Activation of a Sweet Taste Receptor by Oleanane-Type Glycosides from Wisteria sinensis
Abstract
1. Introduction
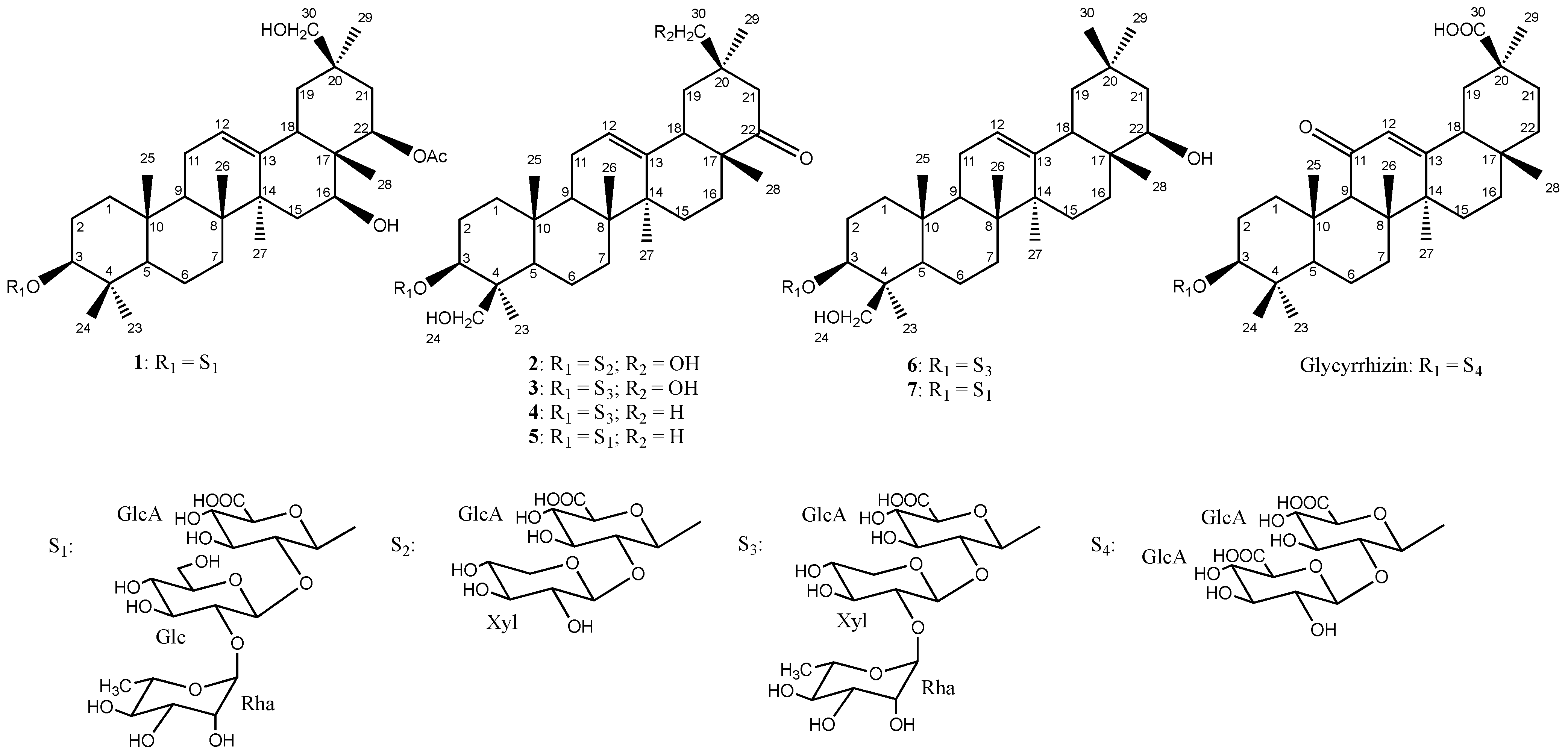
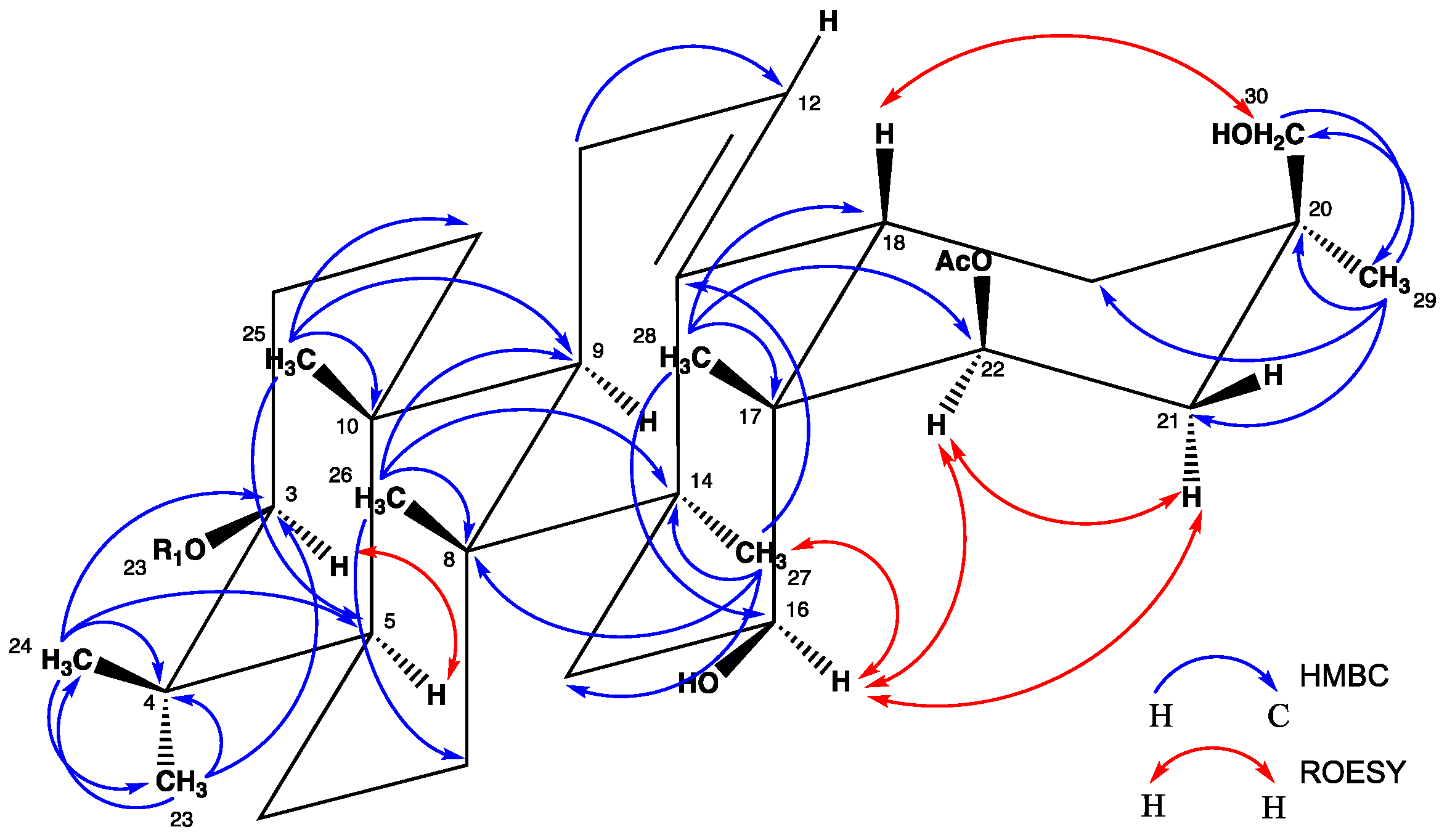
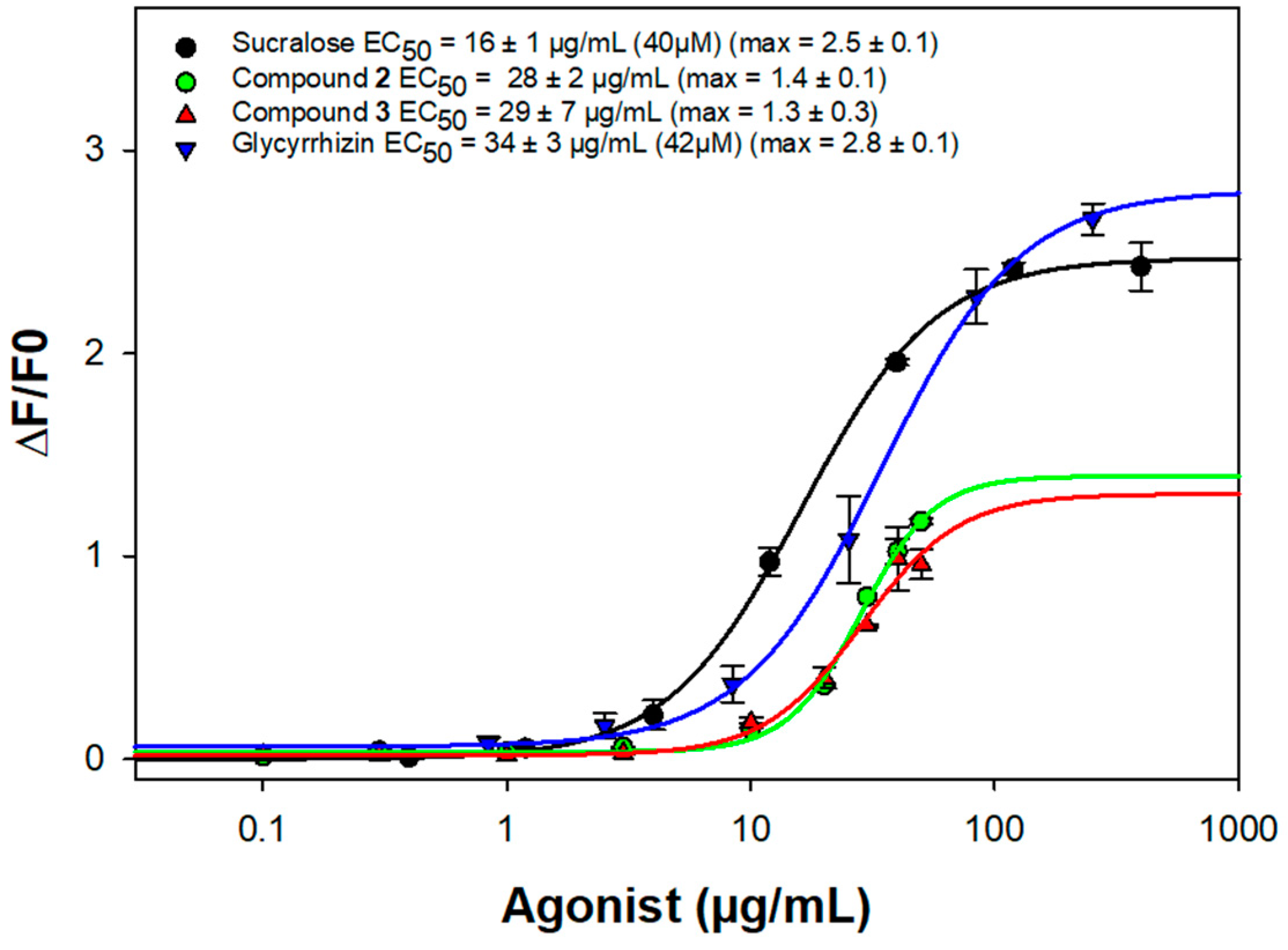
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis and Absolute Configuration Determination
3.5. Bioactivity Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Disse, E.; Bussier, A.-L.; Veyrat-Durebex, C.; Deblon, N.; Pfluger, P.T.; Tschöp, M.H.; Laville, M.; Rohner-Jeanrenaud, F. Peripheral ghrelin enhances sweet taste food consumption and preference, regardless of its caloric content. Physiol. Behav. 