Sophocarpine Alleviates Isoproterenol-Induced Kidney Injury by Suppressing Inflammation, Apoptosis, Oxidative Stress and Fibrosis
Abstract
1. Introduction
2. Results
2.1. Treatment with Sophocarpine (SOP)-Alleviated Isoproterenol (ISO)-Induced Kidney Injury
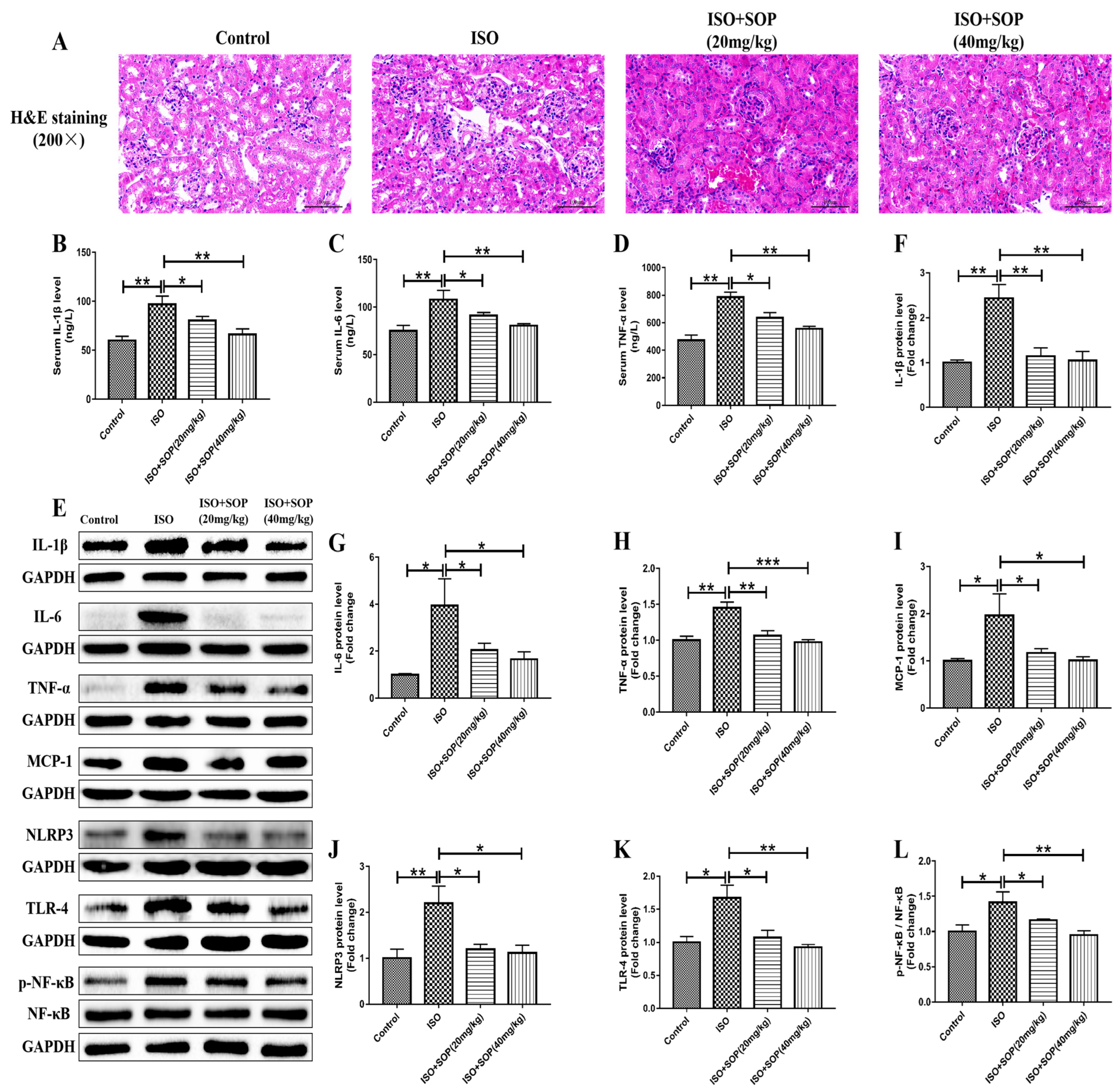
2.2. Treatment with SOP Ameliorated ISO-Induced Inflammatory Response
2.3. Treatment with SOP Hindered ISO-Induced Apoptosis
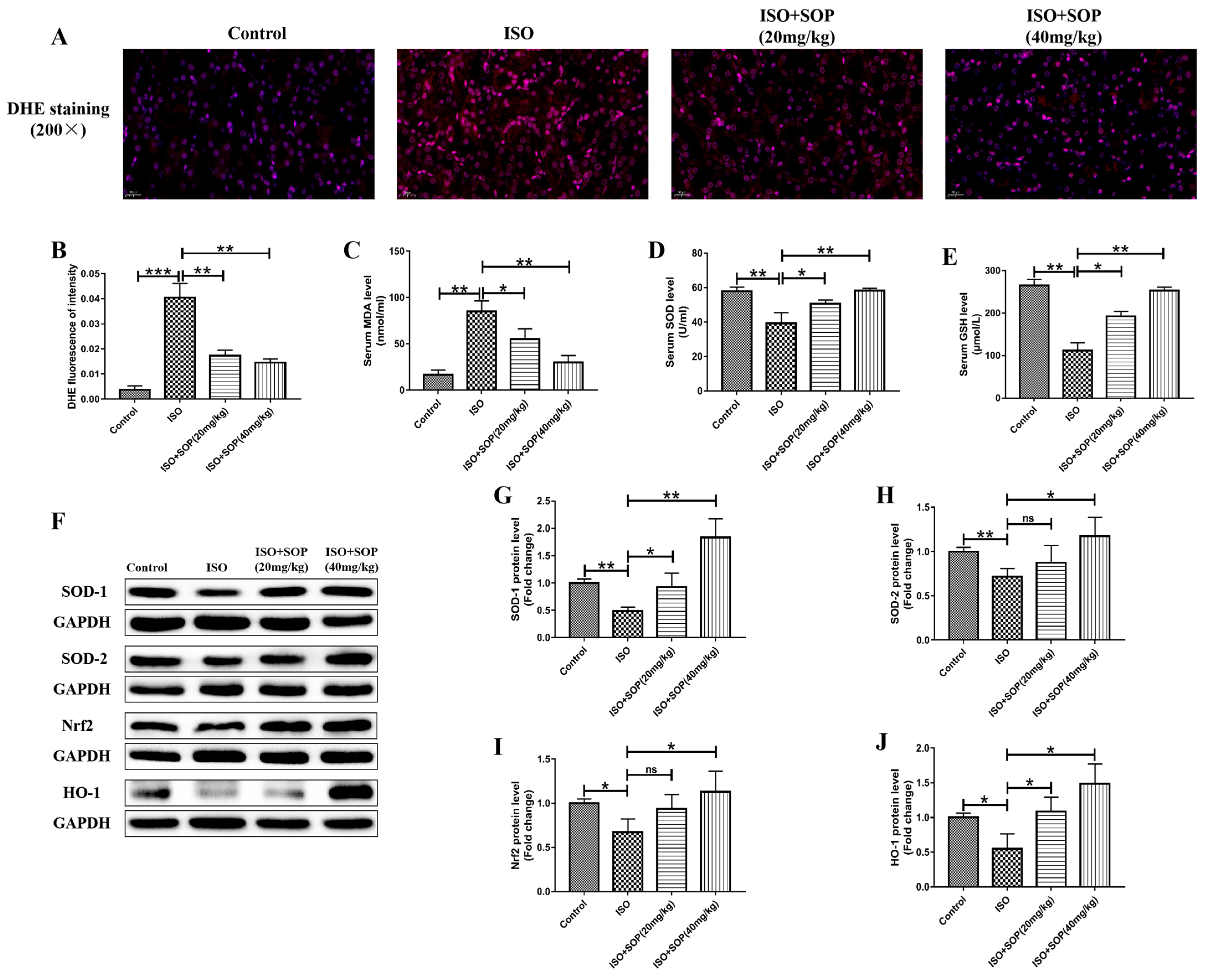
2.4. Treatment with SOP Inhibited ISO-Induced Oxidative Stress
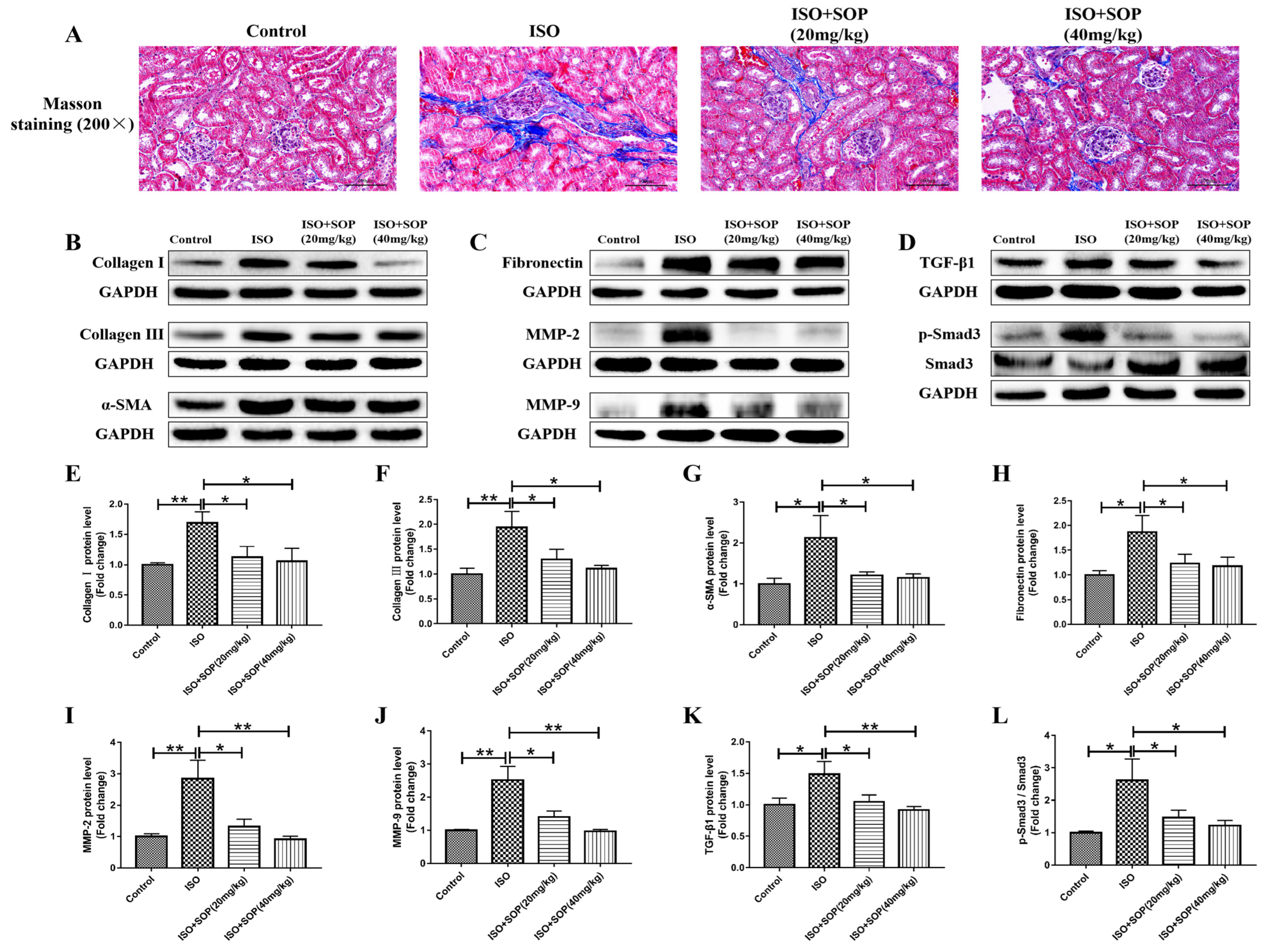
2.5. Treatment with SOP Reduced ISO-Induced Fibrosis
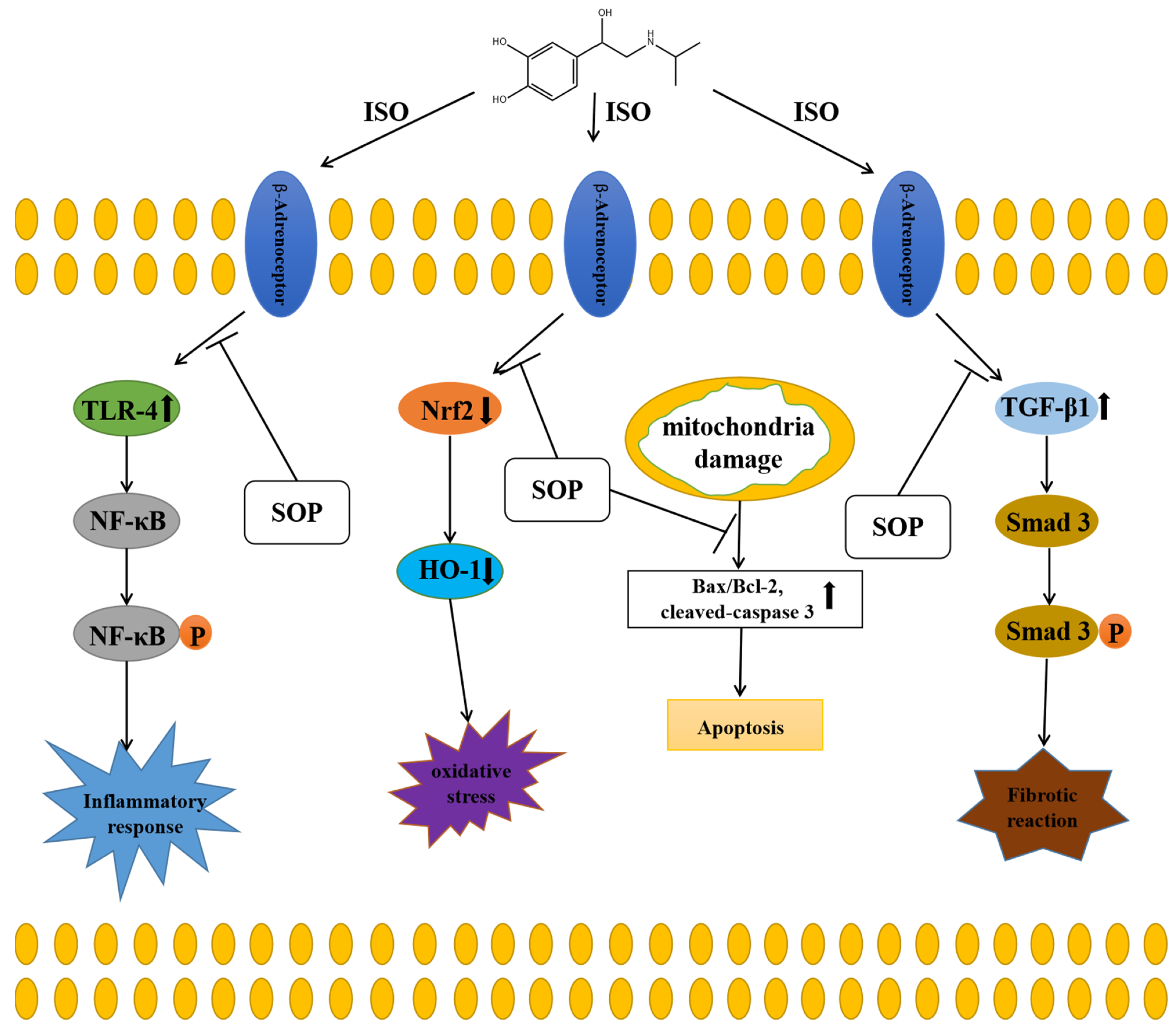
3. Discussion
4. Materials and Methods
4.1. Chemicals and Animals
4.2. Animal Model
4.3. Detection of Serum SCr, BUN, CK-MB and LDH Levels
4.4. Hematoxylin/Eosin (H&E) and Masson Staining
4.5. Measurement of Inflammatory Cytokines by Relevant Enzyme-Linked Immunosorbent Assay (ELISA) Kits
4.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
4.7. Dihydroethidium (DHE) Staining
4.8. Detection of Serum Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Glutathione (GSH) Levels
4.9. Western Blotting
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lei, L.; Li, L.; Zhang, H. Advances in the Diagnosis and Treatment of Acute Kidney Injury in Cirrhosis Patients. Biomed Res. Int. 2017, 2017, 8523649. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Gbadegesin, R.A. Update on prognosis driven classification of pediatric AKI. Front. Pediatr. 2022, 10, 1039024. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Amdur, R.L.; Amodeo, S.; Kimmel, P.L.; Palant, C.E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011, 79, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Liu, J.; Ouyang, L. Quinolizidine alkaloids derivatives from Sophora alopecuroides Linn: Bioactivities, structure-activity relationships and preliminary molecular mechanisms. Eur. J. Med. Chem. 2020, 188, 111972. [Google Scholar] [CrossRef]
- Wang, F.L.; Wang, H.; Wang, J.H.; Wang, D.X.; Gao, Y.; Yang, B.; Yang, H.J.; Ji, Y.B.; Xin, G.S. Analgesic and Anti-Inflammatory Activities of Sophocarpine from Sophora viciifolia Hance. Biomed Res. Int. 2021, 2021, 8893563. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, T.; Zhu, L.; He, N.; Duan, C.; Deng, W.; Zhang, H.; Zhang, X. Sophocarpine Inhibits Tumorgenesis of Colorectal Cancer via Downregulation of MEK/ERK/VEGF Pathway. Biol. Pharm. Bull. 2019, 42, 1830–1838. [Google Scholar] [CrossRef]
- Zhu, X.; Gu, Z.; Yu, Y.; Yang, W.; Li, M.; Li, Y.; Zhang, P.; Wang, J.; Zhao, J. Neuronal Apoptosis Preventive Potential of Sophocarpine via Suppression of Aβ-Accumulation and Down-Regulation of Inflammatory Response. Dokl. Biochem. Biophys. 2021, 497, 116–122. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Tian, J.; Shen, J.; Xing, Y.; Liu, Z. Sophocarpine administration preserves myocardial function from ischemia-reperfusion in rats via NF-κB inactivation. J. Ethnopharmacol. 2011, 135, 620–625. [Google Scholar] [CrossRef]
