The Effect of Vibratory Grinding Time on Moisture Sorption, Particle Size Distribution, and Phenolic Bioaccessibility of Carob Powder
Abstract
1. Introduction
2. Results and Discussion
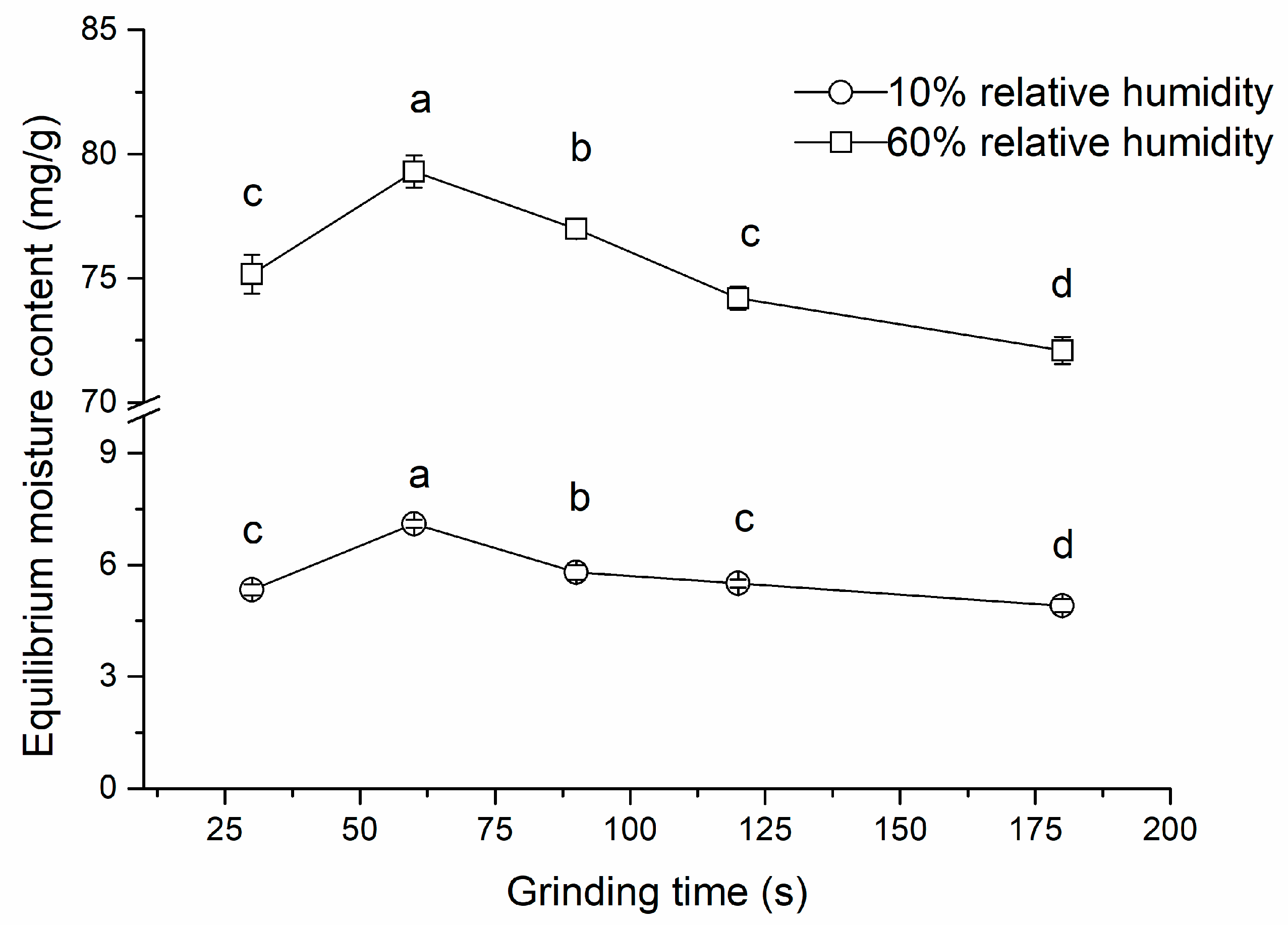
2.1. The Effect of Grinding Time on Particle Size, Colour, and Moisture Adsorption Properties of Carob Powder
2.2. Grinding Time as Affected Antioxidant Properties and Phenolic Content in Carob Powder
2.3. The Effect of Grinding Time on the Bioaccessibility of Antioxidant Properties and Phenolic Content
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Carob Powder Sample
3.3. In Vitro Digestion of Carob Powder
3.4. Particle Size, Colour, Water Activity, and Moisture Sorption Determination
3.5. Antioxidant Properties of Carob Powder
3.6. Phenolic Content in Carob Powder
3.6.1. Spectrophotometric Assays
3.6.2. HPLC Analysis of Phenolic Individuals
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayaz, F.A.; Torun, H.; Glew, R.H.; Bak, Z.D.; Chuang, L.T.; Presley, J.M.; Andrews, R. Nutrient content of carob pod (Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum. Nutr. 2009, 64, 286–292. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Boublenza, I.; El Haitoum, A.; Ghezlaoui, S.; Mahdad, M.; Vasai, F.; Chemat, F. Algerian carob (Ceratonia siliqua L.) populations. Morphological and chemical variability of their fruits and seeds. Sci. Hortic. 2019, 256, e108537. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Adrikopoulos, N.A.; Kaliora, A.C. Carob: A sustainable opportunity for metabolic health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef]
- Issaoui, M.; Flamini, G.; Delgado, A. Sustainability opportunities for Mediterranean food products through new formulations based on carob flour (Ceratonia siliqua L.). Sustainability 2021, 13, 8026. [Google Scholar] [CrossRef]
- Červenka, L.; Frühbauerová, M.; Velichová, H. Functional properties of muffin as affected by substituting wheat flour with carob powder. Potravinarstvo 2019, 13, 212–217. [Google Scholar] [CrossRef]
- Pawlowska, K.; Kuligowski, M.; Jasinska-Kuligowska, I.; Kidon, M.; Siger, A.; Rudzinska, M.; Nowak, J. Effect of replacing cocoa powder by carob powder in the muffins on sensory and physicochemical properties. Plant Foods Hum. Nutr. 2018, 73, 196–202. [Google Scholar] [CrossRef]
- Biernacka, B.; Dziki, D.; Gawlik-Dziki, U.; Różyło, R. Physical, sensorial, and antioxidant properties of common wheat pasta enriched with carob fiber. LWT-Food Sci. Technol. 2017, 77, 186–192. [Google Scholar] [CrossRef]
