Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model
Abstract
1. Introduction
2. Results
2.1. Study of Acute Toxicity
2.1.1. Behavioral Analysis
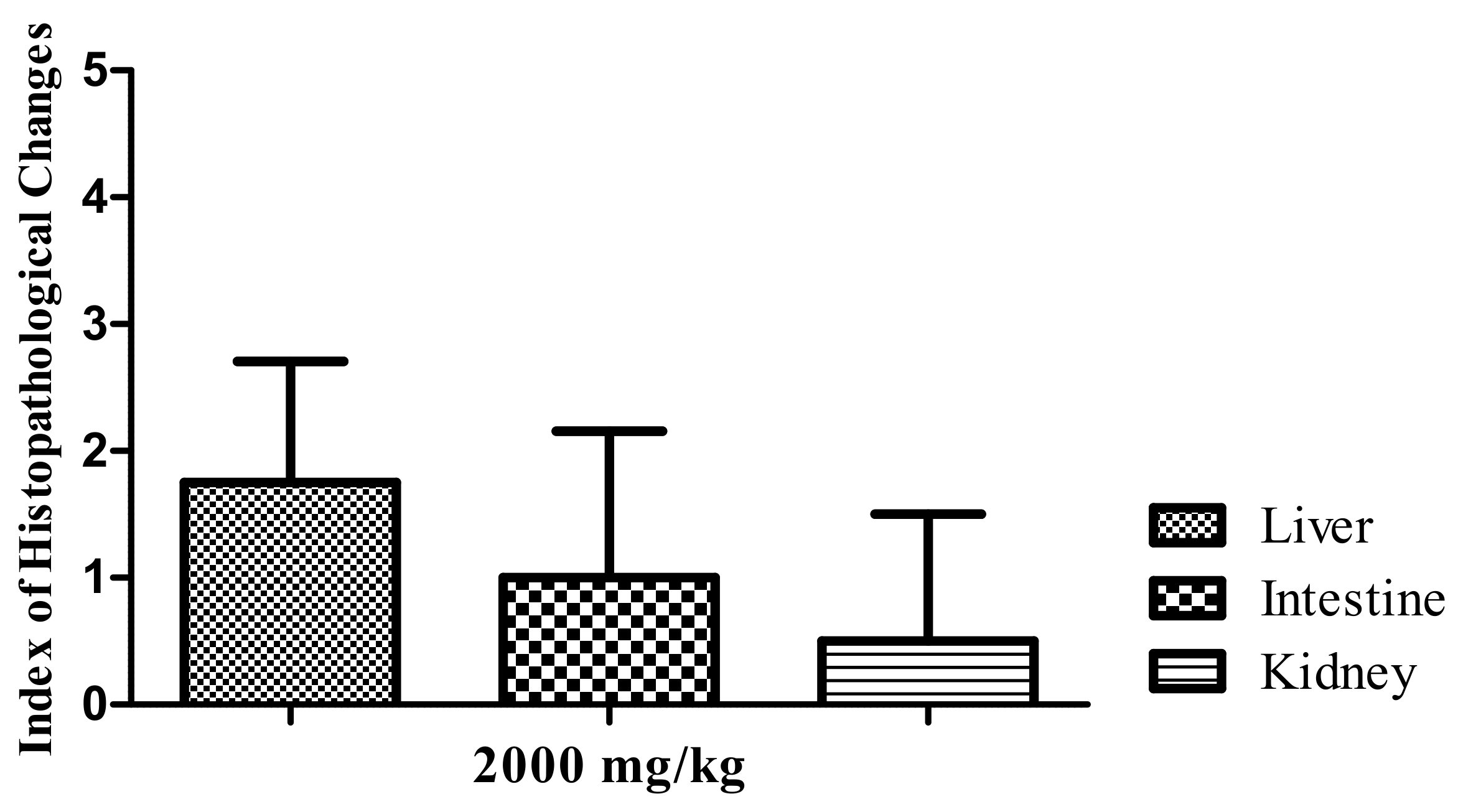
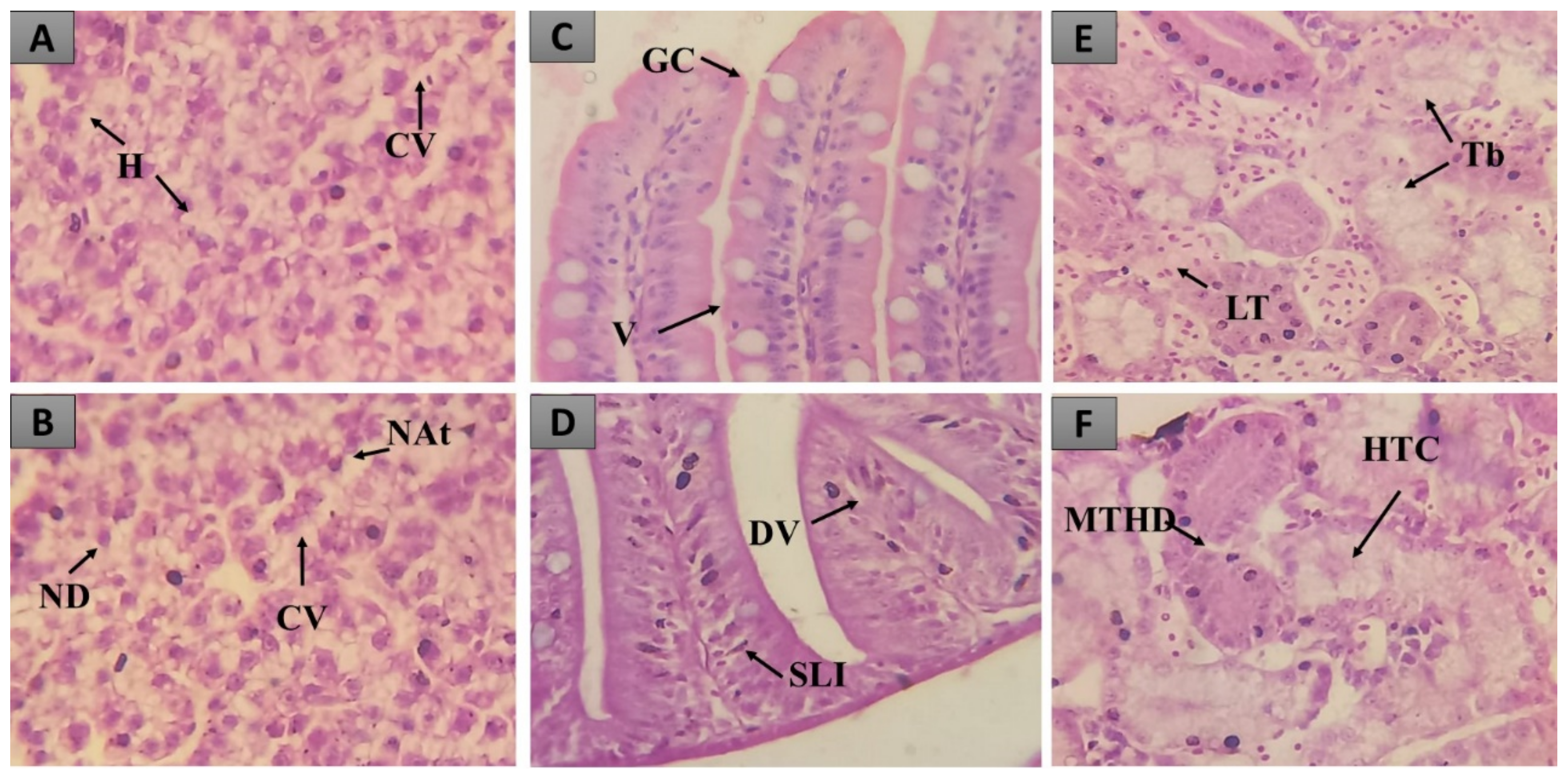
2.1.2. Histopathological Analysis
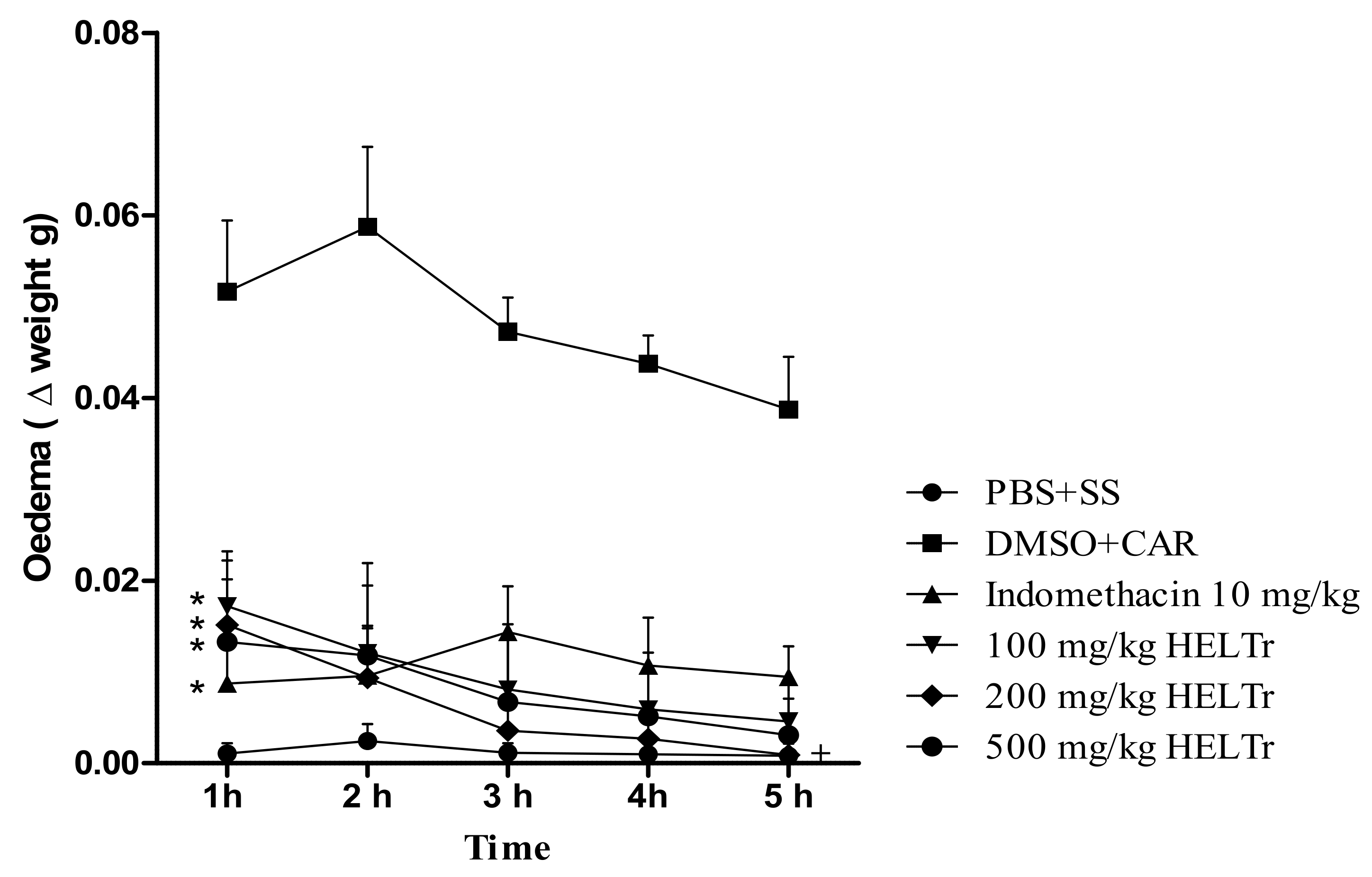
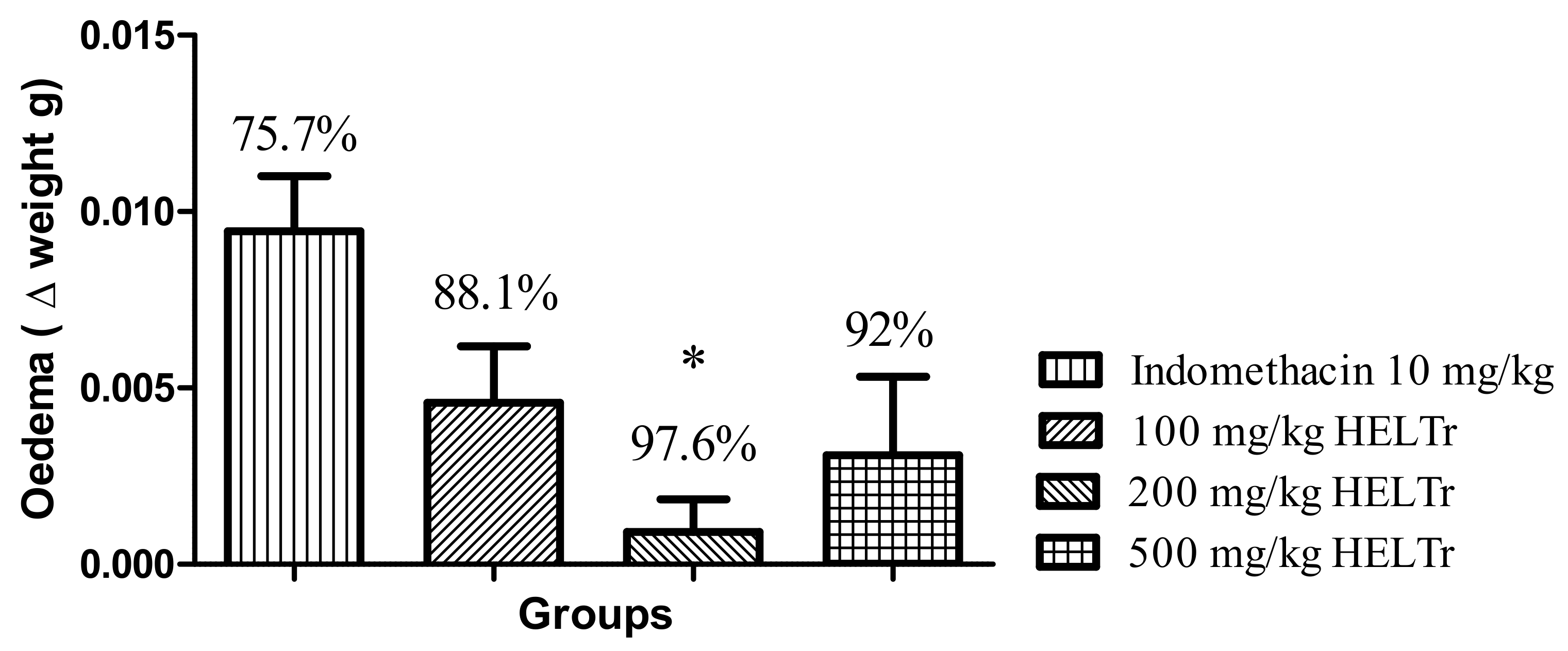
2.2. Effect of the HELTr on Carrageenan-Induced Abdominal Edema
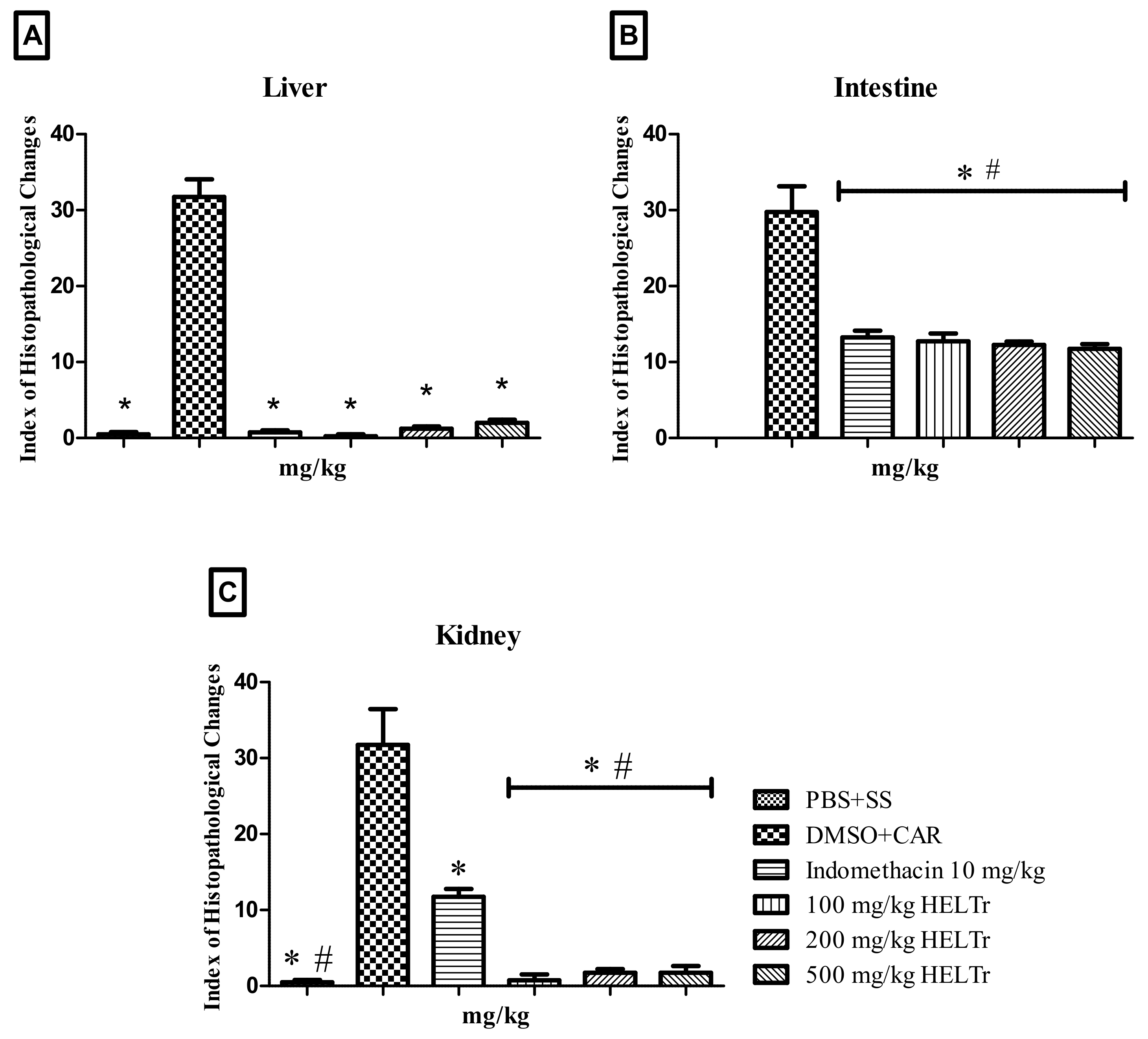
2.3. Histopathological Analysis of the Inflammation
2.3.1. Liver
2.3.2. Intestine
2.3.3. Kidneys
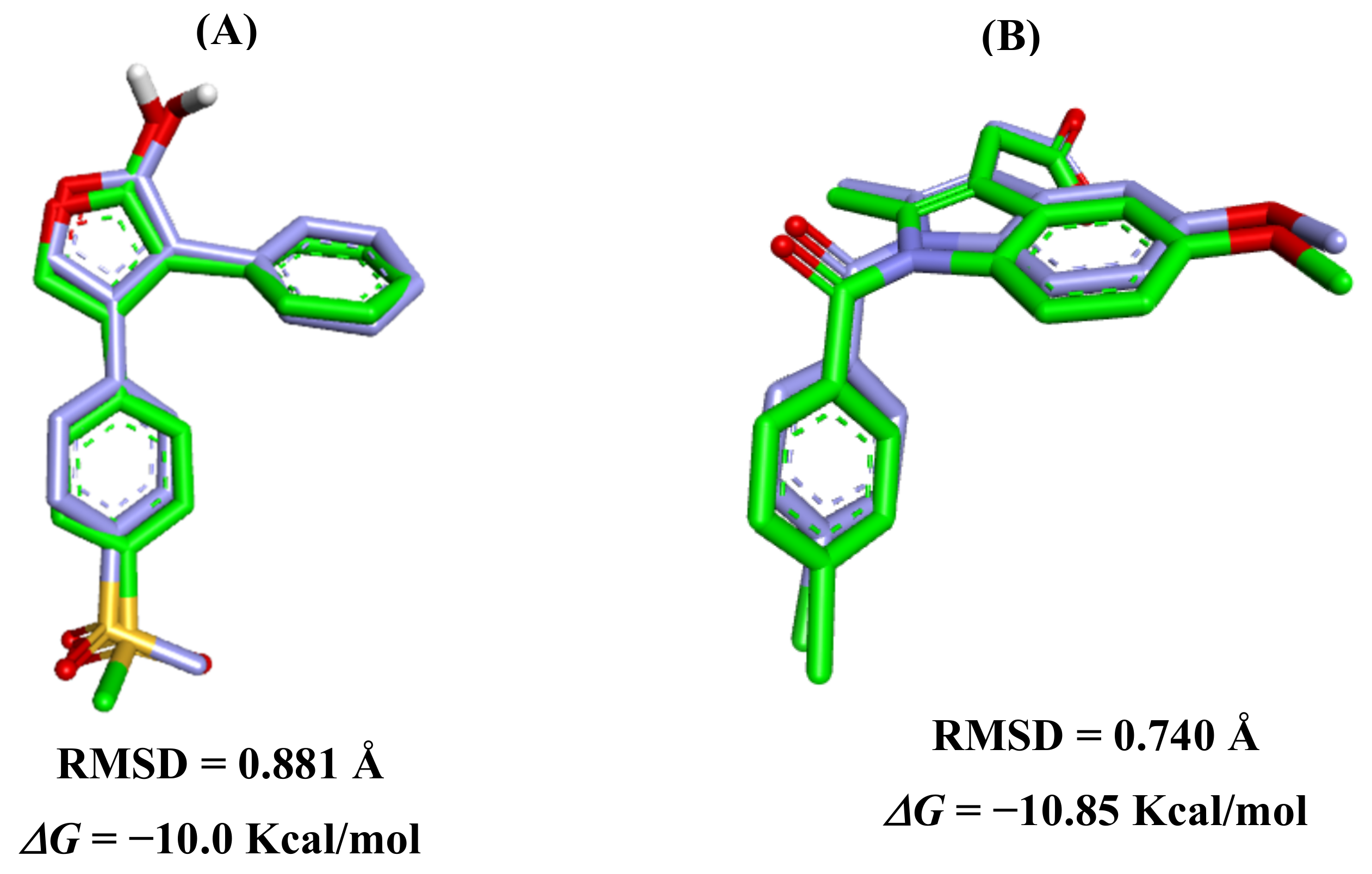
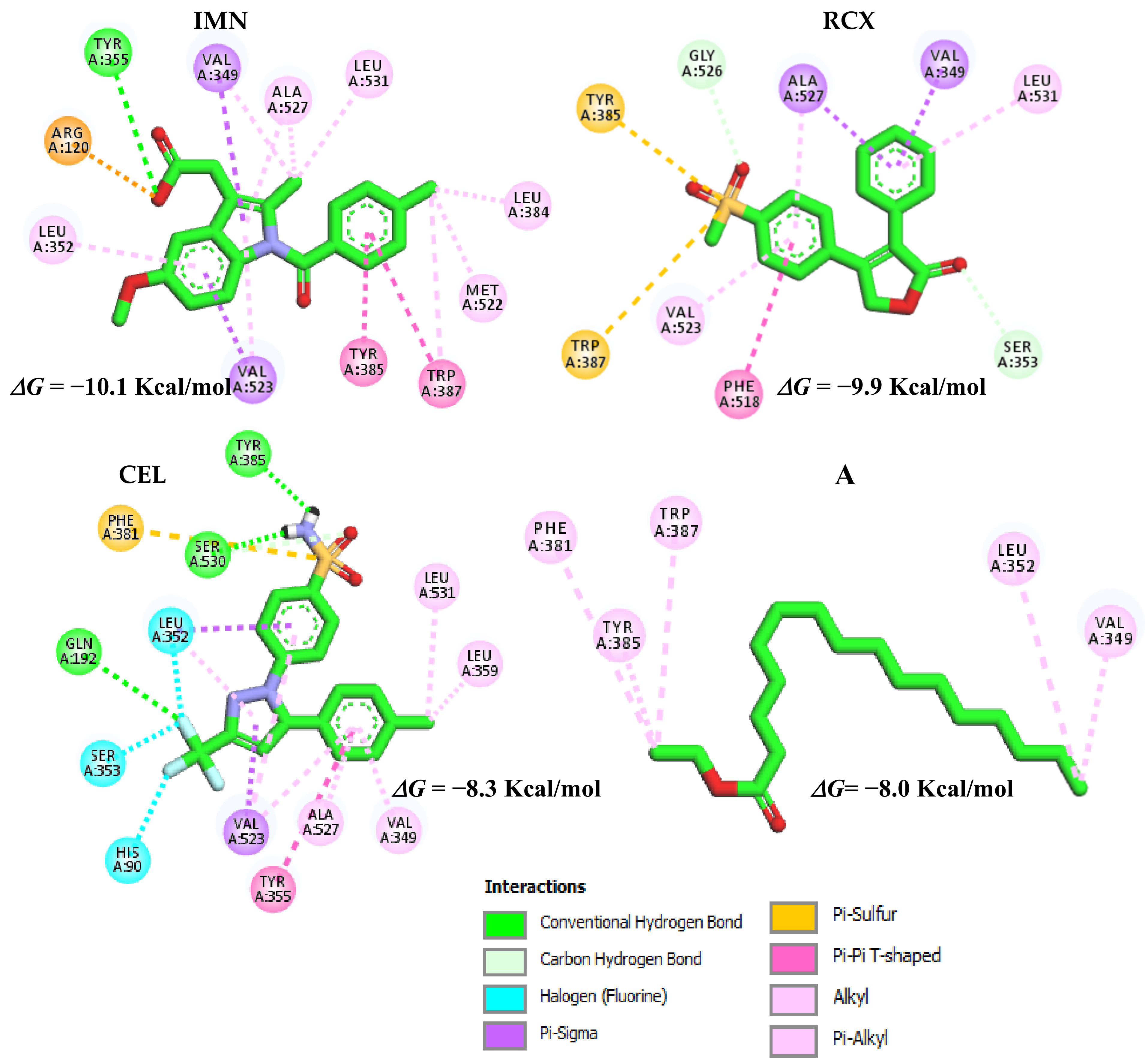
2.4. Analysis for Molecular Docking
Selection of Enzyme and Inhibitor Structure
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of HELTr
4.3. GC-MS Analysis
4.4. Study of the HELTr in Adult Zebrafish
4.4.1. Experimental Animals
4.4.2. Study of Acute Oral Toxicity
4.4.3. Behavioral Analysis and Mortality
4.4.4. Evaluation of the Anti-Inflammatory Activity of the HELTr
4.4.5. Treatment Groups
4.4.6. Induction of Abdominal Edema
4.5. Histopathological Analyses
Assessment of Histopathological Changes
4.6. Statistical Analysis
4.7. Molecular Docking Simulations
4.7.1. Enzyme and Inhibitor Structure Selection
4.7.2. Docking Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Roy, A.; Jauhari, N.; Bharadvaja, N. Medicinal Plants as a Potential Source of Chemopreventive Agents. In Anticancer Plants: Natural Products and Biotechnological Implements; Akhtar, M., Swamy, M., Eds.; Springer: Singapore, 2018; pp. 1089–1139. [Google Scholar]
- Hepzibah, C.J.; Raj, V.D.A. Ocimum sanctum linn: An ethnomedicinal herb as a potential source of anti-carcinogen against various cancer diseases and effective ways to include the basil in everyday diet. Int. J. Health Sci. 2022, 6, 4774–4781. [Google Scholar] [CrossRef]
- Martvall, A.; Lindberg, K. Promotion of Herbal Medicines as a Sustainable Development Strategy. Master’s Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2022. [Google Scholar]
- Souza, A.A.; Ortíz, B.L.S.; Koga, R.C.R.; Sales, P.F.; da Cunha, D.B.; Guerra, A.L.M.; de Souza, G.C.; Carvalho, J.C.T. Secondary metabolites found among the species Trattinnickia rhoifolia Willd. Molecules 2021, 26, 7661. [Google Scholar] [CrossRef]
- Nasir, N.N.; Sekar, M.; Fuloria, S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Begum, M.Y.; Chidambaram, K.; Sathasivam, K.V.; Jeyabalan, S.; et al. Kirenol: A potential natural lead molecule for a new drug design, development, and therapy for inflammation. Molecules 2022, 27, 734. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.T.C.; Barcellos-Silva, I.G.C.; Oliveira Habib-Pereira, N.R.; Antonio, A.S.; Veiga-Junior, V.F. Chemistry, biological activities, and uses of balsams. In Gums, Resins and Latexes of Plant Origin. Reference Series in Phytochemistry; Murthy, H.N., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–35. [Google Scholar]
- Carvalho, L.E.; Pinto, D.S.; Lima, M.P.; Marques, M.O.M.; Facanali, R. The Chemistry of essential oils of Crepidospermum rhoifolium, Trattinnickia rhoifolia and Protium elegans of the Amazon region. J. Essent. Oil Bearing Plants 2009, 12, 92–96. [Google Scholar] [CrossRef]
- Lima, M.P.; Braga, P.A.C.; Macedo, M.L.; Silva, M.F.G.F.; Ferreira, A.G.; Fernandes, J.B.; Vieira, P.C. Phytochemistry of Trattinnickia burserifolia, T. rhoifolia, and Dacryodes hopkinsii: Chemosystematic implications. J. Braz. Chem. Soc. 2004, 15, 385–394. [Google Scholar] [CrossRef]
- DeCarlo, A.; Dosoky, N.S.; Satyal, P.; Sorensen, A.; Setzer, W.N. The essential oils of the burseraceae. In Essential Oil Research; Malik, S., Ed.; Springer Cham: Cham, Switzerland, 2019; pp. 61–145. [Google Scholar]
