Abstract
Dimethindene is a selective histamine H1 antagonist and is commercially available as a racemate. Upon analyzing the synthetic pathways currently available for the industrial preparation of dimethindene, we set up a sustainable approach for the synthesis of this drug, switching from petroleum-based volatile organic compounds (VOCs) to eco-friendly solvents, such as 2-methyltetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME) belonging to classes 3 and 2, respectively. Beyond decreasing the environmental impact of the synthesis (E-factor: 24.1–54.9 with VOCs; 12.2–22.1 with 2-MeTHF or CPME), this switch also improved the overall yield of the process (from 10% with VOCs to 21–22% with 2-MeTHF or CPME) and remarkably simplified the manual operations, working under milder conditions. Typical metrics applied at the first and second pass, according to the CHEM21 metrics toolkit, were also calculated for the whole synthetic procedure of dimethindene, and the results were compared with those of the classical procedure.
1. Introduction
In recent years, growing environmental awareness has led chemists to consider the sustainability of a chemical process as a “key driver for innovation” in the pharmaceutical industry [1]. In order to develop greener and more effective methods to produce active pharmaceutical ingredients (APIs), Anastas and Warner’s 12 Principles of Green Chemistry became the guidelines for building an environmentally responsible manufacturing process [2,3]. Recently, the direct or accidental release of treated solvent waste and toxic chemicals into the environment, as well as hazardous work conditions, have led to the implementation of environmental directives and legislation (Clean Air Act of 1990 and the European Union Solvents Emission Directive 1999/13/EC) and the regulation of the usage of potentially harmful or environmentally damaging chemical substances (Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH)) [4]. These governmental regulations, in addition to many others, have created widespread interest in green chemistry and sustainable technology [5].
During pharmaceutical process development, a solvent selection analysis is pivotal for determining the sustainability of future production lines. Toxic and harmful volatile organic compounds (VOCs) (50–100 °C) (e.g., dichloromethane (DCM) and toluene), traditionally employed as solvents by organic and industrial chemists, have been severely curtailed and blacklisted. In this vein, the whole question of solvents requires rethinking and has become a primary focus, especially in the manufacture of pharmaceuticals because solvents contribute over 80% to the organic waste produced [6,7]. Not only is this “solvent problem” related to their use, but it also has implications with regard to their containment, recovery, and reuse. Thus, increasing attention has been paid to the efficient removal of solvents from manufactured drugs, with the solvents themselves also classified as impurities.
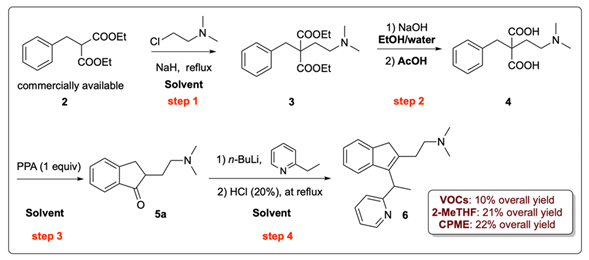
Building on our interest in the use of environmentally responsible solvents in the synthesis of APIs [8,9], we became eager to develop, in cooperation with our industrial partner (Laboratori Alchemia s.r.l.) [10], a sustainable synthesis method for dimethindene (6) (Scheme 1), which is a first-generation selective H1-antihistamine drug and is commercially available as a racemate. It was patented in 1958 and was first introduced for medical use in the 1960s. Because of pressing requests from the Italian and European markets, the synthesis of this drug has attracted a lot of API manufacturers. Currently, the industrial synthesis of dimethindene involves the massive use of genotoxic benzyl chloride (1) and a large amount of polyphosphoric acid (PPA), which are both hazardous to the environment and human health (Scheme 1A) [11,12]. The process involves five steps. In the first one, diethyl malonate reacts with 1, affording the benzyl malonic diethyl ester 2 (a), which is then alkylated with commercially available 2-chloro-N,N-dimethylethan-1-amine in toluene to yield the tertiary amine 3 (b). The subsequent saponification of 3 leads to the amino diacid 4 (c). This is followed by intramolecular Friedel–Craft acylation carried out in PPA to yield the indanone derivative 5a (d). The reaction is finally quenched by cautious addition of ice chips and K2CO3, which causes a vigorous release of CO2. The treatment of 5a with the lithium salt of the commercially available 2-ethylpyridine forms a tertiary alcohol in situ, whose dehydration in refluxing HCl (20%) produces dimethindene (6) (e). The overall yield of the process is around 4–5%.
