Evaluation of a Chaotrope and Kosmotrope in the Multivariate Optimization of PHW-ATPE of Solasodine from Leaves of Solanum mauritianum
Abstract
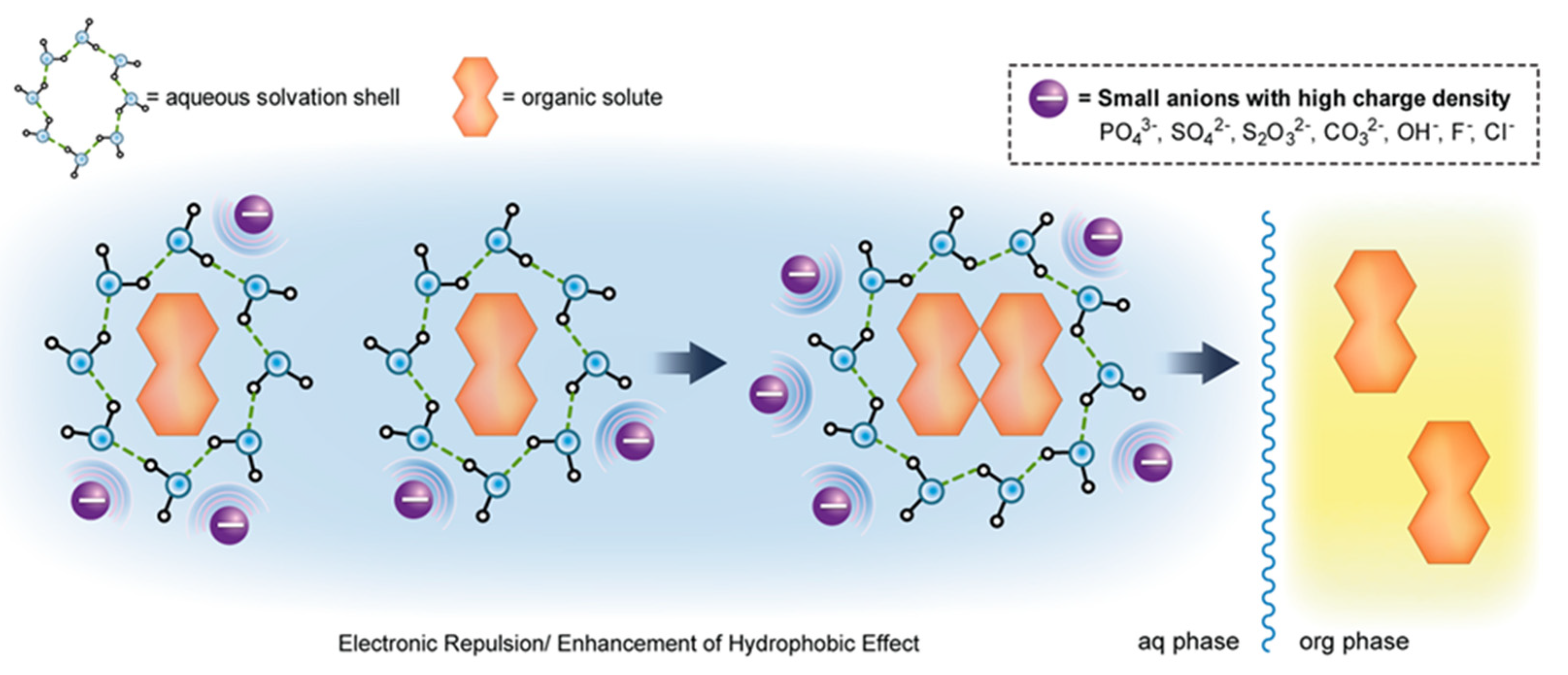
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
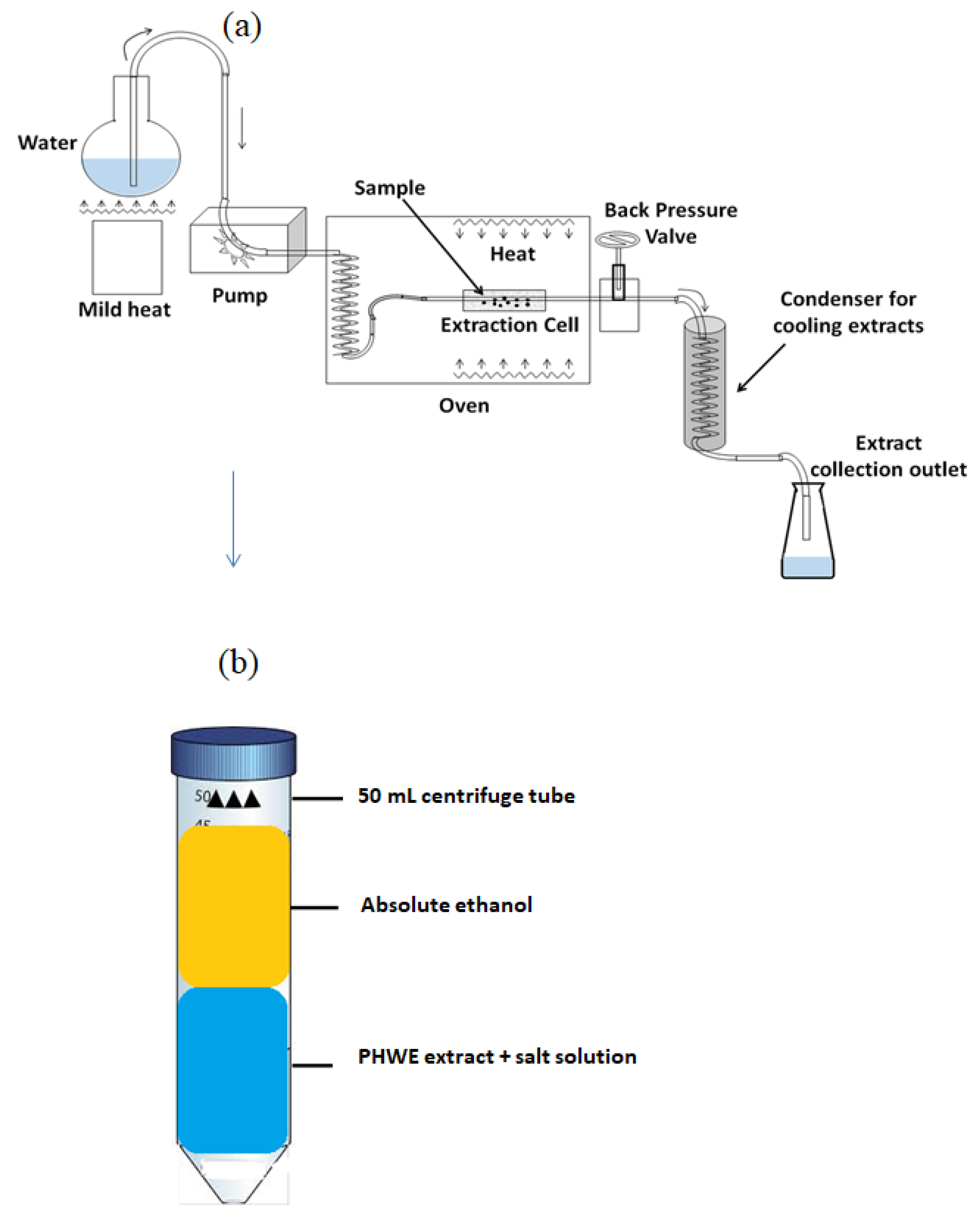
2.2. Sample Collection, Preparation, and ATPE
2.3. Chromatographic and Mass Spectrometry Conditions
2.4. Statistical Analysis
3. Results and Discussion
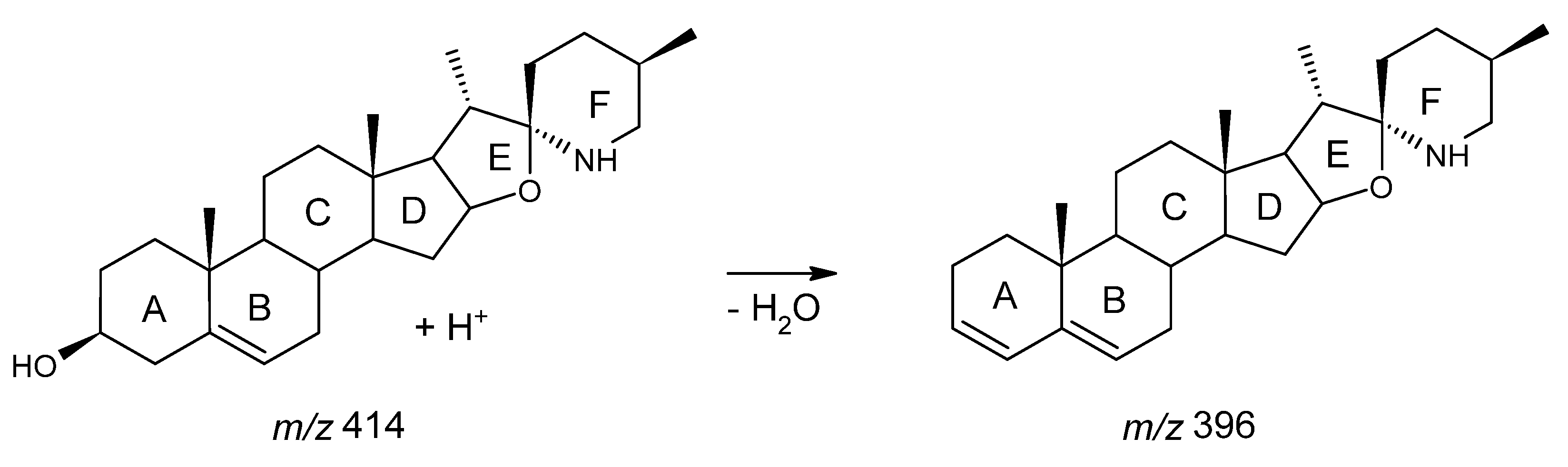
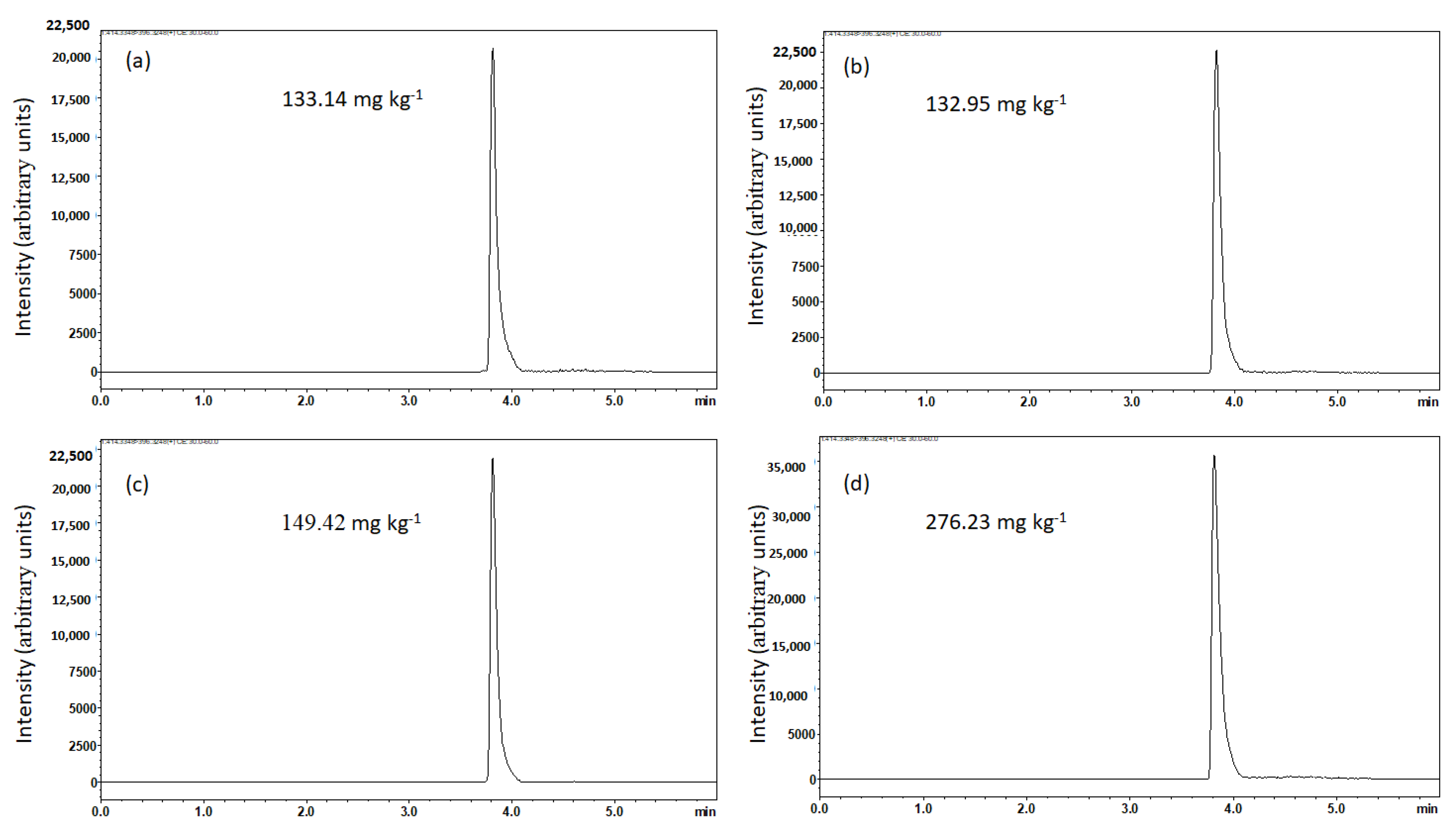
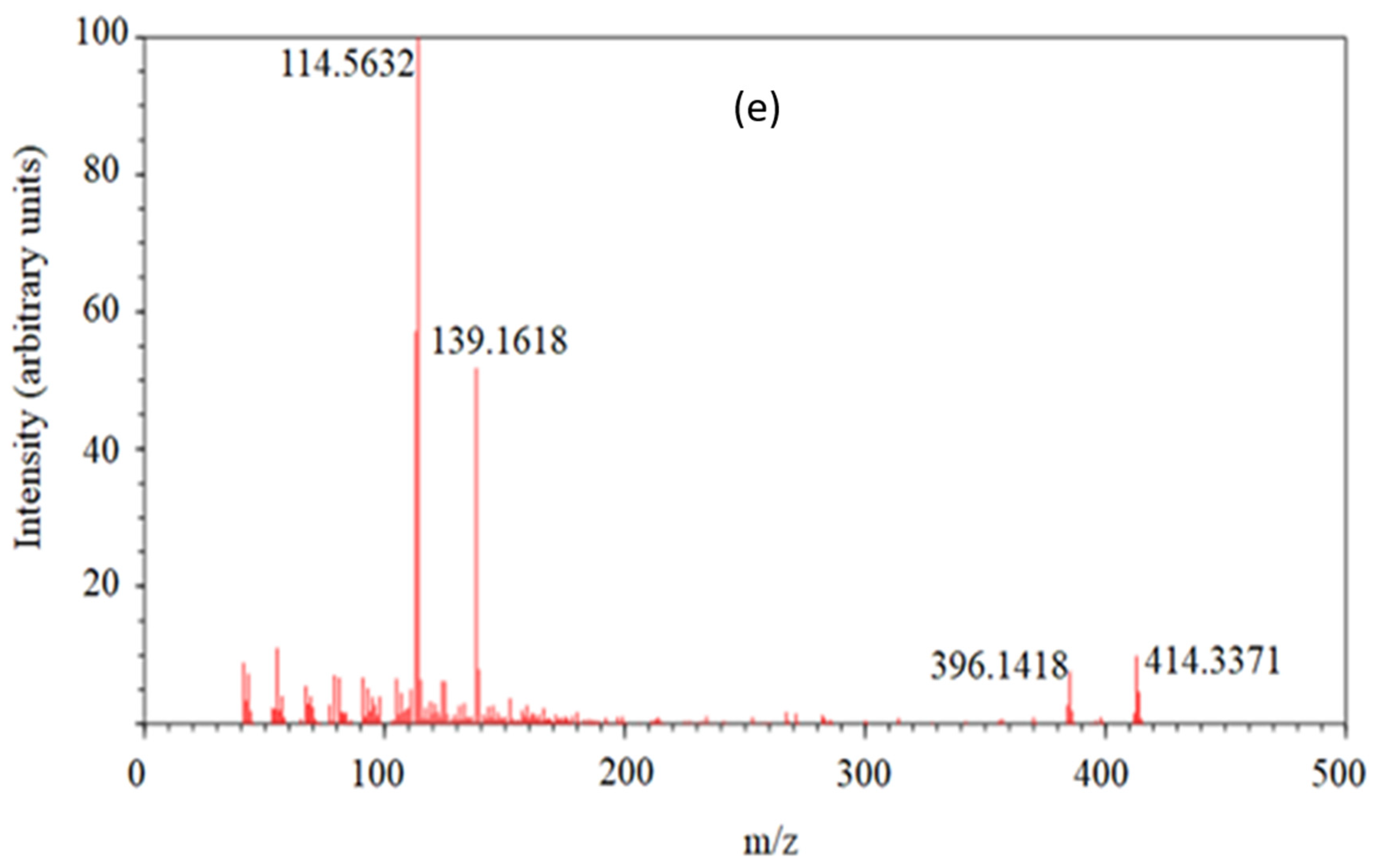
3.1. MRM Quantification of Solasodine Based on the 414 → 396 Transition
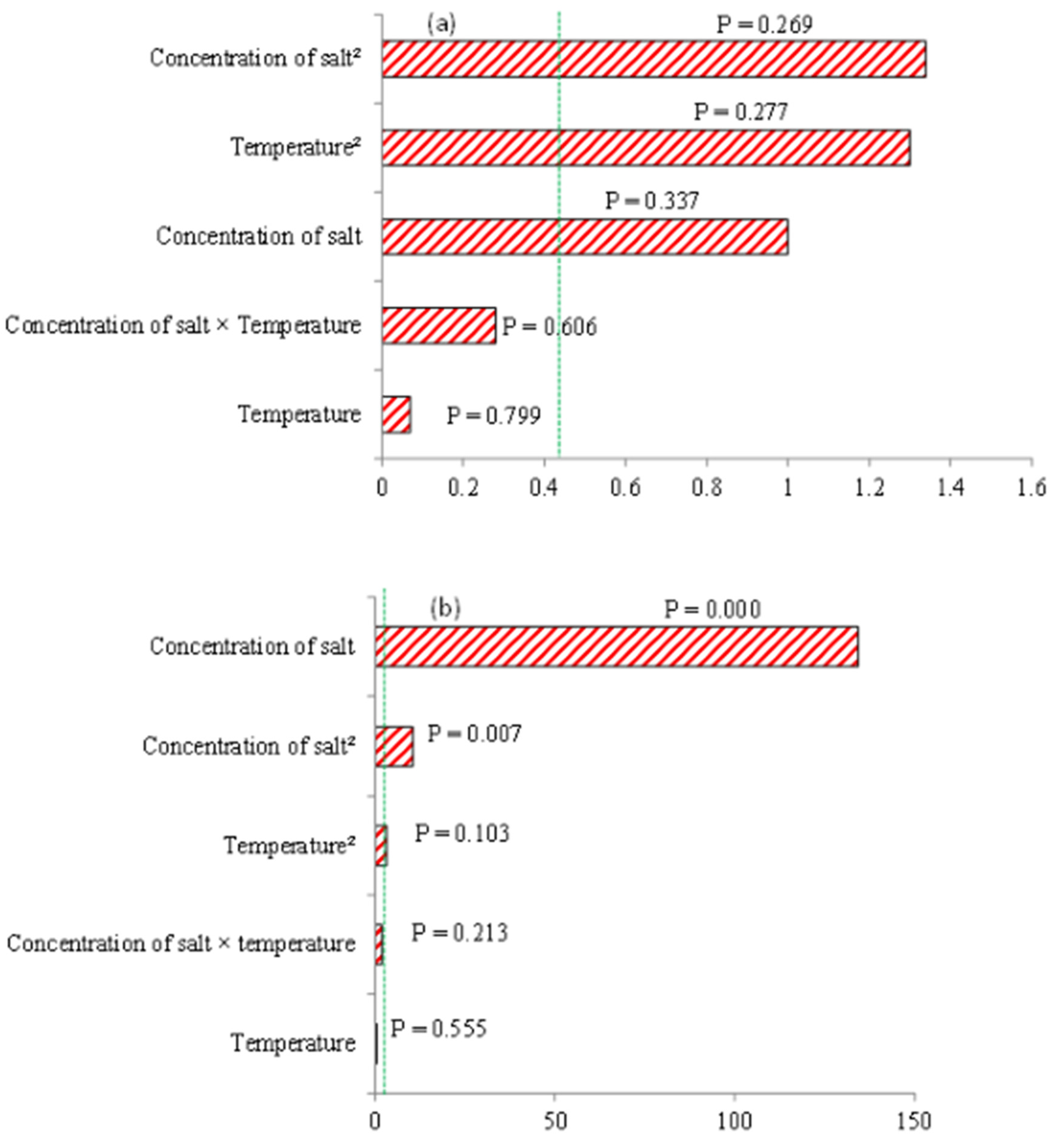
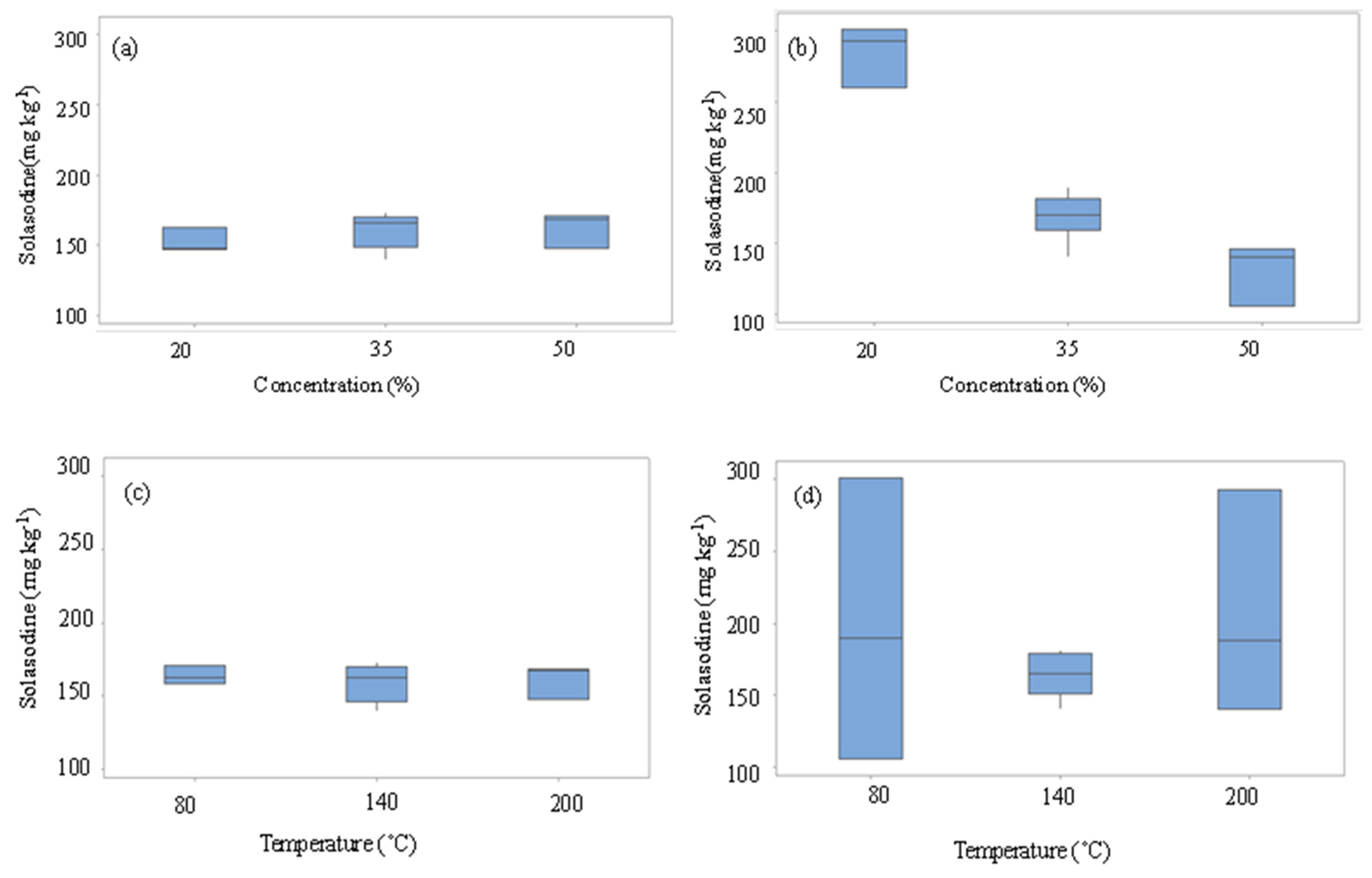
3.2. Fit Statistics and Pareto Chart of Parameter Main Effects and their Interactions Produced from ANOVA and Resultant Box Plots
3.3. Chromatographic Profile of MRM-Based Quantification of Solasodine
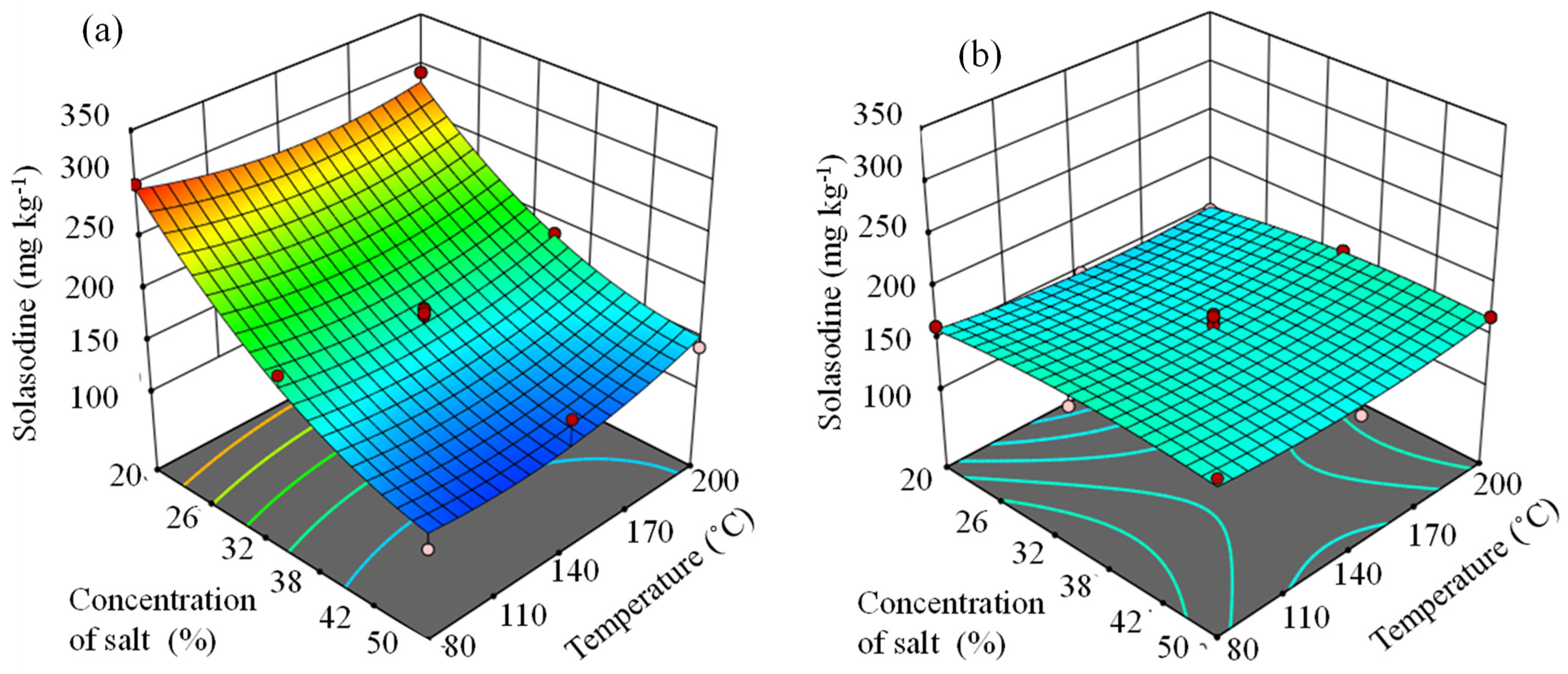
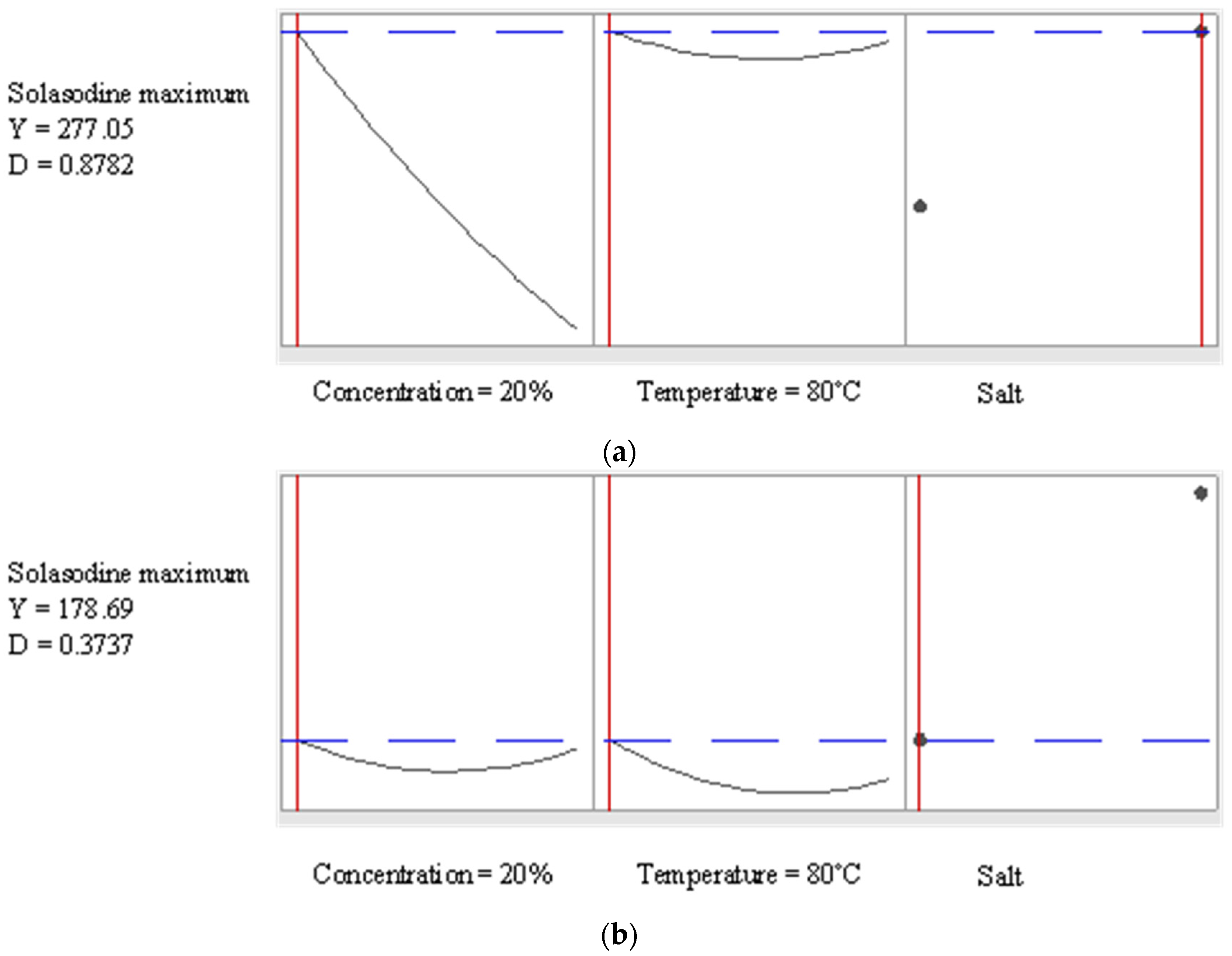
3.4. Optima Obtained from Response Surface Equations with the Aid of NaCl or Na2CO3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shang, X.; Dou, Y.; Zhang, Y.; Tan, J.N.; Liu, X.; Zhang, Z. Tailor-made natural deep eutectic solvents for green extraction of isoflavones from chickpea (Cicer arietinum L.) sprouts. Ind. Crops Prod. 2019, 140, 3–8. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Kuhn, F.; de Azevedo, E.S.; Frazzon, J.; Noreña, C.P.Z. Evaluation of green extraction methods on bioactive compounds and antioxidant capacity from Bougainvillea glabra bracts. Chem. Pharm. 2021, 19, 100362. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Gan, R.Y. Green extraction of antioxidant polyphenols from green tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. Green Chem. Theory Pract. 1998, 29, 1–27. [Google Scholar]
