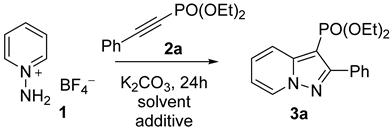
Abstract
A series of pyrazolo[1,5-a]pyridine-3-ylphosphonates were prepared with moderate to good yields by the oxidative [3+2]cycloaddition of 2-subtituted ethynylphosphonates with in situ generated pyridinium-N-imines and their annulated analogs. 2-Aliphatic and 2-Ph acetylenes demonstrate low activity, and the corresponding pyrazolopyridines were achieved with a moderate yield in the presence of 10 mol% Fe(NO3)3·9H2O. At the same time, tetraethyl ethynylbisphosphonate, diethyl 2-TMS- and 2-OPh-ethynylphosphonates possess much greater reactivity and the corresponding pyrazolo[1,5-a]pyridines, and their annulated derivatives were obtained with good to excellent yields without any catalyst. 2-Halogenated ethynylphosphonates also readily reacted with pyridinium-N-imines, forming complex mixtures containing poor amounts of 2-halogenated pyrazolopyridines.
1. Introduction
Pyrazolo[1,5-a]pyridine is an important structural motif in modern medicinal chemistry, as it possesses high metabolic stability and acts as an isostere to indole, purine or other azaindole cores. An anti-inflammatory drug ibudilast (3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine) (Figure 1) has been marketed in Japan for over 25 years and used for the treatment of asthma, post-stroke dizziness and ocular allergies [1,2]. Over the recent years, ibudilast has been paid much attention as an agent for therapy of multiple sclerosis and neurodegenerative disorders [3,4]. Recently, pyrazolo[1,5-a]pyridine scaffolds have been utilized in the design of DDX3X helicase [5], Pan-JAK kinase [6], C-terminal Src kinase [7], human dihydroorotate dehydrogenase [8], p110α-selective PI3 kinase [9], p38 kinase [10], ERK inhibitors [11], 5-HT4 [12], EP1 [13], D3 dopamine receptors antagonists, antitubercular [14] and antimalarial [15] agents, etc. Numerous general approaches have been reported for the construction of this heterocyclic core [16,17]. Among them, the 1,3-dipolar cycloaddition of electron-deficient alkynes and alkenes, such as unsaturated carbonyl compound [18] or nitroalkenes [19,20], with pyridinium-N-imines followed by oxidation is one of the most common methods of pyrazolo[1,5-a]pyridine synthesis.
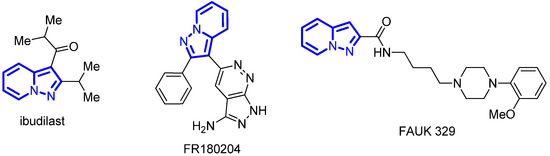
Figure 1.
Biologically active derivatives of pyrazolo[1,5-a]pyridine.
Due to the great importance of phosphonate groups in drug design research [21,22], the combination of a pyrazolo[1,5-a]pyridine moiety with a phosphonate group could be a promising building block for developing new pharmaceuticals. Previously, 2-substituted pyrazolo[1,5-a]pyridine-5-phosphonates were obtained by condensation of pyrazole-5-carbaldehydes with diethyl (3-bromoprop-1-en-1-yl)phosphonate [23] (Scheme 1a). The cycloaddition of EWG-substituted alkynylphosphonates with pyridinium-N-imines led to pyrazolo[1,5-a]pyridine-2-phosphonates [24] and to 3-phosphonates [25] when the EWG was a benzoyl or a perfluoroalkyl group, respectively (Scheme 1b). Since such cycloadditions represent fast and efficient routes to phosphorylated heterocylces, we decided to explore alkynylphosphonates with aliphatic, phenyl, OPh, halogen, TMS and PO(OEt)2-substituents at the triple bond in the reaction with pyridinium-N-imines. In contrast to benzoyl and perfluoroalkyl groups, aliphatic and phenyl substituents show electron donating properties and may reduce the reactivity of acetylenes in such cycloaddition reactions. At the same time, according to the Hammett parameter [26], the PO(OEt)2-group has a slightly stronger electron-withdrawing effect than CO2R. Thus, tetra-alkyl ethynylbisphosphonates have long been recognized to be active in Diels-Alder and 1,3-dipolar cycloaddition reactions [27]. Substituted dienes [28], furane [29], anthracene derivatives [30] and pyrones [31,32] were applied as diene components, and azides [33], nitrile oxides [34] and diazomethane [27] were used as 1,3-dipoles. Dialkyl ethynylphosphonates are also effective dipolarophiles and are often used for the construction of triazole [35,36], pyrazole [37,38] and β-lactam [39] units. However, only several examples of cycloaddition reactions of 2-substituted ethynylphosphonates bearing aryl-, alkyl- or sulfonamide substituent with azides [40,41] or nitrile oxides [42,43] have been reported recently.
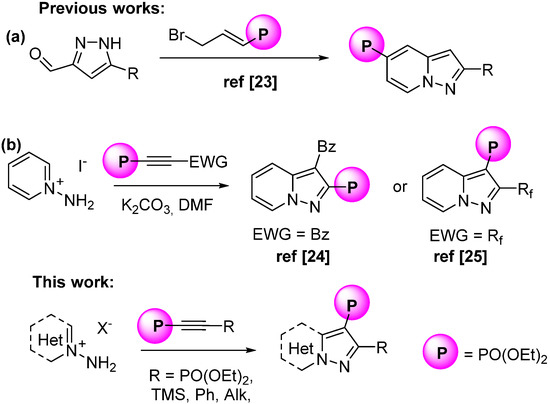
Scheme 1.
Approaches toward pyrazolo[1,5-a]pyridinyl phosphonate synthesis [24,25].
2. Results
At the beginning of our work, N-aminopyridinium tetrafluoroborate 1 and diethyl phenylethynylphosphonate 2a were taken as model substrates for screening optimal conditions. The reaction was followed using 31P NMR to estimate the phosphonate conversion and yield of products. The commonly used K2CO3/MeCN system (Table 1, entry 1) failed to reach the full conversion of 2a, and only a 5-fold excess of salt 1 allowed for achieving the complete consumption of reagent 2a. In the next step, various additives were tried to increase the reactivity of 2a. Initially, we hypothesized that the electron-withdrawing character of the PO(OEt)2-group could be increased by the coordination of metal ions to the phosphonate group oxygen atom. However, strong Lewis acids such as AlCl3 or ZnCl2 preferably interacted with N-imines and, therefore, were not suitable for catalysis. Then, AgNO3 was used, as it was effective in the catalytic hydration of alkynylphosphonates [44]. However, no effect was found with 10 mol% AgNO3 (Table 1, entry 3). Next, we paid our attention to LiCl, which is known to catalyze cycloaddition reactions. The usage of 10 mol% led to notable conversion growth (Table 1, entry 4), but the increased load of LiCl resulted in lowering the conversion (Table 1, entry 5). The addition of Ni and Co salts had no significant effect on the reaction. During further experiments, redox-active additives were employed to accelerate the oxidation step of pyrazolo[1,5-a]pyridine synthesis (see discussion on the mechanism below). The application of chloranil or DDQ was unsuccessful, apparently due to the oxidation reaction with N-imines. Copper salts were previously applied for nitropyrazolo[1,5-a]pyridine synthesis from pyridinium-N-imines and nitrostyrenes [20]. However, in our case, Cu salts completely inhibited the reaction (Table 1, entries 9, 10). Fe(NO3)3 is broadly used as a catalyst in a wide scope of oxidation reactions. Recently, it has been found to be effective in the promotion of 3-acyl-1,2,4-oxadiazole synthesis from alkynes and nitriles [45]. Moreover, iron (III) nitrate mediated synthesis of acylisoxazoles from terminal alkynes was reported [46]. Therefore, we decided to try Fe(NO3)3 in nonahydrate form in our reaction. With an additive level of 10 mol% in CH3CN, we observed an 88% conversion of 2a (Table 1, entry 11). Interestingly, other iron salts, such as FeCl3 and FeSO4, were not as effective as Fe(NO3)3. Further experiments revealed that changing the solvent from CH3CN to the more polar DMSO led to the full conversion of 2a (Table 1, entries 12, 13). No yield changes were observed with an increase in the additive level from 10 to 20 mol%; moreover, 5 mol% Fe(NO3)3 did not lead to the full conversion of phosphonate. Heating had a negative effect on the reaction (Table 1, entry 16). Therefore, the use of Fe(NO3)3 as an additive and DMSO as a solvent were found to be the optimal conditions.

Table 1.
Optimization of the reaction conditions.
With the optimized conditions in hand, the scope of pyridinium salts and alkynylphosphonates was explored (Scheme 2). First, we examined the effect of the substituents in the pyridinium ring of N-aminopyridinium salts on the reaction with phosphonate 2a. The cycloaddition proceeded sluggishly in case of mild donating and moderate withdrawing substituents and required increased equivalents of starting salts. 2-Me-, 4-Me-, and 4-CO2Me-substituted N-aminopyridinium salts showed moderate to good yields of corresponding pyrazolopyridines 3b–e. Despite the bulkiness of phenyl group, a good yield of 7-Ph-substituted pyrazolopyridine 3d was obtained with only 1 eq of salt. Regioselectivity of the cycloaddition of 3d was confirmed using X-ray analysis [47]. Strong donating groups are not favorable and significantly reduce reactivity. Thus, pyrazolopyridine 3f was obtained only in 33% yield with 5 equivalents of 4-OMe-substituted salt. At the same time, 1-amino-4-NMe2-pyridinium mesitylenesulfonate was completely unreactive under used conditions. Such behavior was likely associated with the low acidity of the NH2-group, which could not be deprotonated by K2CO3. The use of DBU or t-BuOK as stronger bases also showed no positive results, apparently due to their reaction with alkyne. Quinolinium and isoquinolinium salts facilitated the dimerization of the corresponding N-imines [48,49] and no cycloaddition products were formed. Subsequently, we investigated the impact of the R-group in 2-R-alkynylphosphonate on the reaction with salt 1. Pyrazolopyridines 3h–j were obtained in moderate yields (30–40%), indicating that both alkyl and cycloalkyl groups led to the decrease in the corresponding phosphonate reactivity. Moreover, the phosphonate bearing the bulky t-Bu-group required a large excess of starting salts to obtain the desired product. α-Hydroxyalkyl substituted acetylenes were also moderately active and formed products 3k,l.