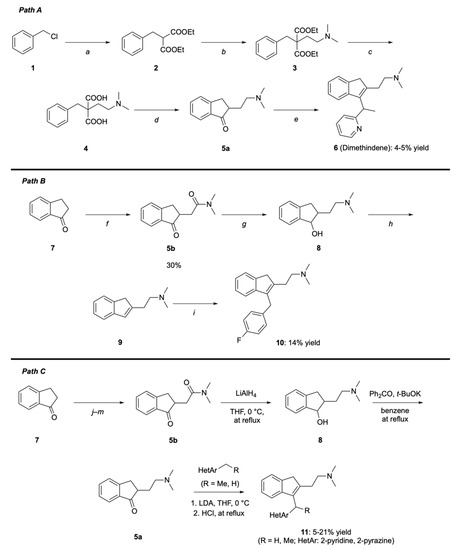
Scheme 1.
Current industrial synthesis of dimethindene (6) using VOCs: Path (A) [11,12]; synthesis of dimethindene analogs 10,11 using VOCs: Paths (B,C) [13,14]. (Path A) Reagents and conditions: (a) diethyl malonate, Na, and EtOH, at reflux. (b) NaH, 2-chloro-N,N-dimethylethan-1-amine, and toluene, at reflux. (c) NaOH and EtOH/water at reflux. (d) PPA, heating. (e) 2-Ethylpyridine, n-BuLi, and Et2O, at −78 °C; HCl (20%), at reflux. Path (B) Reagents and conditions: (f) LDA and THF, at −78 °C; 2-chloro-N,N-dimethylacetamide. (g) LiAlH4 and THF, at reflux. (h) AcOH and HCl, at reflux. (i) 4-Fluorobenzyl bromide, n-BuLi, and THF, at −78 °C. Path (C) Reagents and conditions: (j) glycolic acid (40% in H2O), conc. H2SO4, and dioxane, at reflux; (k) Zn, HOAc, and H2O, at 100 °C; (l) (COCl)2, cat. DMF, and DCM; (m) HNMe2, Et3N, and DCM.
Beaton and co-workers [13] synthesized the intermediate 5b (30% yield) by preliminary treating a THF solution of 1-indanone (7) with LDA at –78 °C and then quenching the resulting enolate with 2-chloro-N,N-dimethylacetamide (f) (Scheme 1B). After the reduction of carbonyl groups of 5b followed by acid-mediated dehydration of secondary alcohol 8 (g,h), n-BuLi and 4-fluorobenzyl bromide were sequentially added to a solution of 9 (i). The desired product 10 (a dimethindene analog) was isolated with an overall yield of 14%. In 2010, the same authors developed another synthetic approach to dimethindene analogs (Scheme 1C) [14]. In this case, 7 was first converted into 5b by a four-step process involving condensation with glycolic acid (j) followed by reduction with Zn in acetic acid (k), acyl chloride formation catalyzed by dimethylformamide (DMF) in DCM (l), and reaction with dimethylamine in DCM (m). Simultaneous reduction of the amide and ketone moieties of 5b with LiAlH4 led to alcohol 8, which was oxidized to produce 5a. Lithium salts from various alkyl heteroaryls, generated by the treatment of alkyl heteroarenes with LDA, were finally added to 5a, followed by acid-mediated dehydration to afford dimethindene analogs 11. Of note, among the various VOCs, experts particularly recommend avoiding dimethylacetamide and DMF, as they bear a risk of nitrosamine formation [15].
With the aim of minimizing the environmental impact of pharmaceutical processes, herein, we report a forward-looking approach for sustainable and more effective synthesis of dimethindene (6). Upon a careful solvent selection based on the replacement of VOCs with more environmentally friendly solvents, such as 2-MeTHF [16] and cyclopentyl methyl ether (CPME) [17], we were able to successfully modify the current industrial process (vide infra), not only decreasing the waste of solvents/materials but also improving the overall yield of the process, which increased from 10% in VOCs to 21–22% in 2-MeTHF/CPME.
2. Results and Discussion
2.1. Alternative Retrosynthetic Approaches to Dimethindene (6)
Inspired by these results, the synthesis of dimethindene was then planned according to the retrosynthetic approach depicted in Scheme 2. It involved two different steps: (i) the addition of 2-ethylpyridine to the carbonyl moiety of 5a, followed by (ii) the dehydration of the corresponding intermediate tertiary alcohol.
