Abstract
Wormlike micelles, which are linear aggregates created by the self-assembly of surfactants, may entangle to form dynamic three-dimensional network-like structures, endowing solutions with considerable macroscopic viscoelasticity. Recently, a pressing need has arisen to research a novel stimuli-responsive worm-like micelle that is efficient and environmentally friendly. CO2 is an inexpensive, abundant, non-toxic, biocompatible, and non-combustible gas, and it is anticipated that CO2 may serve as the trigger for stimuli-responsive worm-like micelles. In this paper, the formation of CO2-switchable pseudo-tetrameric surfactants, which subsequently self-assemble into CO2-switched wormlike micelles, is accomplished using a simple mixing of two commercial reagents, such as stearic acids and cyclen. The rheological characteristics switched by the use of CO2 are cycled between that of a low-viscosity (1.2 mPa·s) fluid and a viscoelastic fluid (worm-like micelles, 3000 mPa·s). This article expands the field of study on stimuli-responsive worm-like micelles.
1. Introduction
Recent years have seen the emergence of wormlike micelles [1] as a significant study path in colloids and soft matter. A wormlike micelle is an equilibrium polymer or living polymer model with static and dynamic properties that differ from those of polymers for both long and short periods relative to its period of existence [2,3,4]. Wormlike micelles are candidates to replace polymers as viscosity enhancers and can be widely used in personal cleaning and care [4], drag-reducing fluid [5,6], solid particle transport [7], and fracturing fluids [5,8], among other industrial applications. A great deal of attention has been focused on the peculiar rheological features of wormlike micelles, which exhibit viscoelasticity like polymers but are not precisely comparable to polymers.
Wormlike micelles are also known as threadlike micelles [7,8,9] or giant micelles [10]. Under certain conditions, rodlike micelles of surfactant continue to grow in one dimension along the non-axial direction to form long flexible columnar micelles [1,2]. These micelles typically have diameters of a few nanometers, and persistent lengths of tens to hundreds of nanometers, while the contour length may reach several micrometers [2], producing micelles resembling worms. Under suitable conditions with proper temperature, concentration, and counterion, wormlike micelles interweave and form a temporary three-dimensional transient network, therefore imparting to the system a macroscopic viscoelasticity comparable to that of polymer solutions. However, wormlike micelles are held together by weak intermolecular non-covalent bonds (bond energy, 40 kJ/mol) [11], whereas polymers are held together by strong covalent bonds (bond energy, 100–900 kJ/mol) [12] between monomers with fixed molecular weight and molecular weight distribution, and their rheological properties are typically shear irreversible, i.e., the solution viscosity cannot be recovered after their molecular chains are broken at high shear rates. At the microscopic level, it is a dynamic equilibrium system, and the size of the wormlike micelles changes in response to temperature, concentration, and type of components. This dynamic equilibrium system imparts shear-reversible viscoelasticity; hence, worm-like micelles are sometimes known as living polymers [1,2], or equilibrium polymers [13,14,15].
Because of their unusual microstructure and modifiable rheological characteristics, stimuli-responsive wormlike micelles have received considerable attention for the control of viscosity. However, the existing triggers are mostly temperature [16,17], pH [18,19], light [20,21], and redox [22,23] responses, and these conventional stimuli often have limits or drawbacks, such as severe application circumstances, contamination of the system by by-products and additives, etc. Therefore, there is a pressing need to study a novel stimulus, and simpler and truly green wormlike micelles. As a commonly accessible, inexpensive, non-toxic, biocompatible, and non-combustible gas, CO2 has been used in surfactants [24], solvents [25], solutes [26], and polymers [27] due to its superior response qualities. Zhang et al. described a new CO2-responsive wormlike micellar system based on natural erucic fatty acid and investigated the rheological properties of its solution [28]. pH-responsive wormlike micelles are micellar systems containing pH-responsive hydrotropes (such as carboxyl or amine groups, etc.) that cause changes in the hydrophilic and lipophilic equilibrium values of surfactant molecules by interacting with H+ or OH− ions, or by structural changes of counter ions, leading to the disruption and reconstruction of the wormlike micelle structure and achieving macroscopic changes in viscoelasticity. When the pH of alkylamidopropyl carboxylate betaine-based surfactant was elevated from near 0 to 4.44, Zhao et al. [29] discovered that the energy storage modulus of the solution increased from 20 Pa to 80 Pa, i.e., the elasticity increased, and a further rise led to gel formation. Hassan’s group [30] also found that when pH increased from 2.9 to 12.3, a solution containing cetrimonium bromide/o-aminobenzoic acid changed from a water-like Newtonian fluid to a shear-thinning fluid with a continuously decreasing critical shear rate. The Feng group [31] produced a viscoelastic wormlike micelle with pH-stimulated responsiveness by combining N-mustard propyl-N,N-dimethyl-tertiary amine and maleic acid (molar ratio, 2:1). When the pH reaches 6.20, the long-chain tertiary amine was protonated to create cationic quaternary ammonium salts; in conjunction with maleic acid, a pseudo-Gemini surfactant structure is generated by electrostatic attraction of non-covalent bonds, self-assembling to form wormlike micelles. When the pH reaches 7.29, the long-chain tertiary amine deprotonates and loses its cationic charge, causing the pseudo-Gemini structure to disappear, and the wormlike micelle network collapses. Consequently, it is anticipated that small-molecule tertiary amines with CO2 stimulation in the protonated state and anionic surfactants will also be capable of forming a novel kind of surfactant with a “baryon-like” structure via non-covalent bonding.