- Banerjee, T.; Valacchi, G.; Ziboh, Z. Inhibition of TNFa-induced cyclooxygenase-2 expression by amentoflavone through suppression of NF-kB activation in A549 cells. Mol. Cell. Biochem. 2002, 238, 5–10. [Google Scholar] [CrossRef]
- Salazar, U.J.; Porcar, R.C. Chemical constituents of the leaves from Trattinickia rhoifolia. Av. Quim. 2010, 5, 63–65. [Google Scholar]
- Wickramaratne, D.B.M.; Mar, W.; Chai, H.; Castillo, J.J.; Farnsworth, N.R.; Soejarto, D.D.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic constituents of Bursera permollis. PMIO 1995, 61, 80–81. [Google Scholar]
- Blancas, J.; Abad-Fitz, I.; Beltrán-Rodríguez, L.; Cristians, S.; Rangel-Landa, S.; Casas, A.; Torres-García, I.; Sierra-Huelsz, J.A. Chemistry, biological activities, and uses of copal resin (Bursera spp.) in Mexico. In Gums, Resins and Latexes of Plant Origin. Reference Series in Phytochemistry; Murthy, H.N., Ed.; Springer Cham: Cham, Switzerland, 2022; pp. 433–446. [Google Scholar]
- Rüdigera, A.L.S.; Siani, A.C.; Veiga, V. The chemistry and pharmacology of the South America genus Protium burm. f. (Burseraceae). PHCOG REV 2007, 1, 93–104. [Google Scholar]
- Jutiviboonsuk, A.; Zhang, H.; Tan, G.T.; Cuiying, M.A.; Hung, N.V.; Cuong, N.M.; Bunyapraphatsara, N.D.; Soejarto, D.; Fong, H.H.S. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry 2005, 66, 2745–2751. [Google Scholar] [CrossRef]
- Hyacienth, B.M.S.; Sánchez-Ortiz, B.L.; Tavares Picanço, K.R.; Pereira, A.C.M.; Sá Hyacienth, D.C.; De Souza, G.C.; Rodrigues Sarquis, R.S.F.; Aduanga, G.M.G.; Navarrete, A.; Carvalho, J.C.T. Endopleura uchi (Huber) Cuatrec.: A medicinal plant for gynecological treatments—Reproductive toxicity assessment in zebrafish (Danio rerio). J. Ethnopharmacol. 2020, 250, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.C.; Duarte, J.L.; Fernandes, C.P.; Moyado, J.A.V.; Navarrete, A.; Carvalho, J.C.T. Obtainment and Study of the Toxicity of Perillyl Alcohol Nanoemulsion on Zebrafish (Danio rerio). JNMR 2016, 4, 1–19. [Google Scholar]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; Mcpherson, J.D.; Johnson, S.L. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef]
- Rico, E.P.; Oliveira, D.L.; Rosemberg, F.B.; Mussulini, B.H.; Bonan, C.D.; Dias, R.D.; Wofchuk, S.; Souza, D.O.; Maurício Reis Bogo, M.R. Expression and functional analysis of Na+-dependent glutamate transporters from zebrafish brain. Brain Res. Bull. 2010, 81, 517–523. [Google Scholar] [CrossRef]
- Goldsmith, P. Zebrafish as a pharmacological tool: The how, why and when. Curr. Opin. Pharmacol. 2004, 4, 504–512. [Google Scholar] [CrossRef]
- Chen, E.; Ekker, S.C. Zebrafish as a genomics research model. Curr. Pharm. Biotechnol. 2004, 5, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Pavadai, S. Anti-inflammatory effect of Naravelia zeylanica DC via suppression of inflammatory mediators in carrageenan-induced abdominal oedema in zebrafish model. Inflammopharmacology 2017, 25, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Thayumanavan, G.; Jeyabalan, S.; Fuloria, S.; Sekar, M.; Ravi, M.; Selvaraj, L.K.; Bala, L.; Chidambaram, K.; Gan, S.H.; Rani, N.I.M.; et al. Silibinin and Naringenin against Bisphenol A-Induced neurotoxicity in zebrafish model—Potential flavonoid molecules for new drug design, development, and therapy for neurological disorders. Molecules 2022, 27, 2572. [Google Scholar] [CrossRef]
- Borges, R.S.; Lima, E.S.; Keita, H.; Ferreira, O.M.; Fernandes, C.P.; Cruz, R.A.S.; Duarte, J.L.; Velázquez-Moyado, J.; Ortiz, B.L.S.; Castro, A.N.; et al. Anti-inflammatory and antialgic actions of a nanoemulsion of Rosmarinus officinalis L. essential oil and a molecular docking study of its major chemical constituents. Inflammopharmacology 2018, 26, 183–195. [Google Scholar] [CrossRef]
