Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Compatibility Studies [23,24]
2.1.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.1.2. Differential Scanning Calorimetry (DSC)
2.2. Preparation of PT-NC Formulation
Experimental Design
- PS
- EE
2.3. Preparation of Optimized Formulation
2.4. PT-NC Morphology
2.5. Dissolution Study
2.6. Short-Term Stability Studies
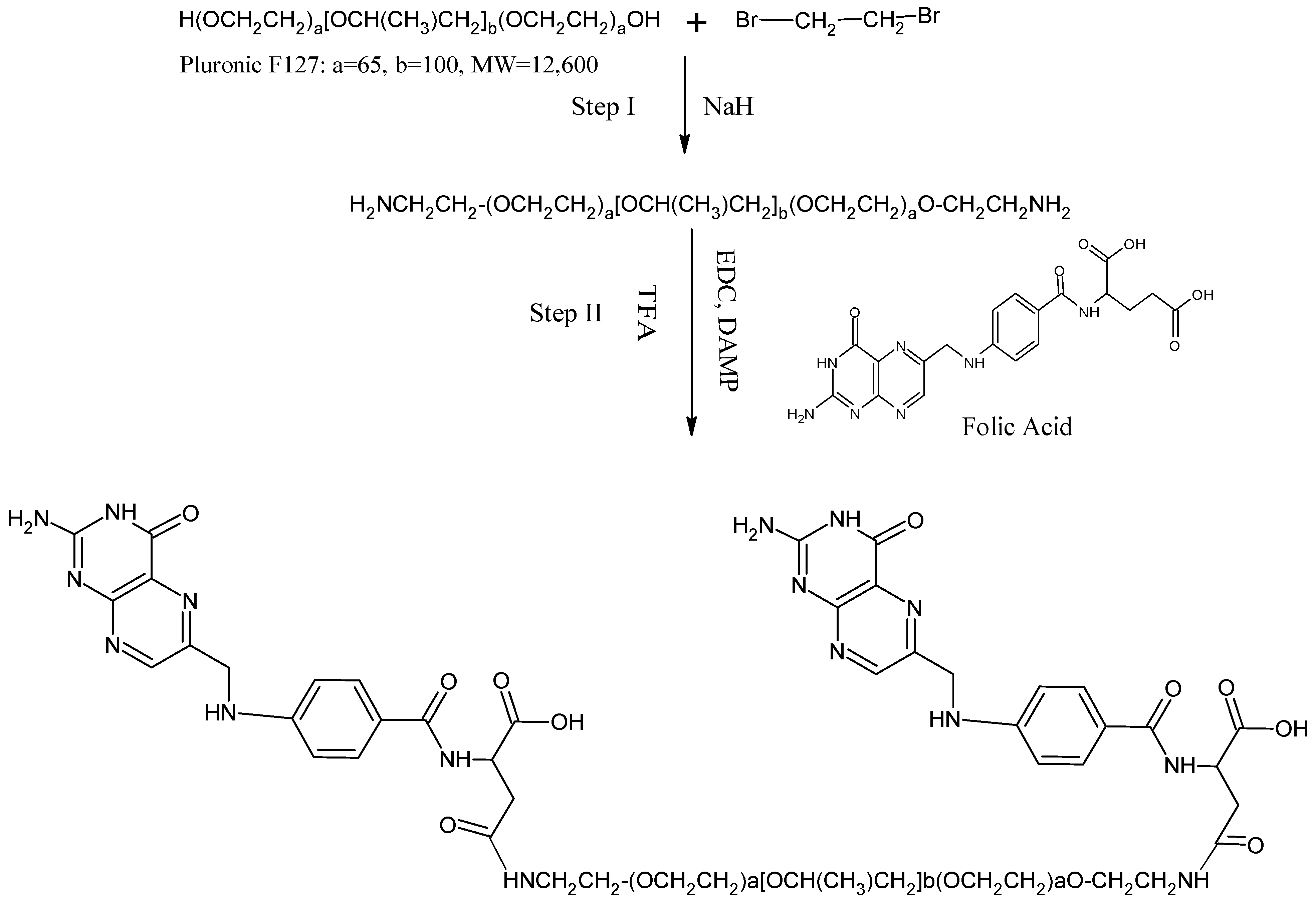
2.7. Production of the FR-Targeted NC and Conjugation of Folic Acid to Pluronic F127
2.8. Evaluation of the Targeted NC in an FR-Positive Tumor Cell Line
2.9. Evaluation of Hemolytic Activity
2.10. Animals and Materials
2.10.1. In Vivo Anti-Cancer Activity
2.10.2. Statistics
3. Results and Discussion
3.1. Compatibility Studies
3.2. Optimization
3.3. Surface Morphology
3.4. Drug Release Study
3.5. Stability Studies
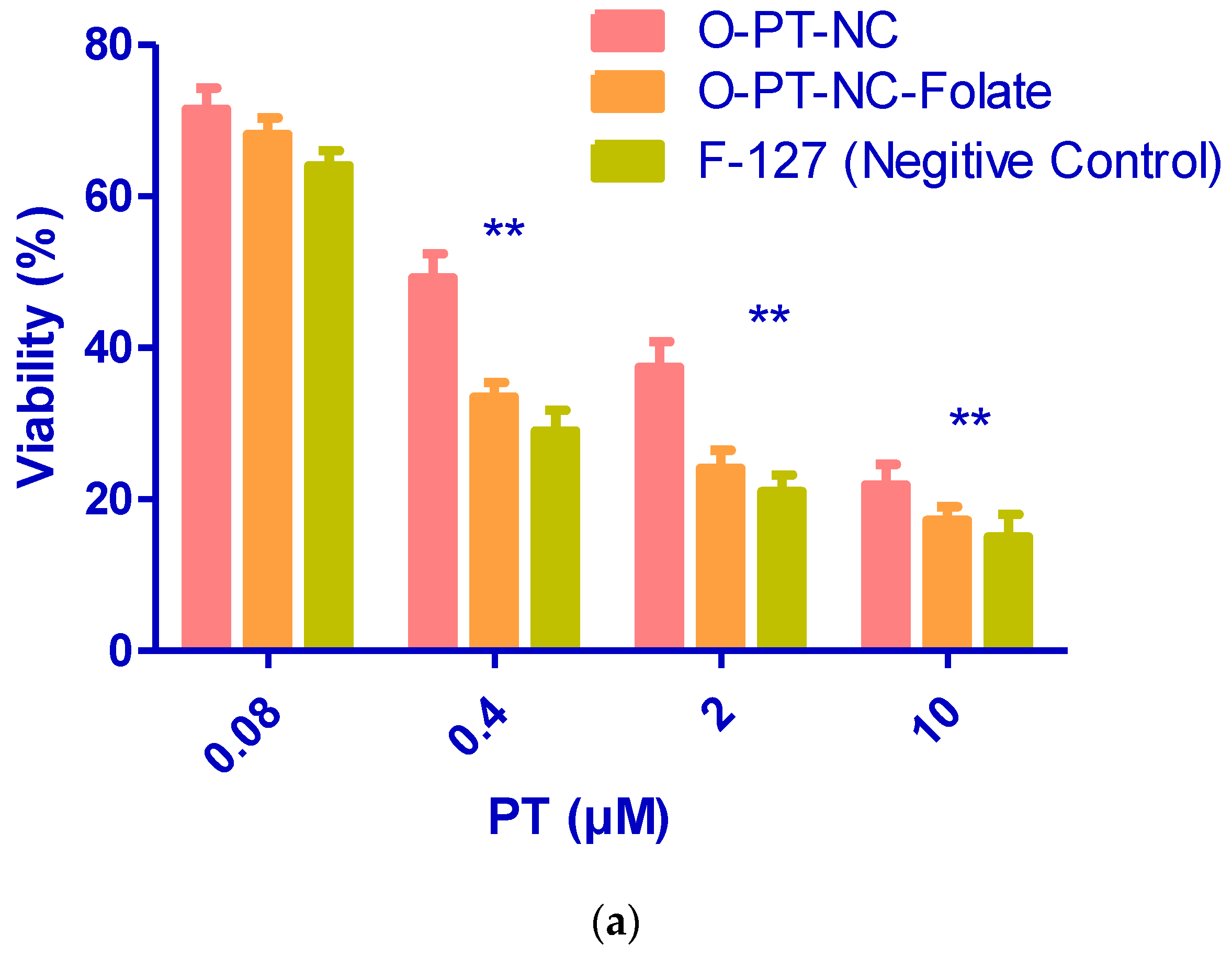
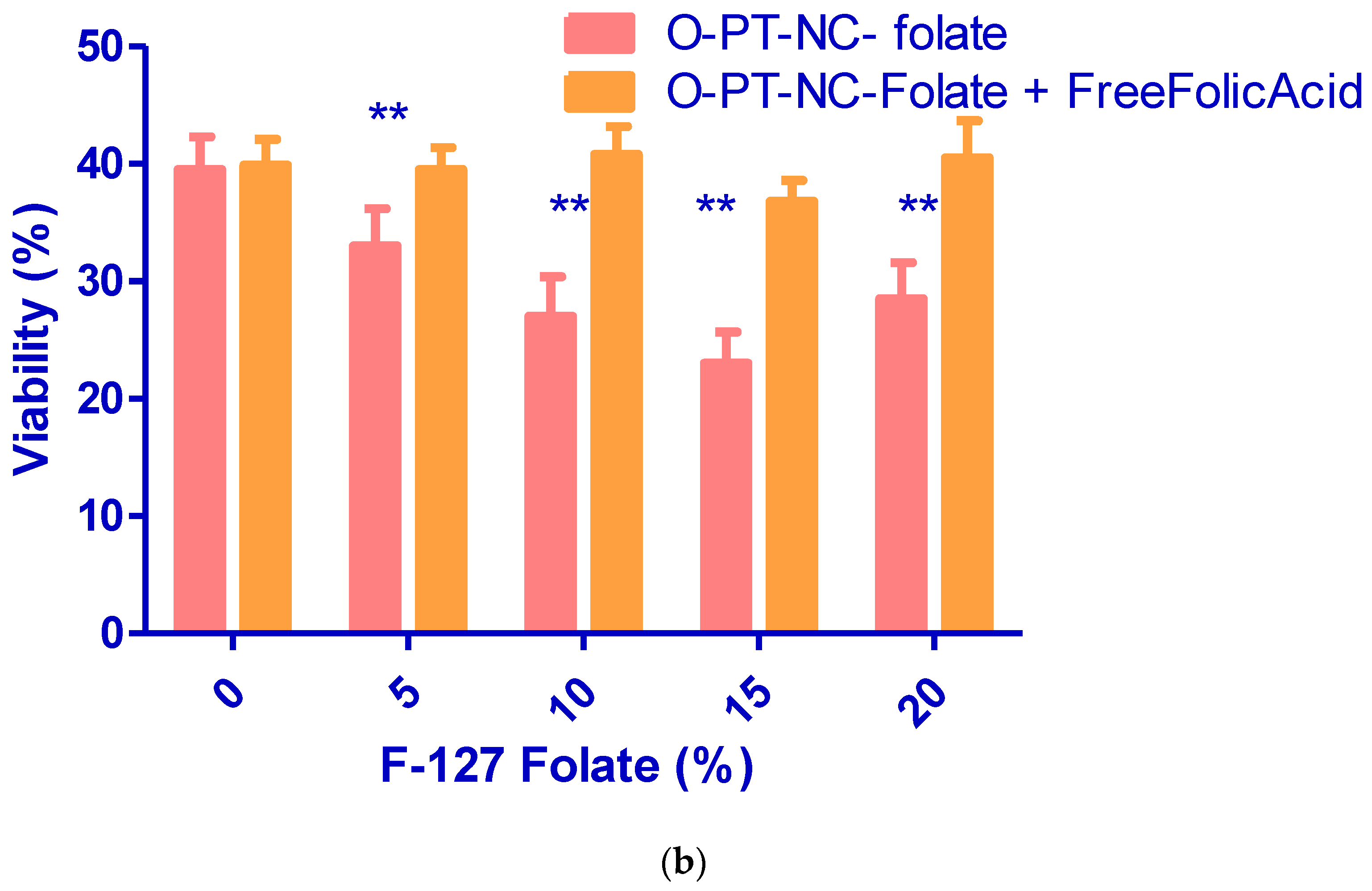
3.6. Folate Receptor-Targeted Nano-Crystals (O-PT-NC-Folate)
3.7. Hemolytic Activity
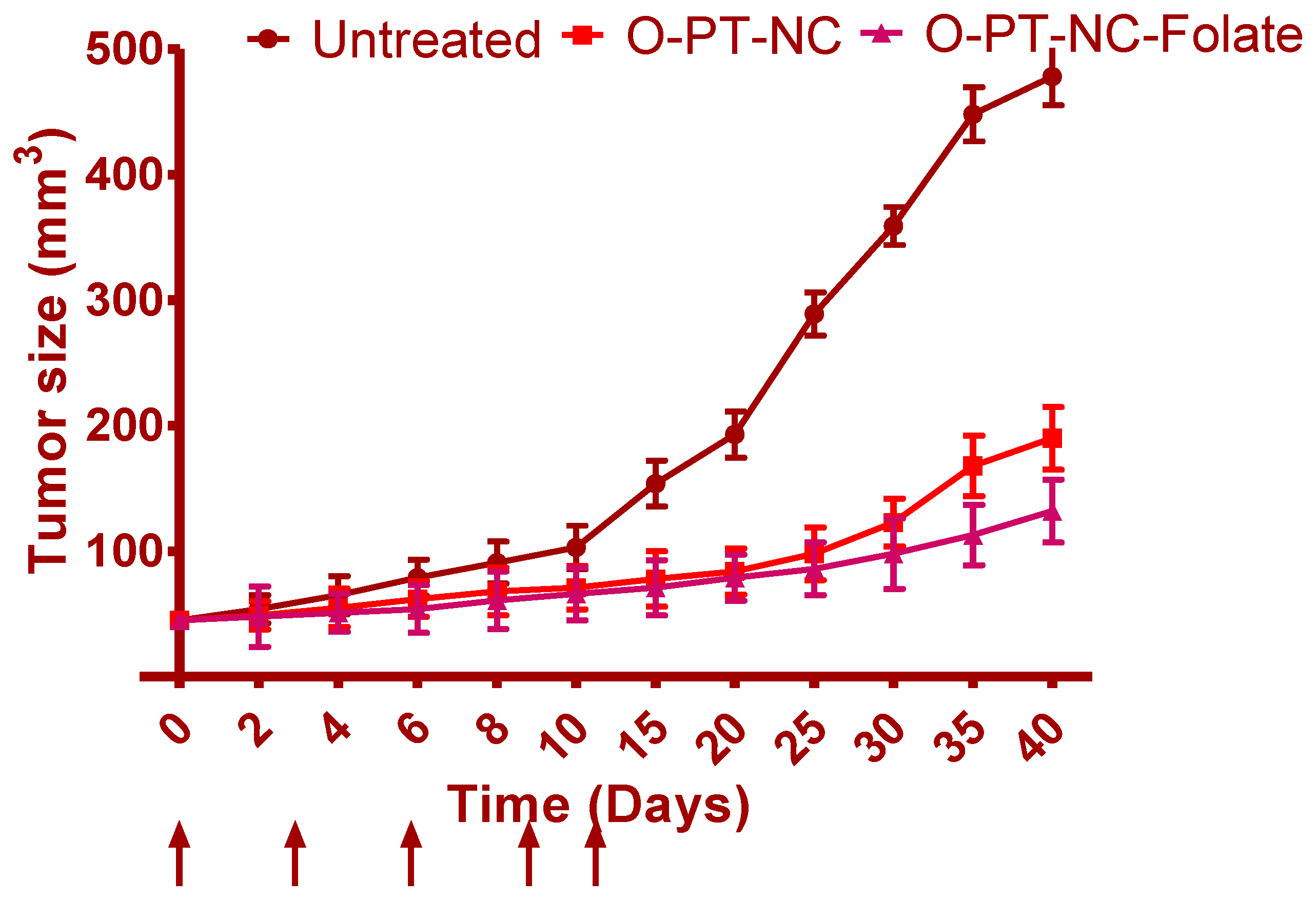
3.8. In Vivo Anti-Cancer Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 1 July 2022).
- Xu, Y.; Liu, X.; Lian, R.; Zheng, S.; Yin, Z.; Lu, Y.; Wu, W. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid-base neutralization. Int. J. Pharm. 2012, 438, 287–295. [Google Scholar] [CrossRef]
- Dong, F.; Xie, Y.; Qi, J.; Hu, F.; Lu, Y.; Li, S.; Wu, W. Bile salt/phospholipid mixed micelle precursor pellets prepared by fluid-bed coating. Int. J. Nanomed. 2013, 8, 1653. [Google Scholar] [CrossRef]
- Wu, W.; Lei, Y.; Lu, Y.; Qi, J.; Nie, S.; Hu, F.; Pam, W. Solid self-nanoemulsifying cyclosporin A pellets prepared by fluid-bed coating: Preparation, characterization and in vitro redispersibility. Int. J. Nanomed. 2011, 6, 795. [Google Scholar] [CrossRef]