- Turner, C.; Ibanez, E. Pressurized hot water extraction and processing. In Enhancing Extraction Processes in the Food Industry; CRC Press: Boca Raton, FL, USA, 2012; pp. 223–254. [Google Scholar]
- Musarurwa, H.; Tavengwa, N.T. Emerging green solvents and their applications during pesticide analysis in food and environmental samples. Talanta 2020, 233, 1–11. [Google Scholar] [CrossRef]
- Cederholm, L.; Xu, Y.; Tagami, A.; Sevastyanova, O.; Odelius, K.; Hakkarainen, M. Microwave processing of lignin in green solvents: A high-yield process to narrow-dispersity oligomers. Ind. Crops Prod. 2020, 145, 112152. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Gbashi, S.; Njobeh, P.B.; De Saeger, S.; De Boevre, M.; Madala, N.E. Development, chemometric-assisted optimization and in-house validation of a modified pressurized hot water extraction methodology for multi-mycotoxins in maize. Food Chem. 2020, 307, 125526. [Google Scholar] [CrossRef]
- Nuapia, Y.; Tutu, H.; Chimuka, L.; Cukrowska, E. Selective extraction of cannabinoid compounds from cannabis seed using pressurized hot water extraction. Molecules 2020, 25, 1335. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, X.; Shirvan, K. System code evaluation of near-term accident tolerant claddings during pressurized water reactor station blackout accidents. Nucl. Eng. Des. 2020, 368, 110814. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Morais, D.R.; Gianvecchio, V.A.; Aquino, E.M.; Cunha, R.L.; Huestis, M.A.; Costa, J.L. Development and validation of a method for quantification of 28 psychotropic drugs in postmortem blood samples by modified Micro-QuEChERS and LC–MS-MS. J. Anal. Toxicol. 