- Quitian-Useche, Y.F.; Sánchez-Ortiz, B.L.; Borges, S.F.; Ramos, B.; Souza, G.C.; Batista, M.A.; Melim, L.I.S.H.; Ferreira, I.M.; Carvalho, J.C.T.; Borges, R.S. Fatty ethanolamide of Bertholletia excelsa triglycerides (Brazil nuts): Anti-inflammatory action and acute toxicity evaluation in Zebrafish (Danio rerio). Inflammopharmacology 2021, 29, 1519–1537. [Google Scholar] [CrossRef]
- Ramos, M.F.S.; Guimarães, A.C.; Siani, A.C. Volatile monoterpenes from the oleoresin of Trattinnickia rhoifolia. Biochem. Syst. Ecol. 2003, 31, 309–311. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Funções em evolução das células endoteliais na inflamação. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Seneme, E.F.; dos Santos, D.C.; Silva, E.M.R.; Franco, Y.E.M.; Longato, G.B. Pharmacological and therapeutic potential of myristicin: A literature review. Molecules 2021, 26, 5914. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.C.; Silva, I.D.R.; Viana, M.D.; Melo, N.C.; Sánchez-Ortiz, B.L.; Oliveira, M.M.R.; Barbosa, W.L.R.; Ferreira, I.M.; Carvalho, J.C.T. Acute toxicity of the hydroethanolic extract of the flowers of Acmella oleracea L. in Zebrafish (Danio rerio): Behavioral and Histopathological Studies. Pharmaceuticals 2019, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Takashima, F.; Hibiya, T. (Eds.) An Atlas of Fish Histology: Normal and Pathogical Features, 2nd ed.; Kodansha Ltd.: Tokyo, Japan, 1995. [Google Scholar]
- Zhang, B.; Li, H.; Yu, K.; Kunqian, Y.; Zhong, J. Molecular docking-based computational platform for high-throughput virtual screening. CCF Trans. HPC 2022, 4, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Veja-teijido, M.; Caracelli, I.; Zukerman-schpector, J. Conformational analyses and docking studies of a series of 5-nitrofuran-and 5-nitrothiophen-semicarbazone derivates in three posible binding sites of trypanothione and glutathione reductases. J. Mol. Graph. Model. 2006, 24, 349–355. [Google Scholar] [CrossRef]
- Santana, A.I.; Vila, R.; Espinosa, A.; Olmedo, D.; Gupta, M.P.; Cañigueral, S. Composition and Biological Activity of Essential Oils from Protium confusum. NPC 2009, 4, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-González, C.; Murià-González, M.J.; Anaya, A.L.; Hernández-Bautista, B.E.; Hernández-Ortega, S.; González, M.C.; Glenn, A.E.; Hanlin, R.T.; Macías-Rubalcava, M.L. Acremoxanthone E, a Novel Member of Heterodimeric Polyketides with a Bicyclo[3.2.2]nonene Ring, Produced by Acremonium camptosporum W. Gams (Clavicipitaceae) Endophytic Fungus. Chem. Biodivers. 2015, 12, 133–147. [Google Scholar] [CrossRef]
- Wejnerowska, G.; Narloch, I. Determination of Benzophenones in Water and Cosmetics Samples: A Comparison of Solid-Phase Extraction and Microextraction by Packed Sorbent Methods. Molecules 2021, 26, 6896. [Google Scholar] [CrossRef]
- Tripathi, T.; Bhatia, A.; Singh, S.; Sarvenda, K.; Khan, A.R.; Sidhu, O.P.; Roy, R. Metabolite Profiling of Commiphora wightii (Guggul) with Respect to Seasons. Nat. Prod. Commun. 2018, 13, 1345–1348. [Google Scholar] [CrossRef]
- Bhatia, A.; Bharti, S.K.; Tripathi, T.; Mishra, A.; Sidhu, O.P.; Roy, R. Metabolic profiling of Commiphora wightii (guggul) reveals a potential source for pharmaceuticals and nutraceuticals. Phytochemistry 2015, 110, 29–36. [Google Scholar] [CrossRef]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Al-hassnwy, S.H.H.; HasanAl-Nomani, R.M. Comparative chemical study for species of the family poaceae. Sys. Rev. Pharm. 2021, 12, 905–920. [Google Scholar]
- Anbuselvi, S.; Rebecca, J.; Manoharan, S.K.S.K.; Senthilvelan, T. GC-MS study of phytochemicals in black gram using two different organic manures. J. Chem. Pharm. Res. 2012, 4, 1246–1250. [Google Scholar]
- Kalaivani, C.S.; Sathish, S.S.; Janakiraman, N.; Johnson, M. GC-MS studies on Andrographis paniculata (Burm. f.) Wall. Ex Nees-a medicinally important plant. Int. J. Med. Arom. Plants 2012, 2, 69–74. [Google Scholar]