- Hollis, C.P.; Li, T. Nanocrystals Production, Characterization, and Application for Cancer Therapy. In Pharmaceutical Sciences Encyclopedia; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Lu, Y.; Chen, Y.; Gemeinhart, R.A.; Wu, W.; Li, T. Developing nanocrystals for cancer treatment. Nanomedicine 2015, 10, 2537–2552. [Google Scholar] [CrossRef]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, D.; Cao, Z.; Zhang, C.; Cheng, D.; Liu, J.; Shuai, X. Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 2015, 3, 1035–1049. [Google Scholar] [CrossRef]
- Li, H.J.; Du, J.Z.; Du, X.J.; Xu, C.F.; Sun, C.Y.; Wang, H.X.; Cao, Z.T.; Yang, X.Z.; Zhu, Y.H.; Nie, S.; et al. Stimuli-responsive clustered nanoparticles for improved tumor penetration and therapeutic efficacy. Proc. Natl. Acad. Sci. USA 2016, 113, 4164–4169. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef]
- Huynh, E.; Zheng, G. Cancer nanomedicine: Addressing the dark side of the enhanced permeability and retention effect. Nanomedicine 2015, 10, 1993–1995. [Google Scholar] [CrossRef]
- Yan, X.; Niu, G.; Lin, J.; Jin, A.J.; Hu, H.; Tang, Y.; Zhang, Y.; Wu, A.; Lu, J.; Zhang, S.; et al. Enhanced fluorescence imaging guided photodynamic therapy of sinoporphyrin sodium loaded graphene oxide. Biomaterials 2015, 42, 94–102. [Google Scholar] [CrossRef]
- Xiong, M.H.; Bao, Y.; Yang, X.Z.; Zhu, Y.H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef]
- Miao, X.; Yang, W.; Feng, T.; Lin, J.; Huang, P. Drug nanocrystals for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1499. [Google Scholar] [CrossRef]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef]