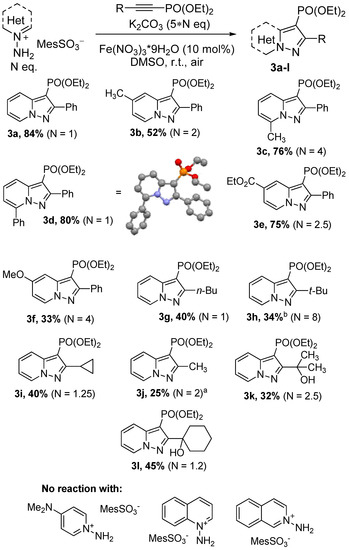
Scheme 2.
Reaction scopes for cycloaddition of N-imines with alkynylphosphonates (isolated yields). a Mixture of diethyl prop-1-yn-1-ylphosphonate and diethyl propa-1,2-diene-1-ylphosphonate was used. b Without the addition of Fe(NO3)3, 0% was observed.
Having succeeded in the Fe(NO3)3-catalyzed alkynylphosphonates cycloaddition, bisphosphonylated acetylene 4a was further studied. In contrast to phosphonate 2a, acetylene 4a showed much greater reactivity and showed pyrazolo[1,5-a]pyridine-2,3-bisphosphonates 5a–j with good to excellent yields without an Fe(NO3)3 additive (Scheme 3). However, pyridinium salts with strong donating groups were still much less reactive. Thus, 4-NMe2-substituted pyridinium salts did not undergo cycloaddition, and in the case of 4-OMe derivatives the corresponding pyrazolopyridine 5e was obtained with a moderate yield in the mixture with the parent 4-OMe-pyridine. Enhanced activity of alkyne 4a made it possible to obtain annulated derivatives 5g–j with relatively high yields.
TMS-substituted ethynylphosphonate 4b also possessed high reactivity toward pyridinium salts (Scheme 3). The loss of the TMS group during the process was observed and corresponding pyrazolopyridines 5k–o were obtained. Annulated derivatives 5p,q were prepared as well, but in the case of quinolinium and isoquinolinium salts only dimers of corresponding N-imines were observed. The higher activity of TMS-phosphonate 4b in contrast to 2-alkyl- and 2-phenylalkynylphosphonates could be explained by the fast removal of the TMS group of 4b in the K2CO3/DMSO medium with the formation of intermediate diethyl ethynylphosphonate.
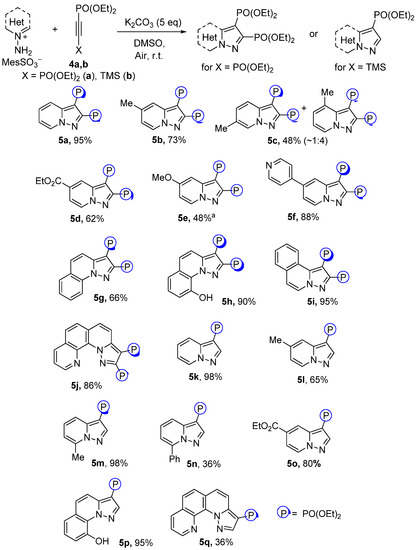
Scheme 3.
Reaction scopes for cycloaddition of N-imines with tetraethyl acetylene bisphosphonate (isolated yields). a The substance was obtained in mixture with 4-metoxypyridine and other unidentified products, yield determined using NMR with CH2Br2 as a standard.
Next, the applicability of 2-halogenated and 2-OPh alkynylphosphonates 4c–f in the cycloaddition reaction with pyridinium-N-imines was studied (Scheme 4). For all halogenated alkynes, the reaction resulted in the formation of complex reaction mixtures from which corresponding 2-Cl and 2-I-pyrazolopyridines 5r,t were isolated with a low yield. Investigation of the reaction mixtures using NMR 31P and GC-MS for alkyne 4c showed the formation of a significant amount of dehalogenated product 5k when DMSO was used as a solvent. Various solvents were screened and the maximum yield of 5r was reached in MeCN with K2CO3 as a base, while the nature of the inorganic base had no remarkable effect on the ratio of 5r:5k. When the excess of salt 1 was taken, formation of 2-aminopyrazolo[1,5-a]pyridine along with products 5k and 5r was also observed. In contrast to halogenated acetylenes 4c–e, the cycloaddition reaction of OPh-substituted phosphonate 4f in THF resulted in a good yield of pyrazolopyridine 5u. The regioselectivity for product 5u was confirmed using X-ray analysis [50].
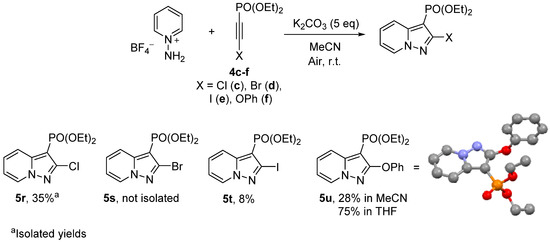
Scheme 4.
Reaction of pyridinium-N-imines with 2-halogenated diethyl acetylenephosphonate.
In terms of previous reports on N-imine cycloadditions [51], a plausible mechanism for the interaction of pyridinium salts with studied alkynes is shown in Scheme 5a. Ylide 6, formed by the deprotonation of salt 1a, reacts with alkynylphosphonates via concerted [3+2]cycloaddition or via Michael addition/intramolecular cyclization, yielding adduct 7. Such intermediates are typically not observable due to the fast rearrangement of dihydropyrazolopyridines 8, which lead to products 3a–l, 5a–q during oxidation. To shed some light on the role of iron (III) nitrate, we provided the reaction between 1a and 2a under an Ar atmosphere (Table 1, entries 19, 20). In the case of 10 mol% additive loading, only a poor yield of 3a was observed, comparable to the value of the additive load. However, when 1 eq of Fe(NO3)3 was taken, a full conversion of 2a was accomplished with a 70% isolated yield of 3a. Additionally, Fe(NO3)3 could affect the reactivity by coordination of Fe3+-ion to the phosphonate group or by nitration of the triple bond (Scheme 5b). Nevertheless, no changes using 31P NMR were found after the addition of iron nitrate to phosphonate 2a in the DMSO even after heating up to 100 °C. Therefore, it could be concluded that iron nitrate serves as a redox mediator in the oxidation of intermediate 8.
Taking into account the low reactivity of 2-alkyl and 2-Ph- acetylenephosphonates, the cycloaddition step in this case is slow and probably reversible, so large amounts of N-imines are lost to degradation. Therefore, additional amounts of N-imines are required. For acetylenes 4a–f, cycloaddition is fast and the oxidation of intermediate 8 is a limiting step.
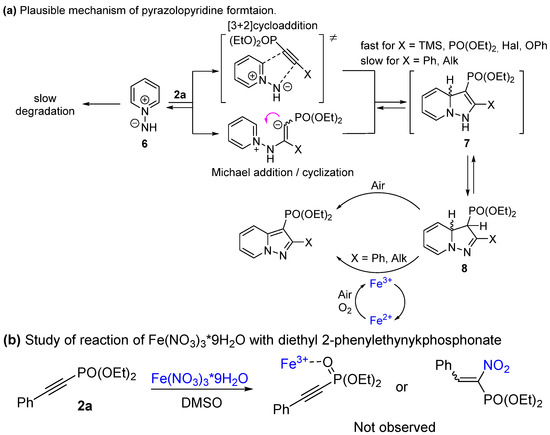
Scheme 5.
Plausible reaction mechanisms.
3. Materials and Methods
Starting materials, unless otherwise noted, were obtained from commercial supplies and used without purification. O-(mesitylenesulfonyl)hydroxylamine (MSH) [52], mesitylenesulfonates of N-aminated heterocycles [52] and 1-aminopyridinium tetrafluoroborate [53] were obtained from previously published procedures. For the preparation of phenyl, alkyl and cycloalkyl alkynylphosphonates, Zhao’s CuSO4-catalysed cross-coupling procedure was applied [54]. Diethyl 2-chloroethynylphosphonate was obtained from a dichloroacetylene solution using the procedure of Svintsitskaya et al. [55] (Caution! Dichloroacetylene is extremely explosive). Tetraethyl acetylenebisphosphonate [56], diethyl 2-bromoethynylphosphonate [57] and diethyl 2-iodoethynylphosphonate [58] were prepared according to previously described methods.
The TLC was carried out on Sorbfil silica plates (UV 254) with further UV light visualization. Flash column chromatography was performed on silica gel (Macherey Nagel, pore size 60 E, 230–400 mesh). Spectral and analytical studies were provided at the Chemical Service Centre of Siberian Branch of the Russian Academy of Sciences. NMR spectra were recorded on Bruker Avance-300 (300.13 MHz for 1H, 121.5 MHz for 31P) and Avance-400 (400.13 MHz for 1H and 100.62 MHz for 13C) spectrometers, using the residual proton and carbon signals of CDCl3 (δH 7.24 ppm; δC 77.16 ppm) as internal standards. The 13C NMR spectra were registered with C-H spin decoupling. Copies of spectra of newly obtained compound are presented in the Supplementary Materials. The masses of molecular ions were determined by HRMS on a DFS Thermo scientific instrument (EI, 70 eV). Melting points were determined using Kofler hot-stage microscope and are uncorrected.