- Jiang, Z.; Meng, Y.; Bo, L.; Wang, C.; Bian, J.; Deng, X. Sophocarpine Attenuates LPS-Induced Liver Injury and Improves Survival of Mice through Suppressing Oxidative Stress, Inflammation, and Apoptosis. Mediat. Inflamm. 2018, 2018, 5871431. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, D.; Liu, J.; Gu, L. Protective effect of sophocarpine on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2019, 70, 180–186. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Hong, H.; Luo, C.; Liu, Z.; Yang, R. Sophocarpine attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. Immunol. Res. 2018, 66, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Allawadhi, P.; Khurana, A.; Sayed, N.; Kumari, P.; Godugu, C. Isoproterenol-induced cardiac ischemia and fibrosis: Plant-based approaches for intervention. Phytother. Res. 2018, 32, 1908–1932. [Google Scholar] [CrossRef] [PubMed]
- Masset, C.; Le Turnier, P.; Bressollette-Bodin, C.; Renaudin, K.; Raffi, F.; Dantal, J. Virus-Associated Nephropathies: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 12014. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.T.; Koyner, J.L. Sepsis associated acute kidney injury. BMJ 2019, 364, k4891. [Google Scholar] [CrossRef]
- Khalifa, A.A.; El Sokkary, N.H.; Elblehi, S.S.; Diab, M.A.; Ali, M.A. Potential cardioprotective effect of octreotide via NOXs mitigation, mitochondrial biogenesis and MAPK/Erk1/2/STAT3/NF-kβ pathway attenuation in isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2022, 925, 174978. [Google Scholar] [CrossRef]
