cheng, H.S.; et al. A Smooth Muscle Cell-Enriched Long Noncoding RNA Regulates Cell Plasticity and Atherosclerosis by Interacting With Serum Response Factor. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2399–2416. [Google Scholar] [CrossRef]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular Smooth Muscle Cells in Atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from Sudden Coronary Death: A Comprehensive Morphological Classification Scheme for Atherosclerotic Lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef]
- Lutgens, E.; De Muinck, E.D.; Kitslaar, P.J.E.H.M.; Tordoir, J.H.M.; Wellens, H.J.J.; Daemen, M.J.A.P. Biphasic Pattern of Cell Turnover Characterizes the Progression from Fatty Streaks to Ruptured Human Atherosclerotic Plaques. Cardiovasc. Res. 1999, 41, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Evan, G.I.; Schwartz, S.M. Apoptosis of Human Vascular Smooth Muscle Cells Derived from Normal Vessels and Coronary Atherosclerotic Plaques. J. Clin. Invest. 1995, 95, 2266–2274. [Google Scholar] [CrossRef]
- Geng, Y.J.; Libby, P. Evidence for Apoptosis in Advanced Human Atheroma: Colocalization with Interleukin-1β-Converting Enzyme. Am. J. Pathol. 1995, 147, 251–266. [Google Scholar] [PubMed]
- Boyle, J.J.; Weissberg, P.L.; Bennett, M.R. Human Macrophage-Induced Vascular Smooth Muscle Cell Apoptosis Requires NO Enhancement of Fas/Fas-L Interactions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1624–1630. [Google Scholar] [CrossRef]
- Clarke, M.C.H.; Figg, N.; Maguire, J.J.; Davenport, A.P.; Goddard, M.; Littlewood, T.D.; Bennett, M.R. Apoptosis of Vascular Smooth Muscle Cells Induces Features of Plaque Vulnerability in Atherosclerosis. Nat. Med. 2006, 12, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, M.R.; Chow, L.W.; Tsihlis, N.D.; Ahanchi, S.S.; Eng, J.W.; Murar, J.; Martinez, J.; Popowich, D.A.; Jiang, Q.; Hrabie, J.A.; et al. Nitric Oxide and Nanotechnology: A Novel Approach to Inhibit Neointimal Hyperplasia. J. Vasc. Surg. 2008, 47, 173–182. [Google Scholar] [CrossRef]
- Tsihlis, N.D.; Oustwani, C.S.; Vavra, A.K.; Jiang, Q.; Keefer, L.K.; Kibbe, M.R. Nitric Oxide Inhibits Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia by Increasing the Ubiquitination and Degradation of UbcH10. Cell Biochem. Biophys. 2011, 60, 89–97. [Google Scholar] [CrossRef]
- Tanner, F.C.; Meier, P.; Greutert, H.; Champion, C.; Nabel, E.G.; Lüscher, T.F. Nitric Oxide Modulates Expression of Cell Cycle Regulatory Proteins: A Cytostatic Strategy for Inhibition of Human Vascular Smooth Muscle Cell Proliferation. Circulation 2000, 101, 1982–1989. [Google Scholar] [CrossRef]
- Li, T.; Ziniel, P.D.; He, P.Q.; Kommer, V.P.; Crowther, G.J.; He, M.; Liu, Q.; Voorhis, W.C.; Williams, D.L.; Wang, M.W. High-Throughput Screening against Thioredoxin Glutathione Reductase Identifies Novel Inhibitors with Potential Therapeutic Value for Schistosomiasis. Infect. Dis. Poverty 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Sayed, A.A.; Simeonov, A.; Thomas, C.J.; Inglese, J.; Austin, C.P.; Williams, D.L. Identification of Oxadiazoles as New Drug Leads for the Control of Schistosomiasis. Nat. Med. 2008, 14, 407–412. [Google Scholar] [CrossRef]