XRD data were obtained on a Bruker Kappa Apex II CCD diffractometer (Mo Kα radiation and a graphite monochromator) at 296K. The structures were solved by direct methods and refined by full-matrix least-squares method against all F2 in anisotropic approximation using the SHELX2014 programs set [59]. The H atoms positions were calculated geometrically and refined with the riding model. Absorption corrections were applied empirically using SADABS programs [60]. The solvent molecule is highly disordered and, therefore, this molecule was removed using the SQUEEZE procedure in the PLATON program [61]. Solvent accessible volume was calculated as 138 Å3 in unit cell.
General procedure for diethyl 2-phenylpyrazolo[1,5-a]pyridine-3-phosphonates synthesis.
N-aminopyridinium salt (N mmol) was dissolved in 5 mL DMSO and K2CO3 (2*N mmol) was added to produce pyridinium-N-imine. The resulting mixture was stirred for 5 min and alkynylphosphonate (1 mmol) was added followed with Fe(NO3)3*9H2O (10 mol%). The solution was kept with stirring overnight at room temperature under air atmosphere. Then, the mixture was diluted in 50 mL water and extracted three times with 20 mL of CH2Cl2. Extracts were combined, washed with water and dried over Na2SO4. Then, solvent was removed in vacuo and the resulting oil was purified by flash chromatography on silica with CHCl3/MeOH (50:1) as the eluent.
Diethyl (2-phenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3a). Yellowish oil, 281mg (85%). NMR 1H (300 MHz, CDCl3): δ = 1.10 (t, J = 7.1 Hz, 6H), 3.89 (dp, J = 10.1 Hz, 7.3 Hz, 2H), 4.03 (dp, J = 10.1 Hz, 7.1 Hz, 2H), 6.89 (td, J = 6.9 Hz, 1.4 Hz, 1H), 7.30 (ddd, J = 9.2 Hz, 6.8 Hz, 1.2 Hz, 1H), 7.41 (m, 3H), 7.85 (m, 2H), 8.20 (d, J = 9.0 Hz, 1H), 8.50 (dd, J = 6.9 Hz, 1.1 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 7.1 Hz), 61.9 (d, J = 4.8 Hz), 93.59 (d, J = 221.0 Hz), 113.89, 119.7, 126.6, 128.2, 128. 8, 129.1, 129.6, 132.6, 145.9 (d, J = 26.3 Hz), 157.6 (d, J = 12.9 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.3 (s). HRMS (m/z): C17H19N2O3P+•, 330.1131 (found), 330.1133 (calc).
Diethyl (5-methyl-2-phenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3b). Yellowish oil, 179mg (52%). NMR 1H (300 MHz, CDCl3): δ = 1.09 (t, J = 6.8 Hz, 6H); 2.39 (s, 3H); 3.83–3.92 (m, 2H); 3.98–4.06 (m, 2H); 6.70 (dd, J = 7.0 Hz, 2.0 Hz, 1H); 7.34–7.41 (m, 3H); 7.81–7.85 (m, 2H); 7.98 (m, 1H); 8.36 (dd, J = 7.0 Hz, 2.2 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 15.8 (d, J = 7.2 Hz), 21.2; 61.4 (d, J = 4.8 Hz), 91.9 (d, J = 221.2 Hz), 115.9, 117.7, 127.6, 127.8, 128.7, 129.2, 132.5, 137.5, 145.9 (d, J = 26.4 Hz), 157.3 (d, J = 12.9 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.8 (s). HRMS (m/z): C18H21N2O3P+•, 344.1277 (found), 344.1284 (calc).
Diethyl (7-methyl-2-phenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3c). Yellowish oil, 261 mg (76%). NMR 1H (300 MHz, CDCl3): δ = 1.09 (t, J = 7.1 Hz, 6H), 2.75 (s, 3H), 3.81–3.94 (m, 2H), 3.96–4.04 (m, 2H), 6.74 (dd, J = 7.0 Hz, 1.1 Hz, 1H), 7.24 (dd, J = 9.1 Hz, 7.0 Hz, 1H), 7.33–7.44 (m, 3H), 7.85–7.91 (m, 2H), 8.11 (dm, J = 9.1 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 15.8 (d, J = 7.2 Hz), 17.8, 61.4 (d, J = 4.8 Hz), 93.1 (d, J = 220.4 Hz), 112.8, 116.7, 126.3, 127.8, 128.6, 129.4, 132.7, 138.5, 145.8 (d, J = 26.5 Hz), 156.6 (d, J = 12.7 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.8 (s). HRMS (m/z): C18H21N2O3P+•, 344.1286 (found), 344.1300 (calc).
Diethyl (2,7-diphenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3d). Yellowish solid, 324 mg (80%). M.p. 122–124 °C. NMR 1H (300 MHz, CDCl3) δ = 1.14 (t, J = 7.1 Hz, 6H), 3.87–4.00 (m, 2H),4.01–4.16 (m, 2H), 7.00 (dd, J = 7.1 Hz, 1.3 Hz, 1H), 7.36–7.52 (m, 7H), 7.87–7.96 (m, 4H), 8.28 (ddd, J = 9.0 Hz, 1.4 Hz, 0.6 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 7.2 Hz), 61.8 (d, J = 4.9 Hz), 93.4 (d, J = 220.0 Hz), 114.5, 118.3, 126.8, 128.0, 128.4, 128.9, 129.5, 129.7, 129.8, 132.8, 133.1 (d, J = 1 Hz), 140.9, 147.0(d, J = 27.1 Hz), 156.8 (d, J = 12.4 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.7 (s). HRMS (m/z): C23H23O3N2P+•, 406.1442 (found), 406.1441 (calc).
Ethyl 3-(diethoxyphosphoryl)-2-phenylpyrazolo[1,5-a]pyridine-5-carboxylate (3e). Yellowish oil, 301 mg (75%). NMR 1H (300 MHz, CDCl3): δ = 1.16 (t, J = 7.1 Hz, 6H), 1.42 (t, J = 7.1 Hz, 3H), 3.89–4.02 (m, 2H), 4.03–4.15 (m, 2H), 4.42 (q, J = 7.1 Hz, 2H), 7.41–7.49 (m, 3H), 7.52 (dd, J = 7.1 Hz, 1.8 Hz, 1H), 7.86–7.94 (m, 2H), 8.55 (ddd, J = 7.1 Hz, 2.1 Hz, 1.0 Hz, 1H), 8.90 (m, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 14.2, 15.9 (d, J = 7.1), 61.8, 61.9 (d, J = 5.3), 96.7 (d, J = 219.8), 112.8, 121.7, 128.1, 128.2, 128.4, 129.2, 129.4, 131.9, 144.5 (d, J = 25.5), 158.3 (d, J = 12.6), 164.6. NMR 31P (121.5 MHz, CDCl3): δ = 13.2 (s). HRMS (m/z): C20H23N2O5P+•, 402.1336 (found), 402.1339 (calc).
Diethyl (5-methoxy-2-phenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3f). Yellowish oil, 118mg (33%). NMR 1H (300 MHz, CDCl3): δ = 1.11 (t, J = 7.1 Hz, 6H), 3.82–3.95 (m, 2H), 3.90 (s, 3H), 3.97–4.10 (m, 2H), 6.58 (dd, J = 7.5 Hz, 2.8 Hz, 1H), 7.36–7.45 (m, 3H), 7.51 (d, J = 2.8 Hz, 1H), 7.80–7.86 (m, 2H), 8.31 (dd, J = 7.5 Hz, 2.0 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): 15.9 (d, J = 7.2 Hz), 55.7, 61.5 (d, J = 5.0 Hz), 91.8 (d, J = 222.4 Hz), 96.5, 108.1, 127.9, 128.8, 129.20, 129.22, 132. 6, 147.6 (d, J = 26.1 Hz), 157.8 (d, J = 12.8 Hz), 158.6. NMR 31P (121.5 MHz, CDCl3): δ = 15.2 (s). HRMS (m/z): C18H21N2O4P+•, 360.1235 (found), 360.1234 (calc).
Diethyl (2-butylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3g). Yellow liquid, 124 mg (40%). NMR 1H (300 MHz, CDCl3): δ = 0.92 (t, J = 7.3 Hz, 3H), 1.28 (t, J = 7.1 Hz, 6H), 1.41 (m, 2H), 1.74 (m, 2H), 2.93 (m, 2H), 3.91–4.02 (m, 2H), 4.03–4.19 (m, 2H), 6.82 (td, J = 6.9 Hz, 1.4 Hz, 1H), 7.24 (ddd, J = 8.9 Hz, 6.8 Hz, 1.2 Hz, 1H), 7.95 (dt, J = 8.9 Hz, 1.3 Hz, 1H), 8.40 (dm, J = 6.8 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 13.8, 16.2 (d, J = 6.9 Hz), 22.6, 27.6, 31.5, 61.4 (d, J = 5.0 Hz), 93.4 (d, J = 223.3 Hz), 112.7, 118.4, 126.0, 128.4, 144.5 (d, J = 26.8 Hz), 160.3 (d, J = 14.7 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 16.3 (s). HRMS (m/z): C15H23N2O3P+•, 310.1441 (found), 310.1445 (calc).