2021, 45, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Lendor, S.; Hassani, S.A.; Boyaci, E.; Singh, V.; Womelsdorf, T.; Pawliszyn, J. Solid phase microextraction-based miniaturized probe and protocol for extraction of neurotransmitters from brains in vivo. Anal. Chem. 2019, 91, 4896–4905. [Google Scholar] [CrossRef]
- Abaroa-Pérez, B.; Caballero-Martel, A.E.; Hernández-Brito, J.J.; Vega-Moreno, D. Solid-Liquid-Liquid Microextraction (μSLLE) Method for Determining Persistent Pollutants in Microplastics. Water Air Soil Pollut. 2021, 232, 171. [Google Scholar] [CrossRef]
- Ciceri, D.; Perera, J.M.; Stevens, G.W. The use of microfluidic devices in solvent extraction. J. Chem. Technol. Biotechnol. 2014, 89, 771–786. [Google Scholar] [CrossRef]
- Agrawal, A.; Keçili, R.; Ghorbani-Bidkorbeh, F.; Hussain, C.M. Green miniaturized technologies in analytical and bioanalytical chemistry. TrAC 2021, 143, 116383. [Google Scholar] [CrossRef]
- Mejia-Carmona, K.; da Silva Burato, J.S.; Borsatto, J.V.B.; de Toffoli, A.L.; Lancas, F.M. Miniaturization of liquid chromatography coupled to mass spectrometry: Current trends on miniaturized LC columns. TrAC 2020, 122, 115735. [Google Scholar] [CrossRef]
- Mokgehle, T.M.; Madala, N.E.; Tavengwa, N.T. Optimization in the Aqueous Two Phase Extraction of Solasodine from Solanum mauritianum and Analysis via UHPLC-qTOF-MS. Chem. Afr. 2022, 5, 651–661. [Google Scholar] [CrossRef]
- Burato, J.S.S.; Medina, D.A.V.; Toffoli, A.L.; Maciel, E.V.S.; Lanças, F.M. Recent advances and trends in miniaturized sample preparation techniques. J. Sep. Sci. 2020, 43, 202–225. [Google Scholar] [CrossRef]