- Lin, Z.R.; Li, Z.Z.; Cao, Y.J.; Yu, W.J.; Ye, J.T.; Liu, P.Q. GDH promotes isoprenaline-induced cardiac hypertrophy by activating mTOR signaling via elevation of α-ketoglutarate level. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 1373–1385. [Google Scholar] [CrossRef]
- Song, W.; Zhang, X.; He, S.; Gai, Y.; Qin, C.; Hu, F.; Wang, Y.; Wang, Z.; Bai, P.; Wang, J.; et al. 68Ga-FAPI PET visualize heart failure: From mechanism to clinic. Eur. J. Nucl. Med. Mol. Imaging 2022, 1–11. [Google Scholar] [CrossRef]
- de Ponte, M.C.; Casare, F.A.M.; Costa-Pessoa, J.M.; Cardoso, V.G.; Malnic, G.; Mello-Aires, M.; Volpini, R.A.; Thieme, K.; Oliveira-Souza, M. The Role of β-Adrenergic Overstimulation in the Early Stages of Renal Injury. Kidney Blood Press. Res. 2017, 42, 1277–1289. [Google Scholar] [CrossRef]
- Ghartavol, M.M.; Gholizadeh-Ghaleh Aziz, S.; Babaei, G.; Hossein Farjah, G.; Hassan Khadem Ansari, M. The protective impact of betaine on the tissue structure and renal function in isoproterenol-induced myocardial infarction in rat. Mol. Genet. Genom. Med. 2019, 7, e00579. [Google Scholar] [CrossRef]
- Sarkaki, A.; Hoseinynejad, S.; Khombi Shooshtari, M.; Rashno, M. Synaptic plasticity and cognitive impairment consequences to acute kidney injury: Protective role of ellagic acid. Iran J. Basic Med. Sci. 2022, 25, 621–628. [Google Scholar] [PubMed]
- Castañeda, R.; Cáceres, A.; Cruz, S.M.; Aceituno, J.A.; Marroquín, E.S.; Barrios Sosa, A.C.; Strangman, W.K.; Williamson, R.T. Nephroprotective plant species used in traditional Mayan Medicine for renal-associated diseases. J. Ethnopharmacol. 