Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing
Abstract
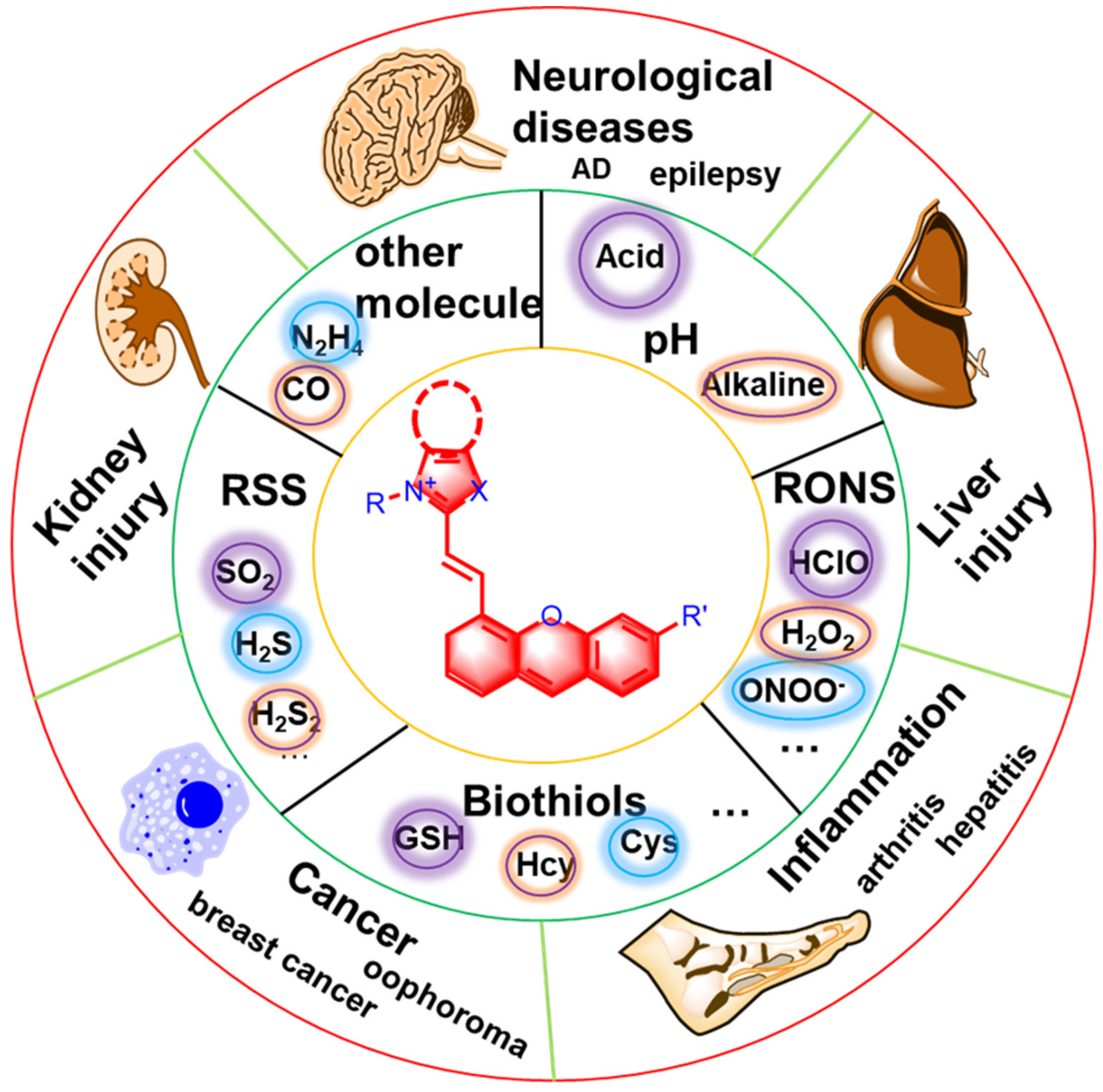
1. Introduction
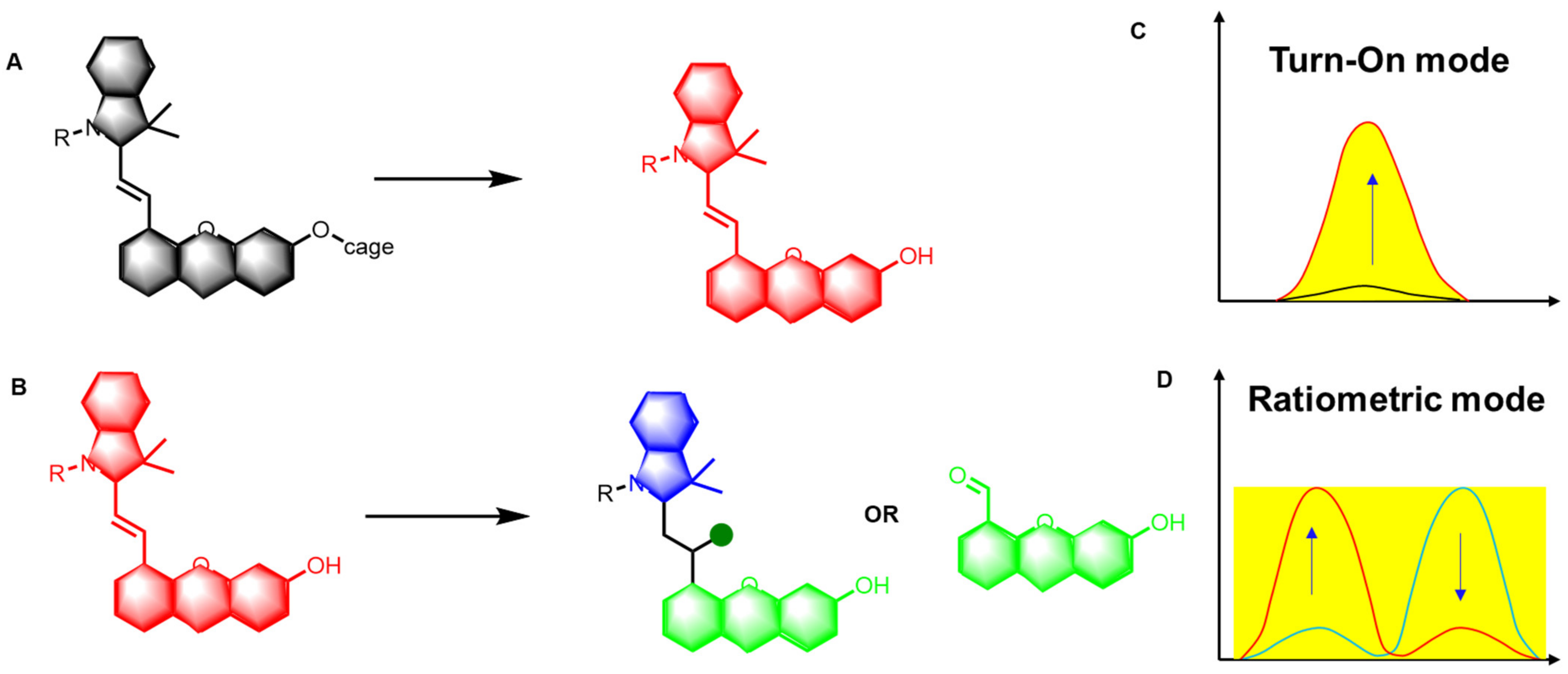
2. The Structure and Design Strategy of Hemicyanine Probes
3. Progress of Hemicyanine-Based Fluorescent Probes
4. Progress in Hemicyanine-Based Fluorescent Probes
4.1. Detecting Protons Using Fluorescent Probes (H+)
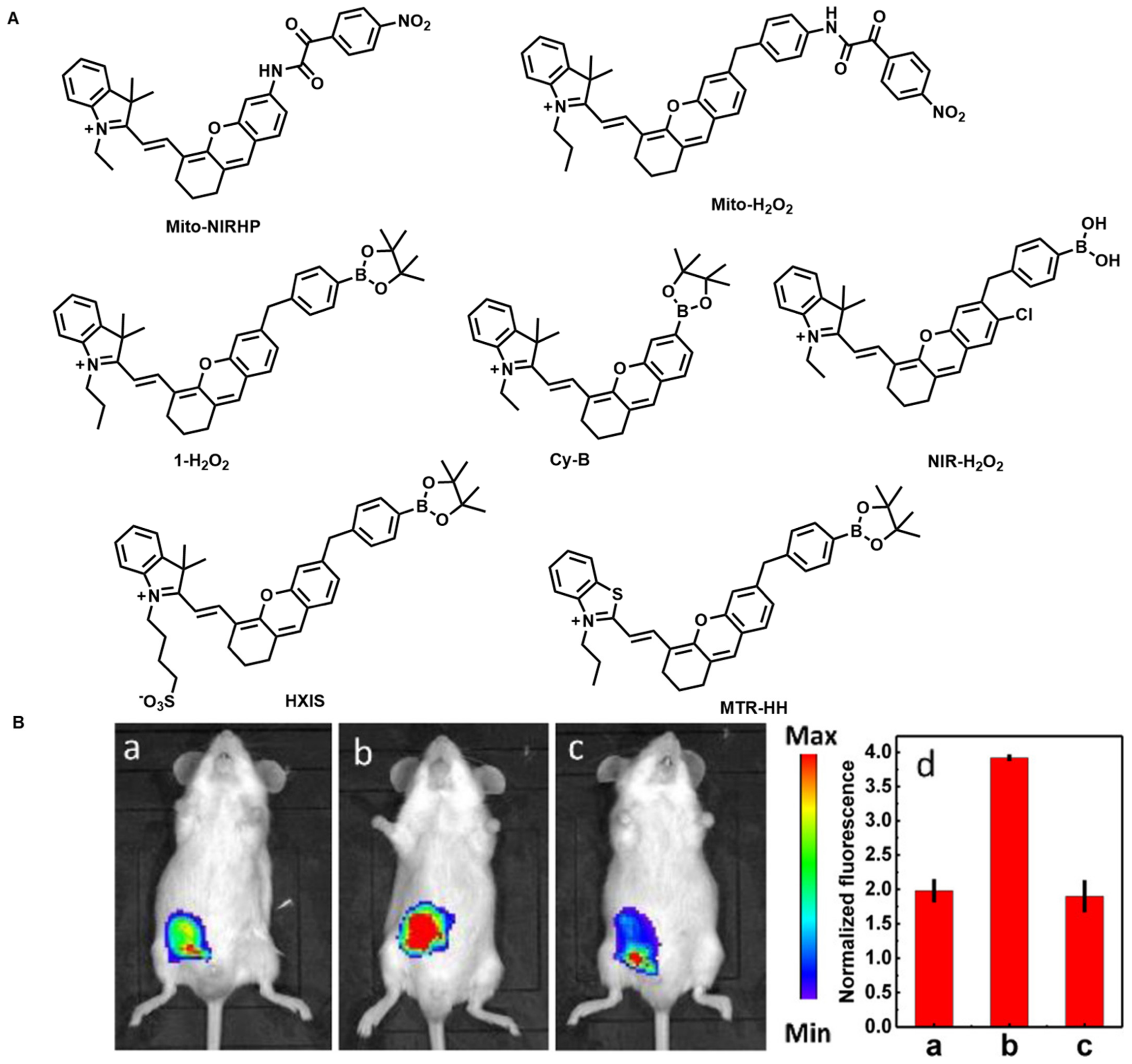
4.2. Hydrogen Peroxide Fluorescent Probes
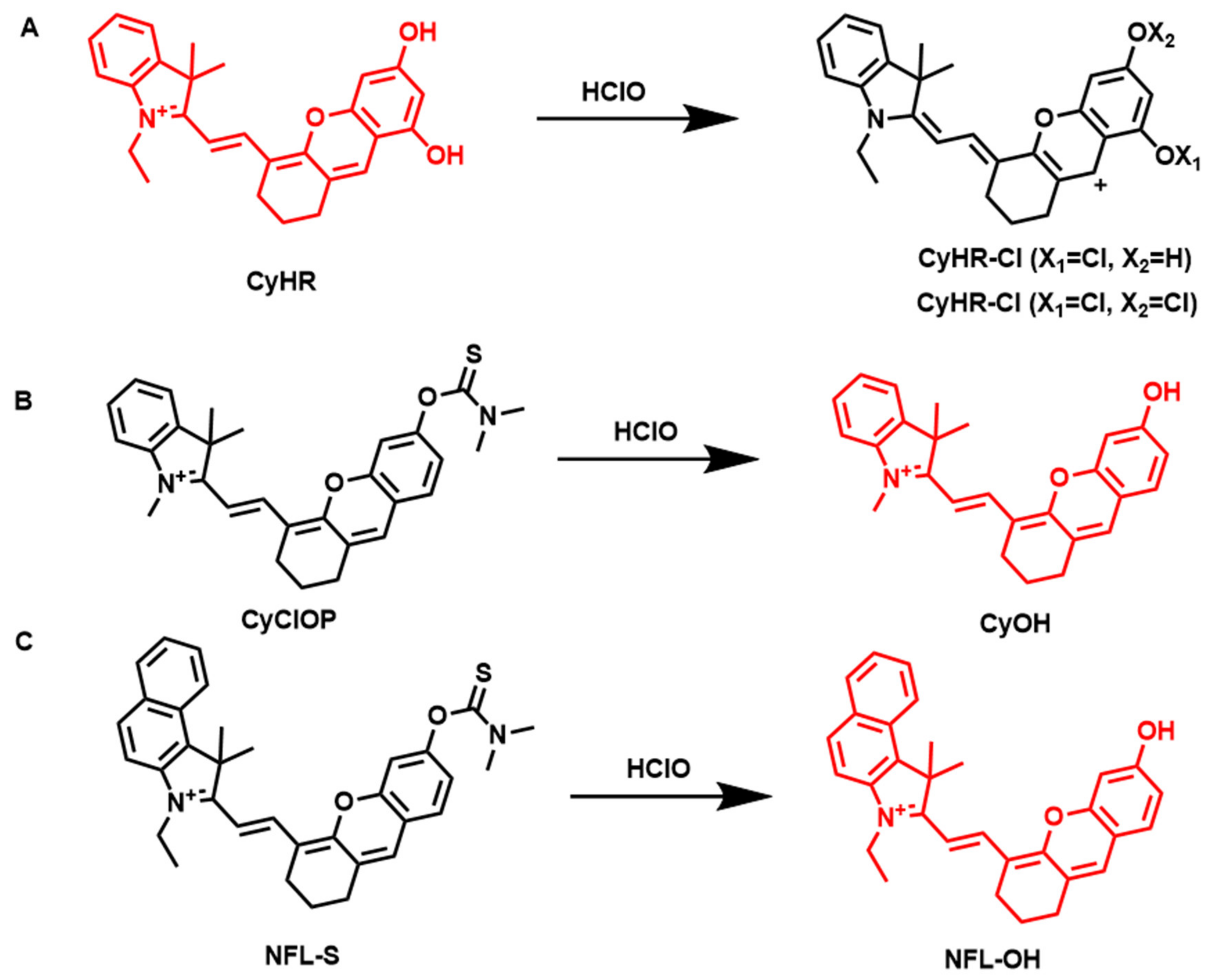
4.3. Fluorescent Probes for the Detection of HClO
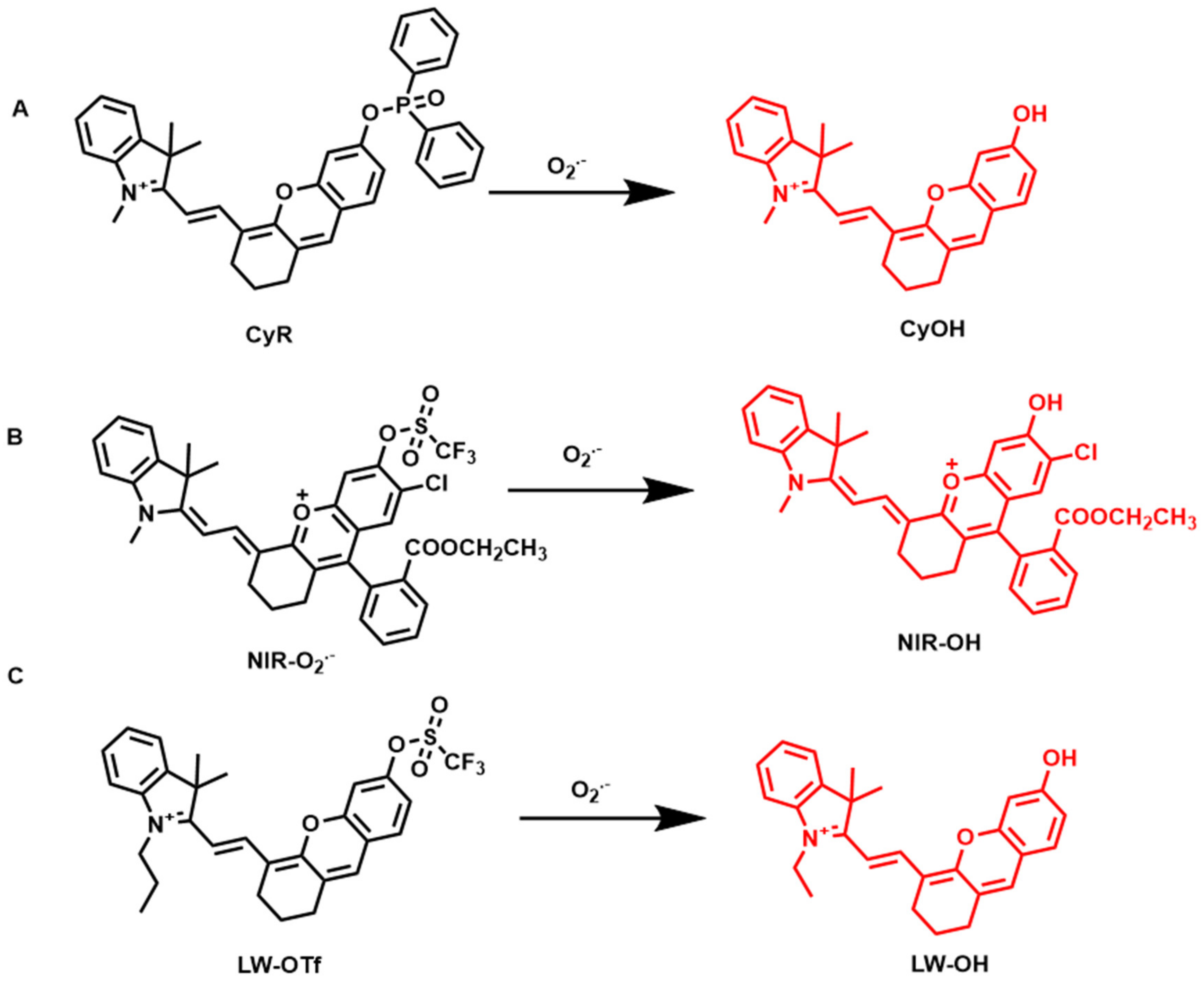
4.4. Fluorescent Probes for Superoxide Anion Detection (O2˙−)
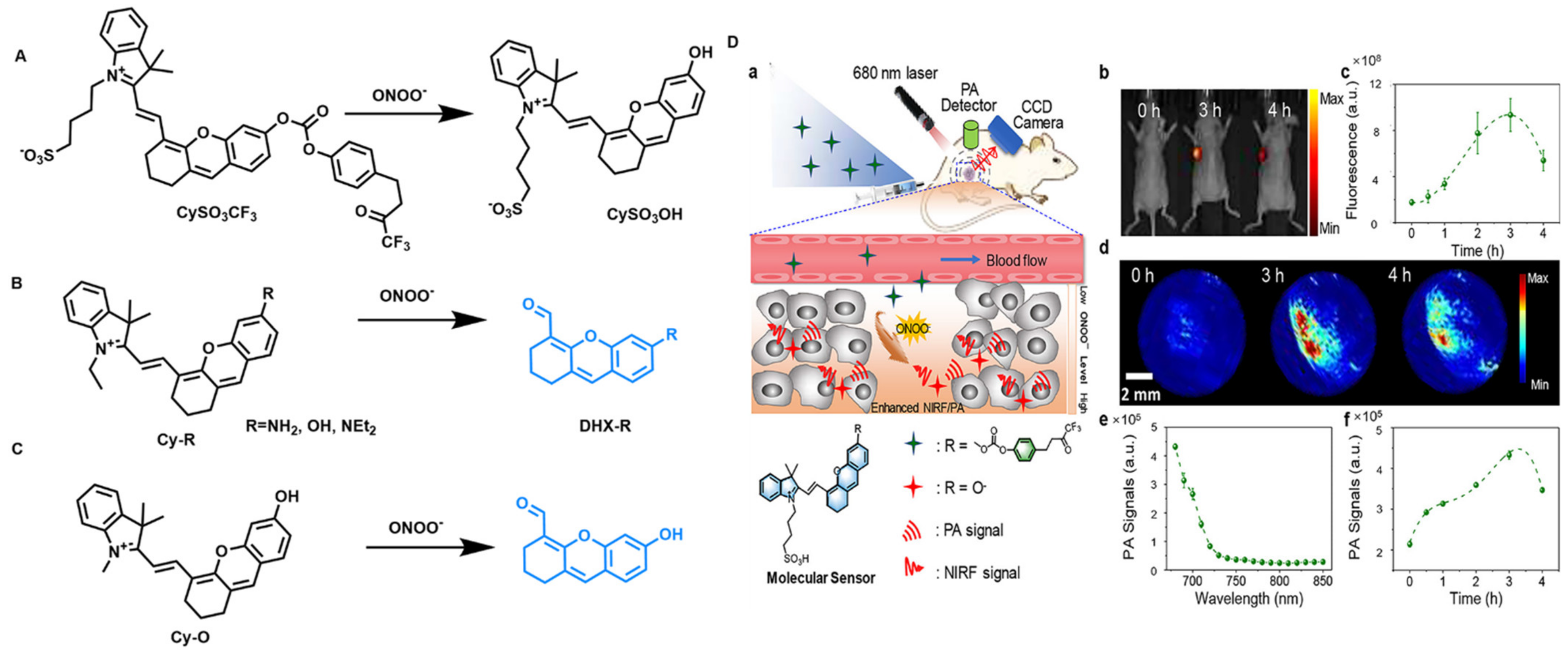
4.5. Fluorescent Probes for Peroxynitrite Detection (ONOO−)
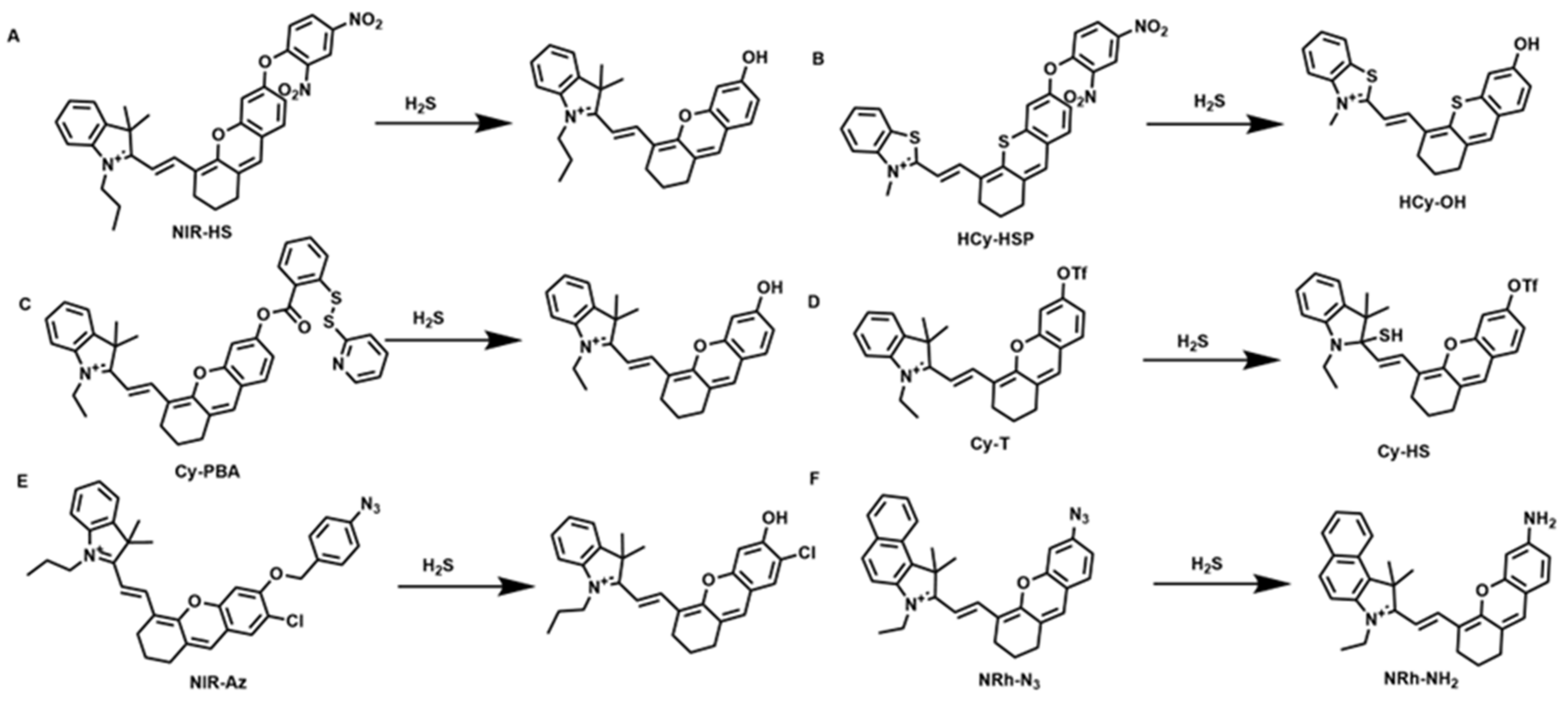
4.6. Fluorescent Probes for the Detection of H2S
4.7. Fluorescent Probes for Hydrogen Polysulfide Detection (H2Sn)
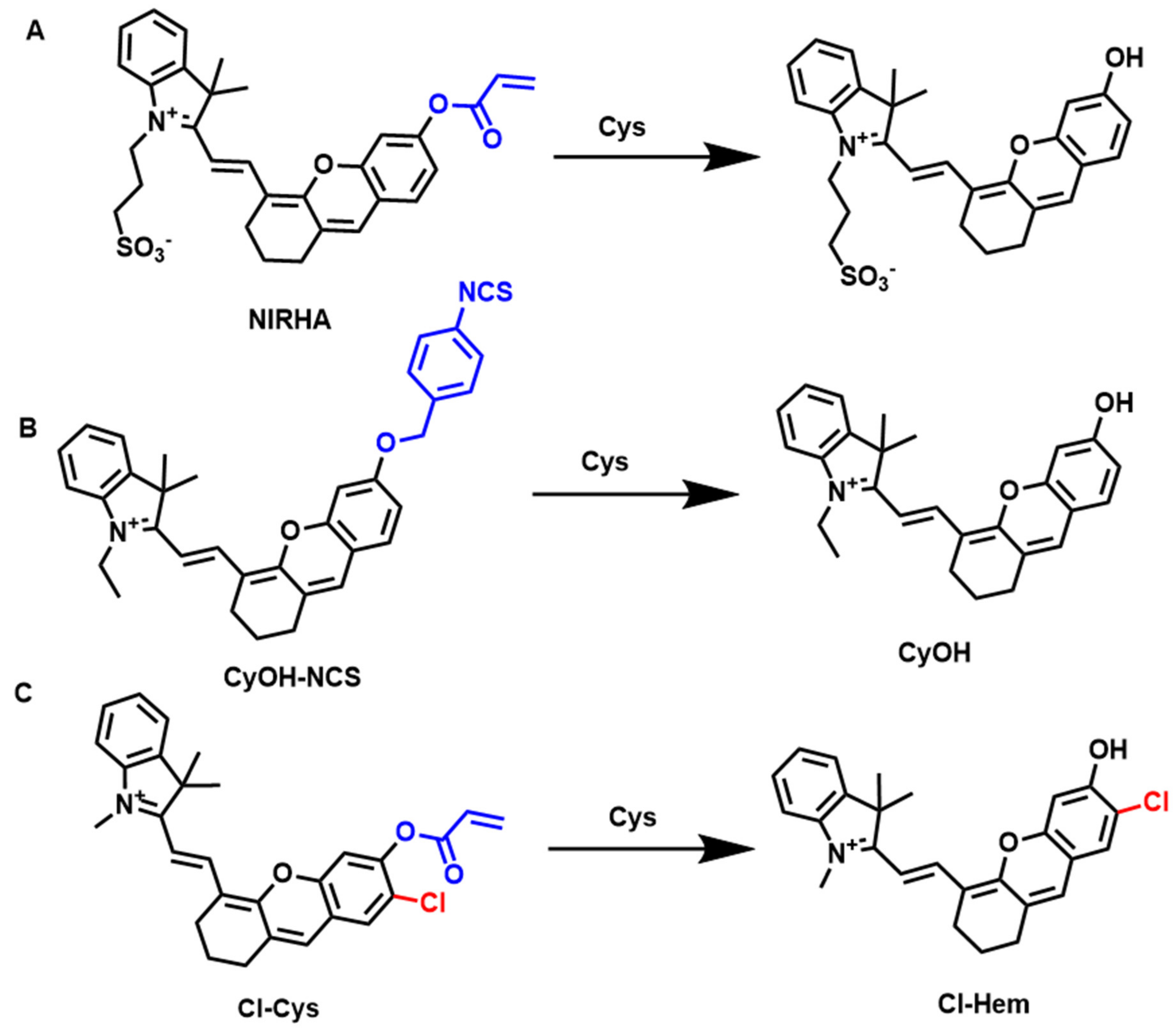
4.8. Fluorescent Probes for the Detection of Cysteine (Cys)
4.9. Fluorescent Probes for the Detection of GSH
4.10. Carbon Monoxide Detection Using Fluorescent Probes (CO)
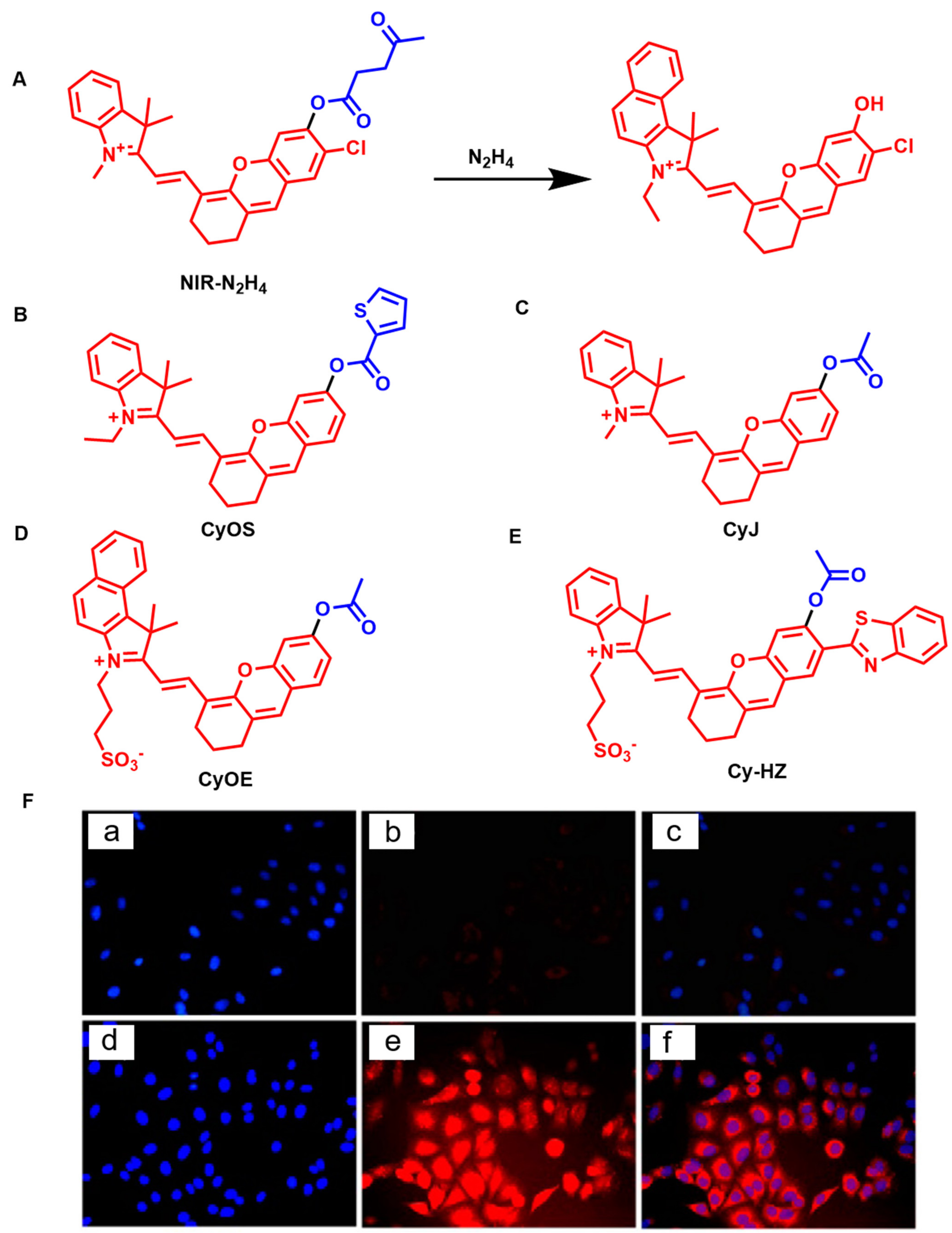
4.11. Fluorescent Probes for the Detection of Hydrazine (N2H4)
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile Types of Inorganic/Organic NIR-IIa/IIb Fluorophores: From Strategic Design toward Molecular Imaging and Theranostics. Chem. Rev. 2021, 122, 209–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Yang, H.; Yu, L.; Xu, Y.; Sharma, A.; Yin, P.; Li, X.; Kim, J.S.; Sun, Y. Advanced biotechnology-assisted precise sonodynamic therapy. Chem. Soc. Rev. 2021, 50, 11227–11248. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.-B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.-H.; Bull, S.D.; He, X.-P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yong, J.; Yuan, J.; Xu, Z.P. Recent advances in the development of responsive probes for selective detection of cysteine. Co-ord. Chem. Rev. 2020, 408, 213182. [Google Scholar] [CrossRef]
- Tanaka, K.; Miura, T.; Umezawa, N.; Urano, Y.; Kikuchi, K.; Higuchi, A.T.; Nagano, T. Rational Design of Fluorescein-Based Fluorescence Probes. Mechanism-Based Design of a Maximum Fluorescence Probe for Singlet Oxygen. J. Am. Chem. Soc. 2001, 123, 2530–2536. [Google Scholar] [CrossRef]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2014, 44, 4596–4618. [Google Scholar] [CrossRef]
- Amatore, C.; Arbault, S.; Bruce, D.; de Oliveira, P.; Erard, L.M.; Vuillaume, M. Characterization of the electrochemical oxidation of peroxynitrite: Relevance to oxidative stress bursts measured at the single cell level. Chemistry 2001, 7, 4171–4179. [Google Scholar] [CrossRef]