- Marzouk, Z.; Chraief, I.; Cheriaa, J.; Bakhrouf, A.; Boukef, K. Effect of fertilization on growth and solasodine content of four natural Solanum sodomeum L. populations. J. Food Agric. Environ. 2005, 3, 341–344. [Google Scholar]
- Fekry, M.I.; Ezzat, S.M.; Salama, M.M.; Alshehri, O.Y.; Al-Abd., A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cham, B.E. First in man topical treatment of melanoma with solasodine glycosides in a formulation curaderm: A case report. J. Cancer Ther. 2020, 11, 617–630. [Google Scholar] [CrossRef]
- Maddala, M.; Gundi, B.; Gudimalla, S. Solasodine phytocompound controlled the food pest Tribolium castaneum growth. J. Entomol. Res. 2020, 44, 227–232. [Google Scholar] [CrossRef]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: A first approach for breast cancer. Metabolomics 2019, 15, 64. [Google Scholar] [CrossRef]
- Shokoohi, R.; Nematollahi, D.; Samarghandi, M.R.; Azarian, G.; Latifi, Z. Optimization of three-dimensional electrochemical process for degradation of methylene blue from aqueous environments using central composite design. Environ. Technol. Innov. 2020, 18, 100711. [Google Scholar] [CrossRef]
- Bas, D.; Boyaci, I.H. Modelling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Arteaga-Crespo, Y.; Radice, M.; Bravo-Sanchez, L.R.; García-Quintana, Y.; Scalvenzi, L. Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon 2019, 6, e03043. [Google Scholar] [CrossRef]
- Jayakumar, K.; Murugan, K. Purified solasodine and caulophyllumine: A from Solanum mauritianum Scop. against MCF-7 breast cancer cell lines in terms of cell growth. Cell cycle and apoptosis. J. Pharmacogn. Phytochem. 2017, 6, 472–478. [Google Scholar]