Diethyl (2-tert-butylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3h). Yellow oil, 105 mg (34%). NMR 1H (300 MHz, CDCl3): δ = 1.25 (t, J = 7.0 Hz, 6H), 1.47 (s, 9H), 3.91–4.17 (m, 4H), 6.79 (td, J = 7.0 Hz, 1.2 Hz, 1H), 7.19 (ddd, J = 9.1 Hz, 6.8 Hz, 1.2 Hz, 1H), 8.01 (dt, J = 9.1 Hz, 1.2 Hz, 1H), 8.38 (ddt, J = 6.9 Hz, 2.1 Hz, 1.1 Hz). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7.1 Hz), 30.2, 34.4, 61.6 (d, J = 5.0 Hz), 91.9 (d, J = 221.7 Hz), 112.7, 118.9, 126.2, 128.7, 145.7 (d, J = 25.4 Hz), 167.5 (d, J = 15.4 Hz), 173.9. NMR 31P (121.5 MHz, CDCl3): δ = 16.1 (s). HRMS (m/z): C15H23N2O3P+•, 310.1442 (found), 310.1445 (calc).
Diethyl (2-cyclopropylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (3i). Yellowish liquid, 118 mg (40%). NMR 1H (300 MHz, CDCl3): δ = 0.98–1.09 (m, 4H), 1.28 (t, J = 7.0 Hz, 6H), 2.35–2.45 (m, 1H), 3.95–4.06 (m, 2H), 4.08–4.19 (m, 2H), 6.79 (td, J = 6.9 Hz, 1.4 Hz, 1H), 7.23 (ddd, J = 8.9 Hz, 6.9 Hz, 1.2 Hz, 1H), 7.92 (dt, J = 8.9 Hz, 1.2 Hz, 1H), 8.35 (dm, J = 6.9 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 8.3, 9.4, 16.1 (d, J = 6.9 Hz), 61.4 (d, J = 4.7 Hz), 93.7 (d, J = 222.2), 112.6, 117.9, 125.9, 128.3, 144.6 (d, J = 26.3 Hz), 161.3 (d, J = 14.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 15.5 (s). HRMS (m/z): C14H19N2O3P+•, 294.1126 (found), 294.1128 (calc).
Diethyl (2-methyl[1,5-a]pyridin-3-yl)phosphonate (3j). Orange oil, 76.0 mg (25 %). NMR 1H (300 MHz, CDCl3): δ = 1.26 (t, J = 7.2Hz, 6H), 2.23 (s, 3H), 3.90–4.04 (m, 2H), 4.04–4.17 (m, 2H), 6.80 (t, J = 6.7Hz, 1H), 7.24 (t, J = 7.8Hz, 1H), 7.92 (d, J = 9.1Hz, 1H), 8.37 (d, J = 6.8Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 13.7, 16.4 (d, J = 7Hz), 61.6 (d, J = 5Hz), 94.2 (d, J = 223Hz), 112.9, 118.4, 126.4, 128.5, 144.8 (d, J = 26.6 Hz), 156.2 (d, J = 14.0 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 15.2 (s). HRMS (m/z): C12H17O3N2P+•, 268.0968 (found), 268.0971 (calc).
Diethyl (2-(2-hydroxypropan-2-yl)pyrazolo[1,5-a]pyridin-3-yl)phosphonate (3k). Recrystallization from ethanol. Yellowish solid, 99.9 mg (32%). M.p. 82–84 °C. NMR 1H (300 MHz, CDCl3): δ = 1.29 (t, J = 7.1 Hz, 6H), 1.72 (s, 6H), 6.95–4.09 (m, 2H), 4.11–4.24 (m, 2H), 6.45 (s, 1H), 6.89 (td, J = 6.9 Hz, 1.4 Hz, 1H), 7.31 (ddd, J = 8.9 Hz, 6.9 Hz, 1.2 Hz, 1H), 7.71 (dt, J = 8.9 Hz, 1.2 Hz, 1H), 8.46 (dm, J = 6.9 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 6.9 Hz), 29.9, 62.1 (d, J = 4.9 Hz), 69.8, 90.9 (d, J = 218.7 Hz), 113.1, 117.8, 126.6, 129.0, 143.9 (d, J = 22.2 Hz), 167.9 (d, J = 16.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 18.3 (s). HRMS (m/z): C14H21N2O4P+•, 312.1241 (found), 312.1243 (calc).
Diethyl (2-(1-hydroxycyclohexyl)pyrazolo[1,5-a]pyridin-3-yl)phosphonate (3l). Recrystallization from ethanol. Orange solid, 158 mg (45%). NMR 1H (300 MHz, CDCl3): δ = 1.25 (t, J = 7.0 Hz, 6H), 1.62 (m, 4H), 1.90 (m, 6H), 2.11 (d, J = 11.0 Hz, 3H), 3.98 (m, 2H), 4.13 (dp, J = 10.1, 7.2, 2H), 6.08 (s, 1H), 6.86 (td, J = 6.9 Hz, 1.4 Hz, 1H), 7.27 (m, 1H), 7.68 (d, J = 8.9 Hz, 1H), 8.43 (d, J = 6.9 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.0 (d, J = 7.0 Hz), 21.6, 25.6, 37.3, 62.0 (d, J = 4.8 Hz), 70.7, 90.9 (d, J = 218.2 Hz), 113.0, 117.8, 126.5, 128.9, 143.8 (d, J = 22.1 Hz), 168.7 (d, J = 16.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 18.6 (s). HRMS (m/z): C17H25N2O4P+•, 352.1545 (found), 352.1547 (calc).
General experimental procedure for synthesis of pyrazolopyridines 5a-r, 5t,u.N-aminopyridinium salt (1 mmol) was dissolved in 5 mL DMSO and K2CO3 (5 mmol) was added to produce pyridinium-N-imine. The resulting mixture was stirred for 5 min and alkynylphosphonate (1 mmol) was added. The solution was kept with stirring overnight at RT under air atmosphere. Then, the mixture was diluted in water and extracted three times with 20 mL of CH2Cl2. The extracts were combined and washed with water and dried over Na2SO4. Then, the solvent was removed in vacuo and the resulting oil was purified by flash chromatography on silica with CHCl3/MeOH (50:1).
Tetraethyl pyrazolo[1,5-a]pyridine-2,3-diylbis(phosphonate) (5a). Yellow oil, 370 mg (95%). NMR 1H (300 MHz, CDCl3): δ = 1.17 (t, J = 7.0 Hz, 6H), 1.22 (t J = 7.0 Hz, 6H), 3.86–420 (m, 8H), 6.86 (tdd, J = 6.9 Hz, 1.4 Hz, 0.7 Hz, 1H), 7.22 (ddd, 9.1 Hz, 6.8 Hz, 1.2 Hz, 1H), 8.13 (dm, J = 9.1, 1H), 8.42 (dm, J = 6.8, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 15.6 (d, J = 7.0 Hz), 15.7 (d, J = 7.0 Hz), 61.5 (d, J = 5.1 Hz), 62.4 (d, J = 5.8 Hz), 99.95 (dd, J = 221.9 Hz, 24.2 Hz), 114.34, 119.58, 126.41, 128.42, 144.30 (dd, J = 25.6 Hz, 9.9 Hz), 146.84 (dd, J = 227.7 Hz, 14.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 7.8 (s), 11.3 (s). HRMS (m/z): C15H24N2O6P2+•, 390.1104 (found), 390.1106 (calc).
Tetraethyl (5-methylpyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) (5b). Orange oil, 194 mg (48%). NMR 1H (300 MHz, CDCl3): δ = 1.28 (t, 7.1 Hz, 6H), 1.32 (t, 7.1 Hz, 6H), 2.38 (s, 3H), 3.98–4.14 (m, 4H), 4.15–4.30 (m, 4H), 6.77 (dd, J = 7.0 Hz, 2.0 Hz, 1H), 8.02 (s, 1H), 8.39 (dd, J = 7.0 Hz, 2.1 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.0 (d, J = 7.1 Hz), 16.1 (d, J = 7.1 Hz), 21.2, 61.9 (d, J = 5.1 Hz), 62.9 (d, J = 5.9 Hz), 98.8 (dd, J = 222.5 Hz, 24.4 Hz), 117.3, 118.3, 127.9, 138.2, 145.1 (dd, J = 25.9 Hz, 9.8 Hz), 147.3 (dd, J = 228.1, 14.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 8.2 (s), 12.0 (s). HRMS (m/z): C16H26N2O6P2+•, 404.1261 (found), 404.1264 (calc).
Tetraethyl (6-methylpyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) and tetraethyl (4-methylpyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) (5c). An inseparable mixture of isomers was obtained. Orange oil, 194 mg (48%). Signals of the heterocyclic part were as follows. For tetraethyl (6-methylpyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) NMR 1H (300 MHz, CDCl3): δ = 2.34 (s, 3H), 7.19 (dd, J = 9.2 Hz, 1.2 Hz, 1H), 8.16 (d, J = 9.2 Hz, 1H), 8.33 (s). For tetraethyl (4-methylpyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) NMR 1H (300 MHz, CDCl3): δ = 2.80.
Ethyl 2,3-bis(diethoxyphosphoryl)pyrazolo[1,5-a]pyridine-5-carboxylate (5d). Orange oil, 286 mg (62%). NMR 1H (300 MHz, CDCl3): δ = 1.35 (t, J = 7.1 Hz, 6H), 1.38 (t, J = 7.1 Hz, 6H), 1.40 (t, J = 7.2 Hz, 3H), 4.10–4.36 (m, 8H), 4.41 (q, J = 7.2 Hz, 2H), 7.57 (ddd, J = 7.3 Hz, 1.9 Hz, 0.8 Hz, 1H), 8.58 (ddd, J = 7.3 Hz, 2.1 Hz, 1.0 Hz, 1H), 8.96 (s, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 14.1, 16.1 (d, J = 6.9 Hz), 16.2 (d, J = 6.9 Hz), 61.9, 62.4 (d, J = 5.4 Hz), 63.3 (d, J = 5.9 Hz), 103.9 (dd, J = 220.8 Hz, 23.8 Hz), 114.0, 122.6, 128.8, 143.8 (dd, J = 24.9 Hz, 10.1 Hz), 148.5 (dd, J = 227.8 Hz, 14.0 Hz), 164.2. NMR 31P (121.5 MHz, CDCl3): δ = 7.2 (s), 10.4 (s). HRMS (m/z): C18H28N2O8P2+•, 462.1312 (found), 462.1315 (calc).