The creation of worm micelles by pseudo-Gemini surfactants has been described, but not the formation of worm micelles by pseudo-tetrameric surfactants. Inspired by the CO2 stimulation response feature of small-molecule amines, this work focuses on the preparation of pseudo-tetrameric surfactants through non-covalent bonding with conventional anionic surfactants. The feasibility and generalizability of creating an anionic worm-like micellar system with a CO2 stimulation response are studied.
2. Results and Discussion
2.1. CO2 Switchable Behavior
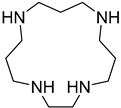
Our team previously investigated a CO2 switchable pseudo-tetrameric surfactant combination produced by mixing oleic acid and four-amine-containing cyclic polyamine (cyclen) [32]. The effect of surfactant hydrophobic tail chain length is examined in this research; the oleic acid is replaced with stearic acid (SA) to avoid the effect of double bonds. The solution of SA/cyclen (300 mM) is produced by combining SA (1200 mM) and cyclen (300 mM) in a precise 4:1 stoichiometric molar ratio (Scheme 1). The as-prepared SA/cyclen solution is viscous; when CO2 is continuously injected into the solution, the viscosity of the SA/cyclen solution gradually decreases.
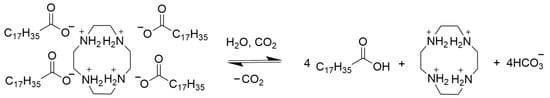
Scheme 1.
The CO2 switching process of the mixed surfactant SA/cyclen.
Figure 1a depicts the viscosity of the SA/cyclen solution in order to explore the distinct flow phenomena of the solution before and after the addition of CO2; the appearance of the SA/cyclen solution is shown in Figure 1b,c. After CO2 is added, the SA/cyclen solution is a standard Newtonian fluid, such as pure water; its shear viscosity is not changed by the shear rate, and its viscosity is just 1.2 mPa·s; the protonated SA is insoluble in water forming the opaque emulsion visible in Figure 1c. After bubbling N2 to eliminate CO2, the viscosity of the SA/cyclen solution rises to 3000 mPa·s, as Figure 1b shows, an increase of about 2500 times, and a viscosity plateau is seen at low shear rates (Figure 1). When the shear rate reaches a critical value, the shear viscosity of the solution gradually decreases with the increase of the shear rate. Typically, this shear-thinning behavior is believed to be the result of the rearrangement of the entangled structure formed by wormlike micelles in solution under stress gradually aligning parallel to the direction of the flow field; thus, it is regarded as one of the most compelling types of evidence for the existence of wormlike micelles [7].
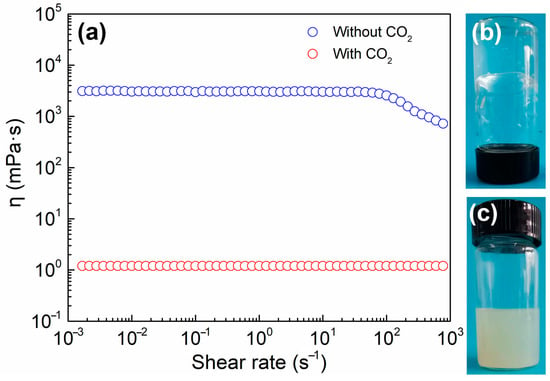
Figure 1.
(a) Steady rheology of SA/cyclen (300 mM) in the absence or presence of CO2 at 25 °C; (b) the physical appearance of SA/cyclen (300 mM) in the absence of CO2 at 25 °C; (c) the physical appearance of SA/cyclen (300 mM) in the presence of CO2 at 25 °C.
