Antioxidants of Amaranth, Quinoa and Buckwheat Wholemeals and Heat-Damage Development in Pseudocereal-Enriched Einkorn Water Biscuits
Abstract
1. Introduction
2. Results and Discussion
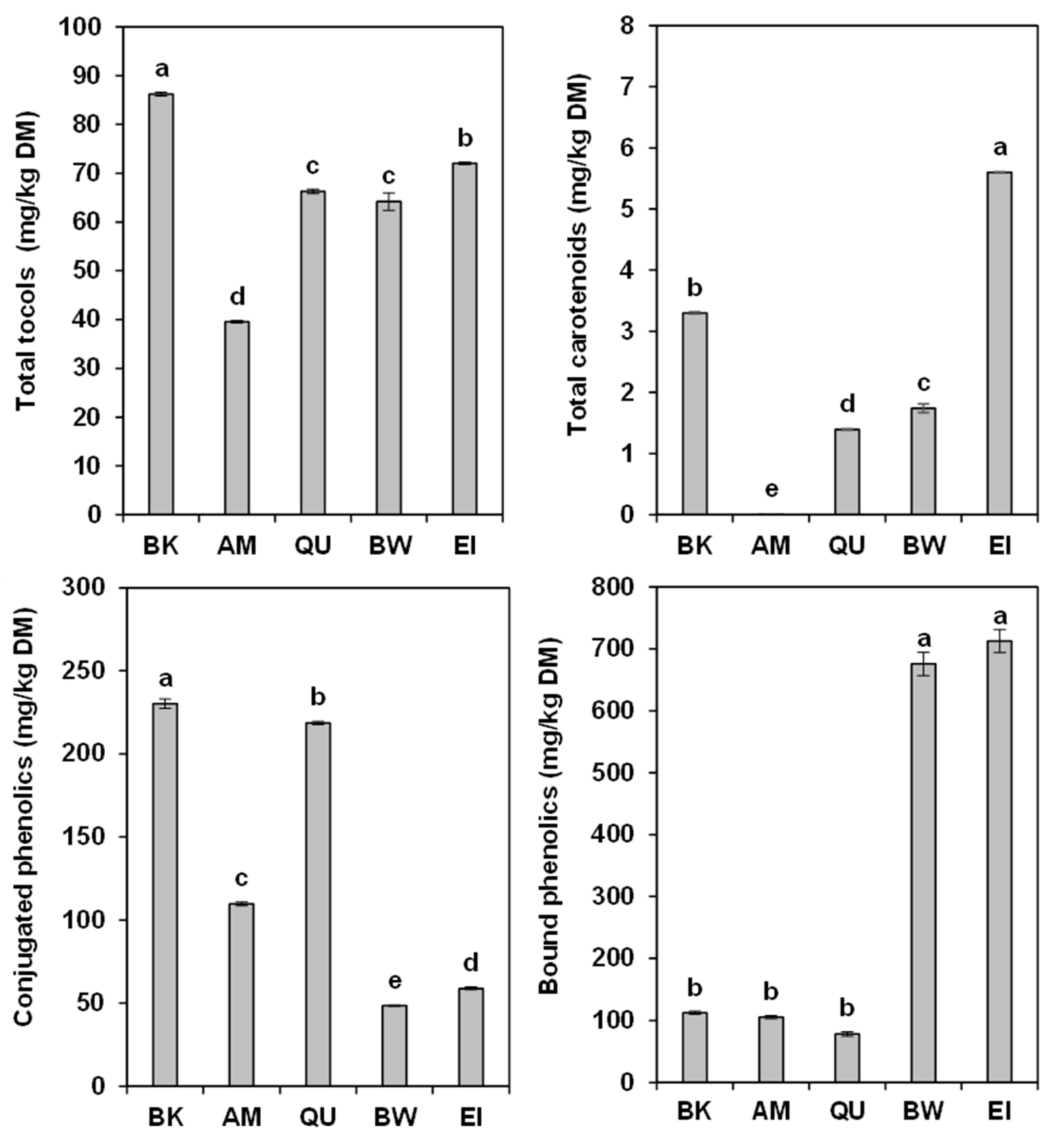
2.1. Tocols
2.2. Carotenoids
2.3. Conjugated Phenolic Acids
2.4. Bound Phenolic Acids
2.5. Antioxidant Capacity
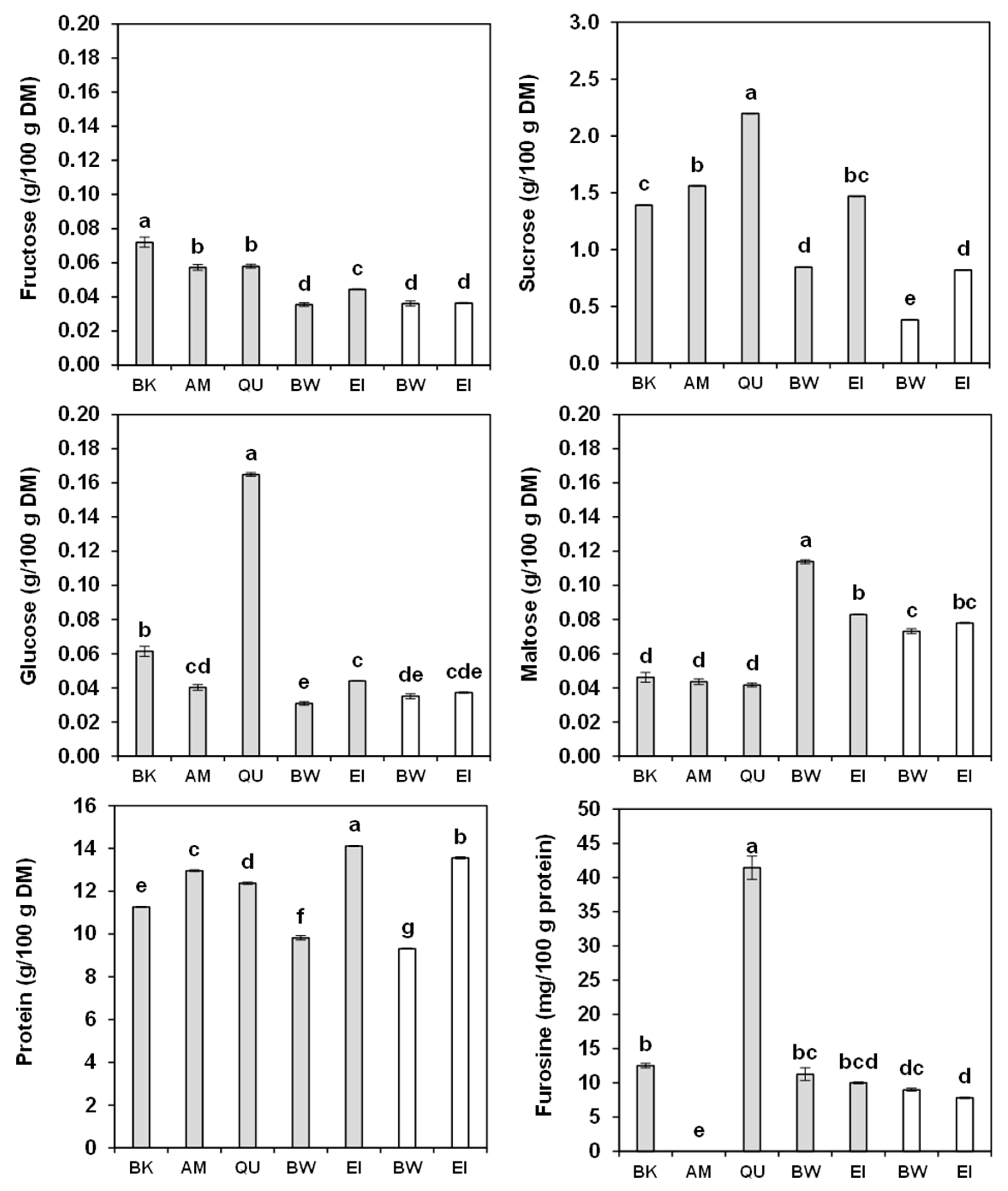
2.6. Sugars
2.7. Protein Content and Amino-Acid Composition
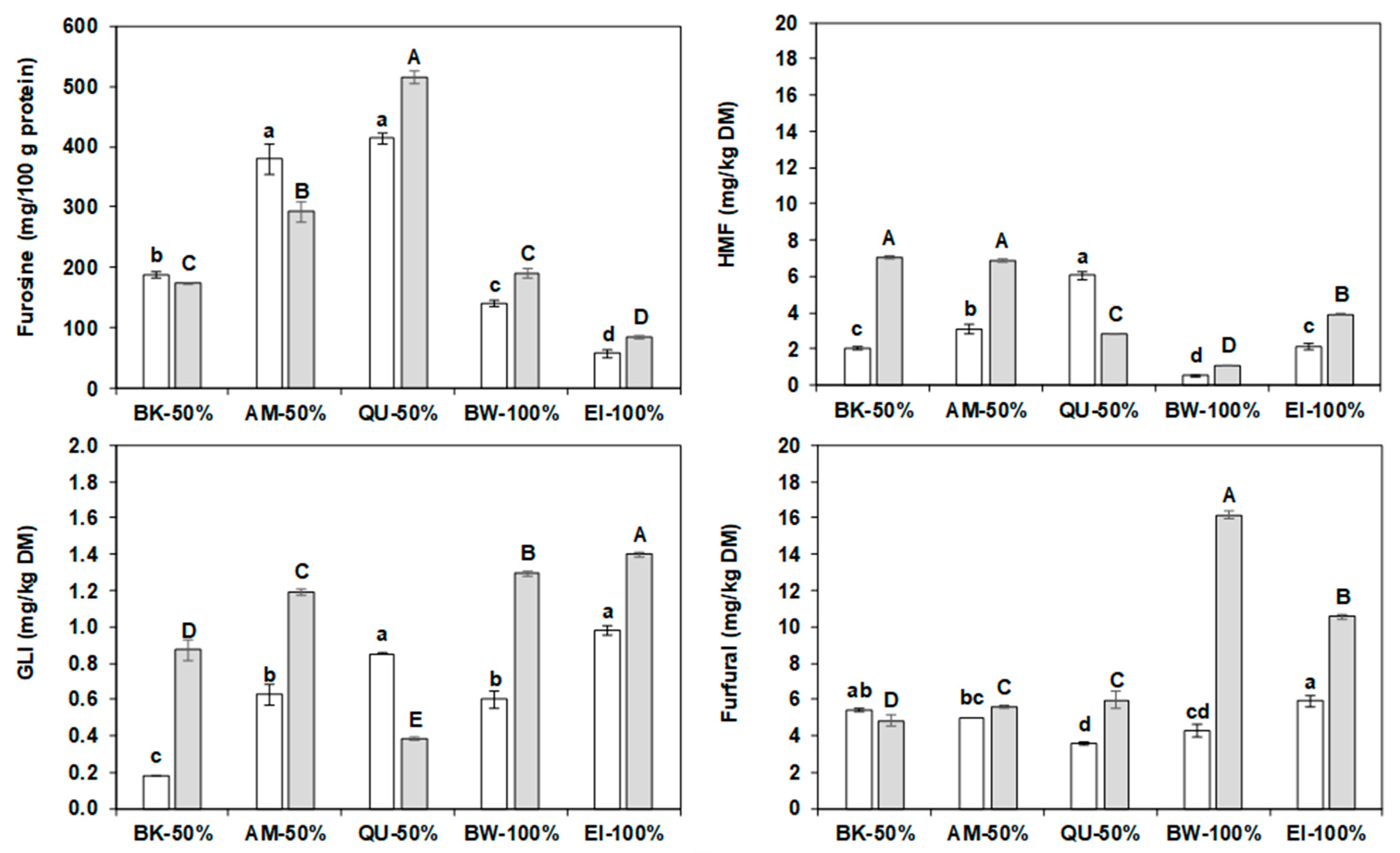
2.8. Furosine
2.9. Water Biscuits Heat Damage
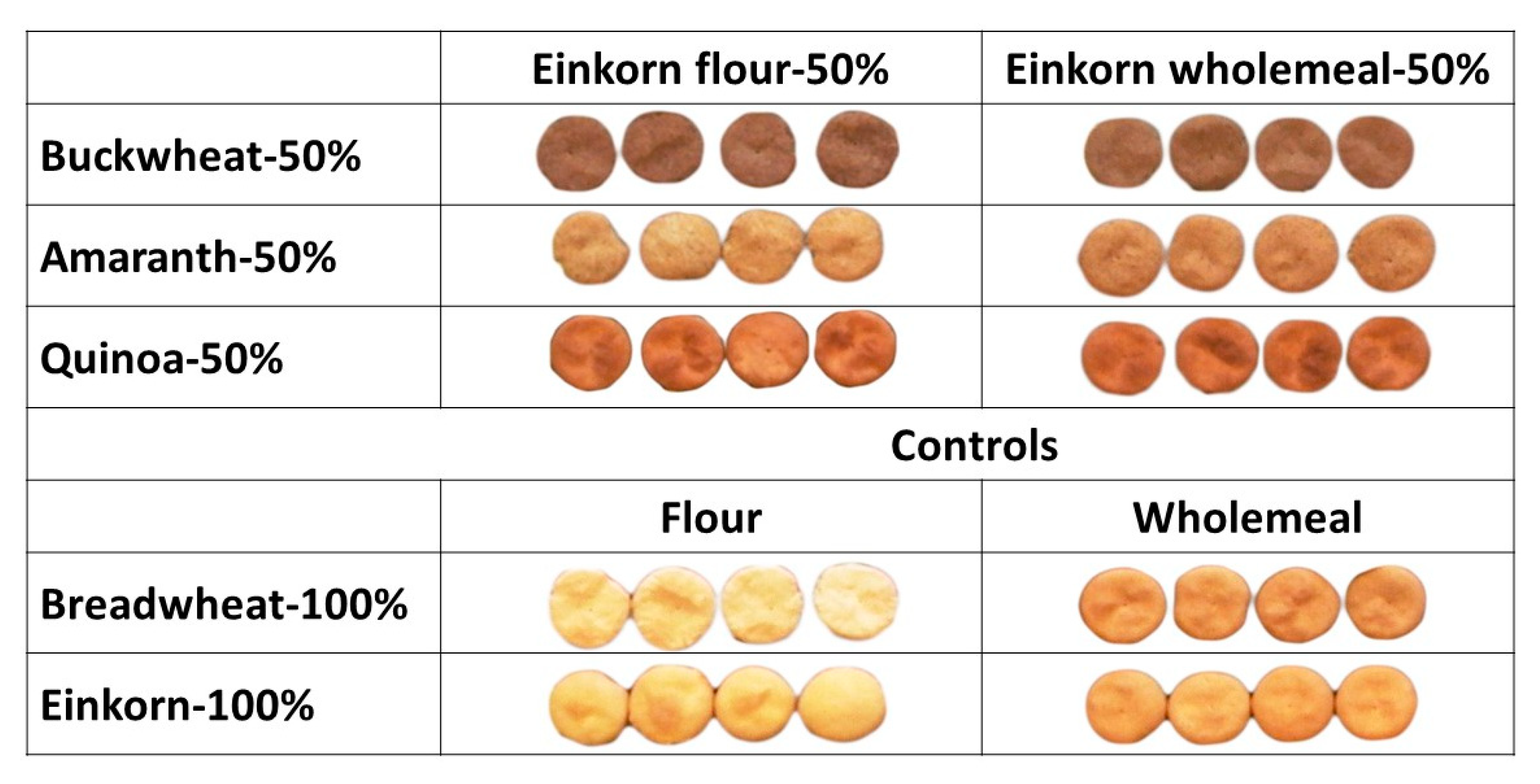
2.10. Colour of Flours and Water Biscuits
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Wholemeal and Refined Flour Preparation
3.2.2. Water Biscuits Production
3.3. Analyses
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Procopet, O.; Oroian, M. Amaranth seed polyphenol, fatty acid and amino acid profile. Appl. Sci. 2022, 12, 2181. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 255, 321–328. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Wiyngaard, H.; Arendt, E.K.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Navruz-Varli, S.; Sanlier, N. Nutritional and health benefits of quinoa (Chenopodium quinoa Willd.). J. Cereal Sci. 2016, 69, 371–376. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 2018, 75, 170–180. [Google Scholar] [CrossRef]
- Ohnishi, O. Discovery of new Fagopyrum species and its implication for the studies of evolution of Fagopyrum and of the origin of cultivated buckwheat. In Proceedings of the 6th International Symposium on Buckwheat, Shinshu, Japan, 24–29 August 1995; pp. 175–190. [Google Scholar]
- Antognoni, F.; Potente, G.; Biondi, S.; Mandrioli, R.; Marincich, L.; Ruiz, K.B. Free and conjugated phenolic profiles and antioxidant activity in quinoa seeds and their relationship with genotype and environment. Plants 2021, 10, 1046. [Google Scholar] [CrossRef]
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the composition of whole grains’ phenolic acids and dietary fibre and their effect on chronic non-communicable diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042. [Google Scholar] [CrossRef]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Cruz, A.G.; Faria, J.A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Villamiel, M. Nonenzymatic Browning for Cookies, Crackers, and Biscuits. In Bakery Products Science and Technology; Hui, Y.H., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 433–442. [Google Scholar]
- Hidalgo, A.; Pompei, C. Hydroxymethylfurfural and furosine reaction kinetics in tomato products. J. Agric. Food Chem. 2000, 48, 78–82. [Google Scholar] [CrossRef]
- Cattaneo, S.; Masotti, F.; Pellegrino, L. Effects of overprocessing on heat damage of UHT milk. Eur. Food Res. Technol. 2008, 226, 1099–1106. [Google Scholar] [CrossRef]
