Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Profile
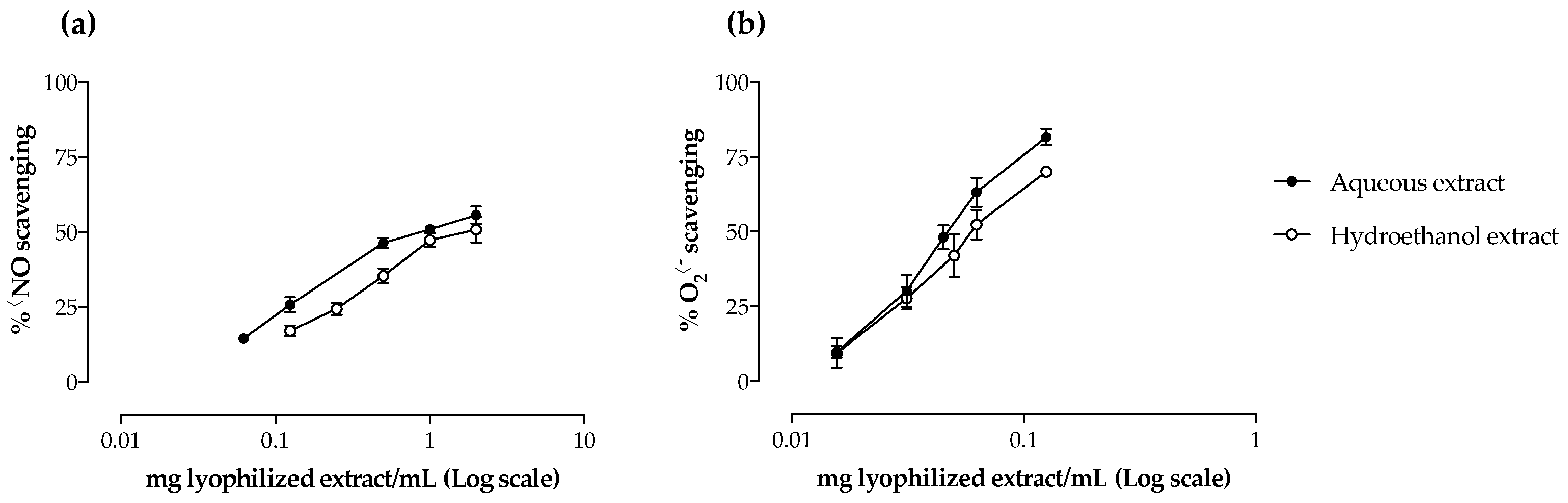
2.2. Antioxidant Activity
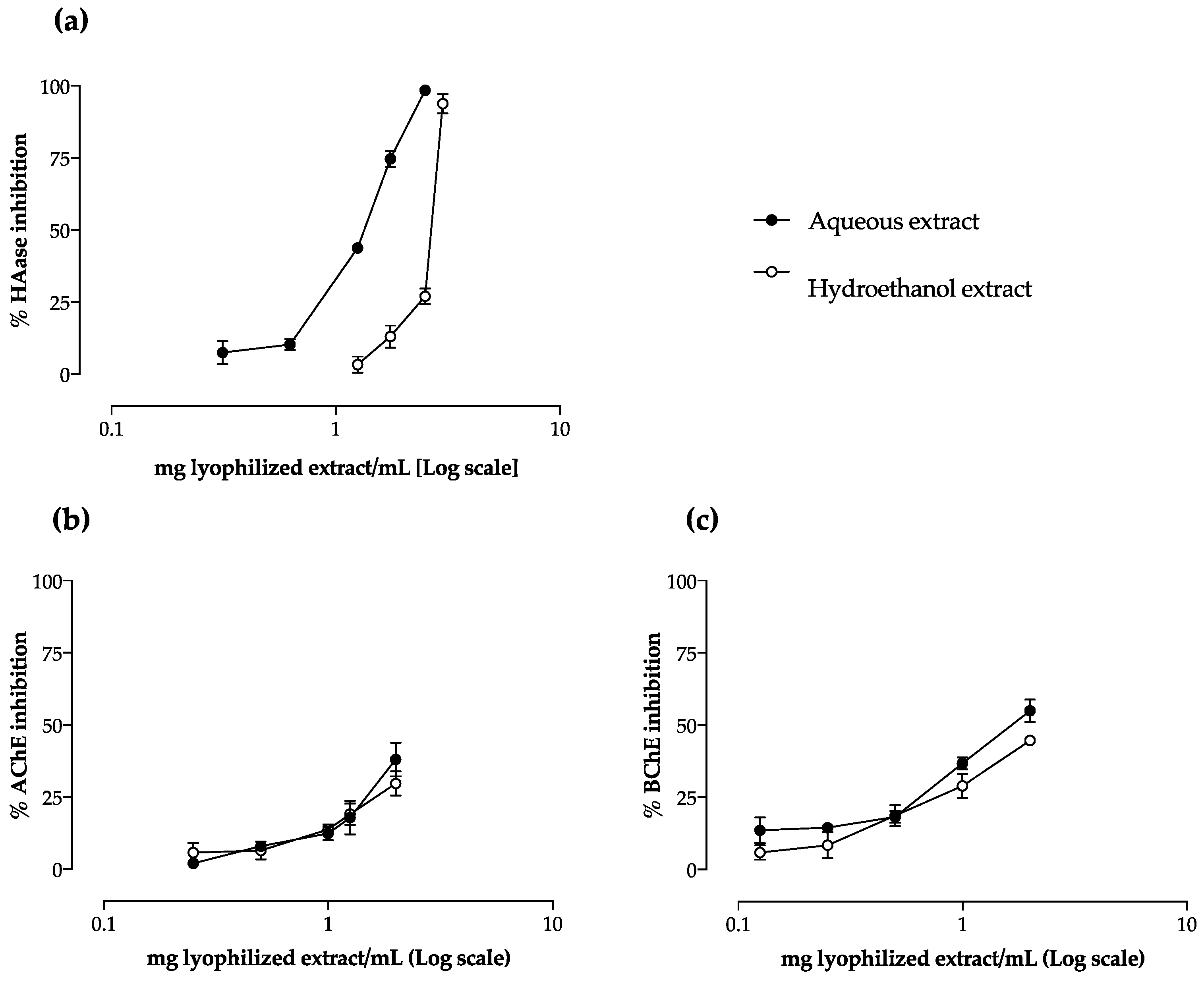
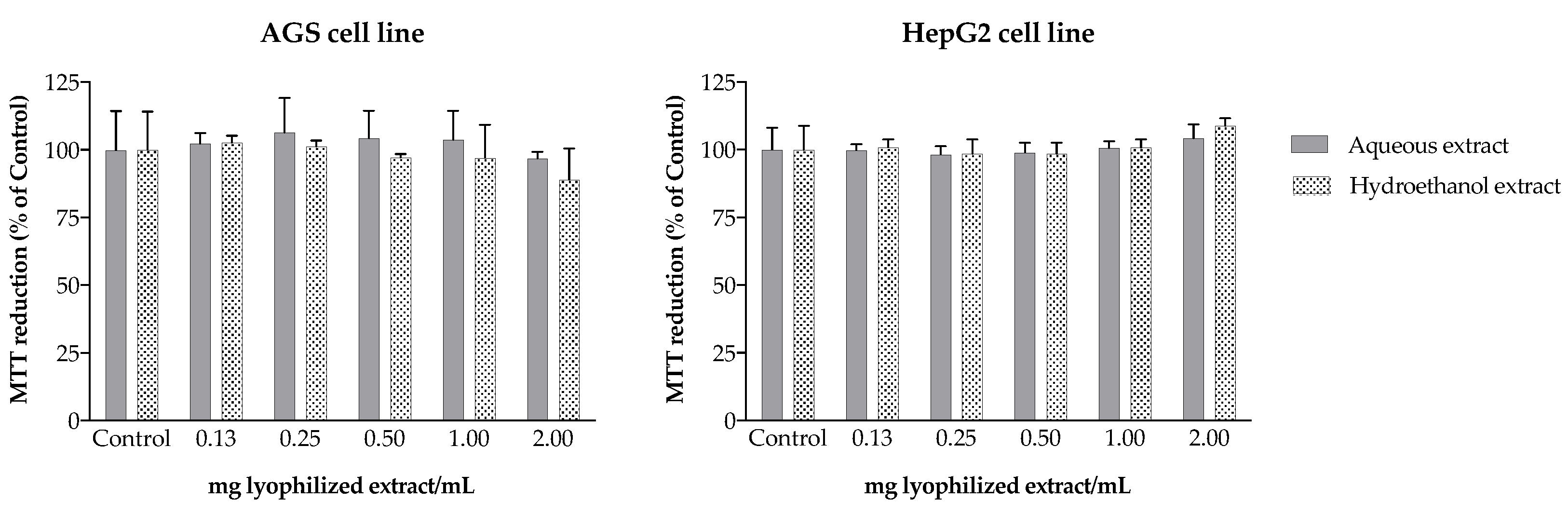
2.3. Anti-Inflammatory Potential
2.4. Effect on AChE and BChE Activity
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material and Extract Preparation
3.3. HPLC-DAD Analysis
3.4. Superoxide Anion Radical (O2•−) Scavenging Assay
3.5. Nitric Oxide Radical (•NO) Scavenging Assay
3.6. HAase Inhibition Assay
3.7. AChE and BChE Inhibition Assays
3.8. Cell Culture and Treatments
3.9. Toxicity to RAW 264.7 Cells
3.10. NO Release by RAW 264.7 Cells
3.11. Toxicity to AGS and HepG2 Cells
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palhares, R.M.; Gonçalves Drummond, M.; dos Santos Alves Figueiredo Brasil, B.; Pereira Cosenza, G.; das Graças Lins Brandão, M.; Oliveira, G. Medicinal plants recommended by the world health organization: DNA barcode identification associated with chemical analyses guarantees their quality. PLoS ONE 2015, 10, e0127866. [Google Scholar]
- Bodeker, G.; Ong, C.-K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; World Health Organization: Geneva, Switzerland, 2005; Volume 1.
- Calixto, J.B.; Campos, M.M.; Otuki, M.F.; Santos, A.R. Anti-inflammatory compounds of plant origin. Part II. Modulation of pro-inflammatory cytokines, chemokines and adhesion molecules. Planta Med. 2004, 70, 93–103. [Google Scholar] [PubMed]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Kim, H.K.; Cheon, B.S.; Kim, Y.H.; Kim, S.Y.; Kim, H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999, 58, 759–765. [Google Scholar] [CrossRef]
- Abot, A.; Fried, S.; Cani, P.D.; Knauf, C. Reactive oxygen species/reactive nitrogen species as messengers in the gut: Impact on physiology and metabolic disorders. Antioxid. Redox Signal. 2022, 37, 394–415. [Google Scholar] [CrossRef]