Tetraethyl (5-methoxypyrazolo[1,5-a]pyridine-2,3-diyl)bis(phosphonate) (5e). Yellow liquid, 202mg. Mixture of 53e and 4-methoxypyridine. NMR 1H (300 MHz, CDCl3): δ = 1.26 (t, J = 7.1 Hz, 6H), 1.30 (t, J = 7.1 Hz, 6H), 3.83 (s, 3H), 3.96–4.28 (m, 8H), 6.59 (dd, J = 7.6, 2.8, 1.0, 1H), 7.50 (d, J = 2.8, 1H), 8.29 (dd, J = 7.6, 2.0, 1H). NMR 13C (100.6 MHz, CDCl3): NMR 31P (121.5 MHz, CDCl3): δ = 8.1 (s), 12.3 (s). HRMS (m/z): C16H26N2O7P2+•, 420.1210 (found), 420.1215 (calc).
Tetraethyl 5-(pyridin-4-yl)pyrazolo[1,5-a]pyridine-2,3-diyldiphosphonate (5f). Brown oil, 411 mg (88 %). NMR 1H (300 MHz, CDCl3): δ = 1.30 (t, J = 7.1 Hz, 6H), 1.35 (t, J = 7.1 Hz, 6H), 4.04–4.33 (m, 8H), 7.25 (d, J = 7.2 Hz, 2.0 Hz, 1H), 7.56 (m, 2H), 8.59–8.68 (m, 4H). NMR 13C (100.6 MHz, CDCl3): δ = 16.0 (d, J = 6.0 Hz), 16.1 (d, J = 6.0 Hz), 62.1 (d, J = 5.1 Hz), 62.9 (d, J = 5.9 Hz), 101.5 (dd, J = 221.4 Hz, 24.0 Hz), 113.5, 117.8, 121.1, 129.2, 136.7, 144.7, 144.8 (dd, J = 25.6 Hz, 10.1 Hz), 148.1 (dd, J =227.6 Hz, 13.9 Hz), 150.3. NMR 31P (121.5 MHz, CDCl3): δ = 5.7 (s), 9.5 (s). HRMS (m/z): C20H27N3O6P2+•, 467.1366 (found), 467.1370 (calc).
Tetraethyl pyrazolo[1,5-a]quinoline-2,3-diyldiphosphonate (5g). Yellow oil, 290 mg (66 %). NMR 1H (300 MHz, CDCl3): δ = 1.32 (t, J = 7.0 Hz, 6H), 1.41 (t, J = 7.1 Hz, 6H), 4.10-4.22 (m, 4H), 4.27-4.38 (m, 4H), 7.52 (ddd, J = 8.3 Hz, 7.2 Hz, 1.2 Hz, 1H), 7.64 (d, J = 9.4 Hz, 1H), 7.70 (ddd, J = 8.4 Hz, 7.2 Hz, 1.4 Hz, 1H), 7.80 (dd, J = 7.8 Hz, 1.4 Hz, 1H), 8.18 (d, J = 9.4 Hz, 1H), 8.64 (dm, J = 8.4 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.0 (d, J = 6.9 Hz), 16.2 (d, J = 6.6 Hz), 62.3 (d, J = 5.2 Hz), 63.2 (d, J = 6.0 Hz), 102.9 (dd, J = 220.5, 24.7 Hz), 116.2 (d, J = 1.3 Hz), 117.1, 123.7, 126.3, 128.1, 128.3, 129.9, 133.87, 143.21 (dd, J = 25.5 Hz, 9.4 Hz), 146.10 (dd, J = 230.8 Hz, 14.1 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 8.6 (s), 12.1 (s). HRMS (m/z): C19H26N2O6P2+•, 440.1258 (found), 440.1261 (calc).
Tetraethyl (9-hydroxypyrazolo[1,5-a]quinoline-2,3-diyl)bis(phosphonate) (5h). Purified by chromatography on Al2O3, with CHCl3 as eluent. Yellow oil, 410 mg (90%). NMR 1H (300 MHz, CDCl3): δ = 1.33 (t, J = 7.1 Hz, 6H), 1.40 (t, J = 7.1 Hz, 6H), 4.07–4.38 (m, 8H), 7.23 (dd, J = 7.9 Hz, 1.2 Hz, 1H), 7.29 (dd, J = 7.9 Hz, 1.2 Hz, 1H), 7.41 (t, J = 7.9 Hz, 1H), 7.65 (d, J = 9.6 Hz, 1H), 8.12 (d, J = 9.6 Hz, 1H), 11.36 (s, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 6.8 Hz), 16.2 (d, J = 6.5 Hz), 62.4 (d, J = 5.2 Hz), 63.3 (d, J = 6.0 Hz), 102.8 (dd, J = 219.5 Hz, 23.1 Hz), 116.6, 116.7, 118.6, 121.6, 125.5, 127.4, 129.4, 142.9 (dd, J = 25.2 Hz, 9.8 Hz), 144.1 (dd, J = 228.3 Hz, 14.8 Hz), 148.4. NMR 31P (121.5 MHz, CDCl3): δ = 6.1 (s), 10.7 (s). HRMS (m/z): C19H26N2O7P2+•, 456.1205 (found), 456.1210 (calc).
Tetraethyl pyrazolo[5,1-a]isoquinoline-1,2-diylbis(phosphonate) (5i). Yellow oil, 418 mg (95 %). NMR 1H (300 MHz, CDCl3): δ = 1.32 (t, J = 7.1 Hz, 6H), 1.40 (t, J = 7.0 Hz, 6H), 4.15–4.38 (m, 8H), 7.21–7.30 (m, 1H), 7.68 (td, J = 7.3 Hz, 2.0 Hz, 2H), 7.77 (dd, J = 7.3 Hz, 2.1 Hz, 1H), 8.34 (dd, J = 7.3 Hz, 2.0 Hz, 1H), 9.33 (dm, J = 7.3 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.3 (d, J = 6.9 Hz), 16.4 (d, J = 6.9 Hz), 62.7 (d, J = 5.6 Hz), 63.3 (d, J = 6.0 Hz), 103.9 (dd, J = 218.5 Hz, 24.5 Hz), 116.2, 124.1, 126.2, 127.5, 127.8, 128.6, 129.7, 130.4, 141.9 (dd, J = 24.9 Hz, 9.1 Hz), 148.3 (dd, J = 229.7 Hz, 13.9 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 8.6 (s), 12.7 (s). HRMS (m/z): C19H26N2O6P2+•, 440.1261 (found), 440.1265 (calc).
Tetraethyl pyrazolo[1,5-a][1,10]phenanthroline-2,3-diylbis(phosphonate) (5j). Purified by chromatography on Al2O3, with CHCl3 as eluent. Yellow oil, 422 mg (86 %). NMR 1H (300 MHz, CDCl3): δ = 1.27 (t, J = 7.1 Hz, 6H), 1.43 (t, J = 7.1 Hz, 6H), 4.05–4.22 (m, 4H), 4.31–4.55 (m, 4H), 7.49 (dd, J = 8.1 Hz, 4.1 Hz, 1H), 7.68 (d, J = 9.3 Hz, 1H), 7.71 (s, 2H), 8.16 (dd, J = 8.2, 1.9, 1H), 8.47 (d, J = 9.3 Hz, 1H), 9.10 (dd, J = 4.2 Hz, 1.9 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 15.9 (d, J = 6.9 Hz), 16.1 (d, J = 6.9 Hz), 61.9 (d, J = 5.0 Hz), 63.3 (d, J = 6.0 Hz), 101.1 (dd, J = 218.4, 24.3), 119.0, 121.9, 125.0, 126.1, 126.3, 127.8, 128.7, 129.9, 135.8, 139.3, 145.6 (dd, J = 27.3, 9.1), 147.3 (dd, J = 232.1, 12.8), 149.8. NMR 31P (121.5 MHz, CDCl3): δ = 8.0 (s), 11.7 (s). HRMS (m/z): C22H27N3O6P2+•, 491.1366 (found), 491.1370 (calc).
Diethyl (pyrazolo[1,5-a]pyridin-3-yl)phosphonate (5k). Without purification. Yellow oil, 110 mg (98%). NMR 1H (300 MHz, CDCl3): δ = 1.34 (t, J = 7.2 Hz, 6H), 4.02–4.25 (m, 4H), 6.97 (td, J = 6.9 Hz, J = 1.2 Hz, 1H), 7.38 (ddd, J = 8.9 Hz, J = 6.9 Hz, J = 1.0 Hz, 1H), 7.98 (dt, J = 8.9 Hz, J = 1.0 Hz, 1H), 8.23 (d, J = 1.9 Hz, 1H), 8.57 (ddt, J = 7.0 Hz, J = 2.1 Hz, J = 1.0 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.4 (d, J = 7 Hz), 62.0 (d, J = 5 Hz), 96.4 (d, J = 224 Hz), 113.7, 118.6, 126.7, 129.3, 142.8 (d, J = 25.0 Hz), 146.4 (d, J = 14.0 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.3 (s). HRMS (m/z): C11H15N2O3P+•, 254.0822 (found), 254.0820 (calc).
Diethyl (5-methylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (5l). Without purification. Brown oil, 75 mg (65%). NMR 1H (300 MHz, CDCl3): δ = 1.30 (t, J = 7.1 Hz, 6H), 2.41 (s, 3H), 3.96–4.20 (m, 4H), 6.74 (dd, J = 7.1 Hz, J = 1.5 Hz, 1H), 7.69 (s, 1H), 8.11 (d, J = 1.6 Hz, 1H), 8.39 (dd, J = 7.15 Hz, J = 1.4 Hz). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7 Hz), 21.2, 61.8 (d, J = 5 Hz), 94.8 (d, J = 224 Hz), 116.1, 116.8, 128.2, 137.9, 142.9 (d, J = 25 Hz), 146.2 (d, J = 14 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.8 (s). HRMS (m/z): C12H17N2O3P+•, 268.0975 (found), 268.0971 (calc).