- Choo, K.S.O.; Bollen, M.; Dykes, G.A.; Coorey, R. Aroma-volatile profile and its changes in Australian grown black Périgord truffle (Tuber melanosporum) during storage. Int. J. Food Sci. Technol. 2021, 56, 5762–5776. [Google Scholar] [CrossRef]
- Ikpa, C.C.B.; Maduka, T.O.D. Antimicrobial Properties of Methanol Extract of Dacryodes edulis Seed and Determination of Phytochemical Composition Using FTIR and GCMS. Chem. Afr. 2020, 3, 927–935. [Google Scholar] [CrossRef]
- Duru, I.A.; Maduka, T.O.D. Profiling and comparison of fatty acids in the oils from the fruits of Dacryodes edulis and Canarium schweinfurthii. J. Med. Plants Stud. 2020, 8, 213–217. [Google Scholar] [CrossRef]
- Murungi, L.K.; Kirwa, H.; Torto, B. Differences in essential oil content of berries and leaves of Solanum sarrachoides (Solanaceae) and the effects on oviposition of the tomato spider mite (Tetranychus evansi). Ind. Crops. Prod. 2013, 46, 73–79. [Google Scholar] [CrossRef]
- Othman, A.R.; Abdullah, N.; Ahmad, S.; Intan Safinar, I.S.; Zakaria, M.P. Elucidation of in-vitro anti-inflammatory bioactive compounds isolated from Jatropha curcas L. plant root. BMC Complement. Altern. Med. 2015, 15, 11. [Google Scholar] [CrossRef]
- Ramos, R.S.; Costa, J.S.; Silva, R.C.; da Costa, G.V.; Rodrigues, A.B.L.; Rabelo, É.M.; Souto, R.N.P.; Taft, C.A.; Silva, C.H.T.d.P.; Rosa, J.M.C. Identification of potential inhibitors from pyriproxyfen with insecticidal activity by virtual screening. Pharmaceuticals 2019, 12, 20. [Google Scholar] [CrossRef]
- Costa, G.V.; Ferreira, E.F.B.; Ramos, R.S.; Silva, B.L.; Sá, E.M.F.; Silva, A.K.P.; Lobato, C.M.; Souto, R.N.P.; Silva, C.H.T.P.; Rosa, J.M.C.; et al. Dos Hierarchical Virtual Screening of Potential Insectides Inhibitors of Acetylcholinesterase and Juvenile Hormone from Temephos. Pharmaceuticals 2019, 12, 61. [Google Scholar] [CrossRef]
- Ramos, R.S.; Macêdo, W.J.C.; Costa, J.S.; Silva, C.H.T.P.; Rosa, J.M.C.; Cruz, J.N.; Oliveira, M.S.; Aguiar Andrade, E.H.; Silva, R.B.L.; Souto, R.N.P. Potential inhibitors of the enzyme acetylcholinesterase and juvenile hormone with insecticidal activity: Study of the binding mode via docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2020, 38, 4687–4709. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.S.; Borges, R.S.; Souza, J.S.N.; Araujo, I.F.; Chaves, M.H.; Santos, C.B.R. Identification of potential antiviral inhibitors from hydroxychloroquine and 1, 2, 4, 5-tetraoxanes analogues and investigation of the mechanism of action in SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 1781. [Google Scholar] [CrossRef]
- Pham, V.C.; Shun, J.-S.; Choi, M.J.; Kim, T.W. Biological evaluation and molecular docking study of 3-(4-sulfamoylphenyl)-4-phenyl-1H-pyrrole-2, 5-dione as COX-2 inhibitor. Bull. Korean Chem. Soc. 2012, 33, 721–724. [Google Scholar] [CrossRef]
- Araújo, P.H.F.; Ramos, R.S.; Cruz, J.N.; Silva, S.G.; Ferreira, E.F.B.; Lima, L.R.; Macêdo, W.J.C.; Espejo-Román, J.M.; Campos, J.M.; Santos, C.B.R. Identification of potenCOX-2 inhibitors for the treatment of inflammatory diseases using molecular modeling approaches. Molecules 2020, 25, 4183. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Palheta, I.C.; Ota, S.S.B.; Morais, R.B.; Barros, V.A.; Ramos, R.S.; Silva, R.C.; Costa, J.D.S.; Silva, C.H.T.P.; Campos, J.M.; et al. Toward of safer phenylbutazone derivatives by exploration of toxicity mechanism. Molecules 2019, 24, 143. [Google Scholar] [CrossRef]