- Jivad, N.; Rabiei, Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac. J. Trop. Biomed. 2014, 4, 780–789. [Google Scholar] [CrossRef]
- Shah, A.; Dar, T.; Dar, P.; Ganie, S.; Kamal, M. A current perspective on the inhibition of cholinesterase by natural and synthetic inhibitors. Curr. Drug Metab. 2017, 18, 96–111. [Google Scholar] [CrossRef]
- Suganthy, N.; Pandian, S.K.; Devi, K.P. Cholinesterase inhibitors from plants: Possible treatment strategy for neurological disorders-a review. Int. J. Biomed Pharm. Sci. 2009, 3, 87–103. [Google Scholar]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3. [Google Scholar] [CrossRef]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 2015, 20, 7438–7453. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, O.S.; Adelowo, F.E.; Ayodele, D.T.; Odelade, K.A. Phytochemistry and pharmacological activities of Cymbopogon citratus: A review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Pavlović, I.; Omar, E.; Drobac, M.; Radenković, M.; Branković, S.; Kovačević, N. Chemical composition and spasmolytic activity of Cymbopogon schoenanthus (L.) Spreng.(Poaceae) essential oil from Sudan. Arch. Biol. Sci. 2017, 69, 409–415. [Google Scholar] [CrossRef]
- Khadri, A.; Neffati, M.; Smiti, S.; Falé, P.; Lino, A.R.L.; Serralheiro, M.L.M.; Araújo, M.E.M. Antioxidant, anti-acetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT-Food Sci. Technol. 2010, 43, 331–336. [Google Scholar] [CrossRef]
- Ben Othman, M.; Han, J.; El Omri, A.; Ksouri, R.; Neffati, M.; Isoda, H. Antistress effects of the ethanolic extract from Cymbopogon schoenanthus growing wild in Tunisia. Evid. Based Complement. Altern. Med. 2013, 2013, 737401. [Google Scholar] [CrossRef][Green Version]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Figueirinha, A.; Cruz, M.T.; Francisco, V.; Lopes, M.C.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaf infusion in lipopolysaccharide-stimulated dendritic cells: Contribution of the polyphenols. J. Med. Food 2010, 13, 681–690. [Google Scholar] [CrossRef]