- Ait Ameur, L.; Rega, B.; Giampaoli, P.; Trystram, G.; Birlouez-Aragon, I. The fate of furfurals and other volatile markers during the baking process of a model cookie. Food Chem. 2008, 111, 758–763. [Google Scholar] [CrossRef]
- Cárdenas Ruiz, J.; Guerra-Hernández, E.; García-Villanova, B. Furosine is a useful indicator in pre-baked breads. J. Sci. Food Agric. 2004, 84, 366–370. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; García-Villanova, B.; Guerra-Hernández, E. Effect of toasting time on the browning of sliced bread. J. Sci. Food Agric. 2001, 81, 513–518. [Google Scholar] [CrossRef]
- Resmini, P.; Pellegrino, L.; Pagani, M.A.; De Noni, I. Formation of 2-acetyl-3-d-glucopyranosylfuran (glucosylisomaltol) from nonenzymatic browning in pasta drying. Ital. J. Food Sci. 1993, 4, 341–353. [Google Scholar]
- Fernández-Artigas, P.; Guerra-Hernández, E.; García-Villanova, B. Changes in sugar profile during infant cereal manufacture. Food Chem. 2001, 74, 499–505. [Google Scholar] [CrossRef]
- Guerra-Hernández, E.; Ramírez-Jiménez, A.; García-Villanova, B. Glucosylisomaltol, a new indicator of browning reaction in baby cereals and bread. J. Agric. Food Chem. 2002, 50, 7282–7287. [Google Scholar] [CrossRef]
- Zielinski, H.; Ciska, E.; Kozlowska, H. The cereal grains: Focus on vitamin E. Czech J. Food Sci. 2001, 19, 182–188. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Holse, M.; Hansen, A.; Arendt, E.K.; Gallagher, E. Impact of baking on vitamin E content of pseudocereals amaranth, quinoa and buckwheat. Cereal Chem. 2009, 86, 511–515. [Google Scholar] [CrossRef]
- Lehmann, J.; Putnam, D.H.; Qureshi, A.A. Vitamin E isomers in grain amaranths (Amaranthus spp.). Lipids 1994, 29, 177–181. [Google Scholar] [CrossRef]
- Skwarylo-Bednarz, B. Assessment of content of fat and tocopherols in seeds of Amaranthus in relation to diversified fertilizazion with macroelements. Ecol. Chem. Eng. 2012, 19, 273–279. [Google Scholar] [CrossRef]
- Ogrodowska, D.; Zadernowski, R.; Czaplicki, S.; Derewiaka, D.; Wronowska, B. Amaranth seeds and products—The source of bioactive compounds. Pol. J. Food Nutr. Sci. 2014, 64, 165–170. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Content of fat, vitamins and minerals in quinoa (Chenopodium quinoa, Willd) seeds. Food Chem. 1993, 48, 131–136. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C.; Piscozzi, R. Carotenoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 2006, 44, 182–193. [Google Scholar] [CrossRef]
- Lampi, A.-M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and tocotrienols in wheat genotypes in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9716–9721. [Google Scholar] [CrossRef]