- Santos, C.B.R.; Silva, R.R.; Ortiz, B.L.S.; Silva, G.M.; Giuliatti, S.; Balderas-Lopez, J.L.; Navarrete, A.; Carvalho, J.C.T. Oil from the fruits of Pterodon emarginatus Vog.: A traditional anti-inflammatory. Study combining in vivo and in silico. J. Ethnopharmacol. 2018, 222, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gaddaguti, V.; Rao, T.V.; Rao, A.P. Potential mosquito repellent compounds of Ocimum species against 3N7H and 3Q8I of Anopheles gambiae. 3 Biotechnol. 2016, 6, 26–33. [Google Scholar] [CrossRef]
- Paramasivam, D.; Balasubramanian, B.; Park, S.; Alagappan, P.; Kaul, T.; Liu, W.; Pachiappan, P. Phytochemical profiling and biological activity of Plectranthus amboinicus (Lour.) mediated by various solvent extracts against Aedes aegypti larvae and toxicity evaluation. Asian Pac J Trop Med 2020, 13, 494–502. [Google Scholar]
- Åkesson, C.; Lindgren, H.; Pero, R.W.; Leanderson, T.; Ivars, F. Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100®. Int. Immunopharmacol. 2005, 5, 219–229. [Google Scholar] [CrossRef]
- Xiang, Z.; Xiaoling, W. Chemical Constituents of Chinese White Olive. Pharm. Chem. J. 2017, 51, 465–470. [Google Scholar] [CrossRef]
- Xiang, Z.; Wu, X.; Liu, X. Chemical Composition and Antioxidant Activity of Petroleum Ether Extract of Canarium album. Pharm. Chem. J. 2017, 51, 606–611. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug. Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar]
- Duan, W.; Tan, S.; Chen, J.; Liu, S.; Jiang, S.; Xiang, H.; Xie, Y. Isolation of Anti-HIV Components from Canarium album Fruits by High-Speed Counter-Current Chromatography. Anal. Lett. 2013, 46, 1057–1068. [Google Scholar] [CrossRef]
- Guevara, P.; Munoz, V.; Llanos, R.E.; Zuniga, B.; Cardenas, R.J.; Contreras, J.L.; Ocampo, F. Flavonoides de trece especies del género Bursera con potencial antioxidante. Polibotánica 2017, 44, 185–193. [Google Scholar]
- Dominguez, F.; Maycotte, P.; Acosta, C.A.; Rodriguez, R.S.; Moreno, D.A.; Ferreres, F.; Flores, A.J.C.; Delgado, L.; Perez, S.M.; Anaya, R.M. Bursera copallifera extracts have cytotoxic and migrationinhibitory effects in breast cancer cell lines. Integr. Cancer Ther. 2018, 17, 654–664. [Google Scholar] [CrossRef]
- Sánchez-Monroy, M.B.; León-Rivera, I.; Llanos-Romero, E.R.; García-Bores, A.M.; Guevara-Fefer, P. Cytotoxic activity and triterpenes content of nine Mexican species of Bursera. Nat. Prod. Res. 2021, 35, 4881–4885. [Google Scholar] [CrossRef]
- Reddy, D.A.; Singh, M.; Babu, A.; Shrivastava, B.; Rohilla, S. An Overview of Benefits of Commiphoramukul: The Indian Bdelliumplant. EJMCM 2020, 7, 4239–4246. [Google Scholar]
- Duwiejua, M.; Zeitlin, I.J.; Waterman, P.J.; Chapman, J.C.J.; Mhango, C.J.; Provan, C.J. Anti-Inflammatory activity of resins from some species of the plant family Burseraceae. PMIO 1993, 59, 12–16. [Google Scholar] [CrossRef]
- Savithramma, N.; Linga Rao, M.; Venkateswarlu, P. Isolation and Identification of Phenolic Compounds from Boswellia ovalifoliolata Bal. & Henry and Their Free Radical Scavenger Activity. J. Drug Deliv. Sci. Technol. 2014, 4, 14–21. [Google Scholar]
- Gross, A.V.; Stolz, E.D.; Müller, L.G.; Rates, S.M.K.; Ritter, M.R. Medicinal plants for the “nerves”: A review of ethnobotanical studies carried out in South Brazil. Acta Bot. Bras. 2019, 33, 269–282. [Google Scholar] [CrossRef]
- Mathur, P.; Lau, B.; Guo, S. Conditioned place preference behavior in zebrafish. Nat. Protoc. 2011, 6, 338–345. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Sousa, T.R.; Arruda, A.S.; Peixoto, N.; Gonçalves, P.J.; Almeida, L.M. Evaluation of cytotoxicity and genotoxicity of Hancornia speciosa latex in Allium cepa root model. Braz. J. Biol. 2016, 76, 245–249. [Google Scholar] [CrossRef]
- Santos, I.V.F.; Souza, G.C.; Santana, G.R.; Duarte, J.L.; Fernandes, C.P.; Keita, H.; Velázquez-Moyado, J.A.; Navarrete, A.; Ferreira, I.M.; Carvalho, H.O.; et al. Histopathology in Zebrafish (Danio rerio) to Evaluate the Toxicity of Medicine: An Anti-Inflammatory Phytomedicine with Janaguba Milk (Himatanthus drasticus Plumel). In Histopathology—An Update; Supriya Srivastava, S., Ed.; IntechOpen: London, UK, 2018; pp. 39–66. [Google Scholar]