- Morakul, B.; Suksiriworapong, J.; Chomnawang, M.T.; Langguth, P.; Junyaprasert, V.B. Dissolution enhancement and in vitro performance of clarithromycin nanocrystals produced by precipitation-lyophilization-homogenization method. Eur. J. Pharm. Biopharm. 2014, 88, 886–896. [Google Scholar] [CrossRef]
- Fateminia, S.M.A.; Wang, Z.; Liu, B. Nanocrystallization: An Effective Approach to Enhance the Performance of Organic Molecules. Small Methods 2017, 1, 1600023. [Google Scholar] [CrossRef]
- Bai, M.; Yang, M.; Gong, J.; Xu, H.; Wei, Z. Progress and Principle of Drug Nanocrystals for Tumor Targeted Delivery. AAPS PharmSciTech 2022, 23, 41. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Liu, F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol. Pharm. 2010, 7, 863–869. [Google Scholar] [CrossRef]
- Stinchcombe, T.E. Nanoparticle albumin-bound paclitaxel: A novel Cremphor-EL®-free formulation of paclitaxel. Nanomedicine 2007, 2, 863–869. [Google Scholar] [CrossRef]
- Sznitowska, M.; Klunder, M.; Placzek, M. Paclitaxel solubility in aqueous dispersions and mixed micellar solutions of lecithin. Chem. Pharm. Bull. 2008, 56, 70–74. [Google Scholar] [CrossRef]
- Lima, N.G.P.B.; Lima, I.P.B.; Barros, D.M.C.; Oliveira, T.S.; Raffin, F.N.; De Lima E Moura, T.F.A.; Medeiros, A.C.D.; Gomes, A.P.B.; Aragão, C.F.S. Compatibility studies of trioxsalen with excipients by DSC, DTA, and FTIR. J. Therm. Anal. Calorim. 2014, 115, 2311–2318. [Google Scholar] [CrossRef]
- Pani, N.R.; Nath, L.K.; Acharya, S.; Bhuniya, B. Application of DSC, IST, and FTIR study in the compatibility testing of nateglinide with different pharmaceutical excipients. J. Therm. Anal. Calorim. 2011, 108, 219–226. [Google Scholar] [CrossRef]