- Tuan, A.T.; Thwe, A.A.; Kim, J.K.; Kim, Y.B.; Lee, S.; Park, S.U.P. Molecular characterisation and the light-dark regulation of carotenoid biosynthesis in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Chem. 2013, 141, 3803–3812. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Wood, P.J.; Rabalski, I.; Hucl, P.; Falk, D.; Frègeau-Reid, J. Einkorn: A potential candidate for developing high lutein wheat. Cereal Chem. 2002, 79, 445–457. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef]
- Konopka, I.; Czaplicki, S.; Rotkiewicz, D. Differences in content and composition of free lipids and carotenoids in flour of spring and winter wheat cultivated in Poland. Food Chem. 2006, 95, 290–300. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Improved normal-phase high-performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef]
- Zandomeneghi, M.; Festa, C.; Carbonaro, L.; Galleschi, L.; Lenzi, A.; Calucci, L. Front-surface absorbance spectra of wheat flour: Determination of carotenoids. J. Agric. Food Chem. 2000, 48, 2216–2221. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Kinetics of carotenoids degradation during the storage of einkorn (Triticum monococcum L. ssp. monococcum) and bread wheat (Triticum aestivum L. ssp. aestivum) flours. J. Agric. Food Chem. 2008, 56, 11300–11305. [Google Scholar] [CrossRef]
- Guo, X.D.; Wu, C.S.; Ma, Y.J.; Parry, J.; Xu, Y.Y.; Liu, H.; Wang, M. Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int. 2012, 49, 53–59. [Google Scholar] [CrossRef]
- Repo-Carrasco, R.; Hellström, J.K.; Pihlava, J.M.; Mattila, P.H. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa, kañiwa and kiwicha. Food Chem. 2010, 120, 128–133. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, B.; Chen, P.X.; Liu, R.; Tsao, R. Characterisation of phenolics, betanins and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 166, 380–388. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acid in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic acids composition, total polyphenols content and antioxidant activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A two-years evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics content and antioxidant activity of tartary buckwheat from different locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, S.; Yao, L.; Wang, L.; Li, C. Free and bound phenolics of buckwheat varieties: HPLC characterization, antioxidant activity, and inhibitory potency towards α-glucosidase with molecular docking analysis. Antioxidants 2019, 8, 606. [Google Scholar] [CrossRef]
- Okarter, N. Phenolic compounds from the insoluble-bound fraction of whole grains do not have any cellular antioxidant activity. Life Sci. Med. Res. 2012, 37, 1–10. [Google Scholar]
- Gòmez Cravaca, A.; Segura Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Simultaneous determination of phenolics compouds and saponins in quinoa (Chenopodium quinoa Willd.) by a liquid chromatography-diode array detection-electrospray ionization-time of flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713. [Google Scholar] [CrossRef]