- Ferreira, D.Q.; Ferraz, T.O.; Araújo, R.S.; Cruz, R.A.S.; Fernandes, C.P.; Souza, G.C.; Ortiz, B.L.S.; Sarquis, R.S.F.R.; Miranda, J.C.M.M.; Rafael Garrett, R.; et al. Libidibia ferrea (jucá), a Traditional Anti-Inflammatory: A study of acute toxicity in adult and embryos zebrafish (Danio rerio). Pharmaceuticals 2019, 12, 175. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Keita, H.; Santana, G.R.; Souza, G.C.; Santos, I.V.F.; Amado, J.R.R.; Kourouma, A.; Prada, A.L.; Carvalho, H.O.; Silva, M.L. Effects of Bothrops alternatus venom in zebrafish: A histopathological study. Inflammopharmacol 2018, 26, 273–284. [Google Scholar] [CrossRef]
- Goksøyr, A. Use of cytochrome P450 lA (CYP1A) in fish as a biomarker of aquatic pollution. Arch. Toxicol. Suppl. 1995, 17, 80–95. [Google Scholar]
- Vliegenthart, A.D.; Tucker, C.S.; Del Pozo, J.; Dear, J.W. Zebrafish as model organisms for studying drug-induced liver injury. Br. J. Clin. Pharmacol. 2014, 78, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Pellitero, P.; Sitjà-Bobadillam, A. Pathology of Myxosporea in marine fish culture. Dis. Aquat. Org. 2011, 17, 229–238. [Google Scholar] [CrossRef]
- Roberts, R.J.; Ellis, A.E. The anatomy and physiology of teleosts. In Fish Pathology, 3rd ed.; Roberts, R.J., Ed.; W. B. Saunders: Philadelphia, PA, USA, 2012; pp. 12–54. [Google Scholar]
- He, Q.; Liu, K.; Wang, S.; Hou, H.; Yuan, Y.; Wang, X. Toxicity induced by emodin on zebrafish embryos. Drug Chem. Toxicol. 2012, 35, 149–154. [Google Scholar] [CrossRef]
- Holden, J.A.; Layfield, L.L.; Matthews, J.L. The Zebrafish: Atlas of Macroscopic and Microscopic Anatomy. Cambridge University Press: Cambridge, UK, 2012; pp. 58–100. [Google Scholar]
- Huang, S.Y.; Feng, C.W.; Hung, H.C.; Chakraborty, C.; Chen, C.H.; Chen, W.F.; Jean., Y.H.; Wang, H.M.D.; Sung, C.S.; Sun, Y.M.; et al. A novel zebrafish model to provide mechanistic insights into the inflammatory events in carrageenan-induced abdominal edema. PLoS ONE 2014, 9, e104414. [Google Scholar]
- Batista, F.L.A.; Lima, L.M.G.; Abrante, O.A.; Araujo, J.I.F.; Batista, F.L.A.; Abrante, O.A.; Magalhaes, E.A.; Lima, D.R.; Loma, M.C.L.; Prado, B.S.; et al. Antinociceptive activity of ethanolic extract of Azadirachta indica A. Juss (Neem, Meliaceae) fruit through opioid, glutamatergic and acid-sensitive ion pathways in adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 108, 408–416. [Google Scholar] [CrossRef]
- Prata, M.N.L.; Charlie-Silva, I.; Gomes, J.M.M.; Barra, A.; Berg, B.B.; Paiva, I.R.; Melo, D.C.; Klein, A.; Romero, M.G.M.C.; Oliveira, C.C.; et al. Anti-inflammatory and immune properties of the peltatoside, isolated from the leaves of Annona crassiflora Mart., in a new experimental model zebrafish. Fish Shellfish Immunol. 2020, 101, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Perazzo, F.F.; Souza, G.H.B.; Lopes, W.; Cardoso, L.G.V.; Carvalho, J.C.T.; Nanayakkara, N.P.D.; Bastos, J.K. Anti-inflammatory and analgesic properties of waterethanolic extract from Pothomorphe umbellata (Piperaceae) aerial parts. J. Ethnopharmacol. 2005, 99, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Favacho, H.A.S.; Olivera, B.R.; Santos, K.C.; Medeiros, B.J.L.; Sousa, P.J.C.; Perazzo, F.F.; Carvalho, J.C.T. Anti-inflammatory and antinociceptive activities of Euterpe oleracea Mart., Arecaceae, oil. Rev. Bras. Farmacogn. 2011, 21, 105–114. [Google Scholar] [CrossRef]