- Figueirinha, A.; Paranhos, A.; Pérez-Alonso, J.J.; Santos-Buelga, C.; Batista, M.T. Cymbopogon citratus leaves: Characterization of flavonoids by HPLC–PDA–ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008, 110, 718–728. [Google Scholar] [CrossRef]
- Francisco, V.; Figueirinha, A.; Costa, G.; Liberal, J.; Lopes, M.C.; García-Rodríguez, C.; Geraldes, C.F.; Cruz, M.T.; Batista, M.T. Chemical characterization and anti-inflammatory activity of luteolin glycosides isolated from lemongrass. J. Funct. Foods 2014, 10, 436–443. [Google Scholar] [CrossRef]
- Francisco, V.; Figueirinha, A.; Neves, B.M.; García-Rodríguez, C.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011, 133, 818–827. [Google Scholar] [CrossRef]
- Sousa, R.; Figueirinha, A.; Batista, M.T.; Pina, M.E. Formulation effects in the antioxidant activity of extract from the leaves of Cymbopogon citratus (Dc) stapf. Molecules 2021, 26, 4518. [Google Scholar] [CrossRef]
- Musa, H.A.A.; Ahmed, E.; Osman, G.; Ali, H.; Müller, J. Microbial load and phytochemicals stability of camel hay (Cymbopogon schoenanthus L.) leaves as affected by gamma irradiation. Agric. Biol. J. N. Am. 2010, 1, 662–670. [Google Scholar]
- Abu-Serie, M.M.; Habashy, N.H.; Maher, A.M. In vitro anti-nephrotoxic potential of Ammi visnaga, Petroselinum crispum, Hordeum vulgare, and Cymbopogon schoenanthus seed or leaf extracts by suppressing the necrotic mediators, oxidative stress and inflammation. BMC Complement. Altern. Med. 2019, 19, 1–16. [Google Scholar] [CrossRef]
- Najjaa, H.; Abdelkarim, B.A.; Doria, E.; Boubakri, A.; Trabelsi, N.; Falleh, H.; Tlili, H.; Neffati, M. Phenolic composition of some Tunisian medicinal plants associated with anti-proliferative effect on human breast cancer MCF-7 cells. EuroBiotech J. 2020, 4, 104–112. [Google Scholar] [CrossRef]