2010, 101, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imada, T.; Nakagita, T.; Okada, S.; Nammoku, T.; Abe, K.; Misaka, T. Sweeteners interacting with the transmembrane domain of the human sweet-taste receptor induce sweet-taste synergisms in binary mixtures. Food Chem. 2012, 130, 561–568. [Google Scholar] [CrossRef]
- Mennella, J.A.; Bobowski, N.K.; Reed, D.R. The development of sweet taste: From biology to hedonics. Rev. Endocr. Metab. Disord. 2016, 17, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Carniel Beltrami, M.; Döring, T.; De Dea Lindner, J. Sweeteners and sweet taste enhancers in the food industry. Food Sci. Technol. 2018, 38, 181–187. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nagasawa, M.; Mogami, H.; Lohse, M.; Ninomiya, Y.; Kojima, I. Multimodal function of the sweet taste receptor expressed in pancreatic β-cells: Generation of diverse patterns of intracellular signals by sweet agonists. Endocr. J. 2013, 60, 1191–1206. [Google Scholar] [CrossRef] [PubMed]
- Belloir, C.; Brulé, M.; Tornier, L.; Neiers, F.; Briand, L. Biophysical and functional characterization of the human TAS1R2 sweet taste receptor overexpressed in a HEK293S inducible cell line. Sci. Rep. 2021, 11, 22238. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny—The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Website. Available online: http://www.mobot.org/MOBOT/Research/APweb/welcome.html (accessed on 7 June 2022).