- Van de Bittner, G.C.; Dubikovskaya, E.A.; Bertozzi, C.R.; Chang, C.J. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. USA 2010, 107, 21316–21321. [Google Scholar] [CrossRef]
- Zou, J.; Cai, H.; Wang, D.; Xiao, J.; Zhou, Z.; Yuan, B. Spectrophotometric determination of trace hydrogen peroxide via the oxidative coloration of DPD using a Fenton system. Chemosphere 2019, 224, 646–652. [Google Scholar] [CrossRef]
- Gui, S.; Huang, Y.; Hu, F.; Jin, Y.; Zhang, G.; Yan, L.; Zhang, D.; Zhao, R. Fluorescence Turn-On Chemosensor for Highly Selective and Sensitive Detection and Bioimaging of Al3+ in Living Cells Based on Ion-Induced Aggregation. Anal. Chem. 2015, 87, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection–deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Liew, S.S.; Wei, X.; Pu, K. Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed. 2021, 60, 26454–26475. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, Z.; Wang, R.; Yu, F. Indication of Dynamic Peroxynitrite Fluctuations in the Rat Epilepsy Model with a Near-Infrared Two-Photon Fluorescent Probe. Anal. Chem. 2021, 93, 2490–2499. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2012, 42, 622–661. [Google Scholar] [CrossRef]
- Bonacchi, S.; Cantelli, A.; Battistelli, G.; Guidetti, G.; Calvaresi, M.; Manzi, J.; Gabrielli, L.; Ramadori, F.; Gambarin, A.; Mancin, F.; et al. Photoswitchable NIR-Emitting Gold Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 11064–11068. [Google Scholar] [CrossRef]
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-Specific Near-Infrared Fluorescence Imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef]
- Hou, J.; Qian, M.; Zhao, H.; Li, Y.; Liao, Y.; Han, G.; Xu, Z.; Wang, F.; Song, Y.; Liu, Y. A near-infrared ratiometric/turn-on fluorescent probe for in vivo imaging of hydrogen peroxide in a murine model of acute inflammation. Anal. Chim. Acta 2018, 1024, 169–176. [Google Scholar] [CrossRef]
- Xie, X.; Yang, X.; Wu, T.; Li, Y.; Li, M.; Tan, Q.; Wang, X.; Tang, B. Rational Design of an α-Ketoamide-Based Near-Infrared Fluorescent Probe Specific for Hydrogen Peroxide in Living Systems. Anal. Chem. 2016, 88, 8019–8025. [Google Scholar] [CrossRef]
- Luo, X.; Wang, R.; Lv, C.; Chen, G.; You, J.; Yu, F. Detection of Selenocysteine with a Ratiometric near-Infrared Fluorescent Probe in Cells and in Mice Thyroid Diseases Model. Anal. Chem. 2019, 92, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kim, H.; Xu, F.; Han, J.; Yao, Q.; Wang, J.; Pu, K.; Peng, X.; Yoon, J. Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: Design strategy, biomedical applications, and outlook. Chem. Soc. Rev. 2022, 51, 1795–1835. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, B.; Tang, Y.; Lin, W. A Unique “Integration” Strategy for the Rational Design of Optically Tunable Near-Infrared Fluorophores. Acc. Chem. Res. 2017, 50, 1410–1422. [Google Scholar] [CrossRef]