- Rocchetti, G.; Alcántara, C.; Bäuerl, C.; García-Pérez, J.V.; Lorenzo, J.M.; Lucini, L.; Collado, M.C.; Barba, F.J. Bacterial growth and biological properties of Cymbopogon schoenanthus and Ziziphus lotus are modulated by extraction conditions. Food Res. Int. 2020, 136, 109534. [Google Scholar] [CrossRef]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.). J. Agric. Food Chem. 2005, 53, 2511–2517. [Google Scholar] [CrossRef]
- Campos, J.; Schmeda-Hirschmann, G.; Leiva, E.; Guzmán, L.; Orrego, R.; Fernández, P.; González, M.; Radojkovic, C.; Zuñiga, F.; Lamperti, L. Lemon grass (Cymbopogon citratus (DC) Stapf) polyphenols protect human umbilical vein endothelial cell (HUVECs) from oxidative damage induced by high glucose, hydrogen peroxide and oxidised low-density lipoprotein. Food Chem. 2014, 151, 175–181. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Scientific validation of synergistic antioxidant effects in commercialised mixtures of Cymbopogon citratus and Pterospartum tridentatum or Gomphrena globosa for infusions preparation. Food Chem. 2015, 185, 16–24. [Google Scholar] [CrossRef][Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.; Zarzuelo, A.; Martínez-Augustin, O.; Medina, F.S.D. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Francisco, V.; Costa, G.; Figueirinha, A.; Marques, C.; Pereira, P.; Neves, B.M.; Lopes, M.C.; García-Rodríguez, C.; Cruz, M.T.; Batista, M.T. Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: Contribution of chlorogenic acid. J. Ethnopharmacol. 2013, 148, 126–134. [Google Scholar] [CrossRef]
- Girish, K.; Kemparaju, K. The magic glue hyaluronan and its eraser hyaluronidase: A biological overview. Life Sci. 2007, 80, 1921–1943. [Google Scholar] [CrossRef]