- Jayakumar, K.; Murugan, K. Purified Solasodine from Solanum mauritianum Scop. and its molecular mechanism of antimetastatic potential. J. Phytopharm. 2017, 6, 251–259. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kohli, S.; Chaudhary, A.S. Isolation of solasodine from the unripe fruits of Solanum xanthocarpum Schrad and Wendl. (Solanaceae) and it’s anti cancer activity against HeLa and U937 cell lines. Aust. J. Cancer Nurs. 2013, 12, 199–213. [Google Scholar]
- Senizza, B.; Rocchetti, G.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Glamocilja, J.; Sokovic, M.; Lobine, D.; Etienne, Q.K.; Lucini, L. The phenolic and alkaloid profiles of Solanum erianthum and Solanum torvum modulated their biological properties. Food Biosci. 2021, 41, 1–11. [Google Scholar]
- Gbashi, S.; Njobeh, P.; Steenkamp, P.; Tutu, H.; Madala, N. The effect of temperature and methanol–water mixture on pressurized hot water extraction (PHWE) of anti-HIV analogoues from Bidens Pilosa. Chem. Cent. J. 2016, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Hyde, A.M.; Zultanski, S.L.; Waldman, J.H.; Zhong, Y.L.; Shevlin, M.; Peng, F. General principles and strategies for salting-out informed by the Hofmeister series. Org. Process Res. Dev. 2017, 21, 1355–1370. [Google Scholar] [CrossRef]
- Tejada-Casado, C.; del Olmo-Iruela, M.; García-Campaña, A.M.; Lara, F.J. Green and simple analytical method to determine benzimidazoles in milk samples by using salting-out assisted liquid-liquid extraction and capillary liquid chromatography. J. Chromatogr. B 2018, 1091, 46–52. [Google Scholar] [CrossRef]
- Hernández-Mesa, M.; Cruces-Blanco, C.; García-Campaña, A.M. Simple and rapid determination of 5-nitroimidazoles and metabolites in fish roe samples by salting-out assisted liquid-liquid extraction and UHPLC-MS/MS. Food Chem. 2018, 252, 294–302. [Google Scholar] [CrossRef]