- Moreira, B.P.; Weber, M.H.W.; Haeberlein, S.; Mokosch, A.S.; Spengler, B.; Grevelding, C.G.; Falcone, F.H. Drug Repurposing and De Novo Drug Discovery of Protein Kinase Inhibitors as New Drugs against Schistosomiasis. Molecules 2022, 27, 1414. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Yeh, E.T.H. SUMO: From Bench to Bedside. Physiol. Rev. 2020, 100, 1599–1619. [Google Scholar] [CrossRef] [PubMed]
- Dehnavi, S.; Sadeghi, M.; Penson, P.E.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. The Role of Protein SUMOylation in the Pathogenesis of Atherosclerosis. J. Clin. Med. 2019, 8, 1856. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Xiao, X.; Hu, C.T.; Dai, Y.; Qu, S.L.; Huang, L.; Zhang, C. SUMOylation in Atherosclerosis. Clin. Chim. Acta. 2020, 508, 228–233. [Google Scholar] [CrossRef]
- Nie, C.J.; Li, Y.H.; Zhang, X.H.; Wang, Z.P.; Jiang, W.; Zhang, Y.; Yin, W.N.; Zhang, Y.; Shi, H.J.; Liu, Y.; et al. SUMOylation of KLF4 Acts as a Switch in Transcriptional Programs That Control VSMC Proliferation. Exp. Cell Res. 2016, 342, 20–31. [Google Scholar] [CrossRef]
- Shankman, L.S.; Gomez, D.; Cherepanova, O.A.; Salmon, M.; Alencar, G.F.; Haskins, R.M.; Swiatlowska, P.; Newman, A.A.C.; Greene, E.S.; Straub, A.C.; et al. KLF4-Dependent Phenotypic Modulation of Smooth Muscle Cells Has a Key Role in Atherosclerotic Plaque Pathogenesis. Nat. Med. 2015, 21, 628–637. [Google Scholar] [CrossRef]
- Yao, Y.; Li, H.; Da, X.; He, Z.; Tang, B.; Li, Y.; Hu, C.; Xu, C.; Chen, Q.; Wang, Q.K. SUMOylation of Vps34 by SUMO1 Promotes Phenotypic Switching of Vascular Smooth Muscle Cells by Activating Autophagy in Pulmonary Arterial Hypertension. Pulm. Pharmacol. Ther. 2019, 55, 38–49. [Google Scholar] [CrossRef]
- Qi, Y.X.; Han, Y.; Jiang, Z.L. Mechanobiology and Vascular Remodeling: From Membrane to Nucleus. Adv. Exp. Med. Biol. 2018, 1097, 69–82. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Z. Vascular Smooth Muscle Cells Mechanosensitive Regulators and Vascular Remodeling. J. Vasc. Res. 2022, 59, 90–113. [Google Scholar] [CrossRef]
- Jamin, A.; Wiebe, M.S. Barrier to Autointegration Factor (BANF1): Interwoven Roles in Nuclear Structure, Genome Integrity, Innate Immunity, Stress Responses and Progeria. Curr. Opin. Cell Biol. 2015, 34, 61–68. [Google Scholar] [CrossRef]
- Haraguchi, T.; Koujin, T.; Osakada, H.; Kojidani, T.; Mori, C.; Masuda, H.; Hiraoka, Y. Nuclear Localization of Barrier-to-Autointegration Factor Is Correlated with Progression of S Phase in Human Cells. J. Cell Sci. 2007, 120, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Cenni, V.; Duchi, S.; Squarzoni, S.; Lopez-Otin, C.; Foisner, R.; Lattanzi, G.; Capanni, C. Barrier-to-Autointegration Factor (BAF) Involvement in Prelamin A-Related Chromatin Organization Changes. Oncotarget 2016, 7, 15662–15677. [Google Scholar] [CrossRef] [PubMed]
- Tajik, A.; Zhang, Y.; Wei, F.; Sun, J.; Jia, Q.; Zhou, W.; Singh, R.; Khanna, N.; Belmont, A.S.; Wang, N. Transcription Upregulation via Force-Induced Direct Stretching of Chromatin. Nat. Mater. 2016, 15, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Cenni, V.; Squarzoni, S.; Loi, M.; Mattioli, E.; Lattanzi, G.; Capanni, C. Emerin Phosphorylation during the Early Phase of the Oxidative Stress Response Influences Emerin-BAF Interaction and BAF Nuclear Localization. Cells 2020, 9, 1415. [Google Scholar] [CrossRef]