- Li, H.; Kim, D.; Yao, Q.; Ge, H.; Chung, J.; Fan, J.; Wang, J.; Peng, X.; Yoon, J. Activity-Based NIR Enzyme Fluorescent Probes for the Diagnosis of Tumors and Image-Guided Surgery. Angew. Chem. Int. Ed. 2020, 60, 17268–17289. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of Chemosensors Based on Cyanine Platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.; Cui, H.; Jiang, W. Development of Unique Xanthene-Cyanine Fused Near-Infrared Fluorescent Fluorophores with Superior Chemical Stability for Biological Fluorescence Imaging. Chem.—A Eur. J. 2014, 21, 733–745. [Google Scholar] [CrossRef]
- Cheng, D.; Peng, J.; Lv, Y.; Su, D.; Liu, D.; Chen, M.; Yuan, L.; Zhang, X. De Novo Design of Chemical Stability Near-Infrared Molecular Probes for High-Fidelity Hepatotoxicity Evaluation In Vivo. J. Am. Chem. Soc. 2019, 141, 6352–6361. [Google Scholar] [CrossRef]
- Jia, X.; Chen, Q.; Yang, Y.; Tang, Y.; Wang, R.; Xu, Y.; Zhu, W.; Qian, X. FRET-Based Mito-Specific Fluorescent Probe for Ratiometric Detection and Imaging of Endogenous Peroxynitrite: Dyad of Cy3 and Cy5. J. Am. Chem. Soc. 2016, 138, 10778–10781. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Li, X.; Ma, H. A Strategy for Specific Fluorescence Imaging of Monoamine Oxidase A in Living Cells. Angew. Chem. Int. Ed. 2017, 56, 15319–15323. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Ma, H. A near-infrared fluorescent probe reveals decreased mitochondrial polarity during mitophagy. Chem. Sci. 2019, 11, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.; Li, X.; Ma, H. Mitochondria-Immobilized Near-Infrared Ratiometric Fluorescent pH Probe To Evaluate Cellular Mitophagy. Anal. Chem. 2019, 91, 11409–11416. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.J.; Deore, P.S.; Al-Abdul-Wahid, M.S.; Aboelnga, M.M.; Wetmore, S.D.; Manderville, R.A. Acceptor Influence on Thiolate Sensing by Hemicyanine Dyes. J. Org. Chem. 2019, 84, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Go, Y.-M.; Jones, D.P. Cysteine/cystine redox signaling in cardiovascular disease. Free Radic. Biol. Med. 2011, 50, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, T.; Dong, Q.; Nice, E.C.; Huang, C.; Wei, Y. Redox homeostasis: The linchpin in stem cell self-renewal and differentiation. Cell Death Dis. 2013, 4, e537. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2016, 387, 95–105. [Google Scholar] [CrossRef]
- Bove, P.F.; Van Der Vliet, A. Nitric oxide and reactive nitrogen species in airway epithelial signaling and inflammation. Free Radic. Biol. Med. 2006, 41, 515–527. [Google Scholar] [CrossRef]