Diethyl (7-methylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (5m). Without purification. Brown oil, 125 mg (98%). NMR 1H (300 MHz, CDCl3): δ = 1.29 (t, J = 7 Hz, 6H), 2.77 (s, 3H), 3.96–4.21 (m, 4H), 6.79 (d, J = 7 Hz, 1H), 7.25–7.32 (m, 1H), 7.82 (d, J = 8.8 Hz, 1H), 8.24 (d, J = 1.6 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7 Hz), 17.9, 61.7 (d, J = 5 Hz), 96.3 (d, J = 224 Hz), 112.8, 115.9, 126.5, 138.9, 145.6 (d, J = 14 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 14.7 (s). HRMS (m/z): C12H17N2O3P+•, 268.0974 (found), 268.0971 (calc).
Diethyl (7phenylpyrazolo[1,5-a]pyridin-3-yl)phosphonate (5m). Purified by chromatography on SiO2, with EtOAc:hexane (1:1) as eluent. Yellow solid, 74mg (36 %). NMR 1H (300 MHz, CDCl3): δ = 1.32 (t, J = 7.1 Hz, 6H), 3.99–4.24 (m, 4H), 6.99 (d, J = 6.9 Hz, 1H), 7.39–7.47 (m, 1H), 7.48–7.57 (m, 3H), 7.79–7.85 (m, 2H), 7.95 (d, J = 8.9 Hz, 1H), 8.22 (s, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7.2 Hz), 61.8 (d, J = 5.2 Hz), 96.4 (dd, J = 227 Hz), 114.2, 117.2, 126.7, 128.4, 129.1, 129.7, 132.8, 141.4, 143.4, 143.8, 145.7, 145.9. NMR 31P (121.5 MHz, CDCl3): δ = 14.5 (s). HRMS (m/z): C17H19N2O3P+•, 330.1125 (found), 330.1133 (calc).
Methyl 3-dietoxyphosphorylpyrazolo[1,5-a]pyridine-5-carboxylate (5o). Without purification. Brown oil, 110 mg (80%). NMR 1H (300 MHz, CDCl3): δ = 1.33 (t, J = 7.1 Hz, 6H), 3.96 (s, 3H), 4.01–4.24 (m, 4H), 7.51 (dd, J = 7 Hz, J = 1 Hz, 1H), 8.27 (d, J = 1.7 Hz, 1H), 8.56 (dm, J = 6.5 Hz, 1H), 8.64 (s, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7 Hz), 52.7, 62.1 (d, J = 5 Hz), 99.9 (d, J =222 Hz), 112.7, 120.9, 128.1, 128.9, 141.6 (d, J = 25 Hz), 147.0 (d, J = 12 Hz), 164.9. NMR 31P (121.5 MHz, CDCl3): δ = 12.8 (s). HRMS (m/z): C13H17N2O5P+•, 312.0864 (found), 312.0871 (calc).
Diethyl (9-hydroxypyrazolo[1,5-a]quinoline-3-yl)phosphonate (5p). Without purification. Colorless crystals, 130 mg (95%). NMR 1H (300 MHz, CDCl3): δ = 1.33 (t, J = 7.2 Hz, 6H), 4.00–4.28 (m, 4H), 7.26 (d, J = 7.8Hz, 1H), 7.31 (d, J = 7.8 Hz, 1H), 7.41 (t, J = 7.9 Hz, 1H), 7.66 (d, J = 9.4 Hz, 1H), 7.81 (d, J = 9.4 Hz, 1H), 8.21 (d, J = 1.6 Hz, 1H), 11.68 (s, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7 Hz), 62.0 (d, J = 5 Hz), 98.8 (d, J = 222 Hz), 115.7, 116.4, 118.4, 122.3, 125.2, 126.8, 129.0, 140.5 (d, J = 25 Hz), 142.5 (d, J = 14 Hz), 148.5. NMR 31P (121.5 MHz, CDCl3): δ = 14.1 (s). HRMS (m/z): C15H17N2O4P+•, 320.0922 (found), 320.0921 (calc).
Diethyl (pyrazolo[1,5-a][1,10]phenantroline-3-yl)phosphonate (5q). Without purification. Brown oil, 115 mg (75%). NMR 1H (300 MHz, CDCl3): δ = 1.28 (t, J = 7 Hz, 6H), 3.98–4.22 (m, 4H), 7.61 (dd, J = 8.2 Hz, J = 4.3 Hz, 1H), 7.75–7.86 (m, 3H), 8.24 (d, J = 9.1 Hz, 1H), 8.29 (dd, J = 8.2 Hz, J = 1.3 Hz, 1H), 9.38 (dd, J = 4.3 Hz, J = 1.4 Hz). NMR 13C (100.6 MHz, CDCl3): δ = 16.2 (d, J = 7.5 Hz), 62.0 (d, J = 5 Hz), 97.9 (d, J = 222 Hz), 118.2, 122.1, 124.9, 126, 126.7, 127.7, 129.2, 130.6, 136.3, 139.8, 143.6, 143.8, 146.4, 146.5, 150.5. NMR 31P (121.5 MHz, CDCl3): δ = 14.8 (s). HRMS (m/z): C18H18N3O3P+•, 355.1078 (found), 355.1080 (calc).
Diethyl (2-chloro[1,5-a]pyridin-3-yl)phosphonate (5r). Purified by chromatography on SiO2, with CHCl3/MeOH (50:1) as the eluent. Yellow oil, 92.4 mg (32%). NMR 1H (300 MHz, CDCl3): δ = 1.33 (t, J = 7.1 Hz, 6H), 3.99-4.24 (m, 4H), 6.95 (t, J = 6.7 Hz, 1H), 7.37 (t, J = 7.5 Hz, 1H), 8.09 (d, J = 8.7 Hz, 1H), 8.41 (d, J = 6.7 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 6.9 Hz), 62.1 (d, J = 6.0 Hz), 94.1 (d, J = 225 Hz), 113.9, 118.7, 127.3, 128.4, 145.2 (d, J = 24 Hz), 145.2 (d, J = 25 Hz), 146.7 (d, J = 9 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 10.4 (s). HRMS (m/z): C11H14O3N235ClP+•, 288.0422 (found), 288.0425 (calc).
Diethyl (2-iodo[1,5-a]pyridin-3-yl)phosphonate (5t). Purified by chromatography on SiO2, with CHCl3/MeOH (50:1) as the eluent. Yellow oil, 57.0 mg (15%). NMR 1H (300 MHz, CDCl3): δ = 1.34 (t, J = 7.1 Hz, 6H), 3.97–4.10 (m, 2H), 4.11–4.24 (m, 2H), 6.88 (td, J = 6.8 Hz, J = 1 Hz, 1H), 7.32 (t, J = 8.5 Hz, 1H), 8.15 (d, J = 8.9 Hz, 1H), 8.46 (d, J = 6.9 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 6.9 Hz), 62.1 (d, J = 6.0 Hz), 101.8 (d, J = 227 Hz), 106.4 (d, J = 12 Hz), 113.6, 118.4, 127.0, 128.1, 145.2 (d, J = 25 Hz). NMR 31P (121.5 MHz, CDCl3): δ = 11.1 (s). HRMS (m/z): C11H14O3N2IP+•, 379.9783 (found), 379.9781 (calc).
Diethyl (2-phenoxy[1,5-a]pyridin-3-yl)phosphonate (5u). THF was used as the solvent instead of MeCN. Purified by chromatography on SiO2, with CH2Cl2/NEt3 (100:1) as the eluent. Yellow crystals, 260.0 mg (75%). NMR 1H (300 MHz, CDCl3): δ = 1.31 (t, J = 7.1 Hz, 6H), 4.02–4.25 (m, 4H), 6.83 (td, J = 7.1 Hz, J = 1.4 Hz, 1H), 7.12–7.30 (m, 4H), 7.30–7.42 (m, 3H), 8.01 (d, J = 9 Hz, 1H), 8.27 (dt, J = 6.9 Hz, J = 1 Hz, 1H). NMR 13C (100.6 MHz, CDCl3): δ = 16.1 (d, J = 6.9 Hz), 62.1 (d, J = 6.0 Hz), 82.3 (d, J = 227 Hz), 112.7, 118.0, 119.5, 124.6, 126.9, 128.8, 129.4, 145.0 (d, J = 24 Hz), 155.2, 164.4 (d, J = 7 Hz). HRMS (m/z): C17H19O4N2P+•, 346.1086 (found), 346.1082 (calc).
4. Conclusions
In summary, an operationally simple procedure toward 3-phosphonylated pyrazolo[1,5-a]pyridines based on the cycloaddition of diethyl 2-alkyl-, 2-cycloalkyl-, phenylethynylphosphonates and N-aminopyridinium salts with catalysis by cheap Fe(NO3)3·9H2O was achieved. The reaction is applicable for mild donating and withdrawing functional groups in the pyridinium ring and for various 2-alkyl and α-hydroxyalkyl acetylenephosphonates. Tetraethyl ethylene-1,2-bis(phosphonate), diethyl 2-TMS- and 2-OPh-ethynylphosphonate reacted smoothly without any catalyst to yield the corresponding 2,3-bisphosphonylated and 3-phosphonylated pyrazolo[1,5-a]pyridines and their annulated analogs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27227913/s1, Figure S1: Crystal structures of pyrazolopyridines 3d (A) and 5u (B). Copies of 1H, 13C and 31P NMR spectra of the obtained compounds.