2022, 301, 115755. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guan, X.; Pan, X.; Sun, X.; Wang, F.; Ji, Y.; Huang, P.; Deng, Y.; Zhang, Q.; Han, Q.; et al. Post-Natal Inhibition of NF-κB Activation Prevents Renal Damage Caused by Prenatal LPS Exposure. PLoS ONE 2016, 11, e0153434. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, J.; Chen, E.; Zheng, X. Rosiglitazone Alleviates Contrast-Induced Acute Kidney Injury in Rats via the PPARγ/NLRP3 Signaling Pathway. Dis. Markers 2022, 2022, 4158692. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, W.; Li, Y.; Gu, W.; Huang, H.; Wei, Q.; Bai, G.; Wang, J.; Rizak, J.D.; Zhou, Z. Evaluation of Klotho gene expression and NGAL levels following acute kidney injury during pregnancy hypertensive disorders. Pregnancy Hypertens. 2022, 30, 161–170. [Google Scholar] [CrossRef]
- Kang, S.; Chen, T.; Hao, Z.; Yang, X.; Wang, M.; Zhang, Z.; Hao, S.; Lang, F.; Hao, H. Oxymatrine Alleviates Gentamicin-Induced Renal Injury in Rats. Molecules 2022, 27, 6209. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Xin, L.; Wen, J.; Zhou, Q.; Chen, X.; Ding, X.; Zhang, X. Xinfeng capsule inhibits inflammation and oxidative stress in rheumatoid arthritis by up-regulating LINC00638 and activating Nrf2/HO-1 pathway. J. Ethnopharmacol. 2022, 301, 115839. [Google Scholar] [CrossRef]
- Inoue, T.; Nakamura, Y.; Tanaka, S.; Kohro, T.; Li, L.X.; Huang, L.; Yao, J.; Kawamura, S.; Inoue, R.; Nishi, H.; et al. Bone marrow stromal cell antigen-1 (CD157) regulated by sphingosine kinase 2 mediates kidney fibrosis. Front. Med. 2022, 9, 993698. [Google Scholar] [CrossRef]