- Fernández-Puente, E.; Palomero, J. Genetically Encoded Biosensors to Monitor Intracellular Reactive Oxygen and Nitrogen Species and Glutathione Redox Potential in Skeletal Muscle Cells. Int. J. Mol. Sci. 2021, 22, 10876. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Zhao, Q.; Tang, C.; Zhang, H.; Qin, Y.; Feng, X.; Zhang, J. Fluorescent probes based on nucleophilic aromatic substitution reactions for reactive sulfur and selenium species: Recent progress, applications, and design strategies. Coord. Chem. Rev. 2020, 427, 213601. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; He, X.-P.; Kim, H.-J.; Yoon, J.; Tian, H. Fluorogenic probes for disease-relevant enzymes. Chem. Soc. Rev. 2018, 48, 683–722. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; di Masi, A.; Sciorati, C.; Clementi, E. Peroxynitrite-An ugly biofactor? BioFactors 2010, 36, 264–273. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A Unique Approach to Development of Near-Infrared Fluorescent Sensors for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134, 13510–13523. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.-A. De novo synthesis of phenolic dihydroxanthene near-infrared emitting fluorophores. Org. Biomol. Chem. 2015, 13, 8169–8172. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Tian, H. Most recent advances on enzyme-activatable optical probes for bioimaging. Aggregate 2021, 2, e32. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, W.; Zhu, J.; Pei, W.; Wang, C.; Huang, L.; Yao, C.; Yan, Q.; Huang, W.; Loo, J.S.C.; et al. Inorganic-Organic Hybrid Nanoprobe for NIR-Excited Imaging of Hydrogen Sulfide in Cell Cultures and Inflammation in a Mouse Model. Small 2014, 10, 4874–4885. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2009, 11, 50–61. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yang, S.; Zhao, Y.; Yuan, L.; Zheng, J.; Yang, R. Hemicyanine-based High Resolution Ratiometric near-Infrared Fluorescent Probe for Monitoring pH Changes in Vivo. Anal. Chem. 2015, 87, 2495–2503. [Google Scholar] [CrossRef]
- Wang, B.-L.; Jiang, C.; Li, K.; Liu, Y.-H.; Xie, Y.; Yu, X.-Q. Molecular engineering of a dual emission near-infrared ratiometric fluorophore for the detection of pH at the organism level. Analyst 2015, 140, 4608–4615. [Google Scholar] [CrossRef]
- Wan, Q.; Chen, S.; Shi, W.; Li, L.; Ma, H. Lysosomal pH Rise during Heat Shock Monitored by a Lysosome-Targeting Near-Infrared Ratiometric Fluorescent Probe. Angew. Chem. Int. Ed. 2014, 53, 10916–10920. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Berezin, M.Y.; Guo, K.; Kao, J.; Achilefu, S. Near-Infrared Fluorescent pH-Sensitive Probes via Unexpected Barbituric Acid Mediated Synthesis. Org. Lett. 2008, 11, 29–32. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, Y.; Meng, X.; Yang, H.; Song, L.; Liu, S.; Xu, A.; Chen, Z.; Huang, W.; Zhao, Q. Lysosome-specific sensing and imaging of pH variations in vitro and in Vivo utilizing a near-infrared boron complex. J. Mater. Chem. B 2019, 7, 3569–3575. [Google Scholar] [CrossRef]