- Coelho-de-Souza, A.N.; Santos, C.F.; Lopes-Filho, L.N.; Holanda, F.R.; Oliveira, A.C.; Gomes-Vasconcelos, Y.A.; Oliveira, K.A.; Ferreira-da-Silva, F.W.; Silva-Alves, K.S.; Leal-Cardoso, J.H. Essential oil of Pterodon polygalaeflorus Benth attenuates nociception in mice. Braz. J. Med. Biol. Res. 2018, 51, e7356. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Furtado, A.A.; Zanatta, A.C.; Torres-Rêgo, M.; Lourenço, E.M.G.; Alves, J.S.F.; Galinari, E.; Rocha, H.A.O.; Guerra, G.C.B.; Vilegas, W.; et al. Mass spectrometry characterization of Commiphora leptophloeos leaf extract and preclinical evaluation of toxicity and anti-inflammatory potential effect. J. Ethnopharmacol. 2021, 10, 113229. [Google Scholar] [CrossRef]
- Otuki, M.F.; Lima, F.V.; Malheiros, A.; Cechinel-Filho, V.; Monache, F.D.; Yunes, R.A.; Calixto, J.B. Evaluation of the antinociceptive action caused by ether fraction and a triterpene isolated from resin of Protium kleinii. Life Sci. 2001, 69, 2225–2236. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Ebringerová, A. Ultrasonic extraction of plant materials—Investigation of hemicellulose release from buckwheat hulls. Ultrason. Sonochemistry 2003, 10, 127–133. [Google Scholar] [CrossRef]
- Melecchi, M.I.S. Caracterização Química de Extratos de Hibiscus Tiliaceus l: Estudo Comparativo de Métodos de Extração. Ph.D. Thesis, Universidade de Porto Alegre, Porto Alegre, Brazil, October 2005. [Google Scholar]
- Fernandes, K.; Santos, E.; Batista, C.; Ribeiro, O.; Piracelli, V.; Solci, M.C.; Duvoisin, S., Jr.; Martin, S.; Souza, R.; Machado, C. WSOC and Its Relationship with BC, Levoglucosan and Transition Metals in the PM2.5 of an Urban Area in the Amazon. J. Braz. Chem. Soc. 2002, 33, 570–581. [Google Scholar] [CrossRef]
- Corrêa, F.S.; Bernar, L.P.; Assunção, F.P.C. Purification of Bio-Oil produced by pyrolise of Açaí (Euterpe Oleracea, Mart) seeds. BJS 2021, 7, 18260–18277. [Google Scholar]
- Leary, S.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Phelps, H.A.; Runft, D.L.; Neely, M.N. Adult zebrafish model of streptococcal infection. Curr. Protoc. Microbiol. 2009, 9, 9D.1. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Secondary Metabolites: Secondary metabolic products consisting of C and H.; C, H, and O.; N, S, and P Elements; and O/N Heterocycles. In Therapeutic Use of Medicinal Plants and their Extracts; Springer Natur: Cham, Switzerland, 2018; pp. 165–309. [Google Scholar]
- Crawford, A.D.; Liekens, S.; Kamuhabwa, O.R.; Maes, J.; Munck, S.; Busson, R.; Rozenski, J.; Esguerra, C.V.; Witte, P.A.M. Zebrafish bioassay-guided natural product discovery: Isolation of angiogenesis inhibitors from East African medicinal plants. PLoS ONE 2011, 6, e14694. [Google Scholar] [CrossRef]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a model for inflammation and drug discovery. Drug Discov. Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef]
- Gierse, J.K.; Koboldt, C.M.; Walker, M.C.; Seibert, K.; Isakson, P.C. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem. J. 1999, 339, 607–614. [Google Scholar] [CrossRef]
- Hayashi, S.; Ueno, N.; Murase, A.; Nakagawa, Y.; Takada, J. Novel acid-type cyclooxygenase-2 inhibitors: Design, synthesis, and structure–activity relationship for anti-inflammatory drug. Eur. J. Med. Chem. 2012, 50, 179–195. [Google Scholar] [CrossRef]
- Poleksic, V.; Mitrovic-Tutundzic, V. Fish gills as a monitor of sublethal and chronic effects of pollution. In Sublethal and Chronic Effects of Pollutants on Freshwater Fish; Müller, R., Lloyd, R., Eds.; Cambridge University Press: Cambridge, UK, 1994; pp. 339–352. [Google Scholar]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Crystal structure of rofecoxib bound to human cyclooxygenase-2. Acta Crystallogr. F Struct. Biol. Commun. 2016, 72, 772–776. [Google Scholar] [CrossRef]