- Ren, Z.; Geng, J.; Xiong, C.; Li, X.; Li, Y.; Li, J.; Liu, H. Downregulation of VRK1 Reduces the Expression of BANF1 and Suppresses the Proliferative and Migratory Activity of Esophageal Cancer Cells. Oncol. Lett. 2020, 20, 1163–1170. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Interaction of Obtusilactone B and Related Butanolide Lactones with the Barrier-to-Autointegration Factor 1 (BAF1). A Computational Study. Curr. Res. Pharmacol. drug Discov. 2021, 2, 100059. [Google Scholar] [CrossRef] [PubMed]
- Sears, R.M.; Roux, K.J. Mechanisms of A-Type Lamin Targeting to Nuclear Ruptures Are Disrupted in LMNA- and BANF1- Associated Progerias. Cells 2022, 11, 865. [Google Scholar] [CrossRef]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef]
- Clarke, M.; Bennett, M.; Littlewood, T. Cell Death in the Cardiovascular System. Heart 2007, 93, 659–664. [Google Scholar] [CrossRef]
- Burgess, J.T.; Cheong, C.M.; Suraweera, A.; Sobanski, T.; Beard, S.; Dave, K.; Rose, M.; Boucher, D.; Croft, L.V.; Adams, M.N.; et al. Barrier-to-Autointegration-Factor (Banf1) Modulates DNA Double-Strand Break Repair Pathway Choice via Regulation of DNA-Dependent Kinase (DNA-PK) Activity. Nucleic Acids Res. 2021, 49, 3294–3307. [Google Scholar] [CrossRef]
- Bolderson, E.; Burgess, J.T.; Li, J.; Gandhi, N.S.; Boucher, D.; Croft, L.V.; Beard, S.; Plowman, J.J.; Suraweera, A.; Adams, M.N.; et al. Barrier-to-Autointegration Factor 1 (Banf1) Regulates Poly [ADP-Ribose] Polymerase 1 (PARP1) Activity Following Oxidative DNA Damage. Nat. Commun. 2019, 10, 5501. [Google Scholar] [CrossRef] [PubMed]
- Puente, X.S.; Quesada, V.; Osorio, F.G.; Cabanillas, R.; Cadiñanos, J.; Fraile, J.M.; Ordóñez, G.R.; Puente, D.A.; Gutiérrez-Fernández, A.; Fanjul-Fernández, M.; et al. Exome Sequencing and Functional Analysis Identifies BANF1 Mutation as the Cause of a Hereditary Progeroid Syndrome. Am. J. Hum. Genet. 2011, 88, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, R.; Cadiñanos, J.; Villameytide, J.A.F.; Pérez, M.; Longo, J.; Richard, J.M.; Álvarez, R.; Durán, N.S.; Illán, R.; González, D.J.; et al. Néstor-Guillermo Progeria Syndrome: A Novel Premature Aging Condition with Early Onset and Chronic Development Caused by BANF1 Mutations. Am. J. Med. Genet. A 2011, 155A, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Min, S.; Jeoun, U.W.; Sim, M.S.; Jung, G.H.; Hong, S.M.; Jee, B.A.; Woo, H.G.; Lee, C.; Yoon, G. Global Spliceosome Activity Regulates Entry into Cellular Senescence. FASEB J. 2021, 35, e21204. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Tiwari, M.; Bansal, V.; Sen, G.L. Regulation of Integrin and Extracellular Matrix Genes by HNRNPL Is Necessary for Epidermal Renewal. PLoS Biol. 2021, 19, e3001378. [Google Scholar] [CrossRef]
- Haemmig, S.; Simion, V.; Feinberg, M.W. Long Non-Coding RNAs in Vascular Inflammation. Front. Cardiovasc. Med. 2018, 5, 22. [Google Scholar] [CrossRef]
- Simion, V.; Haemmig, S.; Feinberg, M.W. LncRNAs in Vascular Biology and Disease. Vascul. Pharmacol. 2019, 114, 145–156. [Google Scholar] [CrossRef]
- Sun, X.; Haider Ali, M.S.S.; Moran, M. The Role of Interactions of Long Non-Coding RNAs and Heterogeneous Nuclear Ribonucleoproteins in Regulating Cellular Functions. Biochem. J. 2017, 474, 2925–2935. [Google Scholar] [CrossRef]