- Li, L.; Lietz, G.; Seal, C.J. Phenolic, apparent antioxidant and nutritional composition of quinoa (Chenopodium quinoa Willd.) seeds. Int. J. Food Sci. Technol. 2021, 56, 3245–3254. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and bioavailability of phenolic compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Lee, L.S.; Choi, E.J.; Kim, C.H.; Sung, J.M.; Kim, Y.B.; Seo, D.H.; Choi, H.W.; Choi, Y.S.; Kum, J.S.; Park, J.D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2015, 68, 181–186. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Číž, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Salas, I.A.; Goshev, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Gross, R.; Koch, F.; Malaga, I.; De Miranda, A.F.; Schoeneberger, H.; Trugo, L.C. Chemical composition and protein quality of some local Andean food sources. Food Chem. 1988, 34, 25–34. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Evaluation of heat damage, sugars, amylases and colour in breads from einkorn, durum and bread wheat flours. J. Cereal Sci. 2011, 54, 90–97. [Google Scholar] [CrossRef]
- Guardianelli, L.M.; Salinas, M.V.; Puppo, M.C. Chemical and thermal properties of flours from germinated amaranth seeds. J. Food Meas. Charact. 2019, 13, 1078–1088. [Google Scholar] [CrossRef]
- Rakszegi, M.; Boros, D.; Kuti, C.; Láng, L.; Bedo, Z.; Shewry, P.R. Composition and end-use quality of 150 wheat lines selected for the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9750–9757. [Google Scholar] [CrossRef] [PubMed]
- Volante, A.; Yilmaz, V.A.; Hidalgo, A.; Brandolini, A. Morpho-physiological and qualitative variation of domesticated einkorn (Triticum monococcum L. ssp. monococcum). Genet. Resour. Crop Evol. 2020, 67, 1493–1502. [Google Scholar] [CrossRef]
- Mota, C.; Santos, M.; Mauro, R.; Samman, N.; Matos, A.S.; Torres, D.; Castanheira, I. Protein content and amino acids profile of pseudocereals. Food Chem. 2016, 193, 55–61. [Google Scholar] [CrossRef]
- De Bock, P.; Daelemans, L.; Selis, L.; Raes, K.; Vermeir, P.; Eeckhout, M.; Van Bockstaele, F. Comparison of the chemical and technological characteristics of whole meal flours obtained from amaranth (Amaranthus sp.), quinoa (Chenopodium quinoa) and buckwheat (Fagopyrum sp.) seeds. Foods 2021, 10, 651. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Bailoni, L.; Zancato, M.; Costa, C.V.; Allegri, G. The content of proteic and nonproteic (free and protein-bound) tryptophan in quinoa and cereal flours. Food Chem. 2007, 100, 1350–1355. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Robbins, G.S. Amino acid composition of Buckwheat. J. Agric. Food Chem. 1972, 20, 270–274. [Google Scholar] [CrossRef]