- Champy, A.-S.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Papini, A.-M.; Lacaille-Dubois, M.-A. Structural analysis of oleanane-type saponins from the roots of Wisteria frutescens: Oleanane-type saponins from Wisteria frutescens. Magn. Reson. Chem. 2017, 55, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Champy, A.-S.; Mitaine-Offer, A.-C.; Paululat, T.; Papini, A.-M.; Lacaille-Dubois, M.-A. Triterpene saponins from Wisteria floribunda “Macrobotrys” and “Rosea”. Nat. Prod. Commun. 2017, 12, 1573–1576. [Google Scholar] [CrossRef]
- Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K. Constituents of leguminous plants, XIII. New triterpenoid saponins from Wistaria brachybotrys. J. Nat. Prod. 1991, 54, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Schmid, C.; Brockhoff, A.; Shoshan-Galeczki, Y.B.; Kranz, M.; Stark, T.D.; Erkaya, R.; Meyerhof, W.; Niv, M.Y.; Dawid, C.; Hofmann, T. Comprehensive structure-activity-relationship studies of sensory active compounds in licorice (Glycyrrhiza glabra). Food Chem. 2021, 364, 130420. [Google Scholar] [CrossRef] [PubMed]
- Konoshima, T.; Kozuka, M.; Haruna, M.; Ito, K.; Kimura, T.; Tokuda, H. Studies on the constituents of leguminous plants. (XII.1) from Wistaria brachybotrys SIEB. et (ZUCC.2) The structures of new triterpenoid saponins. Chem. Pharm. Bull. 1989, 37, 2731–2735. [Google Scholar] [CrossRef][Green Version]
- Mohamed, K.M.; Ohtani, K.; Kasai, R.; Yamasaki, K. Oleanene glycosides from seeds of Trifolium alexandrinum. Phytochemistry 1995, 40, 1237–1242. [Google Scholar] [CrossRef]
- Kitagawa, I.; Wang, H.K.; Yoshikawa, M. Saponin and sapogenol. XXXVII.1) Chemical constituents of Astragali radix, the root of Astragalus membranaceus BUNGE. (4). Astragaloside VII and VIII. Chem. Pharm. Bull. 1983, 31, 716–722. [Google Scholar] [CrossRef]
| Position | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 38.5 | 1.02 m 1.64 m | 38.4 | 1.02 m 1.63 m |
| 2 | 25.4 | 1.72 m 2.13 m | 25.5 | 2.10 m 2.13 m |
| 3 | 90.4 | 3.22 | 90.3 | 3.35 dd (11.7, 2.9) |
| 4 | 39.1 | - | 43.3 | - |
| 5 | 55.5 | 0.80 | 56.0 | 0.95 |
| 6 | 17.9 | 1.44 m 1.59 m | 18.0 | 1.41 m 1.66 m |
| 7 | 32.4 | 1.37 m 1.59 m | 32.6 | 1.40 m 1.58 m |
| 8 | 39.8 | - | 39.4 | - |
| 9 | 46.8 | 1.57 m | 47.8 | 1.62 m |
| 10 | 36.3 | - | 36.1 | - |
| 11 | 23.2 | 1.91 m 1.93 m | 23.4 | 1.90 m 1.92 m |
| 12 | 123.0 | 5.33 br t (3.0) | 123.8 | 5.38 br t (3.0) |
| 13 | 142.3 | - | 141.4 | - |
| 14 | 42.9 | - | 41.6 | - |
| 15 | 35.9 | 1.31 m 1.76 m | 24.8 | 1.11 m 1.79 m |
| 16 | 66.2 | 4.15 dd (11.7, 4.7) | 26.7 | 1.21 m 2.07 m |
| 17 | 41.0 | - | 46.9 | - |
| 18 | 45.7 | 2.33 dd (14.1, 4.1) | 46.9 | 2.46 dd (13.5, 3.5) |
| 19 | 40.5 | 1.34 1.73 | 41.8 | 1.62 1.99 |
| 20 | 34.5 | - | 38.6 | - |