Figure 2 shows the switchable states of SA/cyclen solution (300 mM). The pH value of the solution was in the range of 12.2 to 12.4 at the initial stage and in the absence of CO2, and it decreased to about 8.3 after the full introduction of CO2. The original high viscosity of the solution is restored when the CO2 is added into solution. The viscosity remains the same when the introduction and removal of CO2 are cycled back and forth four times (Figure 2). Figure 3 shows the microstructure of SA/cyclen (300 mM) in the absence of CO2. Before CO2 is introduced, many flexible filamentary aggregates a few nanometers in diameter and a few micrometers in length form a three-dimensional mesh structure. After the CO2 is discharged, it is assumed that the network structure disappears and is replaced by spherical structures. This comparison shows that the substantial variation in the macroscopic rheological characteristics of SA/cyclen solution (300 mM) with CO2 uptake and discharge is attributable to the creation and disruption of an entangled network of wormlike micelles.
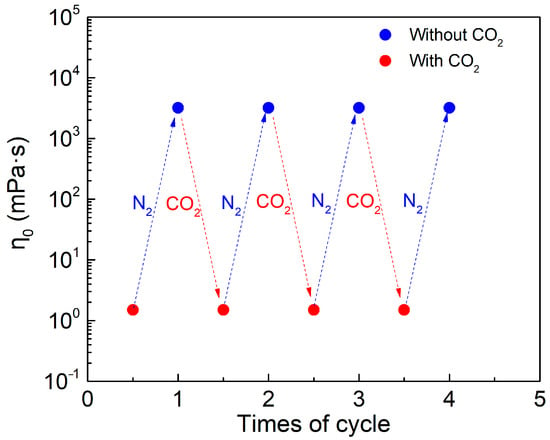
Figure 2.
The reversible switchability of SA/cyclen (300 mM) in the absence or presence of CO2 at 25 °C; the shear rate is 10 s−1.
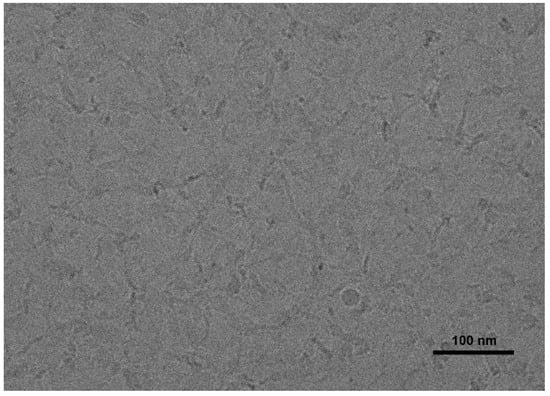
Figure 3.
Cryo-TEM image of SA/cyclen (300 mM) in the absence of CO2; The scale bar is 100 nm.
2.2. Mechanism of CO2 Switching
To further investigate the mechanism of CO2 switching of SA/Cyclen, we measured the pH of the solutions and investigated whether aqueous solutions of SA and cyclen respectively have CO2 switchability. The optimal ratio between SA and cyclen was also investigated.
2.2.1. Testing of pH
To acquire a deeper understanding of the process behind the development and dissolution of the worm-like micellar entanglement network owing to the addition and discharge of CO2, the pH changes of the solution were tested (Figure 4). the results demonstrate that, as the bubbling time of N2 increases, the pH increases from 8.1 to 12.4, indicating that the removal of CO2 causes deprotonation of the SA/cyclen solution (300 mM). Fatty acids with CO2 still reversibly produce worm micelles mostly via pH changes and the reversible protonation of the fatty acids [28,33].
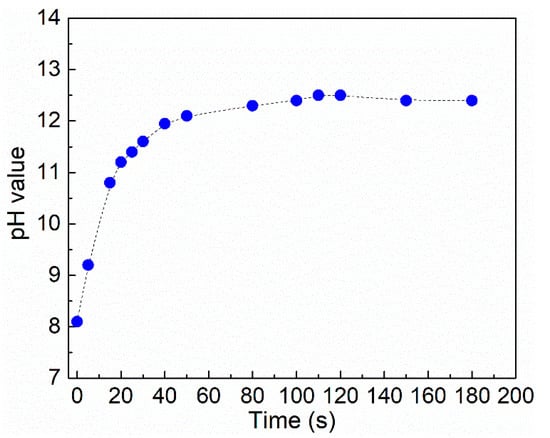
Figure 4.
Evolution of pH of SA/cyclen solution (300 mM) with increasing N2 bubbling time. (Temperature, 25 °C; flow rate of N2, 0.1 L·min−1).
2.2.2. Viscosity of Respective Solutions of SA and Cyclen
Although the molecules of SA and cyclen can be altered by the addition of CO2, the macroscopic properties of the separate solutions of cyclen or SA (concentration of SA, 1200 mM; concentration of cyclen, 300 mM) did not vary appreciably and always showed Newtonian fluid behavior with low viscosity between 0.8 and 1.5 mPa·s, which is quite different from the combination of SA/cyclen. The mixing of SA and cyclen leads to the formation of pseudo-tetrameric surfactants, which are expected to generate micelles that resemble wormlike micelles, as shown in Figure 3.
