Synthesis, Structure and Molecular Docking of New 4,5-Dihydrothiazole Derivatives Based on 3,5-Dimethylpyrazole and Cytisine and Salsoline Alkaloids
Abstract
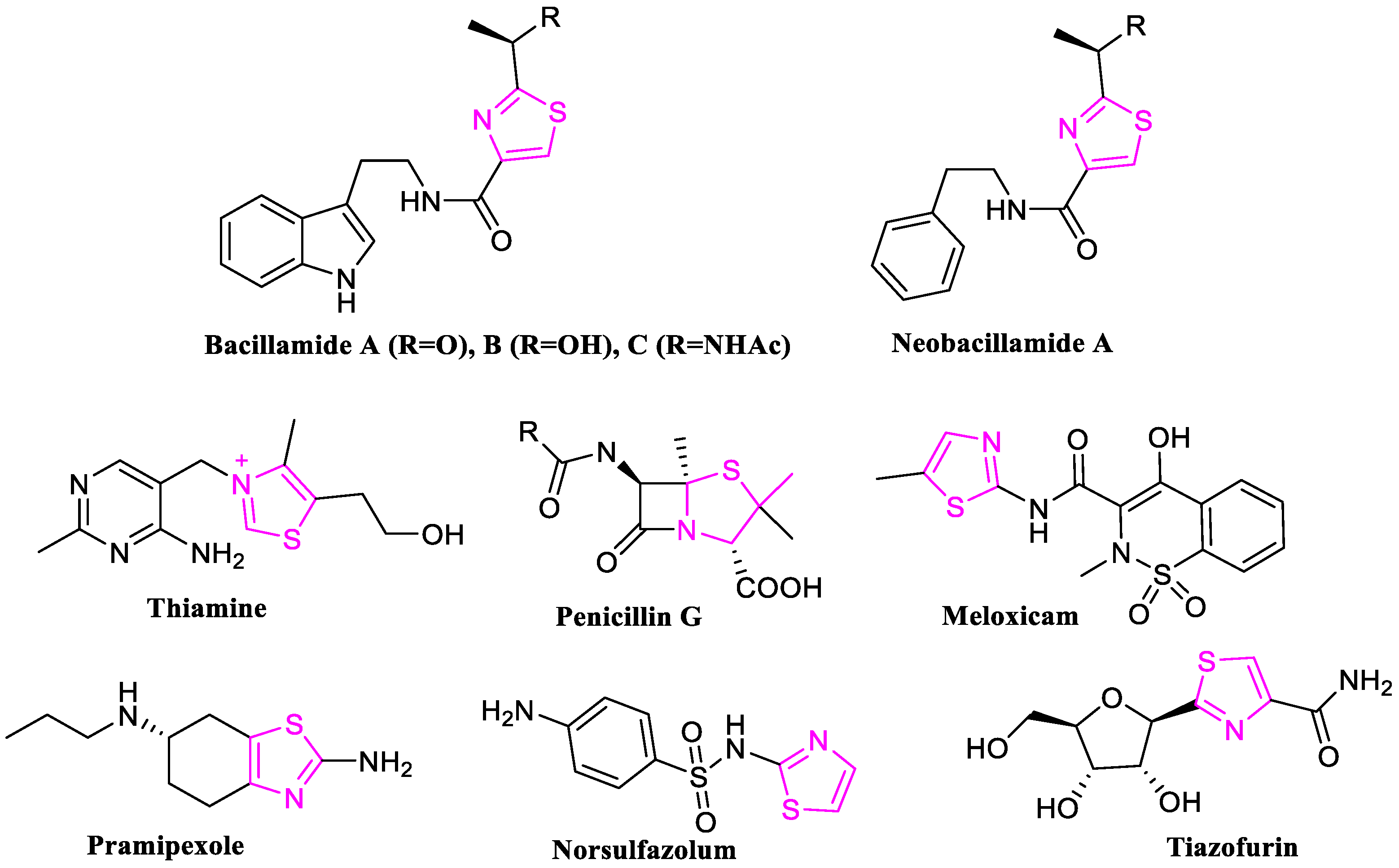
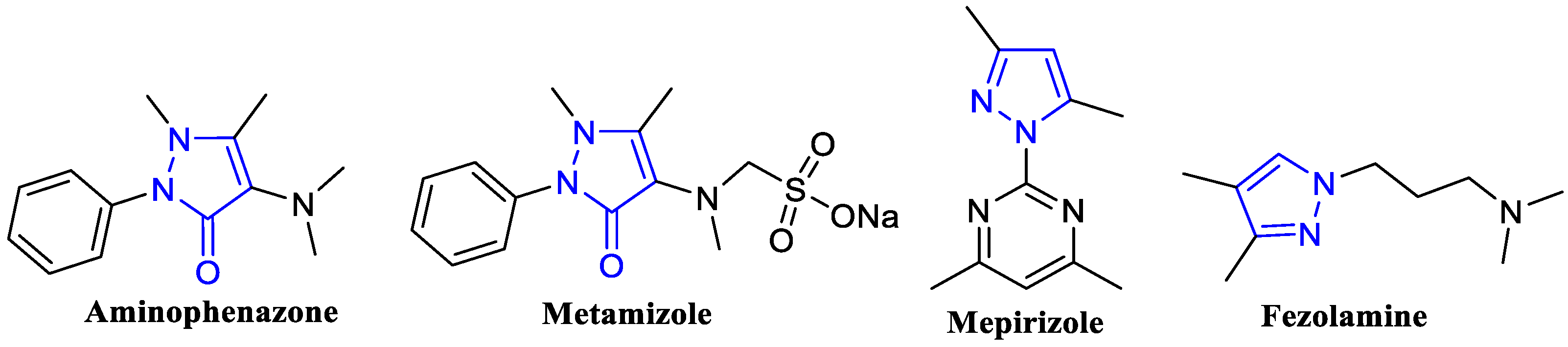
1. Introduction
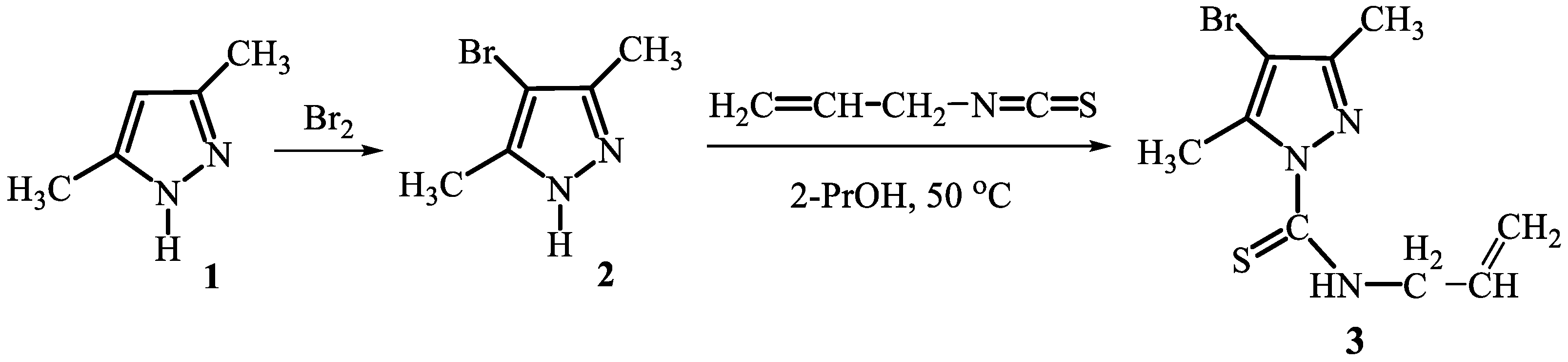
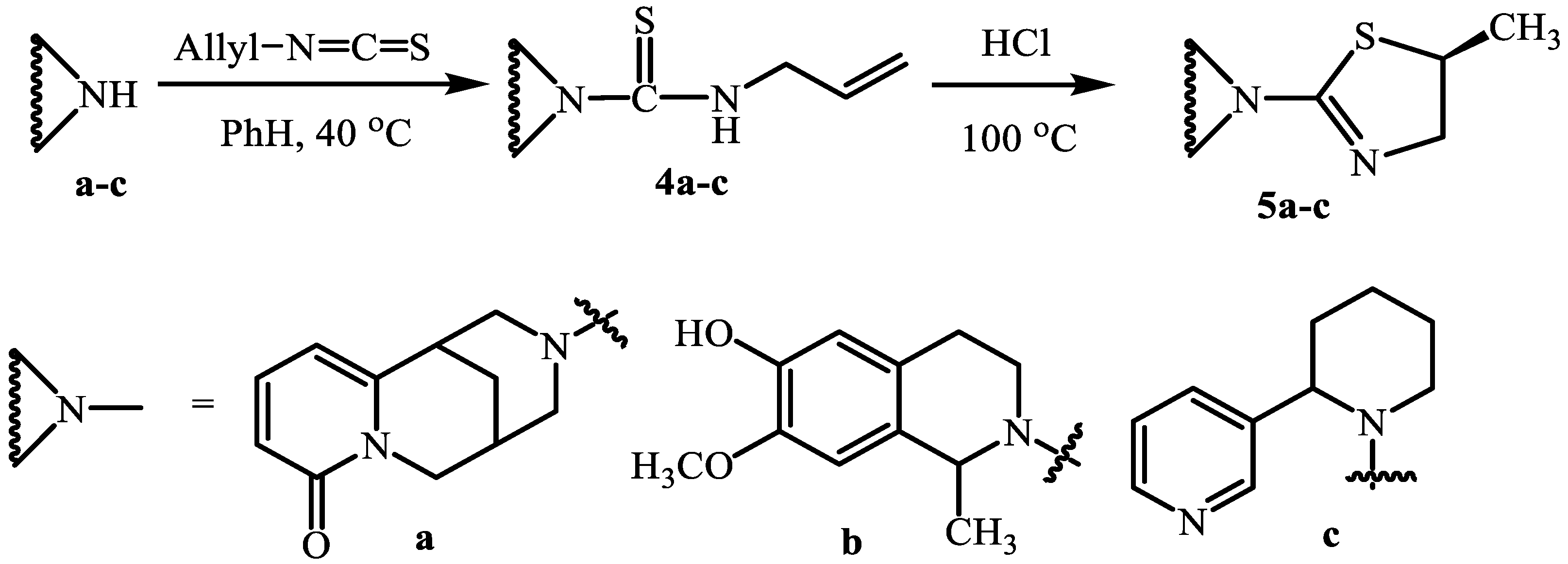
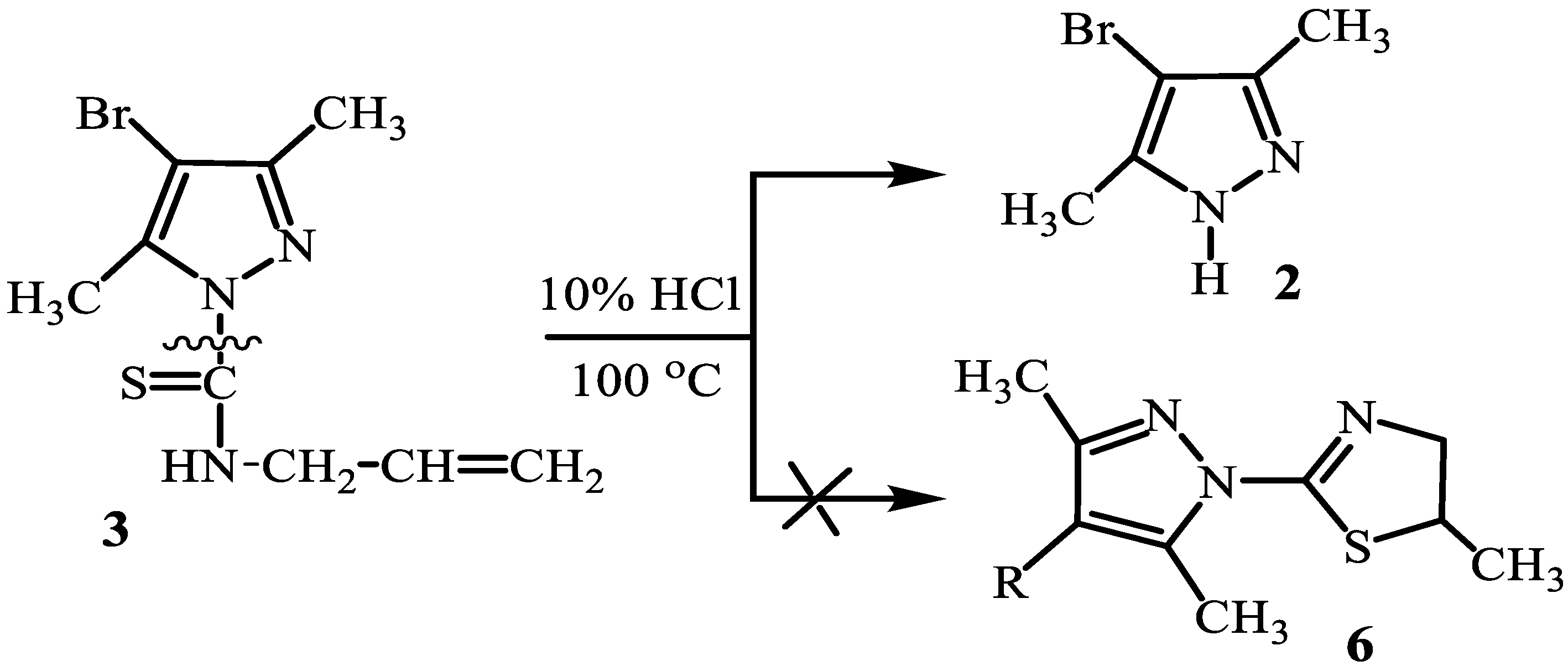
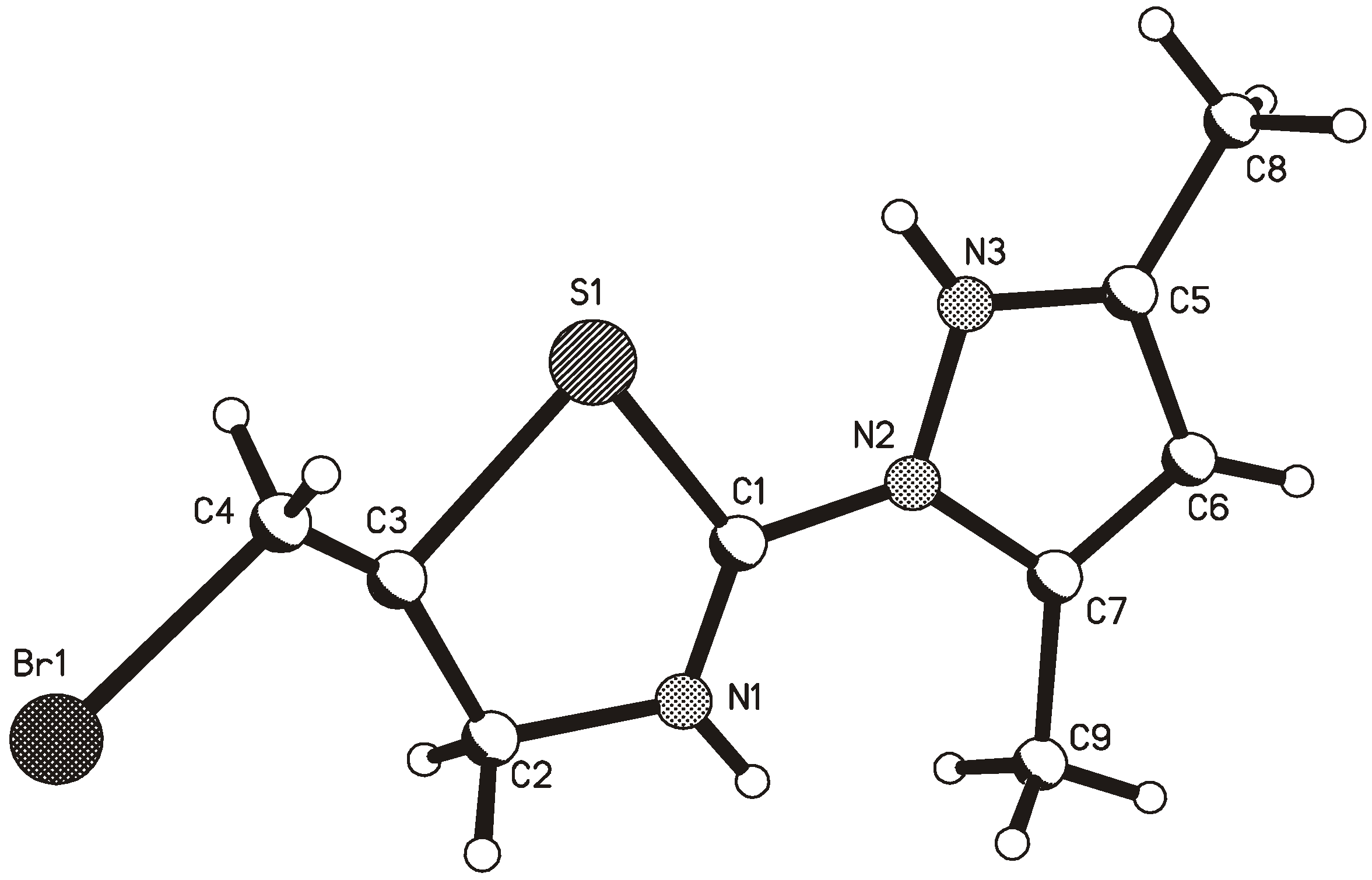
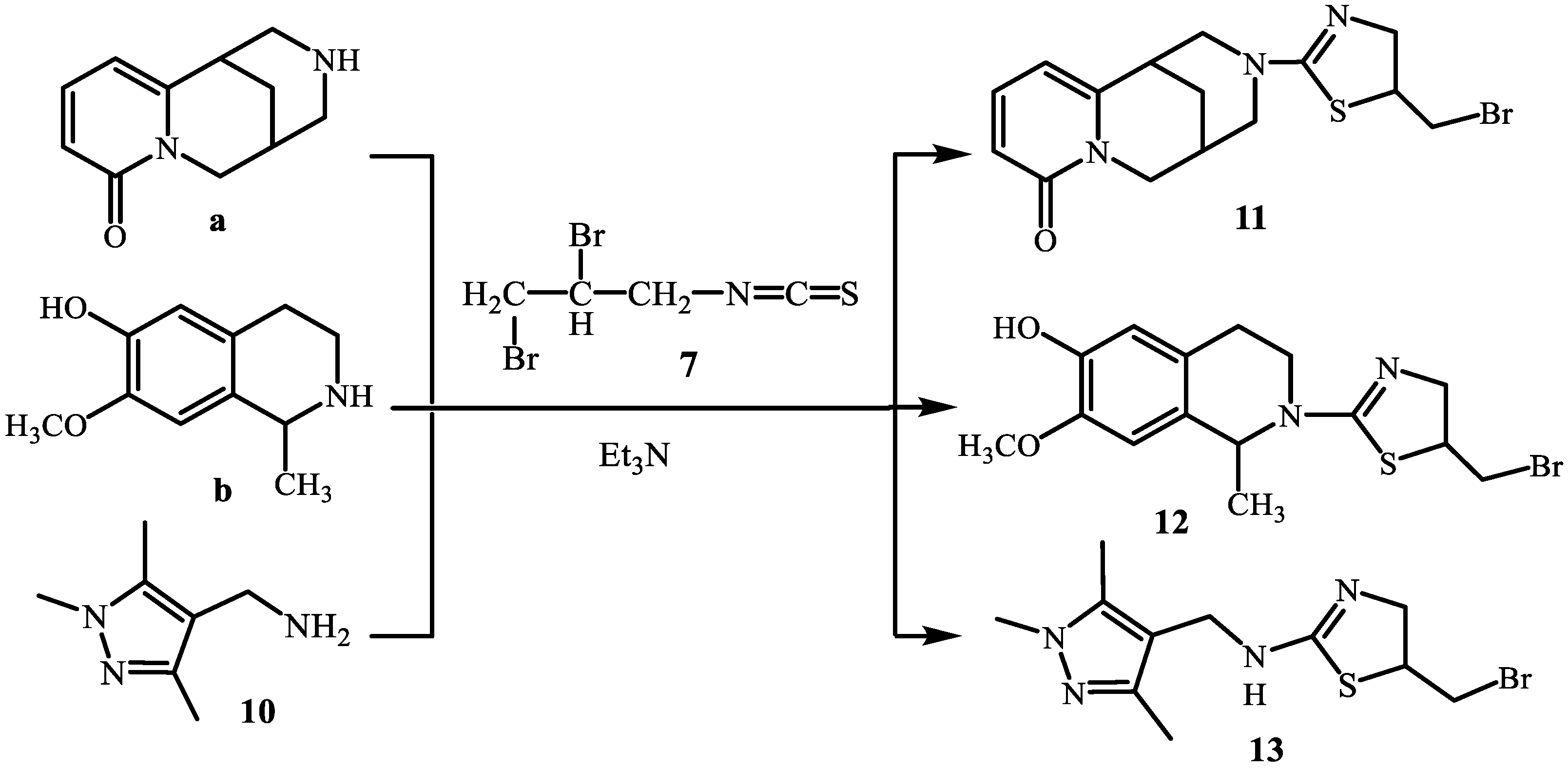
2. Results and Discussion
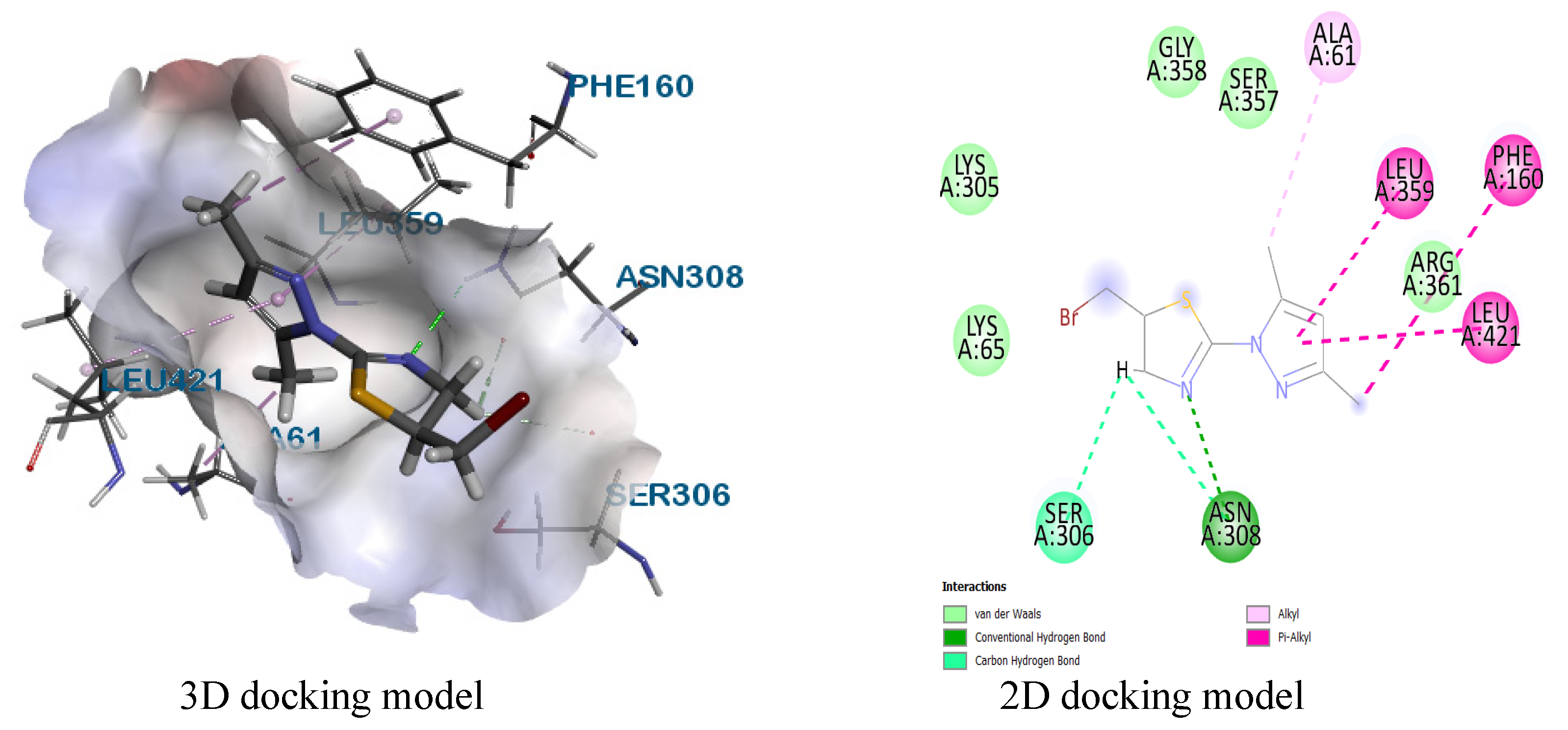
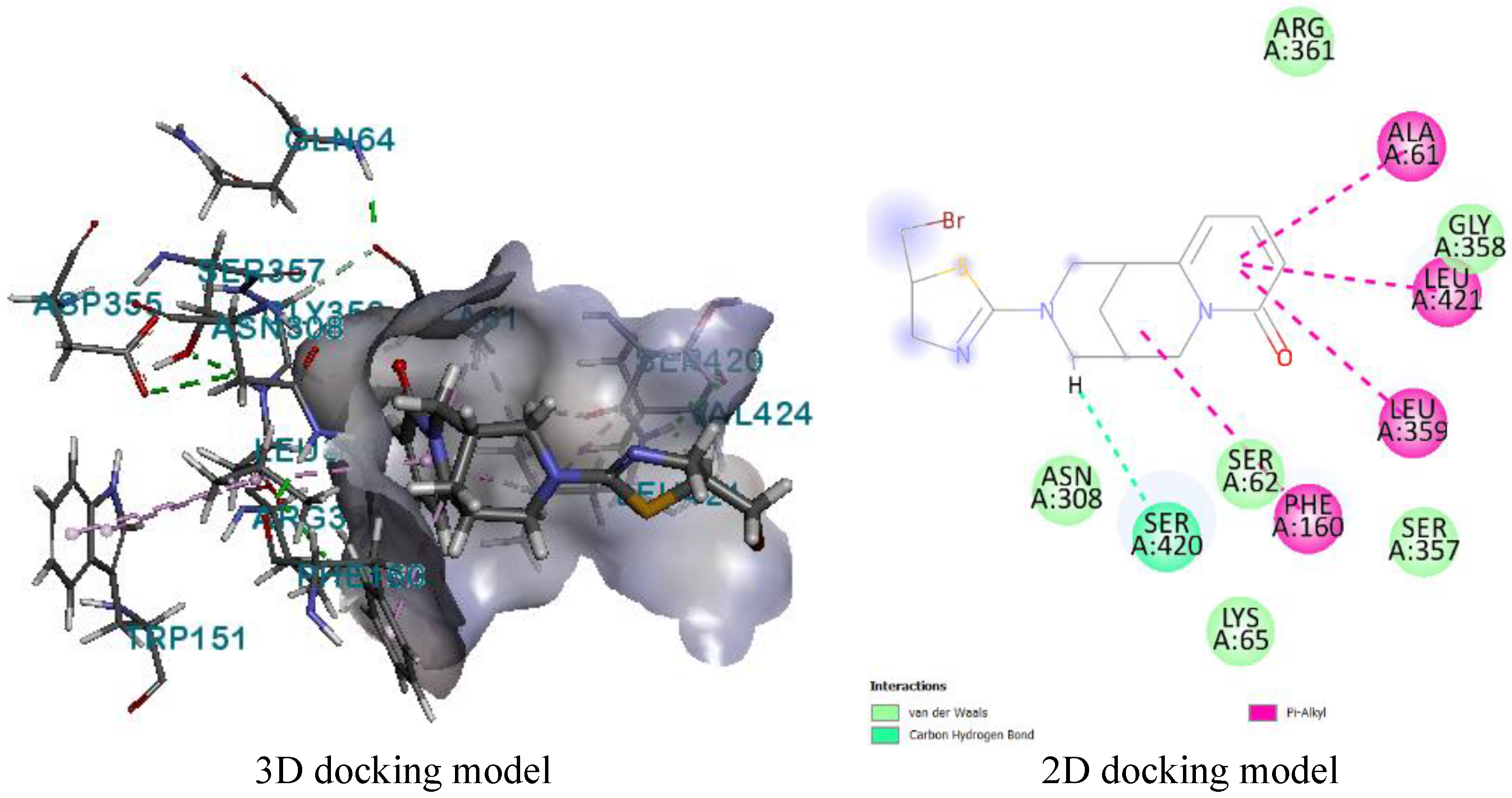
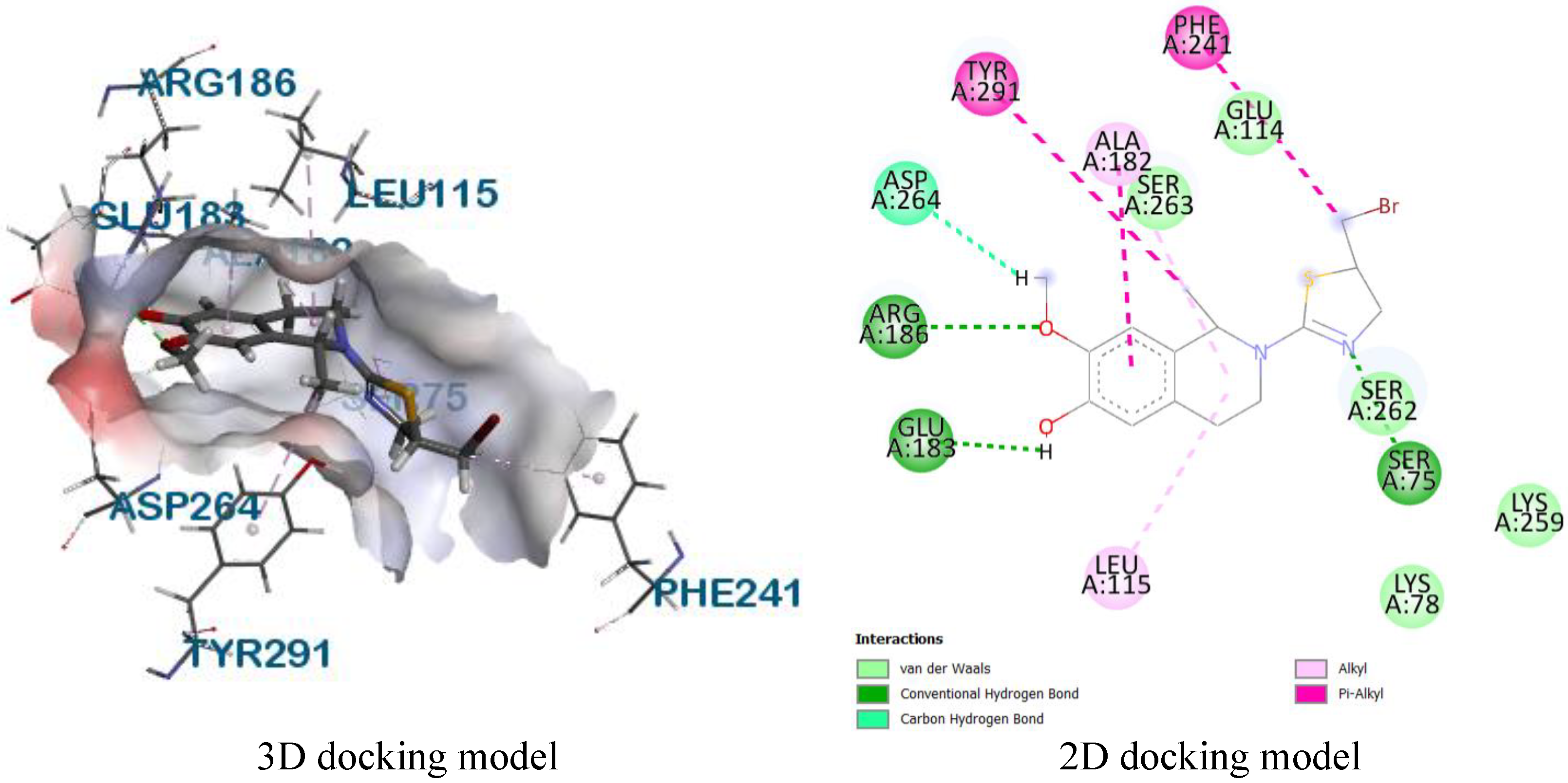
Molecular docking
3. Experimental
Experimental Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hantzsch, A.; Weber, J.H. Ueber verbindungen des thiazols (pyridins der thiophenreihe). Ber. Dsch. Chem. Ges. 1887, 20, 3118–3132. [Google Scholar] [CrossRef]
- Traumann, V. Ueber Amidothiazole und isomere derselben. Justus Liebigs Ann. Chem. 1888, 249, 31–53. [Google Scholar] [CrossRef]
- Aguilar, E.; Meyers, A.I. Reinvestigation of a modified Hantzsch thiazole synthesis. Tetrahedron Lett. 1994, 35, 2473–2476. [Google Scholar] [CrossRef]
- Jacquline, P.; Jebastin, M.; Santhanalakshmi, K.; Muthukumar, S. Green synthesis, biological evaluation and dft calculations of thiazolidinone derivatives—A review. Asian J. Pharm. Clin. Res. 2019, 13, 10–20. [Google Scholar] [CrossRef][Green Version]
- Davyt, D.; Serra, G. Thiazole and oxazole alkaloids: Isolation and synthesis. Mar. Drugs 2010, 8, 2755–2780. [Google Scholar] [CrossRef]
- Sharma, S.; Devgun, M.; Narang, R.; Lal, S.; Rana, A.C. Thiazoles: A retrospective study on synthesis, structure-activity relationship and therapeutic significance. Indian J. Pharm. Educ. Res. 2022, 56, 646–666. [Google Scholar] [CrossRef]