- Sazali, N.H.; Alshishani, A.; Saad, B.; Chew, K.Y.; Chong, M.M.; Miskam, M. Salting-out assisted liquid–liquid extraction coupled with high-performance liquid chromatography for the determination of vitamin D3 in milk samples. R. Soc. Open Sci. 2019, 6, 190952. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D. The influence of phase-forming salt on Cd (II) extraction in aqueous PEG-based two-phase systems. Rev. Roum. Chim. 2008, 53, 141–147. [Google Scholar]
- Neves, C.M.; Rita de Cássia, S.S.; Pereira, M.M.; Freire, M.G.; Coutinho, J.A. Understanding the effect of ionic liquids as adjuvants in the partition of biomolecules in aqueous two-phase systems formed by polymers and weak salting-out agents. Biochem. Eng. J. 2019, 141, 239–246. [Google Scholar] [CrossRef]
- Yajuan, L.; Yanyang, W.K.; Chen, B.W.; Lijun, J.; Jiawen, Z. Partition behavior of spiramycin in an aqueous two-phase system based on polyethylene glycol and sulfates. Sep. Sci. Technol. 2018, 53, 496–502. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Yan, Y.D.; Xu, W.D.; Xue, Y.; Wang, Y.L.; Liu, X.; Li, Y.; Ma, F.; Zhang, M.; Li, S.; et al. Thermal decomposition and oxidation of cation exchange resins with and without Na2CO3–K2CO3 salt. Environ. Technol. Innov. 2022, 28, 102601. [Google Scholar] [CrossRef]
| Factor 1 | Factor 2 | Factor 3 | Solasodine (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|
| Run 1 | % Salt | Temperature (°C) | Salt Type | Run 1 | Run 2 | Mean ± SD | Predicted |
| 1 | 20 | 80 | NaCl | 149.421 | 175.273 | 162.34 ± 18 | 144.95 |
| 2 | 35 | 80 | NaCl | 164.782 | 152.101 | 158.44 ± 9 | 141.47 |