2.2.3. Ratio of SA and Cyclen
Fixing the concentration of SA at 1200 mM and varying the concentration of cyclen (Figure 5), it was found that the viscosity of the mixed solution did not change with varying molar ratio of SA/cyclen in the presence of CO2; whereas, after the removal of CO2, the viscosity reached 3000 mPas and remained nearly constant up to a ratio of SA/cyclen of about 4. This provides more evidence that the tetrameric surfactant exists and that the viscosity of the SA/cyclen solution is mostly determined by the concentration of the tetrameric surfactant.
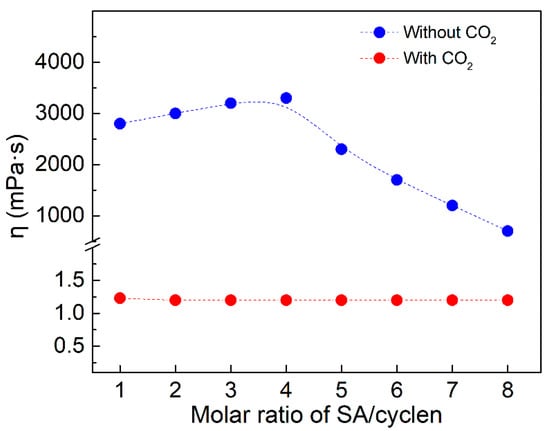
Figure 5.
Change in viscosity with varying molar ratio of CSDS/CTMPDA with SA concentration fixed at 1200 mM. (Temperature, 25 °C; shear rate, 10 s−1).
Combining the above experimental results, the mechanism of the CO2-switchable behavior of SA/cyclen can be summarized as follows: In the presence of CO2, mixed SA/cyclen behaves more like pure SA in water, resulting in low viscosity; however, when N2 is introduced to discharge CO2, SA becomes an anionic surfactant. Under the influence of electrostatic attraction, one molecule of cyclen and four molecules of SA combine to form a pseudo-tetrameric surfactant with non-covalently interaction, which microscopically forms viscoelastic worm-like micelles. When CO2 is added, the carboxyl group of SA is protonated, causing electrostatic attraction to decrease, and the structure of the pseudo-tetrameric surfactant collapses, causing the worm-like micelles to disassemble. The cyclen molecule serves as a spacer for the pseudo-tetrameric surfactant. This mechanism is consistent with the previously reported mechanism of the anionic surfactant sodium dodecyl sulfate and diamines switched by CO2 [34].
2.3. Effect of Sparging Time for CO2
Figure 6 illustrates the variation of η in the SA/cyclen solution (300 mM) with saturated CO2 with the bubbling time of N2 to remove CO2. At the commencement of CO2 removal, η climbs quickly over a short period, then plateaus after around 100 s, showing a maximum value of the viscosity. This turning point correlates with the variation of pH with the period of bubbling CO2, i.e., when η approaches equilibrium, the pH decreases to its lowest. In the presence of CO2, SA is protonated, but in the absence of CO2, SA becomes an anionic surfactant that combines with cyclen to form a pseudo-tetrameric surfactant with non-covalent bonds, resulting in a rapid rise in η. After approximately 60 s, the change in η tends to moderate, meaning a maximum concentration of the pseudo-tetrameric surfactant has been reached and no new networks of worm-like micelles are formed. This demonstrates that the viscosity of the SA/cyclen combination is significantly influenced by the concentration of the pseudo-tetrameric surfactant.
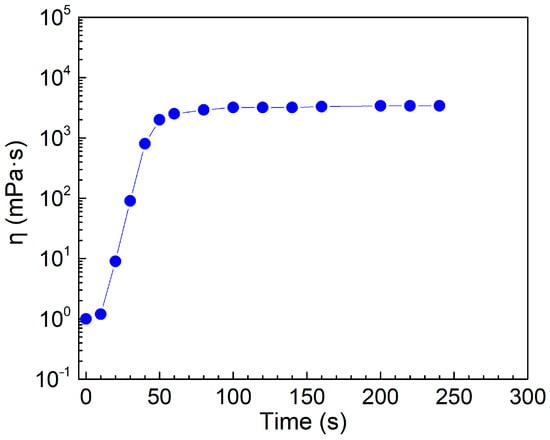
Figure 6.
Change in viscosity of SA/cyclen solution (300 mM) with N2 bubbling time for the removal of CO2 (Temperature, 25 °C; shear rate, 10 s−1; flow rate of N2, 0.1 L·min−1).
2.4. Effect of SA/Cyclen Concentration
Figure 7 demonstrates that, in the presence of CO2, η0 increases very slowly with increasing surfactant concentration, and there is no discernible change in the entire concentration range; after the removal of CO2, the curve is clearly divided into two distinct regions, and the concentration corresponding to the inflection point is the critical overlapping concentration.