- Aguilar, E.B.; de Jesús Albarracín, G.; Uñates, M.A.; Piola, H.D.; Camiña, J.M.; Escudero, N.L. Evaluation of the nutritional quality of the grain protein of new amaranths varieties. Plant Foods Hum. Nutr. 2015, 70, 21–26. [Google Scholar] [CrossRef]
- Juan, P.R.; Pastor, J.; Alaiz, M.; Megías, C.; Vioque, J. Caracterización proteica de las semillas de once especies de amaranto. Grasas Aceites 2007, 58, 49–55. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.E. Nutritional value and use of the Andean crops quinoa (Chenopodium quinoa) and kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Novak, V.; Du, J.; Charrondiere, R. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Wild). Food Chem. 2015, 193, 47–54. [Google Scholar] [CrossRef]
- Wright, K.; Pike, O.; Fairbanks, D.J.; Huber, S. Composition of Atriplex hortensis, sweet and bitter Chenopodium quinoa seeds. Food Chem. Toxicol. 2002, 67, 1383–1385. [Google Scholar] [CrossRef]
- Acquistucci, R.; D’Egidio, M.G.; Vallega, V. Amino acid composition of selected strains of diploid wheat, Triticum monococcum L. Cereal Chem. 1995, 72, 213–216. [Google Scholar]
- Galterio, G.; Borghi, B.; Bucarelli, F.; Cannella, C.; Salamini, F. Breadmaking quality, protein subunits and amino-acid composition of einkorn (Triticum monococcum) compared with other wheat species. In Cereals for Human Health and Preventive Nutrition, Brno (Czech Republic), 7–11 July 1998; Mendelova Zemedelska a Lesnicka Univerzita: Brno, Czech Republic, 1998. [Google Scholar]
- Hidalgo, A.; Brandolini, A. Heat damage of water biscuits from einkorn, durum and bread wheat flours. Food Chem. 2011, 128, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, A.; Ferraretto, A.; De Noni, I.; Bottani, M.; Cattaneo, S.; Galli, S.; Brandolini, A. Bioactive compounds and antioxidant properties of pseudocreals-enriched water biscuits and their in vitro digestates. Food Chem. 2018, 240, 799–807. [Google Scholar] [CrossRef]
- Hellwig, M. The chemistry of protein oxidation in food. Angew. Chem. Int. Ed. 2019, 58, 16742–16763. [Google Scholar] [CrossRef]
- Schouten, M.A.; Tappi, S.; Glicerina, V.; Rocculi, P.; Angeloni, S.; Cortese, M.; Caprioli, G.; Vittori, S.; Romani, S. Formation of acrylamide in biscuits during baking under different heat transfer conditions. LWT 2022, 153, 12541. [Google Scholar] [CrossRef]
- AACC (American Association of Cereal Chemists). Official Methods 44-15A, 46-10. In Approved Methods of the American Association of Cereal Chemists; AACC: Minneapolis, MN, USA, 1995. [Google Scholar]
- Brandolini, A.; Glorio-Paulet, P.; Estivi, L.; Locatelli, N.; Cordova-Ramos, J.S.; Hidalgo, A. Tocopherols, carotenoids and phenolics changes during Andean lupin (Lupinus mutabilis Sweet) seeds processing. J. Food Compos. Anal. 2022, 106, 104335. [Google Scholar] [CrossRef]