- Borisenko, V.E.; Koll, A.; Kolmakov, E.E.; Rjasnyi, A.G. Hydrogen bonds of 2-aminothiazoles in intermolecular complexes (1,1 and 1,2) with proton acceptors in solutions. J. Mol. Struct. 2006, 783, 101–115. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Anwar, C.J.; Ahmad, S. An efficient synthesis of 2-alkyl-4-hydroxy-2h-1,2-benzothiazine-3-carboxamide-1,1-dioxides. Bull. Korean Chem. Soc. 2005, 26, 1771–1775. [Google Scholar] [CrossRef]
- Markus, B.; Salome, V.G.; Christoph, B.; Werner, J.P. Molecular aspects of drug recognition by specific T cells. Curr. Drug Targets 2004, 4, 1–11. [Google Scholar] [CrossRef]
- Glicklich, E.A. Sulfathiazole ointment in the treatment of pyogenic dermatoses. N. Engl. J. Med. 1942, 226, 981–983. [Google Scholar] [CrossRef]
- Popsavin, M.; Torović, L.; Svircev, M.; Kojic, V.; Bogdanovic, G.; Popsavin, V. Synthesis and antiproliferative activity of two new tiazofurin analogues with 2′-amido functionalities. Bioorganic Med. Chem. Lett. 2006, 16, 2773–2776. [Google Scholar] [CrossRef] [PubMed]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D.; Ryan, M.J.; Taylor, D.G.; Connolly, C.J.; Doherty, A.M.; Klutchko, S.R.; Sircar, I. Structure–activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Yadav, R.; Srivastava, S.D. Synthesis and biological activity of 4-oxothiazolidines and their 5-arylidenes. Indian J. Chem. 2004, 43B, 399–405. [Google Scholar] [CrossRef]