| 3 | 50 | 80 | NaCl | 167.337 | 173.475 | 170.40 ± 4 | 152.15 |
| 4 | 20 | 80 | Na2CO3 | 276.235 | 325.356 | 300.79 ± 34 | 268.57 |
| 5 | 35 | 80 | Na2CO3 | 137.631 | 242.474 | 190.05 ± 74 | 169.69 |
| 6 | 50 | 80 | Na2CO3 | 90.256 | 121.386 | 105.82 ± 22 | 94.48 |
| 7 | 20 | 140 | NaCl | 145.755 | 147.766 | 146.76 ± 1 | 131.04 |
| 8 | 35 | 140 | NaCl | 133.142 | 145.833 | 139.48 ± 9 | 124.54 |
| 9 | 35 | 140 | NaCl | 141.55 | 185.129 | 163.33 ± 31 | 145.84 |
| 10 | 35 | 140 | NaCl | 143.837 | 193.206 | 168.52 ± 35 | 150.47 |
| 11 | 35 | 140 | NaCl | 153.087 | 193.257 | 173.17 ± 28 | 154.62 |
| 12 | 35 | 140 | NaCl | 160.403 | 181.400 | 170.90 ± 15 | 152.59 |
| 13 | 35 | 140 | NaCl | 147.844 | 189.184 | 168.51 ± 29 | 150.46 |
| 14 | 35 | 140 | NaCl | 133.210 | 189.690 | 161.45 ± 40 | 144.15 |
| 15 | 35 | 140 | NaCl | 146.783 | 199.122 | 172.95 ± 37 | 154.42 |
| 16 | 35 | 140 | NaCl | 144.264 | 145.378 | 144.82 ± 1 | 129.30 |
| 17 | 35 | 140 | NaCl | 135.107 | 156.155 | 145.63 ± 15 | 130.03 |
| 18 | 50 | 140 | NaCl | 144.261 | 150.600 | 147.43 ± 5 | 131.63 |
| 19 | 20 | 140 | Na2CO3 | 231.064 | 288.117 | 259.59 ± 40 | 231.78 |
| 20 | 35 | 140 | Na2CO3 | 132.953 | 148.042 | 140.49 ± 11 | 125.44 |
| 21 | 35 | 140 | Na2CO3 | 143.273 | 174.470 | 158.87 ± 22 | 141.85 |
| 22 | 35 | 140 | Na2CO3 | 140.993 | 178.487 | 159.74 ± 27 | 142.63 |
| 23 | 35 | 140 | Na2CO3 | 144.551 | 183.655 | 164.10 ± 28 | 146.52 |
| 24 | 35 | 140 | Na2CO3 | 160.268 | 135.875 | 148.07 ± 17 | 132.21 |
| 25 | 35 | 140 | Na2CO3 | 175.495 | 187.357 | 181.42 ± 8 | 161.99 |
| 26 | 35 | 140 | Na2CO3 | 182.617 | 177.587 | 180.10 ± 4 | 160.81 |
| 27 | 35 | 140 | Na2CO3 | 138.686 | 209.858 | 174.27 ± 50 | 155.60 |