- Yilmaz, V.A.; Brandolin, A.; Hidalgo, A. Phenolic acids and antioxidant activity of wild, feral and domesticated wheats. J. Cereal Sci. 2015, 64, 168–175. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hogenboom, J.A.; D’Incecco, P.; Fuselli, F.; Pellegrino, L. Ion-exchange chromatographic method for the determination of the free amino acid composition of cheese and other dairy products: An inter-laboratory validation study. Food Anal. Methods 2017, 10, 3137–3148. [Google Scholar] [CrossRef]
- Bal, L.M.; Kar, A.; Satya, S.; Naik, S.N. Kinetics of colour change of bamboo shoot slices during microwave drying. Int. J. Food Sci. Technol. 2011, 46, 827–833. [Google Scholar] [CrossRef]


| Buck Wheat | Amaranth | Quinoa | Bread Wheat | Einkorn Wheat | |
|---|---|---|---|---|---|
| Tocols | |||||
| α-tocopherol | 3.50 d ± 0.10 | 10.41 c ± 0.04 | 30.18 a ± 0.44 | 12.66 b ± 0.04 | 13.13 b ± 0.15 |
| β-tocopherol | nd d | 29.16 a ± 0.28 | nd d | 9.15 b ± 0.41 | 5.70 c ± 0.05 |
| γ-tocopherol | 77.63 a ± 0.24 | nd c | 36.11 b ± 0.01 | nd c | nd c |
| δ-tocopherol | 4.89 a ± 0.05 | nd b | nd b | nd b | nd b |
| α-tocotrienol | nd c | nd c | nd c | 4.15 b ± 0.08 | 12.38 a ± 0.04 |
| β-tocotrienol | nd c | nd c | nd c | 38.22 b ± 1.27 | 40.81 a ± 0.02 |
| Carotenoids | |||||
| (α+β)-carotene | nd c | nd c | 0.15 b ± 0.00 | 0.16 b ± 0.01 | 0.44 a ± 0.01 |
| Β-cryptoxanthin | nd b | nd b | nd b | nd b | 0.04 a ± 0.00 |
| Lutein | 3.17 b ± 0.01 | nd e | 1.18 d ± 0.00 | 1.43 c ± 0.05 | 4.80 a ± 0.01 |
| Zeaxanthin | 0.13 b ± 0.01 | nd d | 0.06 c ± 0.01 | 0.15 b ± 0.01 | 0.33 a ± 0.01 |
| Conjugated phenolics | |||||
| p-hydroxybenzoic acid | 53.72 a ± 1.18 | 43.30 b ± 0.09 | 32.90 c ± 0.08 | 2.58 d ± 0.02 | 2.51 d ± 0.17 |
| Vanillic acid | nd d | 6.52 c ± 0.18 | 83.42 a ± 1.14 | 7.79 bc ± 0.03 | 8.45 b ± 0.14 |
| Syringic acid | 0.12 c ± 0.00 | 0.12 c ± 0.00 | 3.03 b ± 0.01 | 6.62 a ± 0.26 | 6.26 a ± 0.33 |
| Syringaldehyde | 165.40 a ± 2.13 | nd c | nd c | 1.09 b ± 0.03 | 2.26 b ± 0.11 |
| p-coumaric acid | 10.95 b ± 0.49 | 5.41 c ± 0.10 | 39.47 a ± 0.11 | 1.28 e ± 0.03 | 2.98 d ± 0.08 |
| Ferulic acid | nd e | 54.30 a ± 0.69 | 59.76 a ± 0.26 | 29.13 d ± 0.60 | 36.49 c ± 0.97 |
| Bound phenolics | |||||
| p-hydroxybenzoic acid | 24.46 a ± 0.18 | 6.56 b ± 0.25 | 5.36 c ± 0.36 | 2.44 d ± 0.04 | 3.33 d ± 0.48 |
| Vanillic acid | 7.80 a ± 0.07 | 1.19 e ± 0.05 | 3.60 d ± 0.02 | 6.80 b ± 0.20 | 4.50 c ± 0.07 |
| Syringic acid | 8.61 a ± 0.15 | 3.99 c ± 0.37 | 2.86 d ± 0.05 | 8.66 a ± 0.15 | 5.87 b ± 0.30 |
| Syringaldehyde | 43.04 a ± 0.68 | nd b | nd b | nd b | nd b |
| p-coumaric acid | 17.10 b ± 0.99 | 9.29 c ± 0.22 | 3.82 d ± 0.20 | 16.57 b ± 0.32 | 33.30 a ± 1.01 |
| Ferulic acid | 11.62 c ± 0.65 | 84.60 b ± 2.90 | 62.88 b ± 2.93 | 641.25 a ± 18.11 | 665.51 a ± 18.25 |