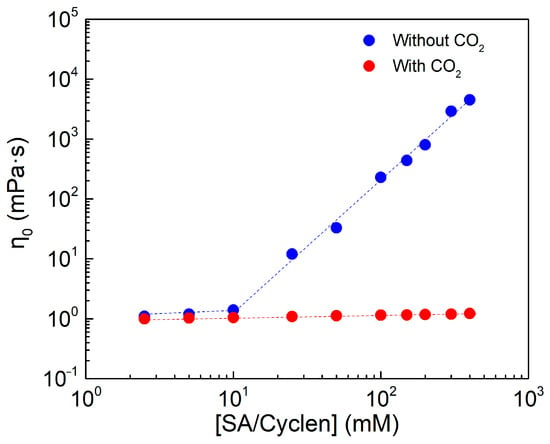
Figure 7.
The effect of concentration on viscosity of SA/cyclen solution without and with CO2 at 25 °C, the shear rate is 10 s−1.
2.5. Effect of Amine Types
The connection between η and concentration for a pseudo-tetrameric surfactant with SA and different types of amines is shown in Table 1 and Figure 8. The curves of viscosity are clearly split into two sections. In the dilute solution zone, η grows slowly with increasing concentration; in the sub-concentrated solution region, η grows exponentially with increasing concentration. Under the same concentration—100 mM for example—η of the SA/cyclen solution has the largest value, indicating that when the spacer consists of two methylene groups, the pseudo-tetrameric surfactant formed has the greatest ability to self-assemble into worm-like micelles. Table 1 and Figure 8 demonstrate that the size of the spacer in the amine molecule is one of the most influential elements influencing the formation of wormlike micelles for pseudo-tetrameric surfactants.

Table 1.
Comparison of the viscosity of SA mixed with different amines (molar ratio, 4:1).
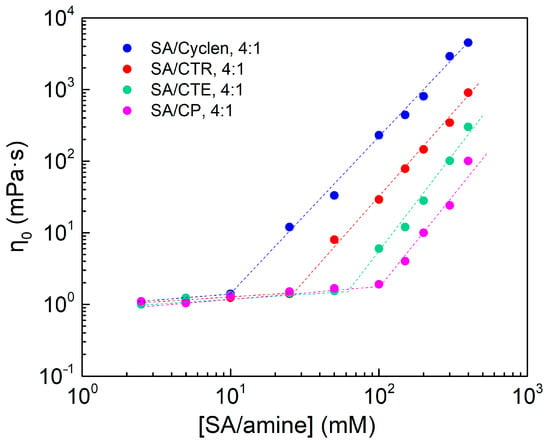
Figure 8.
The effect of concentration on viscosity of SA/amine at 25 °C, the shear rate is 10 s−1.
2.6. Effect of Hydrophobic Chain Length
Figure 9 shows the three distinct hydrophobic tail chain lengths of tetrameric surfactant micelle solutions may also be separated into slow- and rapid-growth regions. Table 2 shows that the longer the hydrophobic chain, the higher the viscosity; and the reason is that a stronger hydrophobic associative force of the surfactant improves the creation of wormlike micelles. There is a possible relationship between the inflection point and the critical micelle concentration (CMC) of the fatty acids. The CMC of the fatty acids increases by a factor of two to eight for every two carbon atoms removed from the alkyl chain [35]. The pattern of CMC is comparable to that of the inflection point in Figure 9.
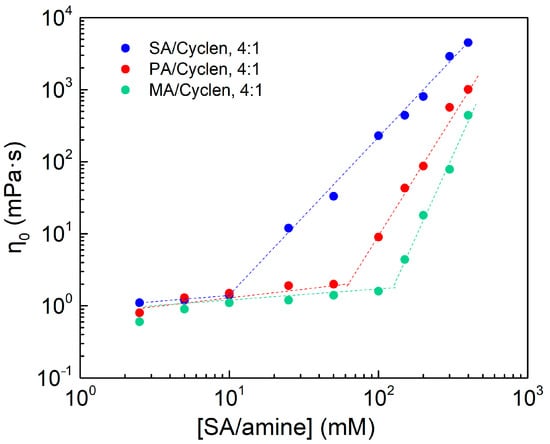
Figure 9.
The effect of concentration on viscosity of long-chain carboxylic acid/cyclen at 25 °C, the shear rate is 10 s −1.

Table 2.
Comparison of different long-chain carboxylic acids mixed with cyclen (molar ratio, 4:1).