- Ding, Y.; Yin, R.; Zhang, S.; Xiao, Q.; Zhao, H.; Pan, X.; Zhu, X. The Combined Regulation of Long Non-Coding RNA and RNA-Binding Proteins in Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 731958. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lin, C.L.; Chuang, J.H.; Chiu, F.Y.; Sun, Y.Y.; Liang, M.C.; Lin, Y. Heterogeneous Nuclear Ribonucleoprotein M Associates with MTORC2 and Regulates Muscle Differentiation. Sci. Rep. 2017, 7, 41159. [Google Scholar] [CrossRef]
- Thomas, P.; Forse, R.A.; Bajenova, O. Carcinoembryonic Antigen (CEA) and Its Receptor HnRNP M Are Mediators of Metastasis and the Inflammatory Response in the Liver. Clin. Exp. Metastasis 2011, 28, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Feil, S.; Fehrenbacher, B.; Lukowski, R.; Essmann, F.; Schulze-Osthoff, K.; Schaller, M.; Feil, R. Transdifferentiation of Vascular Smooth Muscle Cells to Macrophage-like Cells during Atherogenesis. Circ. Res. 2014, 115, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Dautova, Y.; Kapustin, A.N.; Pappert, K.; Epple, M.; Okkenhaug, H.; Cook, S.J.; Shanahan, C.M.; Bootman, M.D.; Proudfoot, D. Calcium Phosphate Particles Stimulate Interleukin-1β Release from Human Vascular Smooth Muscle Cells: A Role for Spleen Tyrosine Kinase and Exosome Release. J. Mol. Cell. Cardiol. 2018, 115, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Shibagaki, Y.; Hattori, S.; Matsuoka, M. C9-ALS/FTD-Linked Proline-Arginine Dipeptide Repeat Protein Associates with Paraspeckle Components and Increases Paraspeckle Formation. Cell Death Dis. 2019, 10, 746. [Google Scholar] [CrossRef]
- Ahmed, A.S.I.; Dong, K.; Liu, J.; Wen, T.; Yu, L.; Xu, F.; Kang, X.; Osman, I.; Hu, G.; Bunting, K.M.; et al. Long Noncoding RNA NEAT1 (Nuclear Paraspeckle Assembly Transcript 1) Is Critical for Phenotypic Switching of Vascular Smooth Muscle Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E8660–E8667. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Zhao, Z.; Li, Y.; Xu, P.; Shi, J.; Li, Z.; Wang, K.; Huang, X.; Ji, J.; Liu, W.; et al. FBXO16-Mediated HnRNPL Ubiquitination and Degradation Plays a Tumor Suppressor Role in Ovarian Cancer. Cell Death Dis. 2021, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chao, T.C.; Chang, K.Y.; Lin, N.; Patil, V.S.; Shimizu, C.; Head, S.R.; Burns, J.C.; Rana, T.M. The Long Noncoding RNA THRIL Regulates TNFα Expression through Its Interaction with HnRNPL. Proc. Natl. Acad. Sci. USA 2014, 111, 1002–1007. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, B.; Qin, Y.; Zhang, B. Emerging Roles and Mechanism of M6A Methylation in Cardiometabolic Diseases. Cells 2022, 11, 1101. [Google Scholar] [CrossRef]
- Ray, P.S.; Jia, J.; Yao, P.; Majumder, M.; Hatzoglou, M.; Fox, P.L. A Stress-Responsive RNA Switch Regulates VEGFA Expression. Nature 2009, 457, 915–919. [Google Scholar] [CrossRef]
- Grosskreutz, C.L.; Anand-Apte, B.; Dupláa, C.; Quinn, T.P.; Terman, B.I.; Zetter, B.; D’Amore, P.A. Vascular Endothelial Growth Factor-Induced Migration of Vascular Smooth Muscle Cells in Vitro. Microvasc. Res. 1999, 58, 128–136. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Khotina, V.A.; Omelchenko, A.V.; Kalmykov, V.A.; Orekhov, A.N. The Role of the VEGF Family in Atherosclerosis Development and Its Potential as Treatment Targets. Int. J. Mol. Sci. 2022, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Cardús, A.; Parisi, E.; Gallego, C.; Aldea, M.; Fernández, E.; Valdivielso, J.M. 1,25-Dihydroxyvitamin D3 Stimulates Vascular Smooth Muscle Cell Proliferation through a VEGF-Mediated Pathway. Kidney Int. 2006, 69, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Gherzi, R.; Chen, C.Y.; Trabucchi, M.; Ramos, A.; Briata, P. The Role of KSRP in MRNA Decay and MicroRNA Precursor Maturation. Wiley Interdiscip. Rev. RNA 2010, 1, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tang, Y.; Yang, D.; Zheng, L. MicroRNA-591 Functions as a Tumor Suppressor in Hepatocellular Carcinoma by Lowering Drug Resistance through Inhibition of Far-Upstream Element-Binding Protein 2-Mediated Phosphoinositide 3-Kinase/Akt/Mammalian Target of Rapamycin Axis. Pharmacology 2019, 104, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chou, C.F.; Liu, S.; Crossman, D.; Yusuf, N.; Wu, Y.; Chen, C.Y. KSRP Modulates Melanoma Growth and Efficacy of Vemurafenib. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 759–770. [Google Scholar] [CrossRef]
- Pan, R.; Cai, W.; Sun, J.; Yu, C.; Li, P.; Zheng, M. Inhibition of KHSRP Sensitizes Colorectal Cancer to 5-Fluoruracil through MiR-501-5p-Mediated ERRFI1 MRNA Degradation. J. Cell. Physiol. 2020, 235, 1576–1587. [Google Scholar] [CrossRef]
- Palzer, K.A.; Bolduan, V.; Käfer, R.; Kleinert, H.; Bros, M.; Pautz, A. The Role of KH-Type Splicing Regulatory Protein (KSRP) for Immune Functions and Tumorigenesis. Cells 2022, 11, 1482. [Google Scholar] [CrossRef]
- Gherzi, R.; Lee, K.Y.; Briata, P.; Wegmüller, D.; Moroni, C.; Karin, M.; Chen, C.Y. A KH Domain RNA Binding Protein, KSRP, Promotes ARE-Directed MRNA Turnover by Recruiting the Degradation Machinery. Mol. Cell 2004, 14, 571–583. [Google Scholar] [CrossRef]
- Boucas, J.; Fritz, C.; Schmitt, A.; Riabinska, A.; Thelen, L.; Peifer, M.; Leeser, U.; Nuernberg, P.; Altmueller, J.; Gaestel, M.; et al. Label-Free Protein-RNA Interactome Analysis Identifies Khsrp Signaling Downstream of the P38/Mk2 Kinase Complex as a Critical Modulator of Cell Cycle Progression. PLoS ONE 2015, 10, e0125745. [Google Scholar] [CrossRef]
- Gou, Q.; Gao, L.; Nie, X.; Pu, W.; Zhu, J.; Wang, Y.; Liu, X.; Tan, S.; Zhou, J.K.; Gong, Y.; et al. Long Noncoding RNA AB074169 Inhibits Cell Proliferation via Modulation of KHSRP-Mediated CDKN1a Expression in Papillary Thyroid Carcinoma. Cancer Res. 2018, 78, 4163–4174. [Google Scholar] [CrossRef]
- Panchenko, M.P.; Silva, N.; Stone, J.R. Up-Regulation of a Hydrogen Peroxide-Responsive Pre-MRNA Binding Protein in Atherosclerosis and Intimal Hyperplasia. Cardiovasc. Pathol. 2009, 18, 167–172. [Google Scholar] [CrossRef][Green Version]
- Zhang, R.Y.; Wu, C.M.; Hu, X.M.; Lin, X.M.; Hua, Y.N.; Chen, J.J.; Ding, L.; He, X.; Yang, B.; Ping, B.H.; et al. LncRNA AC105942.1 Downregulates HnRNPA2/B1 to Attenuate Vascular Smooth Muscle Cells Proliferation. DNA Cell Biol. 2021, 40, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ranjan, P.; Suleiman, Z.G.; Goswami, S.K.; Li, J.; Prasad, R.; Verma, S.K. MRNA Modifications in Cardiovascular Biology and Disease: With a Focus on M6A Modification. Cardiovasc. Res. 2022, 118, 1680–1692. [Google Scholar] [CrossRef]
- Peng, L.; Long, T.; Li, F.; Xie, Q. Emerging Role of m 6 A Modification in Cardiovascular Diseases. Cell Biol. Int. 2022, 46, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cui, X.; Zhang, X.; Cheng, M.; Li, X.; Guo, Z.; Cui, X. The Role of M6A Ribonucleic Acid Modification in the Occurrence of Atherosclerosis. Front. Genet. 2021, 12, 733871. [Google Scholar] [CrossRef]