- Begum, A.; Sindhu, K.; Giri, K.; Umera, F.; Gauthami, G.; Kumar, J.V.; Naveen, N.; Rao, K.N.V.; Ali, S.S.; Sri, K. Pharmacognostical and physio-chemical evaluation of Indian Asparagus officinalis Linn family Lamiaceae. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 327–336. [Google Scholar]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Isolation and assessment of natural mucoadhesive agent isolated from Abelmoschus esculents. J. Pharm. Res. 2017, 11, 438–443. [Google Scholar]
- Martin, B.; Seguin, J.; Annereau, M.; Fleury, T.; Lai-Kuen, R.; Neri, G.; Lam, A.; Bally, M.; Mignet, N.; Corvis, Y. Preparation of parenteral nanocrystal suspensions of etoposide from the excipient free dry state of the drug to enhance in vivo antitumoral properties. Sci. Rep. 2020, 10, 18059. [Google Scholar] [CrossRef]
- Kurakula, M.; Raghavendra Naveen, N. In situ gel loaded with chitosan-coated simvastatin nanoparticles: Promising delivery for effective anti-proliferative activity against tongue carcinoma. Mar. Drugs 2020, 18, 201. [Google Scholar] [CrossRef]
- Naveen, N.R.; Kurakula, M.; Gowthami, B. Process optimization by response surface methodology for preparation and evaluation of methotrexate loaded chitosan nanoparticles. Mater. Today Proc. 2020, 33, 2716–2724. [Google Scholar] [CrossRef]
- Kurakula, M. Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef]
- Liu, F.; Park, J.Y.; Zhang, Y.; Conwell, C.; Liu, Y.; Bathula, S.R.; Huang, L. Targeted cancer therapy with novel high drug-loading nanocrystals. J. Pharm. Sci. 2010, 99, 3542–3551. [Google Scholar] [CrossRef]
- Taillefer, R. The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis. Semin. Nucl. Med. 1999, 29, 16–40. [Google Scholar] [CrossRef]
- Naveen, N.R. Design and characterization of sustained release matrix tablets of glimepiride by using synthetic and natural polymers. Int. J. Drug Discov. Herb. Res. 2013, 3, 573–578. [Google Scholar]