| Asp | Thr | Ser | Glu | Gly | Ala | Val | Cys | Met | Ile | Leu | Tyr | Phe | Lys | His | Arg | Pro | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wholemeal | ||||||||||||||||||
| BK | 92.9 ± 1.5 | 37.5 ± 0.4 | 45.4 ± 0.2 | 170.9 ± 0.3 | 58.9 ± 0.2 | 41.6 ± 0.3 | 51.2 ± 0.0 | 14.9 ± 0.3 | 2.4 ± 0.3 | 38.2 ± 0.1 | 64.9 ± 0.2 | 13.2 ± 0.3 | 47.1 ± 0.6 | 56.9 ± 0.1 | 23.3 ± 0.2 | 98.6 ± 0.3 | 37.2 ± 0.1 | 895.9 ± 2.6 |
| AM | 84.7 ± 0.0 | 36.6 ± 0.2 | 56.2 ± 0.2 | 169.5 ± 1.5 | 78.9 ± 0.1 | 36.5 ± 0.2 | 42.7 ± 0.5 | 15.8 ± 0.2 | 10.0 ± 0.3 | 39.3 ± 0.1 | 56.6 ± 0.0 | 17.1 ± 0.4 | 43.3 ± 0.4 | 58.0 ± 0.6 | 25.1 ± 0.5 | 97.1 ± 0.1 | 38.1 ± 0.7 | 905.9 ± 0.1 |
| QU | 83.1 ± 0.4 | 34.5 ± 0.4 | 39.8 ± 0.1 | 142.1 ± 0.9 | 54.6 ± 0.2 | 41.2 ± 0.4 | 47.2 ± 0.1 | 6.9 ± 0.8 | 5.0 ± 0.2 | 40.5 ± 0.2 | 63.8 ± 0.1 | 14.3 ± 0.4 | 42.1 ± 0.1 | 54.7 ± 0.1 | 26.9 ± 0.2 | 92.4 ± 0.7 | 36.3 ± 0.3 | 825.8 ± 3.7 |
| BW | 52.3 ± 0.0 | 28.1 ± 0.3 | 42.6 ± 0.2 | 288.3 ± 0.1 | 41.0 ± 0.1 | 33.6 ± 0.1 | 43.0 ± 0.6 | 12.7 ± 0.2 | 4.9 ± 0.1 | 34.4 ± 0.2 | 65.2 ± 0.2 | 11.6 ± 0.7 | 45.9 ± 0.6 | 27.3 ± 0.2 | 21.1 ± 0.6 | 48.7 ± 0.0 | 96.1 ± 0.3 | 897.1 ± 2.7 |
| EI | 47.1 ± 0.3 | 24.7 ± 0.0 | 41.5 ± 0.3 | 277.9 ± 0.9 | 31.6 ± 0.2 | 29.0 ± 0.0 | 40.7 ± 0.8 | 14.2 ± 0.1 | 8.3 ± 0.2 | 37.5 ± 0.4 | 61.3 ± 0.0 | 11.3 ± 0.5 | 45.0 ± 0.4 | 23.8 ± 0.2 | 20.2 ± 0.0 | 43.6 ± 0.0 | 86.0 ± 0.0 | 843.9 ± 2.3 |
| Flour | ||||||||||||||||||
| BW | 46.5 ± 0.5 | 27.0 ± 0.7 | 45.8 ± 0.7 | 347.2 ± 3.1 | 39.0 ± 0.3 | 31.3 ± 0.3 | 44.2 ± 0.2 | 10.4 ± 0.5 | 9.4 ± 0.8 | 37.9 ± 0.8 | 70.2 ± 1.1 | 10.6 ± 0.2 | 50.1 ± 1.1 | 23.5 ± 0.2 | 21.3 ± 0.3 | 40.8 ± 2.1 | 117.4 ± 1.8 | 972.9 ± 9.7 |
| EI | 42.4 ± 1.3 | 25.7 ± 0.1 | 47.8 ± 0.4 | 353.5 ± 0.5 | 30.1 ± 0.1 | 27.3 ± 0.2 | 43.8 ± 0.0 | 19.1 ± 0.5 | 11.6 ± 0.3 | 44.4 ± 0.0 | 70.1 ± 0.4 | 11.7 ± 0.3 | 54.0 ± 0.2 | 21.3 ± 0.6 | 21.3 ± 0.2 | 40.3 ± 0.1 | 108.9 ± 0.2 | 973.3 ± 1.0 |
| Water biscuits (before baking) | ||||||||||||||||||
| BK 50% | 70.8 ± 0.5 | 33.0 ± 0.3 | 49.7 ± 0.4 | 288.8 ± 2.3 | 47.0 ± 0.4 | 36.0 ± 0.4 | 50.4 ± 0.4 | 18.1 ± 0.3 | 8.2 ± 0.1 | 44.5 ± 0.7 | 72.7 ± 1.1 | 13.4 ± 0.6 | 54.3 ± 0.5 | 40.5 ± 0.3 | 23.7 ± 0.3 | 71.5 ± 1.9 | 81.6 ± 1.2 | 1007 ± 6.1 |
| AM 50% | 62.7 ± 0.5 | 30.6 ± 0.2 | 51.3 ± 0.5 | 266.1 ± 2.1 | 52.8 ± 0.5 | 31.4 ± 0.3 | 43.1 ± 0.3 | 17.2 ± 0.2 | 10.9 ± 0.2 | 41.8 ± 0.6 | 63.7 ± 1.0 | 14.2 ± 0.6 | 48.7 ± 0.5 | 38.6 ± 0.3 | 22.9 ± 0.3 | 66.7 ± 1.7 | 75.6 ± 1.1 | 938.2 ± 5.6 |
| QU 50% | 67.1 ± 0.5 | 31.9 ± 0.3 | 46.5 ± 0.4 | 265.7 ± 2.1 | 44.8 ± 0.4 | 36.2 ± 0.4 | 48.4 ± 0.4 | 13.7 ± 0.2 | 8.4 ± 0.1 | 45.2 ± 0.7 | 71.6 ± 1.1 | 13.9 ± 0.6 | 51.1 ± 0.5 | 40.5 ± 0.3 | 25.5 ± 0.3 | 70.3 ± 1.8 | 78.1 ± 1.1 | 958.9 ± 5.8 |
| BW | 46.5 ± 0.5 | 27.0 ± 0.7 | 45.8 ± 0.7 | 347.2 ± 3.1 | 39.4 ± 0.3 | 31.3 ± 0.3 | 44.2 ± 0.2 | 10.4 ± 0.5 | 9.4 ± 0.8 | 37.9 ± 0.8 | 70.2 ± 1.1 | 10.6 ± 0.2 | 50.1 ± 1.1 | 23.5 ± 0.2 | 21.3 ± 0.3 | 40.8 ± 2.1 | 117.4 ± 1.8 | 972.9 ± 9.7 |