- Mazi, W.; Yan, Y.; Zhang, Y.; Xia, S.; Wan, S.; Tajiri, M.; Luck, R.L.; Liu, H. A near-infrared fluorescent probe based on a hemicyanine dye with an oxazolidine switch for mitochondrial pH detection. J. Mater. Chem. B 2020, 9, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Wang, T.-R.; Liu, Y.-J.; Shen, S.-L.; Cao, X.-Q. A near-infrared fluorescent probe based on the hemicyanine skeleton for the detection of hydrogen peroxide in vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 266, 120435. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Yao, Q.; Fan, J.; Wang, J.; Peng, X. A NIR fluorescent probe: Imaging endogenous hydrogen peroxide during an autophagy process induced by rapamycin. J. Mater. Chem. B 2016, 4, 7363–7367. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, L.; Li, Z.; Li, D.; Tian, X.; Zhang, C. Near-infrared fluorescence probe for hydrogen peroxide detection: Design, synthesis, and application in living systems. Analyst 2019, 144, 3643–3648. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Z.; Ding, N.; Tang, X.; Zhang, C. A new near-infrared fluorescence probe synthesized from IR-783 for detection and bioimaging of hydrogen peroxide in vitro and in vivo. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 268, 120642. [Google Scholar] [CrossRef]
- Gong, Y.; Feng, D.; Liu, W.; Fang, J.; Feng, S. A self-immolative near-infrared probe based on hemi-benzothiazolecyanine for visualizing hydrogen peroxide in living cells and mice. Dye. Pigment. 2020, 186, 108954. [Google Scholar] [CrossRef]
- Ma, J.; Yan, C.; Li, Y.; Duo, H.; Li, Q.; Lu, X.; Guo, Y. Unusual Hypochlorous Acid (HClO) Recognition Mechanism Based on Chlorine–Oxygen Bond (Cl−O) Formation. Chem.—A Eur. J. 2019, 25, 7168–7176. [Google Scholar] [CrossRef]
- Gao, W.; Ma, Y.; Liu, Y.; Ma, S.; Lin, W. Observation of endogenous HClO in living mice with inflammation, tissue injury and bacterial infection by a near-infrared fluorescent probe. Sens. Actuators B Chem. 2020, 327, 128884. [Google Scholar] [CrossRef]
- Qian, X.; Yu, H.; Zhu, W.; Yao, X.; Liu, W.; Yang, S.; Zhou, F.; Liu, Y. Near infrared fluorescent probe for in vivo bioimaging of endogenous hypochlorous acid. Dye. Pigment. 2021, 188, 109218. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Zhang, R.; Zhang, F.; Liu, W.; Liu, X.; Lee, S.M.-Y.; Zhang, H. A phosphinate-based near-infrared fluorescence probe for imaging the superoxide radical anion in vitro and in vivo. Chem. Commun. 2016, 52, 2679–2682. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Cheng, D.C.D.; Su, D.S.D.; Chen, M.; Yin, B.