- Stark, M.; Bram, E.E.; Akerman, M.; Mandel-Gutfreund, Y.; Assaraf, Y.G. Heterogeneous Nuclear Ribonucleoprotein H1/H2-Dependent Unsplicing of Thymidine Phosphorylase Results in Anticancer Drug Resistance. J. Biol. Chem. 2011, 286, 3741–3754. [Google Scholar] [CrossRef]
- Wang, X.; Luo, D.; Wu, S. Molecular Dysfunctions of Mitochondria-Associated Endoplasmic Reticulum Contacts in Atherosclerosis. Oxid. Med. Cell. Longev. 2021, 2021, 2424509. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial Dysfunction in Atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Talaverón-Rey, M.; Suárez-Carrillo, A.; Munuera-Cabeza, M.; Sánchez-Alcázar, J.A. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines 2021, 9, 258. [Google Scholar] [CrossRef]
- Davis, S.A.; Itaman, S.; Khalid-Janney, C.M.; Sherard, J.A.; Dowell, J.A.; Cairns, N.J.; Gitcho, M.A. TDP-43 Interacts with Mitochondrial Proteins Critical for Mitophagy and Mitochondrial Dynamics. Neurosci. Lett. 2018, 678, 8–15. [Google Scholar] [CrossRef]
- Markin, A.M.; Khotina, V.A.; Zabudskaya, X.G.; Bogatyreva, A.I.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. Disturbance of Mitochondrial Dynamics and Mitochondrial Therapies in Atherosclerosis. Life 2021, 11, 165. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Lu, J.; Siedlak, S.L.; Fujioka, H.; Liang, J.; Jiang, S.; Ma, X.; Jiang, Z.; Da Rocha, E.L.; et al. The Inhibition of TDP-43 Mitochondrial Localization Blocks Its Neuronal Toxicity. Nat. Med. 2016, 22, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.H.; Xu, F.; Li, P.; Guo, J.Q. Transactive Response DNA Binding Protein of 43/Histone Deacetylase 6 Axis Alleviates H 2 O 2 -Induced Retinal Ganglion Cells Injury through Inhibiting Apoptosis and Autophagy. J. Cell. Biochem. 2019, 120, 4312–4320. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef] [PubMed]
- Izumikawa, K.; Nobe, Y.; Yoshikawa, H.; Ishikawa, H.; Miura, Y.; Nakayama, H.; Nonaka, T.; Hasegawa, M.; Egawa, N.; Inoue, H.; et al. TDP-43 Stabilises the Processing Intermediates of Mitochondrial Transcripts. Sci. Rep. 2017, 7, 7709. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiao, L.; Li, J.; Sun, P.; Shang, L.; Zhang, J.; Zhao, Q.; Ouyang, Y.; Li, L.; Gong, K. MicroRNA Profiling of Diabetic Atherosclerosis in a Rat Model. Eur. J. Med. Res. 2018, 23, 55. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Aquilano, K.; Punziano, C.; Minopoli, G.; Faraonio, R. MicroRNAs, Long Non-Coding RNAs, and Circular RNAs in the Redox Control of Cell Senescence. Antioxidants 2022, 11, 480. [Google Scholar] [CrossRef]
- Chen, W.; Yu, F.; Di, M.; Li, M.; Chen, Y.; Zhang, Y.; Liu, X.; Huang, X.; Zhang, M. MicroRNA-124-3p Inhibits Collagen Synthesis in Atherosclerotic Plaques by Targeting Prolyl 4-Hydroxylase Subunit Alpha-1 (P4HA1) in Vascular Smooth Muscle Cells. Atherosclerosis 2018, 277, 98–107. [Google Scholar] [CrossRef]
- Jia, C.; Gao, F.; Zhao, Y.; Ji, S.; Cai, S. Identification and Functional Analysis of Changes to the Ox-LDL-Induced MicroRNA-124-3p/DLX5 Axis in Vascular Smooth Muscle Cells. Adv. Clin. Exp. Med. 2021, 30, 1271–1281. [Google Scholar] [CrossRef]
- Yan, L.; Yang, H.; Duan, H.; Wu, J.; Qian, P.; Fan, X.; Wang, S. MiR-124-3p Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Proliferation and Migration through Targeting STAT3. Int. J. Clin. Exp. Med. 2017, 10, 9198–9205. [Google Scholar]