| EI | 42.4 ± 1.3 | 25.7 ± 0.1 | 47.8 ± 0.4 | 353.5 ± 0.5 | 30.1 ± 0.1 | 27.3 ± 0.2 | 43.8 ± 0.0 | 19.1 ± 0.5 | 11.6 ± 0.3 | 44.4 ± 0.0 | 70.1 ± 0.4 | 11.7 ± 0.3 | 54.0 ± 0.2 | 21.3 ± 0.6 | 21.3 ± 0.2 | 40.3 ± 0.1 | 108.9 ± 0.2 | 973.3 ± 1.0 |
| Water biscuit (after baking) | ||||||||||||||||||
| BK 50% | 66.2 ± 0.2 | 31.7 ± 0.0 | 48.8 ± 0.4 | 291.4 ± 0.1 | 43.8 ± 0.0 | 34.6 ± 0.2 | 49.0 ± 0.5 | 13.0 ± 0.0 | 4.8 ± 0.0 | 42.9 ± 0.0 | 70.0 ± 0.2 | 10.3 ± 0.1 | 51.1 ± 0.0 | 30.8 ± 0.1 | 22.4 ± 0.4 | 58.9 ± 0.1 | 83.6 ± 0.4 | 953.9 ± 0.2 |
| AM 50% | 62.2 ± 0.1 | 30.8 ± 0.1 | 51.8 ± 0.2 | 270.8 ± 0.4 | 52.8 ± 0.2 | 31.8 ± 0.1 | 44.0 ± 0.6 | 14.7 ± 0.1 | 9.9 ± 0.2 | 42.2 ± 0.3 | 64.0 ± 0.1 | 10.5 ± 0.9 | 48.1 ± 0.8 | 28.5 ± 0.2 | 23.4 ± 0.2 | 59.4 ± 0.1 | 77.6 ± 0.3 | 923.0 ± 0.2 |
| QU 50% | 63.1 ± 0.4 | 30.3 ± 0.0 | 43.9 ± 0.5 | 259.2 ± 0.0 | 44.1 ± 0.0 | 36.2 ± 0.1 | 47.5 ± 0.6 | 12.7 ± 0.2 | 7.4 ± 0.0 | 44.1 ± 0.2 | 68.2 ± 0.1 | 10.9 ± 0.2 | 47.1 ± 0.4 | 24.7 ± 0.1 | 23.9 ± 0.2 | 61.0 ± 0.1 | 80.7 ± 0.1 | 905.7 ± 2.3 |
| BW | 41.0 ± 0.2 | 24.5 ± 0.3 | 41.9 ± 0.4 | 319.3 ± 0.5 | 35.5 ± 0.1 | 28.8 ± 0.1 | 40.9 ± 0.2 | 14.7 ± 0.2 | 9.5 ± 0.2 | 35.4 ± 0.2 | 64.2 ± 0.2 | 10.9 ± 0.3 | 46.2 ± 0.2 | 19.6 ± 0.1 | 19.9 ± 0.2 | 39.2 ± 0.5 | 109.8 ± 0.9 | 901.4 ± 0.7 |
| EI | 39.1 ± 0.1 | 22.8 ± 0.2 | 42.4 ± 0.3 | 316.3 ± 0.1 | 27.6 ± 0.1 | 25.2 ± 0.1 | 40.4 ± 0.3 | 15.8 ± 0.0 | 10.4 ± 0.1 | 40.2 ± 0.1 | 62.8 ± 0.2 | 9.6 ± 0.2 | 48.2 ± 0.1 | 17.4 ± 0.0 | 18.9 ± 0.2 | 35.3 ± 0.1 | 98.0 ± 0.3 | 870.8 ± 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estivi, L.; Pellegrino, L.; Hogenboom, J.A.; Brandolini, A.; Hidalgo, A. Antioxidants of Amaranth, Quinoa and Buckwheat Wholemeals and Heat-Damage Development in Pseudocereal-Enriched Einkorn Water Biscuits. Molecules 2022, 27, 7541. https://doi.org/10.3390/molecules27217541
Estivi L, Pellegrino L, Hogenboom JA, Brandolini A, Hidalgo A. Antioxidants of Amaranth, Quinoa and Buckwheat Wholemeals and Heat-Damage Development in Pseudocereal-Enriched Einkorn Water Biscuits. Molecules. 2022; 27(21):7541. https://doi.org/10.3390/molecules27217541
Chicago/Turabian StyleEstivi, Lorenzo, Luisa Pellegrino, Johannes A. Hogenboom, Andrea Brandolini, and Alyssa Hidalgo. 2022. "Antioxidants of Amaranth, Quinoa and Buckwheat Wholemeals and Heat-Damage Development in Pseudocereal-Enriched Einkorn Water Biscuits" Molecules 27, no. 21: 7541. https://doi.org/10.3390/molecules27217541
APA StyleEstivi, L., Pellegrino, L., Hogenboom, J. A., Brandolini, A., & Hidalgo, A. (2022). Antioxidants of Amaranth, Quinoa and Buckwheat Wholemeals and Heat-Damage Development in Pseudocereal-Enriched Einkorn Water Biscuits. Molecules, 27(21), 7541. https://doi.org/10.3390/molecules27217541