- Cruz, J.V.; Neto, M.F.A.; Silva, L.B.; Ramos, R.S.; Costa, J.S.; Brasil, D.S.B.; Lobato, C.C.; Costa, G.V.; Bittencourt, J.A.H.M.; Silva, C.H.T.P.; et al. Identification of novel protein kinase receptor type 2 inhibitors using pharmacophore and structure-based virtual screening. Molecules 2018, 23, 453. [Google Scholar] [CrossRef] [PubMed]
- Leão, R.P.; Cruz, J.V.; Costa, G.V.; Cruz, J.N.; Ferreira, E.F.B.; Silva, R.C.; Lima, L.R.; Borges, R.S.; Santos, G.B.; Santos, C.B.R. Identification of new rofecoxib-based cyclooxygenase-2 inhibitors: A bioinformatics approach. Pharmaceuticals 2020, 13, 209. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.L.B.D.; Cruz, J.N.; Silva, L.B.; Ramos, R.S.; Neto, M.F.A.; Lobato, C.C.; Ota, S.S.B.; Leite, F.H.A.; Borges, R.S.; da Silva, C.H.T.P.; et al. Identification of novel chemical entities for adenosine receptor type 2a using molecular modeling approaches. Molecules 2020, 25, 1245. [Google Scholar] [CrossRef] [PubMed]
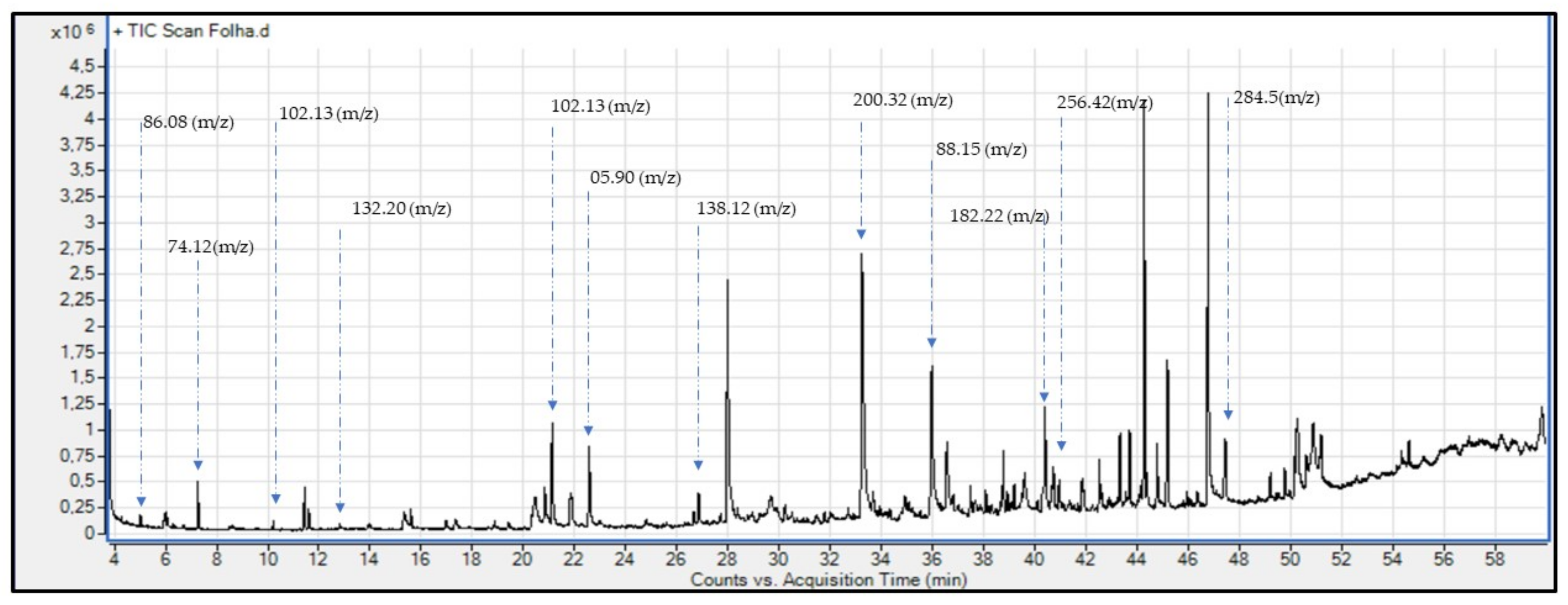
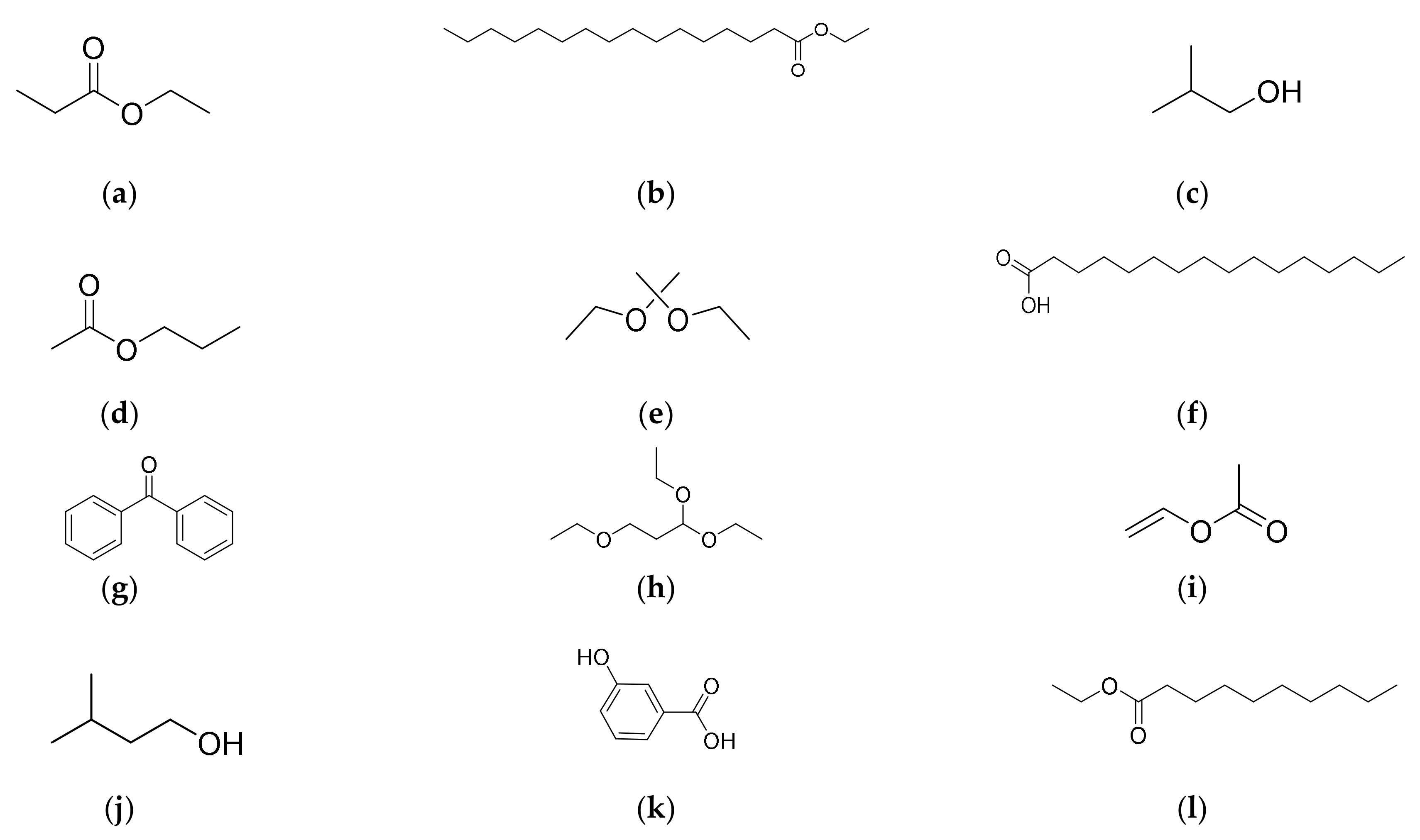



| Names of Compounds | Molecular Formula | Similarity | RT * | Molar Mass (m/z) | Class of Metabolites |
|---|---|---|---|---|---|
| Propanoic acid, ethyl ester | C5H10O2 | 98.0 | 10.41 | 102.06 | Ester |
| Hexadecanoic acid, ethyl ester | C18H36O2 | 95.4 | 47.93 | 284.5 | Fatty acid |
| 1-Propanol 2-methyl | C4H10O | 95.2 | 07.93 | 74.12 | Alcohol |
| n-Propyl acetate | C5H10O2 | 95.0 | 20.61 | 102.13 | Ester |
| Propane, 2,2-diethoxy | C7H16O2 | 94.0 | 12.69 | 132.20 | Acetone |
| n-Hexadecanoic acid | C16H3202 | 90.9 | 41.91 | 256.42 | Fatty acid |
| Benzophenone | C13H10O | 90.0 | 40.38 | 182.22 | Acetone |
| Propane,1,1,3-triethoxy | C9H20O3 | 89.6 | 23.87 | 176.25 | Ester |
| Acetic acid ethenyl ester | C4H6O2 | 80.0 | 05.90 | 86.08 | Ester |
| 1-Butanol, 3-methyl | C5H12O | 80.0 | 36.0 | 88.15 | Alcohol |
| 3-Hydroxy, benzoic acid | C7H6O3 | 71.2 | 27.97 | 138.12 | Benzoic acid |
| Decanoic acid, ethyl ester | C12H24O2 | 70.3 | 33.64 | 200.32 | Ester |
| Group | Stage I | Stage II | Stage III | Total | % |
|---|---|---|---|---|---|
| 2000 mg/kg | 1/3 | 0/2 | 2/4 | 3/9 | 33.3 |
| Control (DMSO) | 1/3 | 0/2 | 1/4 | 2/9 | 22.2 |
| Molecules | MW a | CLogP b | HBA c | HBD d | Ro5 e |
|---|---|---|---|---|---|
| Normal range | <500 | <5 | <10 | <5 | Max.4 |
| RCX | 314.4 | 2.3 | 4 | 0 | 0 |
| IMN | 357.8 | 4.3 | 4 | 1 | 0 |
| CEL | 381.4 | 3.4 | 7 | 1 | 0 |
| B | 182.2 | 3.4 | 1 | 0 | 0 |
| I | 256.4 | 6.4 | 2 | 1 | 1 |
| A | 284.5 | 7.8 | 2 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, A.A.; Ortíz, B.L.S.; Borges, S.F.; Pinto, A.V.P.; Ramos, R.d.S.; Pena, I.C.; Rocha Koga, R.d.C.; Batista, C.E.; de Souza, G.C.; Ferreira, A.M.; et al. Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model. Molecules 2022, 27, 7741. https://doi.org/10.3390/molecules27227741
de Souza AA, Ortíz BLS, Borges SF, Pinto AVP, Ramos RdS, Pena IC, Rocha Koga RdC, Batista CE, de Souza GC, Ferreira AM, et al. Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model. Molecules. 2022; 27(22):7741. https://doi.org/10.3390/molecules27227741
Chicago/Turabian Stylede Souza, Agerdânio Andrade, Brenda Lorena Sánchez Ortíz, Swanny Ferreira Borges, Andria Vanessa Pena Pinto, Ryan da Silva Ramos, Igor Colares Pena, Rosemary de Carvalho Rocha Koga, Carla Estefani Batista, Gisele Custódio de Souza, Adriana Maciel Ferreira, and et al. 2022. "Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model" Molecules 27, no. 22: 7741. https://doi.org/10.3390/molecules27227741
APA Stylede Souza, A. A., Ortíz, B. L. S., Borges, S. F., Pinto, A. V. P., Ramos, R. d. S., Pena, I. C., Rocha Koga, R. d. C., Batista, C. E., de Souza, G. C., Ferreira, A. M., Duvoisin Junior, S., & Tavares Carvalho, J. C. (2022). Acute Toxicity and Anti-Inflammatory Activity of Trattinnickia rhoifolia Willd (Sucuruba) Using the Zebrafish Model. Molecules, 27(22), 7741. https://doi.org/10.3390/molecules27227741