-C.; Yuan, L.; Zhang, X.-B. Visualization of oxidative injury in the mouse kidney using selective superoxide anion fluorescent probes. Chem. Sci. 2018, 9, 7606–7613. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Tian, X.; Groleau, R.R.; Bull, S.D.; Li, P.; Tang, B.; James, T.D. Fluorescent probe for the imaging of superoxide and peroxynitrite during drug-induced liver injury. Chem. Sci. 2021, 12, 3921–3928. [Google Scholar] [CrossRef]
- Zhang, J.; Zhen, X.; Zeng, J.; Pu, K. A Dual-Modal Molecular Probe for Near-Infrared Fluorescence and Photoacoustic Imaging of Peroxynitrite. Anal. Chem. 2018, 90, 9301–9307. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Li, Y.; Jiang, W.-L.; Tian, Y.; Fei, J.; Li, C.-Y. A ratiometric fluorescent probe for peroxynitrite prepared by de novo synthesis and its application in assessing the mitochondrial oxidative stress status in cells and in vivo. Chem. Commun. 2018, 54, 11590–11593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kan, J.; Sun, Y.; Won, M.; Kim, J.H.; Zhang, W.; Zhou, J.; Qian, Z.; Kim, J.S. Nanoliposomal Ratiometric Fluorescent Probe toward ONOO− Flux. ACS Appl. Bio Mater. 2020, 4, 2080–2088. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, X.E.; Zou, F.; Shang, Y.; Meng, W.; Lai, E.; Xu, Z.; Liu, Y.; Zhao, J. A highly selective and sensitive near-infrared fluorescent probe for imaging of hydrogen sulphide in living cells and mice. Sci. Rep. 2016, 6, srep18868. [Google Scholar] [CrossRef]
- Su, W.; Huang, L.; Zhu, L.; Lin, W. A novel fluorescent probe for imagining hydrogen sulfide upregulation in acute lung injury. Sens. Actuators B: Chem. 2022, 369, 132297. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, R.; Duan, G.; Zhou, Z.; Luo, Y.; Li, W.; Zhang, L.; Gu, Y.; Zha, X. A highly sensitive near-infrared fluorescent probe for the detection of hydrogen sulfide and its application in living cells and mice. New J. Chem. 2018, 42, 19795–19800. [Google Scholar] [CrossRef]
- Ma, J.; Li, F.; Li, Q.; Li, Y.; Yan, C.; Lu, X.; Guo, Y. Naked-eye and ratiometric fluorescence probe for fast and sensitive detection of hydrogen sulfide and its application in bioimaging. New J. Chem. 2018, 42, 19272–19278. [Google Scholar] [CrossRef]
- Park, C.S.; Ha, T.H.; Choi, S.-A.; Nguyen, D.N.; Noh, S.; Kwon, O.S.; Lee, C.-S.; Yoon, H. A near-infrared “turn-on” fluorescent probe with a self-immolative linker for the in vivo quantitative detection and imaging of hydrogen sulfide. Biosens. Bioelectron. 2017, 89, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, W.; Zhu, W.; Tiemuer, A.; Zhou, F.; Yang, S.; Yu, H.; Qian, X.; Liu, Y. Near-infrared fluorescent chemodosimeter for real-time in Vivo evaluation of H2S-release efficiency of prodrug. Chem. Commun. 2020, 56, 8111–8114. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Ma, J.; Fan, J.; Li, H.; Yao, Q.; Xu, F.; Wang, J.; Peng, X. A NIR fluorescent chemodosimeter for imaging endogenous hydrogen polysulfides via the CSE enzymatic pathway. J. Mater. Chem. B 2017, 5, 2574–2579. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Liu, J.; Ning, L.; Zhu, X.; Yu, B.; Liu, X.; Yao, X.; Zhang, H. Near-Infrared and Naked-Eye Fluorescence Probe for Direct and Highly Selective Detection of Cysteine and Its Application in Living Cells. Anal. Chem. 2015, 87, 4856–4863. [Google Scholar] [CrossRef]
- Han, C.; Yang, H.; Chen, M.; Su, Q.; Feng, W.; Li, F. Mitochondria-Targeted Near-Infrared Fluorescent Off–On Probe for Selective Detection of Cysteine in Living Cells and in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 27968–27975. [Google Scholar] [CrossRef]
- Li, Y.; He, X.; Huang, Y.; Xu, L.; Zhao, L.; Li, X.; Sun, Y.; Wang, X.; Ma, P.; Song, D. Development of a water-soluble near-infrared fluorescent probe for endogenous cysteine imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 226, 117544. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Wu, M.; Wang, Y.-Q.; He, X.-W.; Li, W.-Y.; Feng, X.-Z. A new hydrothermal refluxing route to strong fluorescent carbon dots and its application as fluorescent imaging agent. Talanta 2013, 117, 196–202. [Google Scholar] [CrossRef]
- Qi, S.; Zhu, L.; Wang, X.; Du, J.; Yang, Q.; Li, Y. Near-infrared turn-on fluorescent probe for discriminative detection of Cys and application in in Vivo imaging. RSC Adv. 2019, 9, 41431–41437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, S.; Fu, Y.; Meng, M.; Jin, H.; Zhao, W. An isothiocyanate-functionalized self-immolative near-infrared fluorescence probe for cysteine sensing and bioimaging in living systems. Sensors Actuators B Chem. 2022, 366, 132013. [Google Scholar] [CrossRef]
- Savani, S.; Onbasli, K.; Gunduz, H.; Aydındogan, E.; Erkısa, M.; Muti, A.; Khan, M.; Sennaroglu, A.; Ulukaya, E.; Acar, H.Y.; et al. Development of a cysteine responsive chlorinated hemicyanine for image-guided dual phototherapy. Bioorg. Chem. 2022, 122, 105725. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; He, P.-Y.; Shi, L.; Pan, S.-L.; Li, M.-Y.; Zhou, Q.; Zhang, H.; Wang, N.; Li, K.; Yu, X.-Q. A near-infrared water-soluble fluorescent probe for the detection of biothiols in living cells and Escherichia coli. Anal. Methods 2018, 11, 821–826. [Google Scholar] [CrossRef]
- Ren, M.; Wang, L.; Lv, X.; Sun, Y.; Chen, H.; Zhang, K.; Wu, Q.; Bai, Y.; Guo, W. A rhodol-hemicyanine based ratiometric fluorescent probe for real-time monitoring of glutathione dynamics in living cells. Analyst 2019, 144, 7457–7462. [Google Scholar] [CrossRef]