- Naveen, N.R.; Gopinath, C.; Kurakula, M. Okra-Thioglycolic Acid Conjugate—Synthesis, Characterization, and Evaluation as a Mucoadhesive Polymer. Processes 2020, 8, 316. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gastroretentive floating tablets: In vitro and in vivo evaluation. Future J. Pharm. Sci. 2017, 3, 140–147. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R.; Patel, B.; Manne, R.; Patel, D.B. Preparation, Optimization and Evaluation of Chitosan-Based Avanafil Nanocomplex Utilizing Antioxidants for Enhanced Neuroprotective Effect on PC12 Cells. Gels 2021, 7, 96. [Google Scholar] [CrossRef] [PubMed]
- Rizg, W.Y.; Naveen, N.R.; Kurakula, M.; Bukhary, H.A.; Safhi, A.Y.; Alfayez, E.; Sindi, A.M.; Ali, S.; Murshid, S.S.; Hosny, K.M. QbD Supported Optimization of the Alginate-Chitosan Nanoparticles of Simvastatin in Enhancing the Anti-Proliferative Activity against Tongue Carcinoma. Gels 2022, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar] [PubMed]
- Rizg, W.Y.; Naveen, N.R.; Kurakula, M.; Safhi, A.Y.; Murshid, S.S.; Mushtaq, R.Y.; Abualsunun, W.A.; Alharbi, M.; Bakhaidar, R.B.; Almehmady, A.M. Augmentation of Antidiabetic Activity of Glibenclamide Microspheres Using S-Protected Okra Powered by QbD: Scintigraphy and In Vivo Studies. Pharmaceuticals 2022, 15, 491. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, N.; Naveen, N.R.; Anitha, P.; Goudanavar, P.S.; Ramkanth, S.; Fattepur, S.; Telsang, M.; Habeebuddin, M.; Answer, M.K. Development of Nanocrystal Compressed Minitablets for Chronotherapeutic Drug Delivery. Pharmaceuticals 2022, 15, 311. [Google Scholar] [CrossRef]
- Naveen, N.R.; Nagaraja, T.S.; Bharathi, D.R.; Reddy, J.N.S. Formulation Design and In Vitro Evaluation for Stomach Specific Drug Delivery System of Anti Retroviral drug–Acyclovir. Int. J. Pharm. Life Sci. 2013, 4, 2506–2510. [Google Scholar]