- Yang, B.V.; Weinstein, D.S.; Doweyko, L.M.; Gong, H.; Vaccaro, W.; Huynh, T.; Xiao, H.-Y.; Doweyko, A.M.; Mckay, L.; Holloway, D.A. Dimethyl-diphenyl-propanamide derivatives as nonsteroidal dissociated glucocorticoid receptor agonists. J. Med. Chem. 2010, 53, 8241–8251. [Google Scholar] [CrossRef] [PubMed]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New series of thiazole derivatives: Synthesis, structural elucidation, antimicrobial activity, molecular modeling and MOE docking. Molecules 2019, 24, 1741. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S. Synthesis, anti-bacterial and antiifungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Spector, F.C.; Liang, L.; Giordano, H.; Sivaraja, M.; Peterson, M.G. Inhibition of herpes simplex virus replication by a 2-amino thiazole via interactions with the helicase component of the UL5-UL8-UL52 complex. J. Virol. 1998, 72, 6979–6987. [Google Scholar] [CrossRef]
- Oniga, O.; Nastasa, C.M.; Oniga, S.; Brindusa, T.; Pârnău, A.; Verite, P.; Crişan, O.; Ioana, I. Synthesis of some 2-(acetophenon-hydrazin)-thiazoles and 2-(4-thiazolyl-methynhydrazin)-thiazoles as potential antibacterial and antifungal agents. Farmacia 2010, 58, 825–833. [Google Scholar]
- Ali, M.; Khan, K.M.; Salar, U.; Ashraf, M.; Taha, M.; Wadood, A.; Hamid, S.; Riaz, M.; Ali, B.; Shamim, S.; et al. Synthesis, in vitro α-glucosidase inhibitory activity, and in silico study of (E) thiosemicarbazones and (E)-2-(2-(arylmethylene)hydrazinyl)-4-arylthiazole derivatives. Mol. Divers. 2018, 22, 841–861. [Google Scholar] [CrossRef]
- Mohammadi-Farani, A.; Foroumadi, A.; Kashani, M.R.; Aliabadi, A. N-Phenyl-2-p-tolylthiazole-4-carboxamide derivatives: Synthesis and cytotoxicity evaluation as anticancer agents. Iran. J. Basic Med. Sci. 2014, 17, 502–508. [Google Scholar] [CrossRef]