- Román, L.; González, A.; Espina, T.; Gómez, M. Degree of roasting of carob flour affecting the properties of gluten-free cakes and cookies. J. Food Sci. Technol. 2017, 54, 2094–2103. [Google Scholar] [CrossRef]
- Akdeniz, E.; Yakışı, E.; Pirouzian, H.R.; Akkın, S.; Turan, B.; Tipigil, E.; Toker, O.S.; Ozcan, O. Carob powder as cocoa substitute in milk and dark compound chocolate formulation. J. Food. Sci. Technol. 2021, 58, 4558–4566. [Google Scholar] [CrossRef]
- Tounsi, L.; Mkaouar, S.; Bredai, S.; Kechaou, N. Valorization of carob by-product for producing an added value powder: Characterization and incorporation into Halva formulation. J. Food Meas. Charact. 2022, 16, 3957–3966. [Google Scholar] [CrossRef]
- Benković, M.; Radić, K.; Čepo, D.V.; Jaškūnas, E.; Janutis, L.; Morkunaite, M.; Srečec, S. Production of cocoa and carob-based drink powders by foam mat drying. J. Food Process Eng. 2018, 41, e12825. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Coelho, N.; Santos-Rufo, A.; Gonçalves, S.; Pérez-Santín, E.; Romano, A. The influence of in vitro gastrointestinal digestion on the chemical composition and antioxidant and enzyme inhibitory capacities of carob liqueurs obtained with different elaboration techniques. Antioxidants 2019, 8, 563. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.; Zou, J.; Yang, Y.; Cui, L.; Chang, X. Effects of different drying and milling methods on the physicochemical properties and phenolic content of hawthorn fruit powders. J. Food Process Preserv. 2020, 44, e14460. [Google Scholar] [CrossRef]
- Araújo, A.L.; Silva Pena, R. Effect of particle size and temperature on the hygroscopic behaviour of cassava flour from dry group and storage time estimation. CyTA J. Food 2020, 18, 178–186. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef]
- Gao, X.; Zhu, D.; Liu, Y.; Zha, L.; Chen, D.; Guo, H. Physicochemical properties and anthocyanin bioaccessibility of downy rose-myrtle powder prepared by superfine grinding. Int. J. Food Prop. 2019, 22, 2022–2032. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Li, J.; Aslam, N.; Sun, H.; Zhao, J.; Wu, Z.; He, S. Effects of particle size on physicochemical and functional properties of superfine black kidney bean (Phaseolus vulgaris L.) powder. PeerJ 2019, 7, e6369. [Google Scholar] [CrossRef]
- An, Y.; Sun, Y.; Zhang, M.; Adhikari, B.; Li, Z. Effect of ball milling time on physicochemical properties of Cordyceps militaris ultrafine particles. J. Food Process Preserv. 2019, 42, e13065. [Google Scholar] [CrossRef]
- Jiang, G.; Ramachandraiah, K.; Wu, Z.; Li, S.; Eun, J.-B. Impact of ball-milling time on the physical properties, bioactive compounds, and structural characteristics of onion peel powder. Food Biosci. 2020, 36, 100630. [Google Scholar] [CrossRef]