3. Experimental Procedures
3.1. Materials
Stearic acid (SA, 95%), palmitic acid (PA, 99%), myristic acid (MA, 99%), cyclen (97%), 1,4,8,12-tetraazacyclopentadecane (CP, 97%), and 1,4,8,11-tetraazacyclotetradecane (CTE, 98%) were purchased from Sigma-Aldrich (Burlington, MA, USA). 1,4,7,10-tetraazacyclotridecane (CTR, 95%) was supplied by BidePharm Co. (Shanghai, China). These reagents are used directly after purchase, without further purification. CO2 (≥99.998%) and N2 (99.998%) were supplied by Xuyuan Chemical Industry Co. Ltd. (Chengdu, China) and were used as received. Ultrapure water (resistivity, 18.25 MΩ·cm−1) was obtained using an ultrapure water purification system (Chengdu Ultrapure Technology Co., Ltd., Chengdu, China).
3.2. Preparation of Solution
Unless otherwise specified, all samples were produced in a 4:1 molar ratio of fatty acid to amine. The fatty acid and amine were accurately weighed, dissolved in ultrapure water, and placed in an oscillating water bath (VWR Sheldon 1217, VWR, Part of Avantor, Radnor, PA, USA) at a constant temperature for at least 24 h to obtain a homogenous clarified solution of the mother liquor. The temperature range of the oscillating water bath was from 5 to 99.9 °C; its temperature sensitivity was ±0.07 °C. A typical example is SA/cyclen as the combination of fatty acid and amine. Concentrations were computed as “SA/cyclen”, i.e., the combination of 4 mol·L−1 of SA and 1 mol·L−1 of cyclen are recorded as 1 mol·L−1 of SA/cyclen. The mother liquor was diluted with ultrapure water to produce the other solutions. The other solutions were produced by diluting the mother liquor with ultrapure water. Then, CO2 was bubbled into the samples at a flow rate of 0.1 L·min−1 while oscillating continuously. Unless otherwise specified, the CO2 was fed through until the pH of the system stopped changing. To remove CO2 from the system, N2 was supplied into the surfactant solution at a flow rate of 0.1 L·min−1 until the pH remained stable. Prior to any measurements, all samples were kept in a water bath at the measurement temperature for at least 24 h.
3.3. pH Testing
The reagent bottle holding the sample to be tested was put in a water bath maintained at a constant temperature. After 30 min, the electrode of a pH meter (PB-10 basic, Sartorius AG, Gottingen, Germany) was submerged in the sample solution until the temperature and pH readings reached equilibrium. Each sample was tested three times in parallel with a relative variation of less than 2%, with the average of the three readings being taken as the pH value of the sample. Before each measurement, the pH meter was calibrated using a standard buffer solution.
3.4. Rheological Performance
The rheological qualities were investigated using a Physica MCR301 rheometer (Anton Paar, Graz, Austria) with a 13.33 mm-radius concentric-axis cylinder CC27 rotor and a rotor (radius, 14.46 mm). The sample temperature was monitored with an accuracy of 0.01 °C utilizing a Peltier thermocouple temperature control device in the rheometer. On the measuring equipment, a solvent trap was used to limit sample evaporation. Each time the instrument was turned on, it was calibrated using Cannon standard oil to decrease measurement mistakes. To ensure that the samples were in equilibrium, they were placed in a water bath at the measuring temperature for more than one hour before being measured and then placed in a revolving cylinder for five minutes. The steady-state shear experiments were performed in rate-controlled mode with shear rates ranging from 10−3 to 102 s−1.
3.5. Cryo-TEM
The microstructure of the micellar solutions was observed by Cryo-TEM (JEM2010, JEOL Ltd., Tokyo, Japan). The working and instrumental conditions were: a working accelerating voltage of 200 kV, the image acquisition system was a CCD imaging system with a Gatan 832 camera, the controlled temperature of the cryo-transfer frame Gatan-626 was not higher than −174 °C, and the microsieve was a Quantifoil 1.2/1.3. The thoroughly dissolved micellar solution was kept stable in a water bath at the set temperature for 1 h, then 3 μL of the sample was quickly pipetted onto the carbon grid. The resulting thin layer of micellar solution applied uniformly to the carbon grid was then quickly immersed in liquid ethane cooled to −175 °C by liquid nitrogen for quenching and cooling, and the treated sample was then stored in liquid nitrogen for Cryo-TEM observation.
4. Conclusions
Electrostatic attraction bonds the quaternary ammonium salt of cyclen to four molecules of SA, generating a pseudo-tetrameric surfactant, increasing the volume of the hydrophobic tail group, and forming a viscoelastic wormlike micelle. After the introduction of CO2, the pseudo-tetrameric surfactant is destroyed and reverted to protonated SA, leading to low viscosity. The enhanced viscosity switched by CO2 stimulation was stable across several cycles in testing. The impacts of several factors on the rheological characteristics of the wormlike micellar structure were analyzed independently, including the introduction time of CO2, surfactant concentration, size of the hydrophobic tail group, and the type of amines. In addition, the pseudo-tetrameric surfactant is generated using only commercially available, affordable anionic surfactants and cyclen in a 4:1 molar ratio in aqueous solution to create CO2-switchable wormlike micelles without complicated chemical synthesis. Moreover, the length of hydrophobic tail chains for SA and the size of spacer groups for cyclen also influence the formation of wormlike micelles, rather than the concentration of the surfactant. A longer hydrophobic chain of the surfactant and shorter spacer group of the amines improve the formation of worm-like micelles.