Author Contributions
Conceptualization, A.V. and I.P.; methodology, A.V.; investigation, I.P.; writing—original draft preparation, A.V. and I.P.; writing—review and editing, A.V.; project administration, A.V.; X-ray analysis, Y.G. and A.S.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Education of Russian Federation, grant number 122040800262-9.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Rolan, P.; Hutchinson, M.; Johnson, K. Ibudilast: A Review of Its Pharmacology, Efficacy and Safety in Respiratory and Neurological Disease. Expert Opin. Pharmacother. 2009, 10, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Ledeboer, A.; Hutchinson, M.R.; Watkins, L.R.; Johnson, K.W. Ibudilast (AV-411): A New Class Therapeutic Candidate for Neuropathic Pain and Opioid Withdrawal Syndromes. Expert Opin. Investig. Drugs 2007, 16, 935–950. [Google Scholar] [CrossRef]
- Fox, R.J.; Coffey, C.S.; Conwit, R.; Cudkowicz, M.E.; Gleason, T.; Goodman, A.; Klawiter, E.C.; Matsuda, K.; McGovern, M.; Naismith, R.T.; et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med. 2018, 379, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.D.; Gyang, T.; Smith, A.D. Ibudilast for the Treatment of Multiple Sclerosis. Expert Opin. Investig. Drugs 2016, 25, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Nogami, M.; Iwatani, M.; Imaeda, T.; Ito, M.; Tanaka, T.; Tawada, M.; Endo, S.; Cary, D.R.; Ohori, M.; et al. Identification of a Selective DDX3X Inhibitor with Newly Developed Quantitative High-Throughput RNA Helicase Assays. Biochem. Biophys. Res. Commun. 2020, 523, 795–801. [Google Scholar] [CrossRef]
- Calbet, M.; Ramis, I.; Calama, E.; Carreño, C.; Paris, S.; Maldonado, M.; Orellana, A.; Calaf, E.; Pauta, M.; De Alba, J.; et al. Novel Inhaled Pan-JAK Inhibitor, LAS194046, Reduces Allergen-Induced Airway Inflammation, Late Asthmatic Response, and PSTAT Activation in Brown Norway Rats. J. Pharmacol. Exp. Ther. 2019, 370, 137–147. [Google Scholar] [CrossRef]
- O’Malley, D.P.; Ahuja, V.; Fink, B.; Cao, C.; Wang, C.; Swanson, J.; Wee, S.; Gavai, A.V.; Tokarski, J.; Critton, D.; et al. Discovery of Pyridazinone and Pyrazolo[1,5-a]Pyridine Inhibitors of C-Terminal Src Kinase. ACS Med. Chem. Lett. 2019, 10, 1486–1491. [Google Scholar] [CrossRef]
- Sainas, S.; Pippione, A.C.; Lupino, E.; Giorgis, M.; Circosta, P.; Gaidano, V.; Goyal, P.; Bonanni, D.; Rolando, B.; Cignetti, A.; et al. Targeting Myeloid Differentiation Using Potent 2-Hydroxypyrazolo[1,5-a]Pyridine Scaffold-Based Human Dihydroorotate Dehydrogenase Inhibitors. J. Med. Chem. 2018, 61, 6034–6055. [Google Scholar] [CrossRef]
- Kendall, J.D.; Giddens, A.C.; Tsang, K.Y.; Marshall, E.S.; Lill, C.L.; Lee, W.-J.; Kolekar, S.; Chao, M.; Malik, A.; Yu, S.; et al. Novel Pyrazolo[1,5-a]Pyridines with Improved Aqueous Solubility as P110α-Selective PI3 Kinase Inhibitors. Bioorganic Med. Chem. Lett. 2017, 27, 187–190. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chu, J.-H.; Li, C.-W.; Hwang, L.-C.; Wu, M.-J. Palladium-Catalyzed Regioselective Arylation of Pyrazolo[1,5-a]Pyridines via C–H Activation and Synthetic Applications on P38 Kinase Inhibitors. Organometallics 2016, 35, 288–300. [Google Scholar] [CrossRef]
- Lechtenberg, B.C.; Mace, P.D.; Sessions, E.H.; Williamson, R.; Stalder, R.; Wallez, Y.; Roth, G.P.; Riedl, S.J.; Pasquale, E.B. Structure-Guided Strategy for the Development of Potent Bivalent ERK Inhibitors. ACS Med. Chem. Lett. 2017, 8, 726–731. [Google Scholar] [CrossRef]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Gagginapally, S.R.; Kancharla, D.M.; Middekadi, V.R.; Bogaraju, N.; Ravella, S.R.; Singh, P.; Birangal, S.R.; et al. Synthesis, Structure–Activity Relationships, and Preclinical Evaluation of Heteroaromatic Amides and 1,3,4-Oxadiazole Derivatives as 5-HT4 Receptor Partial Agonists. J. Med. Chem. 2018, 61, 4993–5008. [Google Scholar] [CrossRef] [PubMed]
- Umei, K.; Nishigaya, Y.; Kondo, A.; Tatani, K.; Tanaka, N.; Kohno, Y.; Seto, S. Novel Pyrazolo[1,5-a]Pyridines as Orally Active EP 1 Receptor Antagonists: Synthesis, Structure-Activity Relationship Studies, and Biological Evaluation. Bioorganic Med. Chem. 2017, 25, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Williams, Z.; Hards, K.; Tang, J.; Cheung, C.-Y.; Aung, H.L.; Wang, B.; Liu, Z.; Hu, X.; Lenaerts, A.; et al. Pyrazolo[1,5-a]Pyridine Inhibitor of the Respiratory Cytochrome Bcc Complex for the Treatment of Drug-Resistant Tuberculosis. ACS Infect. Dis. 2019, 5, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Large, J.M.; Birchall, K.; Bouloc, N.S.; Merritt, A.T.; Smiljanic-Hurley, E.; Tsagris, D.J.; Wheldon, M.C.; Ansell, K.H.; Coombs, P.J.; Kettleborough, C.A.; et al. Potent Inhibitors of Malarial P. Falciparum Protein Kinase G: Improving the Cell Activity of a Series of Imidazopyridines. Bioorganic Med. Chem. Lett. 2019, 29, 509–514. [Google Scholar] [CrossRef]
- Kendall, J.D. Synthesis and Reactions of Pyrazolo[1,5-a]Pyridines and Related Heterocycles. Curr. Org. Chem. 2011, 15, 2481–2518. [Google Scholar] [CrossRef]
- Mohan, D.C.; Ravi, C.; Rao, S.N.; Adimurthy, S. Copper-Mediated Synthesis of Pyrazolo[1,5-a]Pyridines through Oxidative Linkage of C–C/N–N Bonds. Org. Biomol. Chem. 2015, 13, 3556–3560. [Google Scholar] [CrossRef]
- Ravi, C.; Samanta, S.; Mohan, D.; Reddy, N.; Adimurthy, S. Synthesis of Functionalized Pyrazolo[1,5-a]Pyridines: [3+2] Cycloaddition of N-Aminopyridines and α,β-Unsaturated Carbonyl Compounds/Alkenes at Room Temperature. Synthesis 2017, 49, 2513–2522. [Google Scholar] [CrossRef]
- Ravi, C.; Chandra Mohan, D.; Naresh Kumar Reddy, N.; Adimurthy, S. Substrate Selective Synthesis of Pyrazolo[1,5-a]Pyridines through [3+2] Cycloaddition of N-Aminopyridines and β-Nitro Styrenes. RSC Adv. 2015, 5, 42961–42964. [Google Scholar] [CrossRef]
- Motornov, V.A.; Tabolin, A.A.; Nelyubina, Y.V.; Nenajdenko, V.G.; Ioffe, S.L. Copper-Mediated Oxidative [3+2]-Annulation of Nitroalkenes and Pyridinium Imines: Efficient Synthesis of 3-Fluoro- and 3-Nitro-Pyrazolo[1,5-a]Pyridines. Org. Biomol. Chem. 2020, 18, 1436–1448. [Google Scholar] [CrossRef]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The Role of the Phosphorus Atom in Drug Design. ChemMedChem 2018, 14, 190–216. [Google Scholar] [CrossRef] [PubMed]
- Demmer, C.S.; Krogsgaard-Larsen, N.; Bunch, L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011, 111, 7981–8006. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Jia, J.; Xie, Y.-F.; Feng, L.; Xu, H.-Q.; Meng, S.; Zhao, G.-L.; Xu, W.-R.; Ge, Y.-Q. Synthesis of Nitrogen Bridgehead Heterocycles with Phosphonates via a Novel Tandem Process. Heterocycles 2013, 87, 815. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, H.; Zhao, X. Selenium-π-Acid Catalyzed Oxidative Functionalization of Alkynes: Facile Access to Ynones and Multisubstituted Oxazoles. ACS Catal. 2018, 8, 6745–6750. [Google Scholar] [CrossRef]
- Huang, Q.; He, D.; Han, J.; Chen, J.; He, W.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. [3+2] Cycloaddition of N-Aminopyridines and Perfluoroalkynylphosphonates: Facile Synthesis of Perfluoroalkylated Pyrazolo[1,5-a]Pyridines Containing a Phosphonate Moiety. Synthesis 2018, 50, 3731–3737. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Seyferth, D.; Paetsch, J. Diels-Alder Reaction in Organometallic Chemistry. V. Tetramethyl Acetylenediphosphonate and Dimethyl Chloroacetylenephosphonate and Their Reactions with Cyclopentadiene, 1,3-Cyclohexadiene, and Diazomethane. J. Org. Chem. 1969, 34, 1483–1484. [Google Scholar] [CrossRef]