- Aldawsari, H.M.; Raghavendra Naveen, N.; Alhakamy, N.A.; Goudanavar, P.S.; Koteswara Rao, G.; Rani Budha, R.; Nair, A.B.; Badr-Eldin, S.M.; Badr-, S.M. Compression-coated pulsatile chronomodulated therapeutic system: QbD assisted optimization. Drug Deliv. 2022, 29, 2258–2268. [Google Scholar] [CrossRef]
- Hosny, K.M.; Naveen, N.R.; Kurakula, M.; Sindi, A.M.; Sabei, F.Y.; Fatease, A.A.; Jali, A.M.; Alharbi, W.S.; Mushtaq, R.Y.; Felemban, M.; et al. Design and Development of Neomycin Sulfate Gel Loaded with Solid Lipid Nanoparticles for Buccal Mucosal Wound Healing. Gels 2022, 8, 385. [Google Scholar] [CrossRef] [PubMed]
- Bitton, R.J.; Figg, W.D.; Reed, E. A Preliminary Risk-Benefit Assessment of Paclitaxel. Drug Saf. 1995, 12, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Nornoo, A.O.; Osborne, D.W.; Chow, D.S.L. Cremophor-free intravenous microemulsions for paclitaxel. I: Formulation, cytotoxicity and hemolysis. Int. J. Pharm. 2008, 349, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Robbins, J.C.; Bugianesi, R.L.; Ponpipom, M.M.; Shen, T.Y. Modified in vivo behavior of liposomes containing synthetic glycolipids. BBA Gen. Subj. 1981, 674, 19–29. [Google Scholar] [CrossRef]
- Mekhail, T.M.; Markman, M. Paclitaxel in cancer therapy. Expert Opin. Pharmacother. 2002, 3. [Google Scholar]
- Wei, Y.; Pu, X.; Zhao, L. Preclinical studies for the combination of paclitaxel and curcumin in cancer therapy (Review). Oncol. Rep. 2017, 37, 3159–3166. [Google Scholar] [CrossRef]


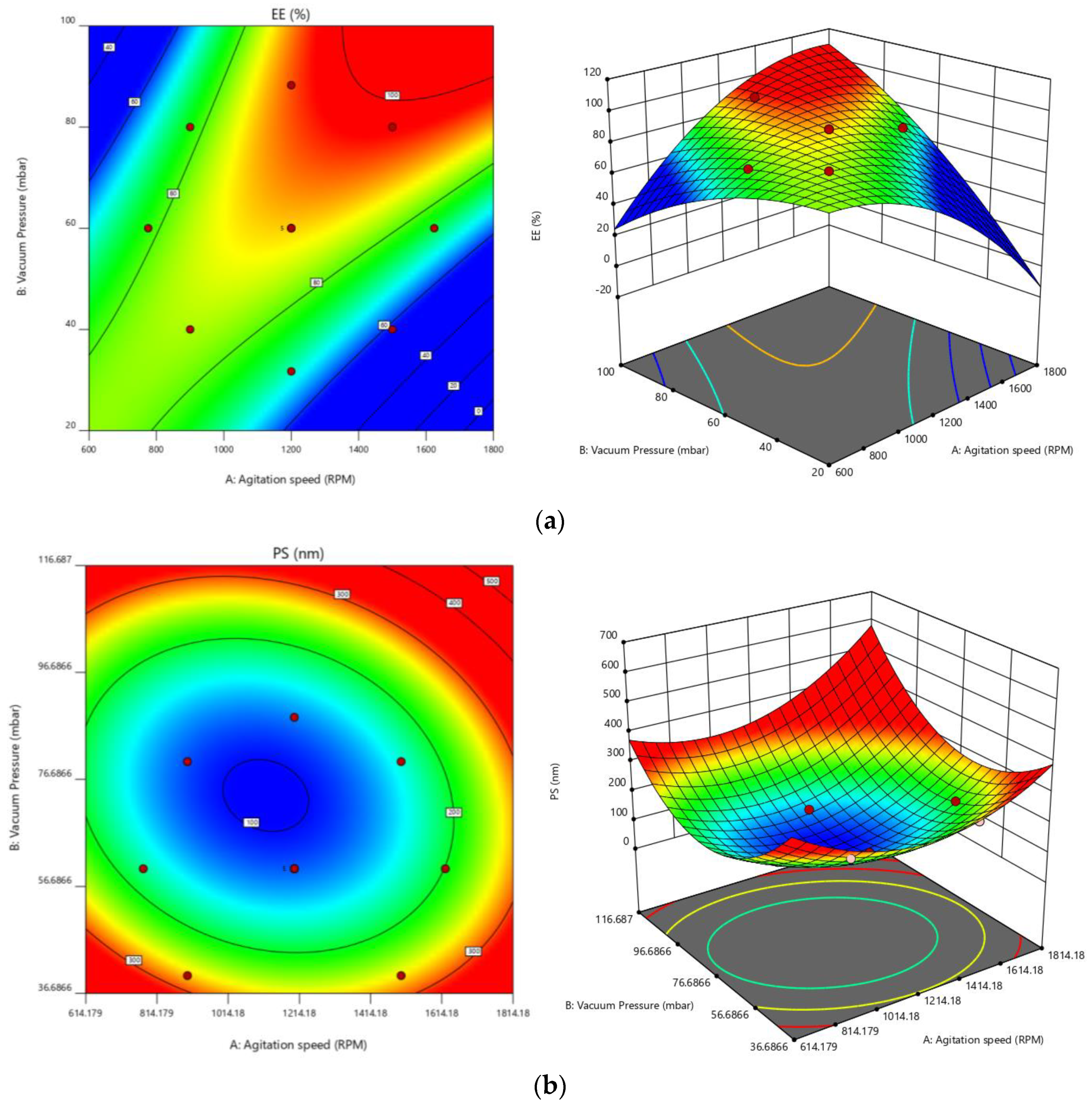
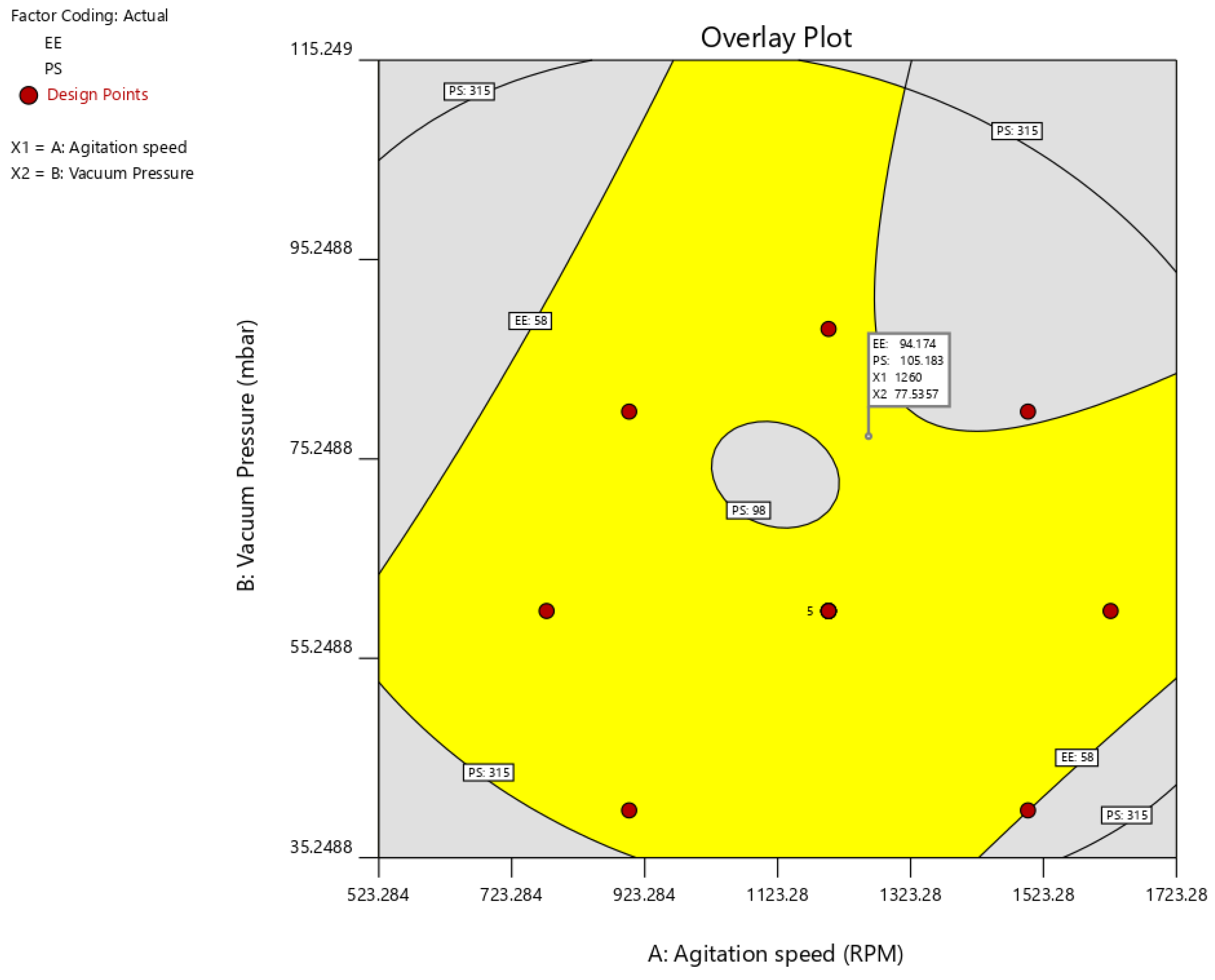



| Factors/Independent Variables | Levels | Responses/Dependent Variables | Constraints | ||||
|---|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | +1 | +1.414 | |||