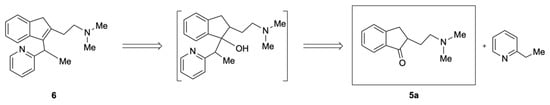
Scheme 2.
An alternative retrosynthetic approach to dimethindene (6).
Several approaches were examined for the synthesis of 5a (Scheme 3). The α-functionalization of 1-indanone (7) with different electrophiles (2-chloro- or 2-iodo-N,N-dimethylethan-1-amine, 2-chloro-N,N-dimethylacetamide, or 2-(dimethylamino)ethyl-4-methylbenzenesulfonate) in various conventional (e.g., THF, toluene, and CPME) and unconventional (e.g., the deep eutectic solvent (DES) choline chloride (ChCl)/urea (1:2 mol mol−1)) solvents [18,19,20,21] using different bases (LDA, KHMDS, NaHMDS, and NaH) proved to be ineffective, leading either to the recovery of the starting material (Scheme 3a,c) or to the isolation of 5a at up to a 30% yield (Scheme 3b; base: LDA; solvent: THF or CPME) (see Supplementary Materials). Ru-catalyzed hydrogen-borrowing C–C bond formation [22,23] between 7 and 2-(dimethylamino)ethan-1-ol (Scheme 3d), the reaction between 2-chloro- or 2-iodo-N,N-dimethylethan-1-amine or 2-chloro-N,N-dimethylacetamide and enamine 12 (prepared in 93% yield from 7 and pyrrolidine in ChCl/urea; see Supplementary Materials) (Scheme 3e), and the reaction between 2-chloro-N,N-dimethylethan-1-amine and β-ketoester 13 (synthesized from 7 and diethyl carbonate in CPME; see Supplementary Materials) using different solvents (THF, toluene, CPME, and DES) and different bases (NaOH, CsCO3, LDA, KHMDS, NaHMDS, and NaH) (Scheme 3f) all led to a null yield of 5a. These unsatisfactory results prompted us to pursue a different approach to dimethindene synthesis.
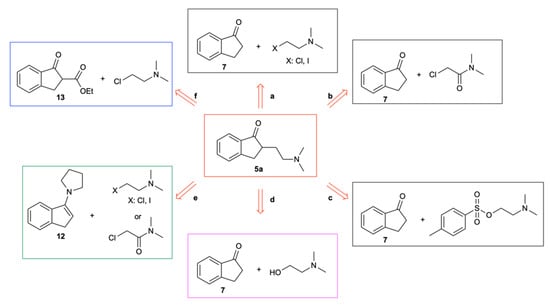
Scheme 3.
Possible retrosynthetic approaches to 5a. (a) α-Functionalization of 1-indanone (7) with 2-chloro- or 2-iodo-N,N-dimethylethan-1-amine; base: LDA, KHMDS, and NaHMDS; solvent: THF and toluene; temperature: −78 °C or −20 °C; (b) α-functionalization of 1-indanone (7) with 2-chloro-N,N-dimethylacetamide; base: LDA and NaH; solvent: THF and CPME; temperature: −78 °C or 0 °C or 25 °C; (c) α-functionalization of 1-indanone (7) with 2-(dimethylamino)ethyl-4-methylbenzenesulfonate; base: LDA and NaH; solvent: THF, toluene, and CPME; temperature: −78 °C or −20 °C or 0 °C; (d) α-functionalization of 1-indanone (7) with 2-(dimethylamino)ethan-1-ol; Ru cat.: [Ru(p-cymene)Cl2]2; ligand: (oxydi-2,1-phenylene)bis(diphenylphosphine); solvent: toluene; base: CsCO3 and NaOH; temperature: 105 °C; 8 h; (e) reaction between enamine 12 and 2-chloro- or 2-iodo-N,N-dimethylethan-1-amine or 2-chloro-N,N-dimethylacetamide; solvent: ChCl/urea; temperature: 25 °C for up to 24 h; (f) reaction between β-ketoester 13 and 2-chloro-N,N-dimethylethan-1-amine; base: LDA, KHMDS, NaMDS, and NaH; solvent: THF, toluene, CPME, and DES; temperature: −78 °C, −20 °C, or 0 °C.