| 28 | 35 | 140 | Na2CO3 | 157.500 | 173.412 | 165.45 ± 11 | 147.73 |
| 29 | 35 | 140 | Na2CO3 | 161.165 | 191.737 | 176.45 ± 21 | 157.55 |
| 30 | 50 | 140 | Na2COLL | 139.682 | 152.258 | 145.97 ± 9 | 130.33 |
| 31 | 20 | 200 | NaCl | 183.968 | 110.836 | 147.40 ± 52 | 131.61 |
| 32 | 35 | 200 | NaCl | 176.443 | 159.208 | 167.82 ± 12 | 149.84 |
| 33 | 50 | 200 | NaCl | 178.835 | 157.696 | 168.26 ± 15 | 150.24 |
| 34 | 20 | 200 | Na2CO3 | 295.727 | 289.355 | 292.54 ± 5 | 261.20 |
| 35 | 35 | 200 | Na2CO3 | 175.636 | 200.448 | 188.04 ± 18 | 167.89 |
| 36 | 50 | 200 | Na2CO3 | 142.454 | 138.034 | 140.24 ± 3 | 125.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgehle, T.M.; Madala, N.E.; Tavengwa, N.T. Evaluation of a Chaotrope and Kosmotrope in the Multivariate Optimization of PHW-ATPE of Solasodine from Leaves of Solanum mauritianum. Molecules 2022, 27, 5547. https://doi.org/10.3390/molecules27175547
Mokgehle TM, Madala NE, Tavengwa NT. Evaluation of a Chaotrope and Kosmotrope in the Multivariate Optimization of PHW-ATPE of Solasodine from Leaves of Solanum mauritianum. Molecules. 2022; 27(17):5547. https://doi.org/10.3390/molecules27175547
Chicago/Turabian StyleMokgehle, Tebogo Mphatlalala, Ntakadzeni Edwin Madala, and Nikita Tawanda Tavengwa. 2022. "Evaluation of a Chaotrope and Kosmotrope in the Multivariate Optimization of PHW-ATPE of Solasodine from Leaves of Solanum mauritianum" Molecules 27, no. 17: 5547. https://doi.org/10.3390/molecules27175547
APA StyleMokgehle, T. M., Madala, N. E., & Tavengwa, N. T. (2022). Evaluation of a Chaotrope and Kosmotrope in the Multivariate Optimization of PHW-ATPE of Solasodine from Leaves of Solanum mauritianum. Molecules, 27(17), 5547. https://doi.org/10.3390/molecules27175547