- Norhidayah, A.; Noriham, A.; Rusop, M. Changes in physical and antioxidant properties of nanostructured Zingiber officinale (ginger) rhizome as affected by milling time. Adv. Mater. Res. 2013, 667, 144–149. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Devahastin, S.; Liu, Y. Influence of low-temperature ball milling time on physicochemical properties, flavor, bioactive compounds contents and antioxidant activity of horseradish powder. Adv. Powder Technol. 2020, 31, 914–921. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Liu, H.; Zhu, Y. Effect of ultrafine grinding time on the functional and flavor properties of soybean isolates. Colloids Surf. B 2020, 196, 111345. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob (Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Labanca, R.A.; Svelander, C.; Alminger, M. Effect of particle size of chia seeds on bioaccessibility of phenolic compounds during in vitro digestion. Cogent Food Agric. 2019, 5, 1694775. [Google Scholar] [CrossRef]
- Savlak, N.; Türker, B.; Yeşilkanat, N. Effects of particle size distribution on some physical, chemical and functional properties of unripe banana flour. Food Chem. 2016, 213, 180–186. [Google Scholar] [CrossRef]
- Capuano, E.; Pellegrini, N. An integrated look at the effect of structure on nutrient bioavailability in plant foods. J. Sci. Food Agric. 2019, 99, 493–498. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT-Food Sci. Technol. 2020, 117, 108623. [Google Scholar] [CrossRef]
- Goulas, V.; Hadjisolomou, A. Dynamic changes in targeted phenolic compounds and antioxidant potency of carob fruit (Ceratonia siliqua L.) products during in vitro digestion. LWT-Food Sci. Technol. 2019, 101, 269–275. [Google Scholar] [CrossRef]
- Frühbauerová, M.; Červenka, L.; Hájek, T.; Pouzar, M.; Palarčík, J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem. 2022, 377, 131968. [Google Scholar] [CrossRef]
- Boublenza, I.; Lazouni, H.A.; Ghaffari, L.; Ruiz, K.; Fabiano-Tixier, A.S.; Chemat, F. Influence of roasting on sensory, antioxidant, aromas, and physicochemical properties of carob pod powder (Ceratonia siliqua L.). J. Food Qual. 2017, 2017, 4193672. [Google Scholar] [CrossRef]
- Ali, H.; Al-Khalifa, A.R.; Aboelsood, W.; Bareh, G.; Farouk, A. Influence of spray-drying on improving the quality of dried carob juice. Qual. Assur. Saf. Crop. 2019, 11, 391–399. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; López, J.; Ah-Hen, K.; Torres, M.J.; Lemus-Mondaca, R. Thermodynamic properties, sorption isotherms and glass transition temperature of cape gooseberry (Physalis peruviana L.). Food Technol. Biotechnol. 2014, 52, 83–92. [Google Scholar]