This approach has numerous benefits, including a single component and ease of preparation. The wormlike micelles described in this paper are more accessible and environmentally friendly than other CO2-switchable micelles because they can be created by merely adjusting the pH of a solution containing a natural fatty acid and an amine, without the requirement for sophisticated organic synthesis or the addition of hydrotropes. It is anticipated that this pseudo-tetrameric surfactant can be employed as a clean fracturing fluid in oil and gas field production enhancement activities; for example, if a dry well cannot be automatically broken, this can be remedied by eliminating CO2 from the surfactant. Moreover, results reveal that this pseudo-tetrameric surfactant was sensitive to CO2 and had potential channel-blocking performance in porous media, which would be efficient for enhanced oil recovery in diverse deposits. Thus, this pseudo-tetrameric surfactant could serve as inspiration for field applications of CO2-sensitive gel technology.
Author Contributions
X.S. conceived and designed the study; X.H. wrote the first draft of the manuscript; X.W. and D.Z. carried out the research; X.W. and D.Z. analyzed the data. All authors contributed to and approved the final draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by the Natural Science Foundation of Sichuan Province (2022NSFSC0197) and the State Key Laboratory of Polymer Materials Engineering (sklpme2022-2-09). This work was also supported by the Open Fund of the Key Lab of Organic Optoelectronics & Molecular Engineering.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this article will be available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cates, M.E.; Candau, S.J. Statics and dynamics of worm-like surfactant micelles. J. Phys.-Condes. Matter 1990, 2, 6869–6892. [Google Scholar] [CrossRef]
- Magid, L.J. The surfactant-polyelectrolyte analogy. J. Phys. Chem. B 1998, 102, 4064–4074. [Google Scholar] [CrossRef]
- Rehage, H.; Hoffmann, H. Viscoelastic surfactant solutions: Model systems for rheological research. Mol. Phys. 1991, 74, 933–973. [Google Scholar] [CrossRef]
- Yang, J. Viscoelastic wormlike micelles and their applications. Curr. Opin. Colloid Interface Sci. 2002, 7, 276–281. [Google Scholar] [CrossRef]
- Zakin, J.L.; Bewersdorff, H.W. Surfactant drag reduction. Rev. Chem. Eng. 1998, 14, 253–320. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Fang, B.; Talmon, Y.; Ge, W.; Raghavan, S.R.; Zakin, J.L. Light-responsive threadlike micelles as drag reducing fluids with enhanced heat-transfer capabilities. Langmuir 2011, 27, 5806–5813. [Google Scholar] [CrossRef]
- Dreiss, C.A. Wormlike micelles: Where do we stand? Recent developments, linear rheology and scattering techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef]
- Ezrahi, S.; Tuval, E.; Aserin, A. Properties, main applications and perspectives of worm micelles. Adv. Colloid Interface Sci. 2006, 128, 77–102. [Google Scholar] [CrossRef]
- Bernheim-Groswasser, A.; Wachtel, E.; Talmon, Y. Micellar growth, network formation, and criticality in aqueous solutions of the nonionic surfactant C12E5. Langmuir 2000, 16, 4131–4140. [Google Scholar] [CrossRef]
- Porte, G. Giant micelles in ideal solutions–either rods or vesicles? J. Phys. Chem. 1983, 87, 3541–3550. [Google Scholar] [CrossRef]
- Turner, M.S.; Marques, C.; Cates, M.E. Dynamics of wormlike micelles: The “bond-interchange” reaction scheme. Langmuir 1993, 9, 695–701. [Google Scholar] [CrossRef]
- Reber, A.C.; Khanna, S.N.; Ottenbrie, R. Thermodynamic stability of polyacrylamide and poly(N,N-dimethyl acrylamide). Polym. Adv. Technol. 2007, 18, 978–985. [Google Scholar] [CrossRef]
- Minagawa, K.; Koyama, K. Electro- and magneto-rheological materials: Stimuli-induced rheological functions. Curr. Org. Chem. 2005, 9, 1643–1663. [Google Scholar] [CrossRef]
- Cates, M.E. Reptation of living-polymers-dynamics of entangled polymers in the presence of reversible chain-scission reactions. Macromolecules 1987, 20, 2289–2296. [Google Scholar] [CrossRef]