2.2. An Environmentally Sustainable Process for the Industrial Synthesis of Dimethindene (6)
A large amount of waste produced (80%) from the API manufacturing process is estimated to be solvent-related [24,25]. Thus, the selection, use, and recovery of solvents would contribute significantly to alleviating this problem. The shift toward greener solvents in the industrial synthesis of APIs is one of the main goals of pharmaceutical companies [26]. According to a recent ICH guidance Q3C (R8) [27], 2-MeTHF and CPME belong to solvent classes 3 and 2, respectively. Thus, they are recognized as “greener alternatives” to VOCs [28,29], and their use has been strongly advocated in the synthesis of drugs and drug intermediates by the ACS Green Chemistry Pharmaceutical Roundtable [30,31,32]. On this basis, we focused on the use of these solvents for the total synthesis of dimethindene (6). Moreover, to quantify the eco-sustainability of the synthesis of 6, when using 2-MeTHF or CPME as the solvent, we made use of the First Pass CHEM21 Metrics Toolkit developed by Clark et al. [33,34,35], calculating atom economy (AE), reaction mass efficiency (RME), optimum efficiency (OE), effective mass yield (EM), and mass intensity (MI) of each step, along with process mass intensity (PMI) metrics, the latter taking into account the reactants, reagents, and solvents of the whole process (PMIRXN) or the solvents and reagents used in the work-up procedure (PMIWU), and we compared these values with the corresponding ones related to the classical synthetic procedure for 6 (see Supplementary Materials for details). In addition, some metrics typical of the Second Pass CHEM21 Metrics Toolkit, which quantify the use of material from renewable sources, such as renewables intensity (RI) and renewables percentage (RP), were also calculated.
The new approach followed for the synthesis of 6 comprised 4 steps and started from commercially available benzyl malonic diethyl ester 2 (Table 1). The latter (1.0 g, 4 mmol) was added to a refluxing suspension of NaH (1 equiv) in CPME or 2-MeTHF (10 mL each). After 1 h, 2-chloro-N,N-dimethylethan-1-amine (1 equiv) was added, and the reaction was refluxed for an additional 6 h. Compound 3 could finally be isolated at an 80% yield from 2-MeTHF and a 90% yield from CPME. Conversely, the yield of 3 was only 63% when using toluene as the solvent and 2 equiv of amine (Table 1, step 1). This allowed a significant improvement in reaction efficiency indicators, such as RME, OE, and E factor (Table 1, step 1). Furthermore, the use of CPME or 2-MeTHF as the reaction solvent and for the work-up step decreased the mass of non-benign reagents (and thus the indicator EM) while improving the recyclability of the process (RI and RP indicators).

Table 1.
Quantitative metrics for each step a of the classical synthesis of dimethindene (6) in VOCs or in CPME or in 2-MeTHF.
The saponification of malonic ester 3 with an aqueous NaOH solution furnished the amino diacid 4 at a 75% yield, and the E factor for this step was very low (9.8). This reaction step was also characterized by a good RP value (90.1%) (Table 1, step 2). The ring closure reaction (Friedel–Craft acylation) to produce the indanone skeleton is generally carried out using an excess of PPA (which also acts as a reaction medium), affording 5a at only a 20% yield. Conversely, a stoichiometric amount of PPA (1 equiv) in CPME or 2-MeTHF was enough to isolate 5a at a 50% yield in CPME and a 55% yield in 2-MeTHF, with a safer and operationally easier work-up procedure, thereby decreasing the amount of mass of non-benign reagents (higher EM value), improving the efficiency of the reaction (higher RME and OE values), and reducing the MIWU to a third compared with that of the classic procedure. The RP indicator for both reactions run in CPME and 2-MeTHF was very high (87–91.8%) (Table 1, step 3).
Finally, by carrying out the nucleophilic addition of the benzylic-type anion of 2-ethylpyridine to the carbonyl moiety of 5a in CPME or 2-MeTHF at 0 °C, followed by dehydration of the corresponding tertiary alcohol in refluxing HCl, dimethindene (6) was isolated at a 65% yield, regardless of whether CPME or 2-MeTHF was used as the solvent. On the other hand, the employment of a VOC, such as Et2O, as an alternative solvent, required the cooling of the system to −78 °C and led to the isolation of 6 at a 60% yield (Table 1, step 4). Again, the use of green solvents improved the efficiency and the sustainability of the reaction as results of the quantitative metrics calculated, particularly for the EM indicator (Table 1, step 4).
In summary, the use of eco-sustainable solvents, such as CPME and 2-MeTHF, made it possible to improve the efficiency and sustainability of the entire synthetic process of dimethindene (6). The overall yield of the isolated product was twice that of the yield obtained through the current synthetic procedure using VOCs, with improvements in all quantitative metrics, especially the RP indicator (Table 2). Furthermore, the use of CPME or 2-MeTHF as the solvent allowed the reaction to be carried out under milder conditions (step 4) and avoided the use of toxic reagents in excess (Table 1, steps 1 and 3).