- Qin, J.; Tian, H.; Kong, F.; Guo, Y.; Du, W.; Zhang, C.; Gu, H.; Li, Y. Construction of GSH activated near-infrared fluorescent and photoacoustic dual-modal probe for in vivo tumor imaging. Sens. Actuators B Chem. 2022, 371, 132522. [Google Scholar] [CrossRef]
- Li, S.-J.; Zhou, D.-Y.; Li, Y.-F.; Yang, B.; Ou-Yang, J.; Jie, J.; Liu, J.; Li, C.-Y. Mitochondria-targeted near-infrared fluorescent probe for the detection of carbon monoxide in vivo. Talanta 2018, 188, 691–700. [Google Scholar] [CrossRef]
- Li, W.; Li, R.; Chen, R.; Ai, S.; Zhu, H.; Huang, L.; Lin, W. Activatable Fluorescent-Photoacoustic Integrated Probes with Deep Tissue Penetration for Pathological Diagnosis and Therapeutic Evaluation of Acute Inflammation in Mice. Anal. Chem. 2022, 94, 7996–8004. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, W.; Yuan, L. Development of a near-infrared fluorescent probe for monitoring hydrazine in serum and living cells. Anal. Methods 2013, 5, 3450–3453. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Zhang, J.; She, M.; Liu, P.; Zhang, S.; Li, J. A highly sensitive and selective near-infrared fluorescent probe for imaging hydrazine in living tissues and mice. Sens. Actuators B Chem. 2018, 261, 418–424. [Google Scholar] [CrossRef]
- Zhang, J.; Ning, L.; Liu, J.; Wang, J.; Yu, B.; Liu, X.; Yao, X.; Zhang, Z.; Zhang, H. Naked-Eye and Near-Infrared Fluorescence Probe for Hydrazine and Its Applications in In Vitro and In Vivo Bioimaging. Anal. Chem. 2015, 87, 9101–9107. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, X.-L.; Zhang, Q.; Wang, K.-P.; Chen, S.; Tang, L.; Hu, Z.-Q. A hemicyanine-based near-infrared fluorescent probe for vapor-phase hydrazine detection and bioimaging in a complete aqueous media. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121406. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Zhu, J.; Yuan, Z.; Xu, H.; Ran, J. Monitoring isoniazid metabolism in Vivo using a near-infrared fluorescent probe. Anal. Methods 2022, 14, 2284–2292. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, P.; Wang, M.; Liu, W.; Liu, L.; Xu, P. Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing. Molecules 2022, 27, 7750. https://doi.org/10.3390/molecules27227750
Luo P, Wang M, Liu W, Liu L, Xu P. Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing. Molecules. 2022; 27(22):7750. https://doi.org/10.3390/molecules27227750
Chicago/Turabian StyleLuo, Pan, Min Wang, Wenguang Liu, Lin Liu, and Peng Xu. 2022. "Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing" Molecules 27, no. 22: 7750. https://doi.org/10.3390/molecules27227750
APA StyleLuo, P., Wang, M., Liu, W., Liu, L., & Xu, P. (2022). Activity-Based Fluorescent Probes Based on Hemicyanine for Biomedical Sensing. Molecules, 27(22), 7750. https://doi.org/10.3390/molecules27227750