- Khadri, A.; Serralheiro, M.; Nogueira, J.; Neffati, M.; Smiti, S.; Araújo, M. Antioxidant and antiacetylcholinesterase activities of essential oils from Cymbopogon schoenanthus L. Spreng. Determination of chemical composition by GC–mass spectrometry and 13C NMR. Food Chem. 2008, 109, 630–637. [Google Scholar] [CrossRef]
- Goes, T.C.; Ursulino, F.R.C.; Almeida-Souza, T.H.; Alves, P.B.; Teixeira-Silva, F. Effect of lemongrass aroma on experimental anxiety in humans. J. Altern. Complement. Med. 2015, 21, 766–773. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Azeez, F.A. Evaluation of antioxidant and acetylcholinesterase-inhibitory properties of methanol extracts of Nauclealatifolia, Cymbopogon citratus and Cocos nucifera: An in vitro study. Br. J. Med. Med. Res. 2014, 4, 2156–2170. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to control hyperglycemia and diabetes-related vascular complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Ferreres, F.; Andrade, P.B.; Pereira, D.M.; Gil-Izquierdo, Á.; Valentão, P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria Secondary Metabolites as Biotechnological Ingredients in Natural Anti-Aging Cosmetics: Potential to Overcome Hyperpigmentation, Loss of Skin Density and UV Radiation-Deleterious Effects. Mar. Drugs 2022, 20, 183. [Google Scholar] [CrossRef]
- Bernardo, J.; Ferreres, F.; Gil-Izquierdo, Á.; Valentao, P.; Andrade, P.B. Medicinal species as MTDLs: Turnera diffusa Willd. Ex Schult inhibits CNS enzymes and delays glutamate excitotoxicity in SH-SY5Y cells via oxidative damage. Food Chem. Toxicol. 2017, 106, 466–476. [Google Scholar] [CrossRef]
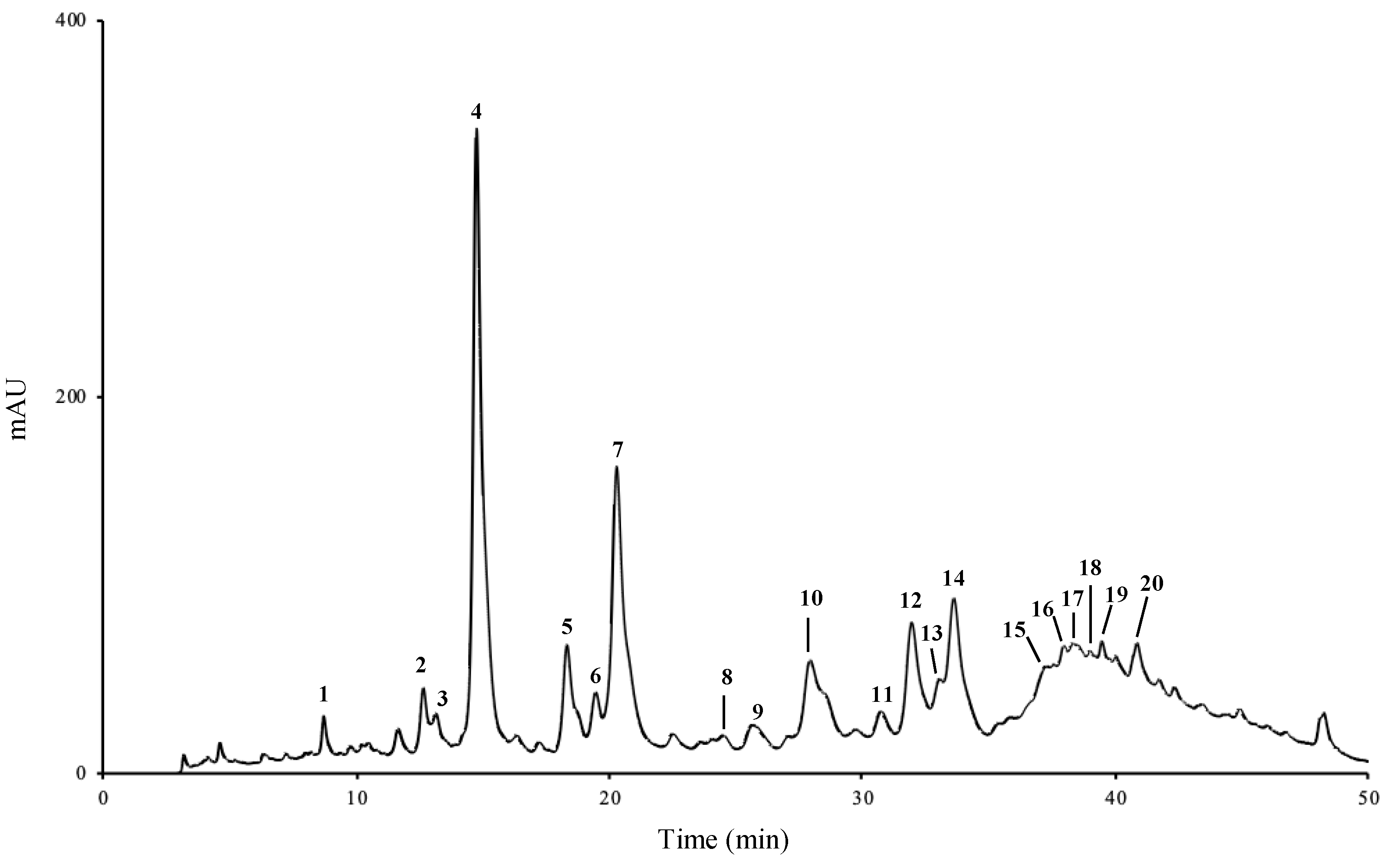

| Compounds | Rt (min) | Aqueous Extract | Hydroethanol Extract | |
|---|---|---|---|---|
| Hydroxycinnamic acids | ||||
| 1 | 3-Caffeoylquinic acid | 8.66 | 0.03 ± < 0.01 | 0.08 ± < 0.01 |