- Bharti, S.K.; Singh, S.K. Design, synthesis and biological evaluation of some novel benzylidene-2-(4-phenylthiazol-2-yl) hydrazines as potential anti-inflammatory agents. Med. Chem. Res. 2014, 23, 1004–1015. [Google Scholar] [CrossRef]
- Ling, S.; Xin, Z.; Qing, Z.; Jian-Bing, L.; Zhong, J.; Jian-Xin, F. Synthesis, structure, and biological activity of novel 1H-1,2,4-triazol-1-yl-thiazole derivatives. Synth. Commun. 2007, 37, 199–207. [Google Scholar] [CrossRef]
- Bulut, I.; Chávez, P.; Mirloup, A.; Huaulmé, Q.; Hébraud, A.; Heinrich, B.; Fall, S.; Méry, S.; Ziessel, R.; Heiser, T.; et al. Thiazole-Based Scaffolding for High Performance Solar Cells. J. Mater. Chem. C 2016, 4, 4296–4303. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, H.; Li, Y.; Zhan, X. Thiazole-based organic semiconductors for organic electronics. Adv. Mater. 2012, 24, 3087–3106. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.D.; Amato, A.A.; de Oliveira, T.B.; Iannini, K.B.R.; da Silva, A.L.; da Silva, T.G.; Leite, E.S.; Hernandes, M.Z.; de Lima, M.d.C.A.; da Rocha Pitta, I.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARγ ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; White, M.S.; Villanueva, E.B.; El-Ashmawy, I.M.; Klegeris, A. Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorganic Med. Chem. 2010, 18, 2019–2028. [Google Scholar] [CrossRef]
- Alegaon, S.G.; Alagawadi, K.R. New thiazolidinedione-5-acetic acid amide derivatives: Synthesis, characterization and investigation of antimicrobial and cytotoxic properties. Med. Chem. Res. 2012, 21, 816–824. [Google Scholar] [CrossRef]
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolyl thiazolidinedione derivatives. Arab. J. Chem. 2019, 12, 413–419. [Google Scholar] [CrossRef]