| The agitation speed—X1 | 775.736 | 900 | 1200 | 1500 | 1624.26 | EE | Maximum |
| Vacuum Pressure—X2 | 31.7157 | 40 | 60 | 80 | 88.284 | PS | Minimum |
| Factor 1 | Factor 2 | Response 1 | Response 2 | ||
|---|---|---|---|---|---|
| Std | Run | A:Agitation speed | B:Vacuum Pressure | EE | PS |
| RPM | mbar | % | nm | ||
| 7 | 4 | 1200 | 31.72 | 69 | 315 |
| 1 | 8 | 900 | 40 | 84 | 265 |
| 2 | 12 | 1500 | 40 | 58 | 258 |
| 5 | 1 | 775.736 | 60 | 79 | 187 |
| 11 | 2 | 1200 | 60 | 88 | 110 |
| 12 | 7 | 1200 | 60 | 87 | 114 |
| 9 | 9 | 1200 | 60 | 89 | 116 |
| 10 | 10 | 1200 | 60 | 88 | 125 |
| 13 | 11 | 1200 | 60 | 89 | 125 |
| 6 | 13 | 1624.26 | 60 | 76 | 212 |
| 3 | 5 | 900 | 80 | 75 | 98 |
| 4 | 6 | 1500 | 80 | 94 | 145 |
| 8 | 3 | 1200 | 88.28 | 96 | 145 |
| Response | Models | R2 | Adju.R2 | Pred.R2 | Adequate Precision | Sequential p-Value | Remarks |
|---|---|---|---|---|---|---|---|
| EE | Linear | 0.3977 | 0.2772 | −0.2036 | ---- | 0.0793 | |
| 2 FI | 0.7658 | 0.6877 | 0.3704 | 27.8130 | 0.0045 | ||
| Quadratic | 0.9785 | 0.9632 | 0.8587 | --- | 0.0002 | Suggested | |
| Cubic | 0.9906 | 0.9774 | 0.5256 | --- | 0.1270 | Aliased | |
| PS | Linear | 0.5762 | 0.4915 | 0.3042 | --- | 0.0022 | |
| 2 FI | 0.5884 | 0.4512 | 0.2250 | --- | 0.0137 | ||
| Quadratic | 0.9756 | 0.9582 | 0.8435 | 19.8857 | <0.0001 | Suggested | |
| Cubic | 0.9789 | 0.9495 | −0.1583 | --- | 0.6942 |
| Intercept | A | B | AB | A² | B² | |
|---|---|---|---|---|---|---|
| EE | 88.2 | −1.40533 | 8.14797 | 11.25 | −5.9125 | −3.4125 |
| p-values | 0.0041 | <0.0001 | <0.0001 | 0.0001 | 0.0032 | |
| PS | 118 | 9.41942 | −65.052 | 13.5 | 34.9375 | 50.1875 |
| p-values | 0.1078 | <0.0001 | 0.1039 | 0.0004 | <0.0001 |
| TEST | INITIAL | 25 °C ± 2 °C + 60% ± 5% RH | 40 °C ±2 °C + 75% ±5% RH | ||