- Czubinski, J.; Wroblewska, K.; Czyzniejewski, M.; Górnaś, P.; Kachlicki, P.; Siger, A. Bioaccessibility of defatted lupin seed phenolic compounds in a standardized static in vitro digestion system. Food Res. Int. 2019, 19, 1126–1134. [Google Scholar] [CrossRef]
- Zeng, Q.; Xu, Z.; Dai, M.; Cao, X.; Xiong, X.; He, S.; Yuan, Y.; Zhang, M.; Dong, L.; Zhang, R.; et al. Effects of simulated digestion on the phenolic composition and antioxidant activity of different cultivars of lychee pericarp. BMC Chem. 2019, 13, 27. [Google Scholar] [CrossRef]
- Sun, Y.; Tao, W.; Huang, H.; Ye, X.; Sun, P. Flavonoids, phenolic acids, carotenoids and antioxidant activity of fresh eating citrus fruits, using the coupled in vitro digestion and human intestinal HepG2 cells model. Food Chem. 2019, 279, 321–327. [Google Scholar] [CrossRef]
- Djaoudene, O.; Mansinhos, I.; Gonçalves, S.; Jara-Palacios, M.J.; Bey, M.B.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory capacities of fruit and seed extracts from different Algerian cultivars of date (Phoenix dactylifera L.) were affected by in vitro simulated gastrointestinal digestion. S. Afr. J. Bot. 2021, 137, 133–148. [Google Scholar] [CrossRef]
- Panagopoulou, E.A.; Chiou, A.; Kasimatis, T.-D.; Bismpikis, M.; Mouraka, P.; Karathanos, V.T. Dried dates: Polar phenols and their fate during in vitro digestion. J. Food Meas. Charact. 2021, 15, 1899–1906. [Google Scholar] [CrossRef]
- Barros, R.G.C.; Pereira, U.C.; Andrade, J.K.S.; Santo de Oliveira, C.; Vasconcelos, S.V.; Narain, N. In vitro gastrointestinal digestion and probiotics fermentation impact on bioaccessbility of phenolics compounds and antioxidant capacity of some native and exotic fruit residues with potential antidiabetic effects. Food Res. Int. 2020, 136, 109614. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.G.; Xie, Y.Q.; Chen, F.; Zhao, Y.; Luo, C.X.; Gao, Y.Q. Total phenolic, flavonoid and antioxidant activity of 23 edible flowers subjected to in vitro digestion. J. Funct. Foods 2015, 17, 243–259. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Wang, L.; Li, Z. Effect of particle size on the release behavior and functional properties of wheat bran phenolic compounds during in vitro gastrointestinal digestion. Food Chem. 2022, 367, 130751. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Baraniak, B.; Gawlik-Dziki, U. In vitro digestibility and starch content, predicted glycemic index and potential in vitro antidiabetic effect of lentil sprouts obtained by different germination techniques. Food Chem. 2013, 138, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour, 4th ed.; John Wiley & Sons: Chichester, UK, 2011; pp. 41–72. [Google Scholar] [CrossRef]