- Liu, X.F.; Zheng, C.J.; Sun, L.P.; Liu, X.K.; Piao, H.R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011, 46, 3469–3473. [Google Scholar] [CrossRef]
- Song, Z.C.; Ma, G.Y.; Lv, P.C.; Li, H.Q.; Xiao, Z.P.; Zhu, H.L. Synthesis, structure and structure–activity relationship analysis of 3-tert-butoxycarbonyl-2-arylthiazolidine-4-carboxylic acid derivatives as potential antibacterial agents. Eur. J. Med. Chem. 2009, 44, 3903–3908. [Google Scholar] [CrossRef]
- Kumar, A.; Chawla, A.; Jain, S.; Kumar, P.; Kumar, S. 3-Aryl-2-{4-[4-(2,4-dioxothiazolidin-5-ylmethyl)phenoxy]-phenyl}-acrylic acid alkyl ester: Synthesis and antihyperglycemic evaluation. Med. Chem. Res. 2011, 20, 678–686. [Google Scholar] [CrossRef]
- Lesyk, R.; Zimenkovsky, B.S. 4-Thiazolidones: Centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2005, 8, 1547–1577. [Google Scholar] [CrossRef]
- Kar, K.; Krithika, U.; Mithuna Basu, P.; Santhosh Kumar, S.; Reji, A.; Prashantha Kumar, B.R. Design, synthesis and glucose uptake activity of some novel glitazones. Bioorg. Chem. 2014, 56, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Srinivasa Reddy, T.; Thummuri, D.; Senwar, K.R.; Praveen Kumar, N.; Naidu, V.G.M.; Bhargava, S.K.; Shankaraiah, N. Synthesis and biological evaluation of new benzimidazole-thiazolidinedione hybrids as potential cytotoxic and apoptosis inducing agents. Eur. J. Med. Chem. 2016, 124, 608–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Rao, W.; Parikh, H.; Li, Q.; Guo, T.L.; Grant, S.; Kellogg, G.E.; Zhang, S. 3,5-Disubstituted-thiazolidine-2,4-dione analogs as anticancer agents: Design, synthesis and biological characterization. Eur. J. Med. Chem. 2012, 47, 125–137. [Google Scholar] [CrossRef]