|---|---|---|---|---|---|
| 3 M | 6 M | 3 M | 6 M | ||
| Physical characteristics | Complies | Complies | Complies | Complies | Complies |
| PS | 106.21 | 105.78 | 104.24 | 104.34 | 103.52 |
| PDI | 0.18 | 0.19 | 0.20 | 0.20 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreeharsha, N.; Prasanthi, S.; Mahalakshmi, S.V.V.N.S.; Goudanavar, P.S.; Naveen, N.R.; Gowthami, B.; Fattepur, S.; Meravanige, G.; Asdaq, S.M.B.; Anwer, M.K.; et al. Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study. Molecules 2022, 27, 7914. https://doi.org/10.3390/molecules27227914
Sreeharsha N, Prasanthi S, Mahalakshmi SVVNS, Goudanavar PS, Naveen NR, Gowthami B, Fattepur S, Meravanige G, Asdaq SMB, Anwer MK, et al. Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study. Molecules. 2022; 27(22):7914. https://doi.org/10.3390/molecules27227914
Chicago/Turabian StyleSreeharsha, Nagaraja, Samathoti Prasanthi, Satyavarapu Veera Venkata Naga Satya Mahalakshmi, Prakash S. Goudanavar, Nimbagal Raghavendra Naveen, Buduru Gowthami, Santosh Fattepur, Girish Meravanige, Syed Mohammed Basheeruddin Asdaq, Md. Khalid Anwer, and et al. 2022. "Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study" Molecules 27, no. 22: 7914. https://doi.org/10.3390/molecules27227914
APA StyleSreeharsha, N., Prasanthi, S., Mahalakshmi, S. V. V. N. S., Goudanavar, P. S., Naveen, N. R., Gowthami, B., Fattepur, S., Meravanige, G., Asdaq, S. M. B., Anwer, M. K., Aldhubiab, B., Islam, M. M., Habeebuddin, M., Telsang, M., Gharsan, M. A., & Haroun, M. (2022). Enhancement of Anti-Tumoral Properties of Paclitaxel Nano-Crystals by Conjugation of Folic Acid to Pluronic F127: Formulation Optimization, In Vitro and In Vivo Study. Molecules, 27(22), 7914. https://doi.org/10.3390/molecules27227914