- Sochor, J.; Ryvolová, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Malik, S. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2018, 9, 449–454. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Sun, B.; da Silva, J.R.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
| Carob Samples | ||||||
|---|---|---|---|---|---|---|
| CP30 | CP60 | CP90 | CP120 | CP180 | ||
| D10 | 17.8 ± 1.1 b | 15.2 ± 0.5 c | 16.3 ± 2.1 bc | 21.4 ± 0.8 a | 19.4 ± 0.3 a | ** |
| D50 | 141.3 ± 2.5 a | 87.9 ± 1.7 d | 110.1 ± 3.5 c | 129.7 ± 2.9 b | 135.1 ± 1.8 b | *** |
| D90 | 427.7 ± 12.5 d | 572.7 ± 8.1 b | 494.6 ± 7.9 c | 810.6 ± 21.3 a | 429.7 ± 5.6 d | ** |
| Span | 2.9 | 6.3 | 4.3 | 6.1 | 3.0 | |
| aw | 0.380 ± 0.005 d | 0.374 ± 0.002 d | 0.403 ± 0.001 c | 0.417 ± 0.001 b | 0.433 ± 0.003 a | *** |
| L* | 47.1 ± 0.5 d | 51.1 ± 0.4 a | 49.4 ± 0.2 b | 48.0 ± 0.3 c | 47.0 ± 0.3 d | *** |
| a* | 6.3 ± 0.4 bc | 5.8 ± 0.6 c | 6.3 ± 0.3 bc | 6.8 ± 0.4 ab | 6.9 ± 0.2 a | *** |
| b* | 19.0 ± 0.5 b | 17.9 ± 0.4 c | 18.7 ± 0.3 b | 20.1 ± 0.4 a | 19.8 ± 0.3 a | *** |
| Carob Samples | ||||||
|---|---|---|---|---|---|---|
| CP30 | CP60 | CP90 | CP120 | CP180 | ||
| w/(μg g−1) | ||||||
| Vanillic acid | 3.95 ± 0.40 ab | 4.17 ± 0.40 ab | 3.58 ± 0.43 b | 4.08 ± 0.26 ab | 4.41 ± 0.08 a | n.s. |
| Ferulic acid | 8.64 ± 0.04 d | 10.77 ± 0.22 b | 10.85 ± 0.43 b | 10.09 ± 0.51 c | 11.28 ± 0.09 ab | *** |
| Cinnamic acid | 45.20 ± 1.20 c | 50.30 ± 1.13 b | 47.80 ± 1.64 bc | 50.00 ± 2.15 b | 54.28 ± 1.42 a | *** |
| Luteolin | 13.62 ± 1.82 b | 16.11 ± 1.44 b | 15.17 ± 1.74 b | 16.97 ± 1.00 b | 20.52 ± 0.57 a | ** |
| Naringenin | 2.76 ± 0.06 c | 6.77 ± 0.25 ab | 6.67 ± 0.44 b | 7.28 ± 0.30 a | 7.28 ± 0.13 a | *** |
| Apigenin | 1.29 ± 0.19 d | 1.94 ± 0.05 c | 2.17 ± 0.06 ab | 1.97 ± 0.11 bc | 2.27 ± 0.04 a | *** |
| w/(mg g−1) | ||||||
| TPC as GAE | 4.73 ± 0.21 d | 5.84 ± 3.98 ab | 5.41 ± 2.19 c | 5.72 ± 0.20 b | 6.07 ± 0.15 a | *** |
| TFC as QUE | 0.19 ± 0.15 b | 0.33 ± 0.13 a | 0.27 ± 0.14 a | 0.32 ± 0.07 a | 0.39 ± 0.05 a | n.s. |
| CC as CAT | 0.33 ± 0.13 a | 0.36 ± 0.03 a | 0.35 ± 0.12 a | 0.44 ± 0.06 a | 0.46 ± 0.19 a | n.s. |
| DPPH as TEAC | 9.29 ± 3.16 b | 11.91 ± 0.51 a | 9.80 ± 0.53 b | 9.59 ± 1.62 b | 11.93 ± 0.74 a | * |
| FRAP as TEAC | 13.78 ± 1.33 c | 16.11 ± 1.80 b | 13.12 ± 1.28 c | 16.95 ± 1.08 ab | 16.52 ± 0.65 a | *** |
| Carob Samples | ||||||
|---|---|---|---|---|---|---|
| CP30 | CP60 | CP90 | CP120 | CP180 | ||
| w/(μg g−1) | ||||||