- Singh, S.P.; Parmar, S.S.; Raman, K.; Stenberg, V.I. Chemistry and biological activity of thiazolidinones. Chem. Rev. 1981, 81, 175–203. [Google Scholar] [CrossRef]
- Shih, M.-H.; Ke, F.-Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef]
- Pandey, Y.; Singh, A.; Sharma, P.K.; Kumar, N. Biological activities of thiazolidine-a review. J. Curr. Pharma Res. 2011, 1, 192–196. [Google Scholar]
- Kayukova, L.A.; Praliev, K.D. Main directions in the search for new antituberculous drugs. Pharm. Chem. J. 2000, 34, 11–18. [Google Scholar] [CrossRef]
- Soldatenkov, A.T.; Kolyadina, N.M.; Shendrik, I.V. Fundamentals of Organic Chemistry of Medicinal Substances; Chemistry: Moscow, Russia, 2001; 192p. (In Russian) [Google Scholar]
- Asif, M. A mini review: Biological significances of nitrogen hetero atom containing heterocyclic compounds. Int. J. Bioorganic Chem. 2017, 2, 146–152. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef] [PubMed]
- Krasovitsky, B.M.; Afanasiadi, L.M. Preparative Chemistry Organic luminophores; Folio: Kharkov, Ukraine, 1997; p. 117. (In Russian) [Google Scholar]
- Kobayashi, K.; Chono, S.; Yamada, H. Mepirizole, a non-steroidal antiinflammatory compound, its ulcerogenicity and inhibitory action on lesions induced by acidic antiinflammatory agents in the rat stomach. Gastroenterol. Jpn. 1980, 15, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Fezolamine: An effective and rapidly acting antidepressant in patients with major depressive disorders. Inpharma Wkly. 1988, 648, 5. [CrossRef]
- Available online: https://go.drugbank.com/drugs/DB01424 (accessed on 19 September 2022).