- Tverdomed, S.N.; Röschenthaler, G.-V.; Kalinovich, N.; Lork, E.; Dogadina, A.V.; Ionin, B.I. New α-Substituted Alkylbenzene- and Dialkylbenzene-1,2-Diphosphonates: Side-Chain Metalation of Tetraethyl 4-Methyl- and 4,5-Dimethylbenzene-1,2-Diphosphonates. Tetrahedron 2008, 64, 5306–5313. [Google Scholar] [CrossRef]
- Mahajna, M.; Quistad, G.B.; Casida, J.E. Retro-Diels−Alder Reaction: Possible Involvement in the Metabolic Activation of 7-Oxabicyclo[2.2.1]Hepta-2(3),5(6)-Diene-2,3-Dicarboxylates and a Phosphonate Analog. Chem. Res. Toxicol. 1996, 9, 241–246. [Google Scholar] [CrossRef]
- Selmani, S.; Schipper, D.J. Orientation Control of Molecularly Functionalized Surfaces Applied to the Simultaneous Alignment and Sorting of Carbon Nanotubes. Angew. Chem. 2018, 130, 2423–2427. [Google Scholar] [CrossRef]
- Kyba, E.P.; Rines, S.P.; Owens, P.W.; Chou, S.-S.P. A Novel Synthesis of 1,2-Diphosphorylbenzenes. Tetrahedron Lett. 1981, 22, 1875–1878. [Google Scholar] [CrossRef]
- Ziegler, T.; Layh, M.; Effenberger, F. Darstellung Hochsubstituierter Aromaten Über Diels-Alder-Reaktionen Mit 2H-Pyran-2-onen. Chem. Ber. 1987, 120, 1347–1355. [Google Scholar] [CrossRef]
- Artyushin, O.I.; Matveeva, E.V.; Bushmarinov, I.S.; Odinets, I.L. Water as a Promoting Media for 1,3-Dipolar Cycloaddition of Phosphorylated Azides to Internal Alkynes. Arkivoc 2012, 2012, 252–263. [Google Scholar] [CrossRef]
- Vereshchagina, Y.A.; Alimova, A.Z.; Sharova, E.V.; Artyushin, O.I.; Chachkov, D.V.; Ishmaeva, E.A. Polarity and Structure of Diphosphorus-Substituted Isoxazole and 1,2,3-Triazole. Russ. J. Org. Chem. 2013, 49, 1369–1372. [Google Scholar] [CrossRef]
- Mukai, S.; Flematti, G.R.; Byrne, L.T.; Besant, P.G.; Attwood, P.V.; Piggott, M.J. Stable Triazolylphosphonate Analogues of Phosphohistidine. Amino Acids 2012, 43, 857–874. [Google Scholar] [CrossRef]
- Lukáč, M.; Hocková, D.; Keough, D.T.; Guddat, L.W.; Janeba, Z. Novel Nucleotide Analogues Bearing (1H-1,2,3-Triazol-4-Yl)Phosphonic Acid Moiety as Inhibitors of Plasmodium and Human 6-Oxopurine Phosphoribosyltransferases. Tetrahedron 2017, 73, 692–702. [Google Scholar] [CrossRef]
- Matoba, K.; Yonemoto, H.; Fukui, M.; Yamazaki, T. Structural modification of bioactive compounds. II. Syntheses of aminophosphonoic acids. Chem. Pharm. Bull. 1984, 32, 3918–3925. [Google Scholar] [CrossRef][Green Version]
- Heimgartner, H.; Mlostoń, G.; Pipiak, P. [3+2] Cycloadditions of N-Protected ‘(S)-Diazoproline’ with Selected Acetylenes. Heterocycles 2017, 95, 223. [Google Scholar] [CrossRef]
- Kowalski, M.K.; Mlostoń, G.; Obijalska, E.; Heimgartner, H. Application of Diethyl Ethynephosphonate for the Synthesis of 3-Phosphonylated β-Lactams via Kinugasa Reaction. Arkivoc 2016, 2017, 59–67. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Y.; Li, P.; Bi, W.; Chen, X.; Zhao, Y. Synthesis of Novel Phosphorylated Chrysin Derivatives by 1,3-Dipolar Cycloaddition Reaction. Phosphorus Sulfur Silicon Relat. Elem. 2017, 192, 1–8. [Google Scholar] [CrossRef]
- Song, W.; Zheng, N.; Li, M.; Ullah, K.; Zheng, Y. Rhodium(I)-Catalyzed Azide-Alkyne Cycloaddition (RhAAC) of Internal Alkynylphosphonates with High Regioselectivities under Mild Conditions. Adv. Synth. Catal. 2018, 360, 2429–2434. [Google Scholar] [CrossRef]
- Perez, V.; Fadel, A.; Rabasso, N. Synthesis of N-Sulfonyl Ynamido-Phosphonates: Valuable Partners for Cycloadditions. Synthesis 2017, 49, 4035–4044. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, H.; Sun, J. Ru-Catalyzed [3+2] Cycloaddition of Nitrile Oxides and Electron-Rich Alkynes with Reversed Regioselectivity. Org. Lett. 2021, 23, 2431–2436. [Google Scholar] [CrossRef]
- Xiang, J.; Yi, N.; Wang, R.; Lu, L.; Zou, H.; Pan, Y.; He, W. Synthesis of β-Ketophosphonates via AgNO3-Catalyzed Hydration of Alkynylphosphonates: A Rate-Enhancement Effect of Methanol. Tetrahedron 2015, 71, 694–699. [Google Scholar] [CrossRef]
- Bian, Q.; Wu, C.; Yuan, J.; Shi, Z.; Ding, T.; Huang, Y.; Xu, H.; Xu, Y. Iron Nitrate-Mediated Selective Synthesis of 3-Acyl-1,2,4-Oxadiazoles from Alkynes and Nitriles: The Dual Roles of Iron Nitrate. J. Org. Chem. 2020, 85, 4058–4066. [Google Scholar] [CrossRef]
- Lai, Z.; Li, Z.; Liu, Y.; Yang, P.; Fang, X.; Zhang, W.; Liu, B.; Chang, H.; Xu, H.; Xu, Y. Iron-Mediated Synthesis of Isoxazoles from Alkynes: Using Iron(III) Nitrate as a Nitration and Cyclization Reagent. J. Org. Chem. 2018, 83, 145–153. [Google Scholar] [CrossRef]
- The Crystallographic Data Was Deposited at the Cambridge Crystallographic Data Center, CCDC 200118. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 October 2022).
- Tsuchiya, T.; Kurita, J.; Snieckus, V. General Photochemical Synthesis of 1H-1,2-Benzodiazepines from N-Iminoquinolinium Ylide Dimers. J. Org. Chem. 1977, 42, 1856–1862. [Google Scholar] [CrossRef]
- Huisgen, R.; Grashey, R.; Krischke, R. 1,3-Dipolare Cycloadditionen, 84. Additionen mit Chinolinium-, Isochinolinium- und Phenanthridinium-N-imid2). Justus Liebigs Ann. Chem. 1977, 1977, 506–527. [Google Scholar] [CrossRef]
- The Crystallographic Data Was Deposited at the Cambridge Crystallographic Data Center, CCDC 2122366. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 28 October 2022).
- Supranovich, V.I.; Vorob’ev, A.Y.; Borodkin, G.I.; Gatilov, Y.V.; Shubin, V.G. Study on Selectivity in the Reaction of 2-Substituted Pyridinium- N-Imines with Dimethyl Acetylenedicarboxylate. Tetrahedron Lett. 2016, 57, 1093–1096. [Google Scholar] [CrossRef]
- Tamura, Y.; Minamikawa, J.; Ikeda, M. O-Mesitylenesulfonylhydroxylamine and Related Compounds-Powerful Aminating Reagents. Synthesis 1977, 1–17. [Google Scholar] [CrossRef]
- Vorob’ev, A.Y.; Supranovich, V.I.; Borodkin, G.I.; Shubin, V.G. New approach toward the synthesis of deuterated pyrazolo[1,5-a]pyridines and 1,2,4-triazolo[1,5-a]pyridines. Beilstein J. Org. Chem. 2017, 13, 800–805. [Google Scholar] [CrossRef]
- Qu, Z.; Chen, X.; Yuan, J.; Qu, L.; Li, X.; Wang, F.; Ding, X.; Zhao, Y. CuSO4·5H2O-catalyzed alkynylphosphonates formation—An efficient coupling reaction of terminal alkynes with H-phosphonates. Can. J. Chem. 2012, 90, 747–752. [Google Scholar] [CrossRef]
- Egorova, A.V.; Viktorov, N.B.; Starova, G.L.; Svintsitskaya, N.I.; Garabadziu, A.V.; Dogadina, A.V. BF3·Et2O catalyzed intramolecular cyclization of diethyl 2-(dialkoxyphosphorylethynyl)-2-arylaminomalonates to 3-phosphonylated indoles. Tetrahedron Lett. 2017, 58, 2997–3001. [Google Scholar] [CrossRef]
- Kruglov, S.V.; Ignat’ev, V.M.; Ionin, B.I.; Petrov, A.A. Synthesis of Symmetrical and Mixed Diphosphonic Esters. J. General Chem. USSR 1973, 43, 1470–1480. [Google Scholar]
- Oakdale, J.S.; Sit, R.K.; Fokin, V.V. Ruthenium-Catalyzed Cycloadditions of 1-Haloalkynes with Nitrile Oxides and Organic Azides: Synthesis of 4-Haloisoxazoles and 5-Halotriazoles. Chem.-Eur. J. 2014, 20, 11101–11110. [Google Scholar] [CrossRef]
- Marian, A.; Maas, G. Diethyl (iodoethynyl)phosphonate and (iodoethynyl)diphenylphosphane oxide: Crystal structures and some cycloaddition reactions. Z. Nat. B 2020, 75, 529–536. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS 1996, Program for Empirical Adsorption Correction. Available online: https://www.scienceopen.com (accessed on 28 October 2022).
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Cryst. Sect. A Found. Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).