| 2 | 4-Caffeoylquinic acid | 12.57 | 0.04 ± < 0.01 | 0.01 ± < 0.01 |
| 3 | Caffeic acid derivative | 13.09 | 0.02 ± < 0.01 | 0.01 ± < 0.01 |
| 4 | Chlorogenic acid | 14.66 | 0.49 ± 0.04 | 0.02 ± 0.01 |
| 5 | Caffeic acid derivative | 18.22 | 0.04 ± > 0.01 | nq |
| 6 | Caffeic acid derivative | 19.33 | 0.02 ± > 0.01 | nq |
| 7 | p-Coumaric acid | 20.21 | 0.18 ± 0.01 | 0.16 ± < 0.01 |
| 8 | Caffeic acid derivative | 24.45 | 0.00 ± < 0.01 | nq |
| 9 | Ferulic acid | 25.51 | 0.01 ± < 0.01 | 0.05 ± 0.01 |
| Σ | 0.83 a ± 0.05 | 0.33 b ± 0.02 | ||
| Flavonoids | ||||
| 10 | Apigenin glycoside | 28.50 | 0.06 ± < 0.01 | 0.03 ± < 0.01 |
| 11 | Apigenin glycoside | 30.60 | 0.01 ± < 0.01 | nq |
| 12 | Isoorientin | 31.81 | 0.13 ± 0.01 | 0.08 ± 0.01 |
| 13 | Luteolin glycoside | 32.89 | 0.07 ± 0.01 | nq |
| 14 | Luteolin-3′,7-di-O-glucoside | 33.46 | 0.31 ± 0.03 | 0.22 ± 0.01 |
| 15 | Apigenin glycoside | 37.10 | 0.09 ± 0.01 | 0.03 ± 0.02 |
| 16 | Apigenin glycoside | 37.91 | 0.03 ± < 0.01 | 0.06 ± 0.01 |
| 17 | Apigenin glycoside | 38.27 | 0.07 ± 0.01 | 0.06 ± 0.01 |
| 18 | Apigenin glycoside | 38.96 | 0.09 ± 0.01 | 0.04 ± 0.01 |
| 19 | Apigenin glycoside | 39.90 | 0.05 ± 0.01 | 0.03 ± 0.01 |
| 20 | Apigenin glycoside | 40.73 | 0.11 ± 0.01 | 0.07 ± 0.01 |
| Σ | 1.02 a ± 0.1 | 0.62 b ± 0.09 | ||
| Total | 1.85 a ± 0.15 | 0.95 b ± 0.11 | ||
| Aqueous Extract | Hydroethanol Extract | |
|---|---|---|
| •NO scavenging | 0.93 ± 0.19 a | 1.27 ± 0.20 b |
| O2•− scavenging | 0.05 ± < 0.01 | 0.06 ± 0.01 |
| NO reduction in RAW 264.7 cells | 1.32 ± 0.17 | 1.38 ± 0.04 |
| HAase | 1.40 ± 0.07 a | 2.57 ± 0.17 b |
| AChE 2 | 1.49 ± 0.17 | 1.69 ± 0.21 |
| BChE2 | 0.68 ± 0.02 a | 0.82 ± 0.12 b |
| Compounds | Antioxidant Activity | Enzyme Inhibition | |||
|---|---|---|---|---|---|
| •NO Scavenging | O2•− Scavenging | HAase | AChE | ||
| Hydroxycinnamic acids | |||||