- Sun, W.; Tian, B.X.; Wang, S.H.; Liu, P.J.; Wang, Y.C. The Function of SEC22B and Its Role in Human Diseases. Cytoskeleton 2020, 77, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mai, W.; Liao, Y. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Rheaume, E.; Tardif, J.C. Examining the Role of and Treatment Directed at IL-1β in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Claude-Taupin, A.; Bissa, B.; Jia, J.; Gu, Y.; Deretic, V. Role of Autophagy in IL-1β Export and Release from Cells. Semin. Cell Dev. Biol. 2018, 83, 36–41. [Google Scholar] [CrossRef]
- New, J.; Thomas, S.M. Autophagy-Dependent Secretion: Mechanism, Factors Secreted, and Disease Implications. Autophagy 2019, 15, 1682–1693. [Google Scholar] [CrossRef]
- Kimura, T.; Jia, J.; Kumar, S.; Choi, S.W.; Gu, Y.; Mudd, M.; Dupont, N.; Jiang, S.; Peters, R.; Farzam, F.; et al. Dedicated SNAREs and Specialized TRIM Cargo Receptors Mediate Secretory Autophagy. EMBO J. 2017, 36, 42–60. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.; Mun, S.; Kwak, Y.; Suh, K.S.; Choi, S.Y.; Han, K. Whole-Exome Sequencing Reveals Rare Genetic Variations in Ovarian Granulosa Cell Tumor. Bosn. J. basic Med. Sci. 2022, 22, 403–411. [Google Scholar] [CrossRef]
- Corsini, A.; Arnaboldi, L.; Raiteri, M.; Quarato, P.; Faggiotto, A.; Paoletti, R.; Fumagalli, R. Effect of the New HMG-CoA Reductase Inhibitor Cerivastatin (BAY W 6228) on Migration, Proliferation and Cholesterol Synthesis in Arterial Myocytes. Pharmacol. Res. 1996, 33, 55–61. [Google Scholar] [CrossRef]
- Ferri, N.; Arnaboldi, L.; Orlandi, A.; Yokoyama, K.; Gree, R.; Granata, A.; Hachem, A.; Paoletti, R.; Gelb, M.H.; Corsini, A. Effect of S(-) Perillic Acid on Protein Prenylation and Arterial Smooth Muscle Cell Proliferation. Biochem. Pharmacol. 2001, 62, 1637–1645. [Google Scholar] [CrossRef]
- Raiteri, M.; Arnaboldi, L.; Mcgeady, P.; Gelb, M.H.; Verri, D.; Tagliabue, C.; Quarato, P.; Ferraboschi, P.; Santaniello, E.; Paoletti, R.; et al. Pharmacological Control of the Mevalonate Pathway: Effect on Arterial Smooth Muscle Cell Proliferation. J. Pharmacol. Exp. Ther. 1997, 281, 1144–1153. [Google Scholar]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A Peptide Search Engine Integrated into the MaxQuant Environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. 1D and 2D Annotation Enrichment: A Statistical Method Integrating Quantitative Proteomics with Complementary High-Throughput Data. BMC Bioinform. 2012, 13 (Suppl. S16), S12. [Google Scholar] [CrossRef] [PubMed]
- Mann, M. Functional and Quantitative Proteomics Using SILAC. Nat. Rev. Mol. Cell Biol. 2006, 7, 952–958. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Vantaggiato, L.; Shaba, E.; Carleo, A.; Bezzini, D.; Pannuzzo, G.; Luddi, A.; Piomboni, P.; Bini, L.; Bianchi, L. Neurodegenerative Disorder Risk in Krabbe Disease Carriers. Int. J. Mol. Sci. 2022, 23, 13537. [Google Scholar] [CrossRef]
- Bianchi, L.; Gagliardi, A.; Landi, C.; Focarelli, R.; De Leo, V.; Luddi, A.; Bini, L.; Piomboni, P. Protein Pathways Working in Human Follicular Fluid: The Future for Tailored IVF? Expert Rev. Mol. Med. 2016, 18, e9. [Google Scholar] [CrossRef]