- Available online: https://go.drugbank.com/drugs/DB04817 (accessed on 19 September 2022).
- Soliman, R.; Darwish Suzan, A.S. Antidiabetic activity of some 1-substituted 3,5-dimethylpyrazoles. J. Med. Chem. 1983, 26, 1659–1663. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.B.; Dulin, W.; Markillie, J.H. The antidiabetic activity of 3,5-dimethylpyrazoles. J. Med. Chem. 1964, 7, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Soldatenkov, A.T.; Anh, L.T.; Zubkov, F.I.; Van Boy, L.; Hieu, T.H.; Polyansky, K.B. Applied Stereochemistry of Biologically Active Substances; Soldatenkov, A.T., Ed.; Knowledge Publishing House: Hanoi, Vietnam, 2015; 326p. (In Russian) [Google Scholar]
- Tiran, A.L.; Stables, J.P.; Kohn, H. Functionalized amino acid anticonvulsants: Synthesis and pharmacological evaluation of conformationally restricted analogues. Bioorg. Med. Chem. 2001, 9, 2693–2708. [Google Scholar] [CrossRef]
- Somsák, L.; Kovács, L.; Tóth, M.; Osz, E.; Szilágyi, L.; Györgydeák, Z.; Dinya, Z.; Docsa, T.; Tóth, B.; Gergely, P. Synthesis of and a comparative study on the inhibition of muscle and liver glycogen phosphorylases by epimeric pairs of d-gluco- and d-xylopyranosylidene-spiro-(thio)hydantoins and n-(d-glucopyranosyl) amides. J. Med. Chem. 2001, 44, 2843–2848. [Google Scholar] [CrossRef]
- Lerchen, H.-G.; Baumgarten, J.; Bruch, K.; Lehmann, T.E.; Sperzel, M.; Kempka, G.; Fiebig, H.-H. Design and optimization of 20-o-linked camptothecin glycoconjugates as anticancer agents. J. Med. Chem. 2001, 44, 4186–4195. [Google Scholar] [CrossRef]
- Mashkovskii, M.D. Drugs of the 20th Century; Novaya Volna: Moscow, Russia, 1998; 320p. [Google Scholar]
- Rahman, F.U.; Bibi, M.; Khan, E.; Shah, A.B.; Muhammad, M.; Tahir, M.N.; Shahzad, A.; Ullah, F.; Zahoor, M.; Alamery, S.; et al. Thiourea derivatives, simple in structure but efficient enzyme inhibitors and mercury sensors. Molecules 2021, 26, 4506. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Turdybekov, D.M. Synthesis and crystal structure of 5-methyl-2-(N-Anabasinyl)-5,6-dihydro-1,3-thiazin-4-one from the alkaloid anabasine. Chem. Nat. Compd. 2010, 46, 586–589. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Talipov, S.A.; Shulgau, Z.T.; Seilkhanov, T.M. Synthesis, structure, and antiradical activity of new methano [1,3]thiazolo [2,3-d][1,3,5]benzoxa-diazocine derivatives. Chem. Heterocycl. Compd. 2015, 50, 1477–1485. [Google Scholar] [CrossRef]
- Alimbayeva, A.S.; Nurkenov, O.A.; Zhakina, A.K.; Kulakov, I.V.; Turdybekov, D.M.; Turdybekov, K.M. Synthesis and spatial structure of 4-(2-hydroxyethyl)-5-(2-hydroxyphenyl)-2H-1,2,4-triazolo-3(4H)-thione. Russ. J. Gen. Chem. 2009, 79, 1532–1536. [Google Scholar] [CrossRef]
- Kulakov, I.V. Synthesis and heterocyclization of β-N-(methacryloylthiocarbamoyl)-isonicotinohydrazide. Chem. Heterocycl. Compd. 2008, 44, 889–890. [Google Scholar] [CrossRef]
- Gazaliev, A.M.; Nurkenov, O.A.; Turdybekov, K.M.; Fazylov, S.D.; Ibraev, M.K.; Turdybekov, D.M.; Issabaeva, M.B. Synthesis and crystal structure of (4S,R)-3,4-dimethyl-5-phenyl-2-(hydroxyethylimino)-1,3-thiazolidine. Mendeleev Commun. 2006, 16, 243–244. [Google Scholar] [CrossRef]
- Mozolis, V.V.; Iokubaitite, S.P. Preparation of N-Substituted Thiourea. Russ. Chem. Rev. 1973, 42, 587–595. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Akhmetova, S.B.; Seidakhmetova, R.B.; Zhambekov, Z.M. Synthesis and antibacterial and antifungal activities of thiourea derivatives of the alkaloid anabasine. Pharm. Chem. J. 2011, 45, 15–18. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Turdybekov, D.M.; Ibragimov, B.T.; Talipov, S.A.; Zhambekov, Z.M.; Ainabaev, A.A.; Turdybekov, K.M. Synthesis of thiourea derivatives of the alkaloid anabasine and crystal structure of N-(anabasino1-thiocarbonyl)furan-2-carboxamide. Chem. Nat. Compd. 2009, 45, 209–212. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Arinova, A.E.; Turdybekov, D.M.; Talipov, S.A.; Ibragimov, B.T. Synthesis of acetylated glycosyl-containing thiourea derivatives based on the alkaloids cytisine and anabasine and the molecular structure of N-cytisino-N′-(2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyl)thiocarbamide. Chem. Nat. Compd. 2011, 47, 777–780. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Turdybekov, D.M.; Ainabaev, A.A.; Turdybekov, K.M.; Gazaliev, A.M. Synthesis and crystal structure of cytisino-N-(2-hydroxyethyl)-thiocarbamide. Chem. Nat. Compd. 2009, 45, 66–68. [Google Scholar] [CrossRef]
- Sokolov, V.B.; Aksinenko, A.Y.; Pushin, A.N.; Martynov, I.V. Intramolecular cyclization of 1-allyl- and 1-methallyl-6-amino-2-thiouracils. Russ. Chem. Bull. 2005, 54, 1744–1746. [Google Scholar] [CrossRef]
- Tkachenko, S.E.; Pushin, A.N.; Sokolov, V.B.; Fedoseev, V.M.; Martynov, I.V. Cyclization of n-allylthiourea derivatives by the action of α-chloronitrosoalkanes. Chem. Heterocycl. Compd. 1998, 34, 347–350. [Google Scholar] [CrossRef]
- Sayed, A.R.; Gomha, S.M.; Abdelrazek, F.M.; Farghaly, M.S.; Hassan, S.A.; Metz, P. Design, efficient synthesis and molecular docking of some novel thiazolyl-pyrazole derivatives as anticancer agents. BMC Chem. 2019, 13, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, J.J.; Johnson, H.W., Jr. Mechanism of the alkylative decarboxylation of N-carbalkoxypyrazoles. J. Org. Chem. 1974, 39, 1909–1915. [Google Scholar] [CrossRef]
- Kulakov, I.V.; Nurkenov, O.A.; Turdybekov, D.M.; Turdybekov, K.M. Synthesis and intramolecular heterocyclization of N-allylthiocarbamide derivatives of the alkaloids cytisine and anabasine into 1,3-thiazoline derivatives and features of their molecular structures. Chem. Nat. Compd. 2010, 46, 257–261. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Gazaliev, A.M.; Ainabaev, A.A.; Kulakov, I.V. Synthesis and intramolecular heterocyclization of N-allylcytisine-12-carbothioamide. Russ. J. Gen. Chem. 2006, 76, 1181–1182. [Google Scholar] [CrossRef]