| Vanillic acid | 0.91 ± 0.01 a (23) † | 0.57 ± 0.03 b (14) | 0.56 ± 0.00 b (15) | 0.55 ± 0.01 b (13) | 0.67 ± 0.23 b (12) | *** |
| Ferulic acid | 4.45 ± 2.12 ab (51) | 4.28 ± 0.17 b (40) | 4.68 ± 0.28 ab (43) | 4.65 ± 0.24 ab (46) | 4.71 ± 0.18 a (42) | n.s. |
| Cinnamic acid | 17.69 ± 0.37 b (39) | 18.34 ± 0.38 a (36) | 17.35 ± 0.35 b (36) | 16.61 ± 0.28 c (33) | 18.02 ± 0.14 a (33) | *** |
| Luteolin | 1.15 ± 0.03 a (8) | 1.16 ± 0.04 a (7) | 1.16 ± 0.03 a (8) | 1.05 ± 0.04 b (6) | 1.05 ± 0.07 b (5) | ** |
| Naringenin | 0.80 ± 0.02 a (29) | 0.81 ± 0.02 a (12) | 0.83 ± 0.01 a (12) | 0.83 ± 0.03 a (11) | 0.80 ± 0.03 a (11) | n.s. |
| Apigenin | 0.08 ± 0.00 c (6) | 0.09 ± 0.01 c (5) | 0.12 ± 0.00 a (6) | 0.10 ± 0.01 b (5) | 0.10 ± 0.01 b (5) | *** |
| w/(mg g−1) | ||||||
| TPC as GAE | 5.10 ± 0.10 c (109) | 5.51 ± 0.27 a (94) | 5.09 ± 0.12 c (94) | 5.66 ± 0.33 a (99) | 5.32 ± 0.29 b (88) | ** |
| TFC as QUE | 0.24 ± 0.02 ab (126) | 0.28 ± 0.05 a (85) | 0.20 ± 0.01 b (74) | 0.23 ± 0.03 b (72) | 0.19 ± 0.02 b (49) | * |
| CC as CAT | 0.59 ± 0.23 a (148) | 0.52 ± 0.18 a (141) | 0.50 ± 0.13 a (143) | 0.57 ± 0.18 a (129) | 0.53 ± 0.16 a (116) | n.s. |
| DPPH as TEAC | 10.24 ± 0.47 b (110) | 12.17 ± 1.23 a (102) | 11.21 ± 0.64 ab (114) | 11.12 ± 1.16 ab (116) | 11.09 ± 1.26 ab (93) | * |
| FRAP as TEAC | 13.97 ± 0.26 (101) c | 16.12 ± 1.43 a (100) | 15.07 ± 0.47 b (115) | 16.05 ± 0.69 a (95) | 14.70 ± 0.31 bc (89) | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Červenka, L.; Frühbauerová, M.; Palarčík, J.; Muriqi, S.; Velichová, H. The Effect of Vibratory Grinding Time on Moisture Sorption, Particle Size Distribution, and Phenolic Bioaccessibility of Carob Powder. Molecules 2022, 27, 7689. https://doi.org/10.3390/molecules27227689
Červenka L, Frühbauerová M, Palarčík J, Muriqi S, Velichová H. The Effect of Vibratory Grinding Time on Moisture Sorption, Particle Size Distribution, and Phenolic Bioaccessibility of Carob Powder. Molecules. 2022; 27(22):7689. https://doi.org/10.3390/molecules27227689
Chicago/Turabian StyleČervenka, Libor, Michaela Frühbauerová, Jiří Palarčík, Sali Muriqi, and Helena Velichová. 2022. "The Effect of Vibratory Grinding Time on Moisture Sorption, Particle Size Distribution, and Phenolic Bioaccessibility of Carob Powder" Molecules 27, no. 22: 7689. https://doi.org/10.3390/molecules27227689
APA StyleČervenka, L., Frühbauerová, M., Palarčík, J., Muriqi, S., & Velichová, H. (2022). The Effect of Vibratory Grinding Time on Moisture Sorption, Particle Size Distribution, and Phenolic Bioaccessibility of Carob Powder. Molecules, 27(22), 7689. https://doi.org/10.3390/molecules27227689