- Bianchi, L.; Altera, A.; Barone, V.; Bonente, D.; Bacci, T.; De Benedetto, E.; Bini, L.; Tosi, G.M.; Galvagni, F.; Bertelli, E. Untangling the Extracellular Matrix of Idiopathic Epiretinal Membrane: A Path Winding among Structure, Interactomics and Translational Medicine. Cells 2022, 11, 2531. [Google Scholar] [CrossRef]
- Bini, L.; Schvartz, D.; Carnemolla, C.; Besio, R.; Garibaldi, N.; Sanchez, J.C.; Forlino, A.; Bianchi, L. Intracellular and Extracellular Markers of Lethality in Osteogenesis Imperfecta: A Quantitative Proteomic Approach. Int. J. Mol. Sci. 2021, 22, 429. [Google Scholar] [CrossRef]

| Product Number | Structure | Vasodilating Activity EC50 ± SE (µM) | Antiproliferative Activity IC50 (µM) |
|---|---|---|---|
| 1 |  | 41 ± 6 | n.c. 1 |
| 2 | 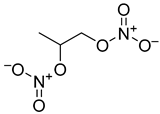 | 0.24 ± 0.03 | >100 |
| 3 | 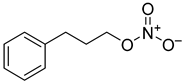 | 0.16 ± 0.03 | n.c. |
| 4 | 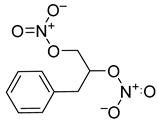 | 0.063 ± 0.010 | n.c. |
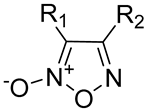 | ||||
|---|---|---|---|---|
| Product Number | R1 | R2 | Vasodilating Activity EC50 ± SE (µM) | Antiproliferative Activity IC50 (µM) |
| 5 | PhSO2 | OEt | 0.012 ± 0.002 | 0.294 |
| 6 | PhSO2 | Ph | 0.024 ± 0.003 | 1.89 |
| 7 | CH3 | Ph | 146 ± 31 | n.c. |
| 8 | Cl | Ph | 0.98 ± 0.20 | 1.28 |
| 9 | NO2 | Ph | 0.043 ± 0.006 | 2.82 |
| 10 | NH2 | Ph | 14 ± 1 | n.c. |
| 11 | COOCH3 | Ph | 0.16 ± 0.02 | 10.08 |
| 12 | CN | Ph | 0.0018 ± 0.0004 | 0.84 |
| 13 | Ph | Ph | 5.0 ± 0.7 | 47.36 |
| 14 | CONH2 | Ph | 0.78 ± 0.08 | 14.83 |
| 15 | Ph | PhSO2 | 0.053 ± 0.005 | >100 |
| 16 | Ph | CN | 0.0043 ± 0.0005 | >100 |
| 17 | Ph | Cl | 0.088 ± 0.011 | >100 |
| 18 | Ph | NO2 | 0.53 ± 0.09 | Ineffective |
| 19 | Ph | COOCH3 | 1.4 ± 0.2 | Ineffective |
| 4-Ph Furoxan Number and IC50 (µM) | 3-Ph Furoxan Number and IC50 (µM) | Furazan Number and IC50 (µM) |
|---|---|---|
| 5/0.294 | - | 20/uneffective |
| 6/1.89 | 15/>100 | 21/uneffective |
| 8/1.28 | 17/>100 | 22/uneffective |
| 9/2.82 | 18/uneffective | 23/uneffective |
| 11/10.08 | 19/uneffective | 24/uneffective |
| 12/0.84 | 16/176.7 | 25/uneffective |
| 13/47.36 | - | 26/uneffective |
| 14/14.83 | - | 27/uneffective |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzarato, L.; Bianchi, L.; Andolfo, A.; Granata, A.; Lombardi, M.; Sinelli, M.; Rolando, B.; Carini, M.; Corsini, A.; Fruttero, R.; et al. Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms. Molecules 2023, 28, 5724. https://doi.org/10.3390/molecules28155724
Lazzarato L, Bianchi L, Andolfo A, Granata A, Lombardi M, Sinelli M, Rolando B, Carini M, Corsini A, Fruttero R, et al. Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms. Molecules. 2023; 28(15):5724. https://doi.org/10.3390/molecules28155724
Chicago/Turabian StyleLazzarato, Loretta, Laura Bianchi, Annapaola Andolfo, Agnese Granata, Matteo Lombardi, Matteo Sinelli, Barbara Rolando, Marina Carini, Alberto Corsini, Roberta Fruttero, and et al. 2023. "Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms" Molecules 28, no. 15: 5724. https://doi.org/10.3390/molecules28155724
APA StyleLazzarato, L., Bianchi, L., Andolfo, A., Granata, A., Lombardi, M., Sinelli, M., Rolando, B., Carini, M., Corsini, A., Fruttero, R., & Arnaboldi, L. (2023). Proteomics Studies Suggest That Nitric Oxide Donor Furoxans Inhibit In Vitro Vascular Smooth Muscle Cell Proliferation by Nitric Oxide-Independent Mechanisms. Molecules, 28(15), 5724. https://doi.org/10.3390/molecules28155724