- Lin, Y.; Qiao, Y.; Yan, Y.; Huang, J. Thermo-responsive viscoelastic wormlike micelle to elastic hydrogel transition in dual-component systems. Soft Matter 2009, 5, 3047–3053. [Google Scholar] [CrossRef]
- Abe, M.; Tobita, K.; Sakai, H.; Kamogawa, K.; Momozawa, N.; Kondo, Y.; Yoshino, N. Thermoresponsive viscoelasticity of concentrated solutions with a fluorinated hybrid surfactant. Colloid Surf. A-Physicochem. Eng. Asp. 2000, 167, 47–60. [Google Scholar] [CrossRef]
- Salkar, R.A.; Hassan, P.A.; Samant, S.D.; Valaulikar, B.S.; Kumar, V.V.; Kern, F.; Candau, S.J.; Manohar, C. A thermally reversible vesicle to micelle transition driven by a surface solid-fluid transition. Chem. Commun. 1996, 1223–1224. [Google Scholar] [CrossRef]
- Klijn, J.E.; Stuart, M.C.A.; Scarzello, M.; Wagenaar, A.; Engberts, J.B.F.N. pH-dependent phase behavior of carbohydrate-based gemini surfactants. Effect of the length of the hydrophobic spacer. J. Phys. Chem. B 2006, 110, 21694–21700. [Google Scholar] [CrossRef]
- Johnsson, M.; Wagenaar, A.; Stuart, M.C.A.; Engberts, J. Sugar-based gemini surfactants with pH-dependent aggregation behavior: Vesicle–to–micelle transition, critical micelle concentration, and vesicle surface charge reversal. Langmuir 2003, 2003 19, 4609–4618. [Google Scholar] [CrossRef]
- Sakai, H.; Orihara, Y.; Kodashima, H.; Matsumura, A.; Ohkubo, T.; Tsuchiya, K.; Abe, M. Photoinduced reversible change of fluid viscosity. J. Am. Chem. Soc. 2005, 127, 13454–13455. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, X.; Qiao, Y.; Yu, C.; Li, Z.; Yan, Y.; Huang, J. Creation of photo-modulated multi-state and multi-scale molecular assemblies via binary-state molecular switch. Soft Matter 2010, 6, 902–908. [Google Scholar] [CrossRef]
- Yang, Y. Novel redox-responsive surfactant. Electrochem. Soc. Interface 2006, 15, 58–59. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Orihara, Y.; Kondo, Y.; Yoshino, N.; Ohkubo, T.; Sakai, H.; Abe, M. Control of viscoelasticity using redox reaction. J. Am. Chem. Soc. 2004, 126, 12282–12283. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Schuur, B.; Nijland, M.; Blahušiak, M.; Juan, A. CO2-switchable solvents as entrainer in fluid separations. ACS Sustain. Chem. Eng. 2018, 6, 10429–10435. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.M.; Jessop, P.G. ”Switchable water”: Aqueous solutions of switchable ionic strength. ChemSusChem 2010, 3, 467–470. [Google Scholar] [CrossRef]
- Cunningham, M.C.; Jessop, P.G. Carbon dioxide-switchable polymers: Where are the future opportunities? Macromolecules 2019, 52, 6801–6816. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Feng, Y. CO2-responsive anionic wormlike micelles based on natural erucic acid. Green Mat. 2014, 2, 95–103. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, G. Visco-elastic properties of VES diverting acid for carbonate reservoirs. Chin. J. Chem. Eng. 2010, 18, 511–514. [Google Scholar] [CrossRef]
- Verma, G.; Aswal, V.K.; Hassan, P. pH-Responsive self-assembly in an aqueous mixture of surfactant and hydrophobic amino acid mimic. Soft Matter 2009, 5, 2919–2927. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y. pH Switchable wormlike micelles. Chem. Commun. 2010, 46, 9028–9030. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, S.; Tang, L.; Yang, S.; Ma, T.; Su, X. Application of CO2-switchable oleic-acid-based surfactant for reducing viscosity of heavy oil. Molecules 2021, 26, 6273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, Y.; Chu, Z.; He, S.; Zhang, J.; Feng, Y. Thermally induced structural transitions from fluids to hydrogels with pH-switchable anionic wormlike micelles. J. Colloid Interface Sci. 2013, 394, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Wang, Y.; Li, X. CO2-switchable viscoelastic fluids based on a pseudogemini surfactant. Langmuir 2013, 29, 4187–4192. [Google Scholar] [CrossRef]
- Hossain, M.S.; Berg, S.; Bergström, C.A.S.; Larsson, P. Aggregation behavior of medium chain fatty acids studied by coarse-grained molecular dynamics simulation. AAPS PhamSciTech 2019, 20, 61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).