| 1 | 3-Caffeoylquinic acid | 0.981 ** | |||
| 2 | 4-Caffeoylquinic acid | −0.816 * | −0.944 ** | ||
| 3 | Caffeic acid derivative | −0.872 * | −0.827 * | ||
| 4 | Chlorogenic acid | −0.978 ** | |||
| 5 | Caffeic acid derivative | −0.974 ** | |||
| 6 | Caffeic acid derivative | −0.952 ** | |||
| 8 | Caffeic acid derivative | −0.998 * | |||
| 9 | Ferulic acid | 0.904 * | 0.961 ** | ||
| Σ | −0.989 ** | ||||
| Flavonoids | |||||
| 10 | Apigenin glycoside | −0.888 * | 0.965 ** | ||
| 12 | Isoorientin | −0.932 ** | |||
| 13 | Luteolin glycoside | −0.998 * | |||
| 14 | Luteolin-3′,7-di-O-glucoside | −0.864 * | |||
| 15 | Apigenin glycoside | −0.828 * | −0.898 ** | ||
| 16 | Apigenin glycoside | 0.889 * | 0.896 ** | ||
| 17 | Apigenin glycoside | −0.928 ** | |||
| 18 | Apigenin glycoside | −0.871 ** | −0.930 ** | ||
| 19 | Apigenin glycoside | −0.940 ** | −0.896 ** | ||
| 20 | Apigenin glycoside | −0.941 * | −0.868 * | ||
| Σ | −0.965 ** | ||||
| Total | −0.814 * | −0.974 ** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, G.; Gomes, E.; Barbosa, M.; Bernardo, J.; Valentão, P. Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions. Molecules 2022, 27, 7707. https://doi.org/10.3390/molecules27227707
Lopes G, Gomes E, Barbosa M, Bernardo J, Valentão P. Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions. Molecules. 2022; 27(22):7707. https://doi.org/10.3390/molecules27227707
Chicago/Turabian StyleLopes, Graciliana, Elisabete Gomes, Mariana Barbosa, João Bernardo, and Patrícia Valentão. 2022. "Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions" Molecules 27, no. 22: 7707. https://doi.org/10.3390/molecules27227707
APA StyleLopes, G., Gomes, E., Barbosa, M., Bernardo, J., & Valentão, P. (2022). Camel Grass Phenolic Compounds: Targeting Inflammation and Neurologically Related Conditions. Molecules, 27(22), 7707. https://doi.org/10.3390/molecules27227707