| 21 | 32.8 | 1.49 1.75 | 46.0 | 2.18 d (14.4) 2.36 d (14.4) |
| 22 | 74.2 | 5.24 dd (3.5, 1.2) | 218.0 | - |
| 23 | 15.4 | 0.88 s | 21.3 | 1.18 s |
| 24 | 27.3 | 1.11 s | 62.5 | 3.23 d (12.0) 4.06 d (12.0) |
| 25 | 14.7 | 0.98 s | 14.7 | 0.88 s |
| 26 | 16.1 | 1.02 s | 16.0 | 0.97 s |
| 27 | 26.5 | 1.26 s | 24.3 | 1.24 s |
| 28 | 13.8 | 0.79 s | 19.9 | 0.97 s |
| 29 | 27.0 | 0.91 s | 25.4 | 0.93 s |
| 30 | 67.3 | 3.49 d (14.1) 3.51 d (14.1) | 67.4 | 3.28 d (12.9) 3.34 d (12.9) |
| at C-22 | ||||
| COCH3 | 171.1 | - | ||
| CH3CO | 20.0 | 2.02 (3H, s) | ||
| GlcA-1 | 104.3 | 4.42 d (7.6) | 103.5 | 4.45 d (7.6) |
| 2 | 79.6 | 3.70 dd (9.4, 7.6) | 79.6 | 3.49 dd (9.4, 7.6) |
| 3 | 77.3 | 3.60 t (9.4) | 76.7 | 3.61 t (9.4) |
| 4 | 72.6 | 3.42 t (9.4) | 72.1 | 3.48 |
| 5 | 75.1 | 3.52 | 75.3 | 3.60 |
| 6 | 175.3 | - | 175.7 | - |
| Xyl-1 | 103.7 | 4.63 d (7.6) | ||
| 2 | 74.1 | 3.19 dd (9.4, 7.6) | ||
| 3 | 77.0 | 3.28 t (9.4) | ||
| 4 | 69.4 | 3.49 m | ||
| 5 | 65.5 | 3.12 dd (11.1, 9.4) 3.81 dd (11.1, 5.3) | ||
| Glc-1 | 100.7 | 4.88 d (7.6) | ||
| 2 | 78.2 | 3.37 dd (9.4, 7.6) | ||
| 3 | 77.9 | 3.44 t (9.4) | ||
| 4 | 71.3 | 3.05 t (9.4) | ||
| 5 | 76.7 | 3.22 m | ||
| 6 | 62.2 | 3.82 dd (11.7, 2.4) 3.52 dd (11.7, 7.6) | ||
| Rha-1 | 100.6 | 5.19 br s | ||
| 2 | 70.9 | 3.91 br s | ||
| 3 | 70.8 | 3.75 dd (9.4, 2.9) | ||
| 4 | 72.8 | 3.39 t (9.4) | ||
| 5 | 68.2 | 4.12 dq (9.4, 5.9) | ||
| 6 | 16.9 | 1.25 d (5.9) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobloss, S.; Bruguière, A.; Pertuit, D.; Miyamoto, T.; Tanaka, C.; Belloir, C.; Lacaille-Dubois, M.-A.; Briand, L.; Mitaine-Offer, A.-C. Activation of a Sweet Taste Receptor by Oleanane-Type Glycosides from Wisteria sinensis. Molecules 2022, 27, 7866. https://doi.org/10.3390/molecules27227866
Hobloss S, Bruguière A, Pertuit D, Miyamoto T, Tanaka C, Belloir C, Lacaille-Dubois M-A, Briand L, Mitaine-Offer A-C. Activation of a Sweet Taste Receptor by Oleanane-Type Glycosides from Wisteria sinensis. Molecules. 2022; 27(22):7866. https://doi.org/10.3390/molecules27227866
Chicago/Turabian StyleHobloss, Samir, Antoine Bruguière, David Pertuit, Tomofumi Miyamoto, Chiaki Tanaka, Christine Belloir, Marie-Aleth Lacaille-Dubois, Loïc Briand, and Anne-Claire Mitaine-Offer. 2022. "Activation of a Sweet Taste Receptor by Oleanane-Type Glycosides from Wisteria sinensis" Molecules 27, no. 22: 7866. https://doi.org/10.3390/molecules27227866
APA StyleHobloss, S., Bruguière, A., Pertuit, D., Miyamoto, T., Tanaka, C., Belloir, C., Lacaille-Dubois, M.-A., Briand, L., & Mitaine-Offer, A.-C. (2022). Activation of a Sweet Taste Receptor by Oleanane-Type Glycosides from Wisteria sinensis. Molecules, 27(22), 7866. https://doi.org/10.3390/molecules27227866