- Wei, J.; Shan, Y.; Xiao, Z.; Wen, L.; Tao, Y.; Fang, X.; Luo, H.; Tang, C.; Li, Y. Anp32e promotes renal interstitial fibrosis by upregulating the expression of fibrosis-related proteins. Int. J. Biol. Sci. 2022, 18, 5897–5912. [Google Scholar] [CrossRef]
- Li, M.; Tan, H.; Gao, T.; Han, L.; Teng, X.; Wang, F.; Zhang, X. Gypensapogenin I Ameliorates Isoproterenol (ISO)-Induced Myocardial Damage through Regulating the TLR4/NF-κB/NLRP3 Pathway. Molecules 2022, 27, 5298. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Fu, Y.; Xu, J.-S. Sophocarpine Alleviates Isoproterenol-Induced Kidney Injury by Suppressing Inflammation, Apoptosis, Oxidative Stress and Fibrosis. Molecules 2022, 27, 7868. https://doi.org/10.3390/molecules27227868
Zhou W, Fu Y, Xu J-S. Sophocarpine Alleviates Isoproterenol-Induced Kidney Injury by Suppressing Inflammation, Apoptosis, Oxidative Stress and Fibrosis. Molecules. 2022; 27(22):7868. https://doi.org/10.3390/molecules27227868
Chicago/Turabian StyleZhou, Wei, Yang Fu, and Jin-Song Xu. 2022. "Sophocarpine Alleviates Isoproterenol-Induced Kidney Injury by Suppressing Inflammation, Apoptosis, Oxidative Stress and Fibrosis" Molecules 27, no. 22: 7868. https://doi.org/10.3390/molecules27227868
APA StyleZhou, W., Fu, Y., & Xu, J.-S. (2022). Sophocarpine Alleviates Isoproterenol-Induced Kidney Injury by Suppressing Inflammation, Apoptosis, Oxidative Stress and Fibrosis. Molecules, 27(22), 7868. https://doi.org/10.3390/molecules27227868