- Kulakov, I.V. Synthesis and intramolecular heterocyclization of the n-allylthiocarbamide of the alkaloid salsoline. Chem. Nat. Compd. 2015, 51, 1204–1205. [Google Scholar] [CrossRef]
- Fedoseev, V.M.; Filippovich, I.V. S-Thiourea derivatives. X. Preparation of 2-amino-5-bromo-d2-dihydro-1,3-thiazine. Zhurnal Org. Khimii 1964, 34, 1556–1561. (In Russian) [Google Scholar]
- Fedoseev, V.M.; Filippovich, I.V. S-Derivatives of thiourea. XI. On the product of the reaction of 2,3-dibromopropylamine bromide with potassium thiocyanate. Zhurnal Org. Khimii 1964, 34, 1561–1565. (In Russian) [Google Scholar]
- Fedoseev, V.M.; Litvinov, L.N. S-Thiourea derivatives. VIII. Synthesis of 2-hydroxy-5-isothiuroniummethylthiazoline. Zhurnal Org. Khimii 1964, 34, 557–560. (In Russian) [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by x-ray and neutron diffraction. Part 1. bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Rouden, J.; Lasne, M.C.; Blanchet, J.; Baudoux, J. (-)-Cytisine and derivatives: Synthesis, reactivity, and applications. Chem. Rev. 2014, 114, 712. [Google Scholar] [CrossRef] [PubMed]
- Mravec, B. Salsolinol, a Derivate of Dopamine, Is a Possible Modulator of Catecholaminergic Transmission: A Review of Recent Developments. Physiol. Res. 2006, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kishida, H.; Roper, D.I.; Lloyd, A.; Park, S.-Y.; Tame, J.R.H. Crystal structure of penicillin binding protein 4 (dacb) from escherichia coli, both in the native form and covalently linked to various antibiotics. J. Biochem. 2006, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Navratna, V.; Nadig, S.; Sood, V.; Prasad, K.; Arakere, G.; Gopal, B. Molecular basis for the role of staphylococcus aureus penicillin binding protein 4 in antimicrobial resistance. J. Bacteriol. 2010, 192, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.rcsb.org/ (accessed on 2 September 2022).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comp. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Dassault Systemes. BIOVIA Discovery Studio 2015—Discovery Studio Modelling Environment; Release 4.5; Dassault Systemes: San Diego, CA, USA, 2015. [Google Scholar]
- Available online: https://go.drugbank.com/drugs/DB00446 (accessed on 19 September 2022).
- Available online: https://go.drugbank.com/drugs/DB00456 (accessed on 19 September 2022).


| Binding Energy (kcal/mol) | ||
|---|---|---|
| Ligand | Receptor | |
| 2EXB | 3HUN | |
| Cephalotin | −7.0 | - |
| Chloramphenicol | - | −6.8 |
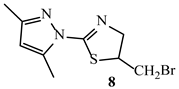 | −5.2 | −5.6 |
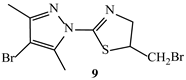 | −5.2 | −6.0 |
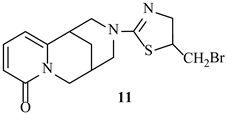 | −6.6 | −7.5 |
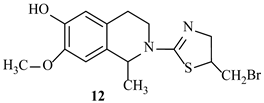 | −6.3 | −7.5 |
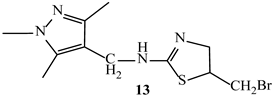 | −5.6 | −6.4 |
| Compound | Receptor | H-Bond | Residual Amino Acid Interactions | |
|---|---|---|---|---|
| Pi-Alkyl, Alkyl, Pi-Sigma, Pi-Pi Stacked | Van-Der Walls Interactions | |||
| 8 | 2EXB | SER306, ASN308 | LEU421, PHE160, LEU359, ALA261 | LYS305, LYS65, ARG361, SER357, GLY358 |
| 9 | SER306 | PHE160, LEU359, LEU421 | LYS305, LYS65, ASN308, ALA61, ARG361 | |
| 11 | SER420 | PHE160, LEU359, LEU421, ALA61 | ASN308, LYS65, SER62, SER357, GLY358, ARG361 | |
| 12 | ASN308, PHE160 | PHE160, ALA61, LEU421, LEU359 | ARG361, GLY358, SER357, LYS65, SER306 | |
| 13 | SER420, SER306 | PHE160, ALA61, LEU421, LEU359 | ASN308, LYS417, LYS305, GLY358, ARG361 | |
| 8 | 3HUN | SER116, SER75 | ALA182, LEU115, PHE241 | ARG186, ASN141, LYS78, SER262, SER139, LYS259, SER263, TYR291 |
| 9 | SER116, SER75 | PHE241, LEU115, ALA182 | LYS259, SER139, ASN141, LYS78, ARG186 | |
| 11 | SER116, SER262, SER75 | LEU115, PHE241 | ALA182, GLU183, ARG186, GLU114, TYR291 | |
| 12 | ASP264, ARG186, GLU183, SER75 | LEU115, PHE241, ALA182, TYR291 | ASN141, LYS78, LYS259, SER116, SER262, SER139, SER263, GLU114 | |
| 13 | - | ALA74, ALA182, PHE241 | ARG186, ASN72, THR180, GLY181, ASN141, SER75, LYS78, LYS259, SER139, SER116, LEU115 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrayev, M.K.; Nurkenov, O.A.; Rakhimberlinova, Z.B.; Takibayeva, A.T.; Palamarchuk, I.V.; Turdybekov, D.M.; Kelmyalene, A.A.; Kulakov, I.V. Synthesis, Structure and Molecular Docking of New 4,5-Dihydrothiazole Derivatives Based on 3,5-Dimethylpyrazole and Cytisine and Salsoline Alkaloids. Molecules 2022, 27, 7598. https://doi.org/10.3390/molecules27217598
Ibrayev MK, Nurkenov OA, Rakhimberlinova ZB, Takibayeva AT, Palamarchuk IV, Turdybekov DM, Kelmyalene AA, Kulakov IV. Synthesis, Structure and Molecular Docking of New 4,5-Dihydrothiazole Derivatives Based on 3,5-Dimethylpyrazole and Cytisine and Salsoline Alkaloids. Molecules. 2022; 27(21):7598. https://doi.org/10.3390/molecules27217598
Chicago/Turabian StyleIbrayev, Marat K., Oralgazy A. Nurkenov, Zhanar B. Rakhimberlinova, Altynaray T. Takibayeva, Irina V. Palamarchuk, Dastan M. Turdybekov, Assel A. Kelmyalene, and Ivan V. Kulakov. 2022. "Synthesis, Structure and Molecular Docking of New 4,5-Dihydrothiazole Derivatives Based on 3,5-Dimethylpyrazole and Cytisine and Salsoline Alkaloids" Molecules 27, no. 21: 7598. https://doi.org/10.3390/molecules27217598
APA StyleIbrayev, M. K., Nurkenov, O. A., Rakhimberlinova, Z. B., Takibayeva, A. T., Palamarchuk, I. V., Turdybekov, D. M., Kelmyalene, A. A., & Kulakov, I. V. (2022). Synthesis, Structure and Molecular Docking of New 4,5-Dihydrothiazole Derivatives Based on 3,5-Dimethylpyrazole and Cytisine and Salsoline Alkaloids. Molecules, 27(21), 7598. https://doi.org/10.3390/molecules27217598