Table 2.
Quantitative metrics for the process of synthesis of dimethindene (6) in VOCs or in CPME or in 2-MeTHF.
3. Materials and Methods
3.1. General Methods
The deep eutectic solvent ChCl/urea (1:2 mol/mol) was prepared by heating under stirring at 60–80 °C for 10–30 min the corresponding individual components until a clear solution was obtained. For 1H NMR (600 MHz) and 13C NMR (150 MHz), CDCl3 or D2O was used as the solvent; chemical shifts are reported in parts per million (δ). FT-IR spectra were recorded on a Perkin–Elmer 681 spectrometer. GC analyses were performed on an HP 6890 model Series II using an HP1 column (methyl siloxane; 30 m × 0.32 mm × 0.25 μm film thickness). Analytical thin-layer chromatography (TLC) was carried out on precoated 0.25 mm thick plates of a Kieselgel 60 F; visualization was accomplished by UV light (254 nm) or by spraying a solution of 5% (w/v) ammonium molybdate and 0.2% (w/v) cerium(III) sulfate in 100 mL 17.6% (w/v) aq. sulfuric acid and heating to 473 K until blue spots appeared. Chromatography was run using silica gel 60 with a particle size distribution of 40–63 μm and 230–400 ASTM. GC–MS analyses were performed on an HP 5995C model. Cyclopentyl methyl ether (CPME) was provided by Zeon Europe GmbH. High-resolution mass spectrometry (HRMS) analyses were performed using a Bruker microTOF QII mass spectrometer equipped with an electrospray ion source (ESI). Other reagents and solvents, unless otherwise specified, were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA) and were used without further purification. The following abbreviations are used to explain the multiplicities: s = singlet, d = doublet, t = triplet, m = multiplet, br = broad, dd = double doublet. RT = room temperature.
3.2. Synthesis of Dimethindene 6 in Green Solvents
3.2.1. Step 1: Synthesis and Characterization Data of 2-Benzyl-2-[2-(Dimethylamino)ethyl]malonic Acid Diethyl Ester (3)
To a refluxing suspension of NaH (0.096 g, 4 mmol, 1 equiv) in CPME or 2-MeTHF (10 mL), 2-benzylmalonic acid diethyl ester 2 (1.0 g, 4 mmol, 1 equiv) was added dropwise. The reaction mixture was refluxed for 1 h to yield a clear yellow solution. After this time, 2-chloro-N,N-dimethylethan-1-amine (0.43 g, 4 mmol, 1 equiv) was added dropwise, and the reaction mixture was refluxed for 6 h. The resulting suspension was extracted with HCl (5%) (3 × 1 mL). The aqueous layers were combined, basified with NH4OH (pH = 11–12), and extracted with CPME or 2-MeTHF (3 × 2 mL). The combined organic layers were dried (Na2SO4) and concentrated to produce compound 3 as a yellow oil at a 90% yield (1.157 g) in CPME and an 80% yield (1.028 g) in 2-MeTHF.
2-Benzyl-2-[2-(dimethylamino)ethyl]malonic acid diethyl ester (3): 1H NMR (600 MHz, CDCl3) δ 1.24 (t, J = 7.3 Hz, 6 H), 1.95–1.99 (m, 2 H), 2.21 (s, 6 H), 2.31–2.35 (m, 2 H), 3.24 (s, 2 H), 4.17 (q, J = 7.3 Hz, 4 H), 7.09–7.11 (m, 2 H), 7.20–7.26 (m, 3 H); 13C NMR (150 MHz, CDCl3) δ 13.9, 29.9, 38.6, 45.4, 54.8, 57.7, 61.1, 126.9, 128.2, 129.9, 136.2, 171.1; \FT-IR (film, cm−1): 2835, 1730, 1581, 1393, 1355, 1285, 1298, 1048, 900, 854, 762, 686; GC/MS (70 eV) m/z (%): 321 (M+, 100), 228 (38), 227 (98), 185 (42), 183 (40), 158 (27), 155 (23), 149 (41), 91 (22), 89 (47), 77 (31). HRMS (ESI) m/z calcd for [C18H27NO4 + H]+: 322.2013; found: 322.2012.
3.2.2. Step 2: Synthesis and Characterization Data of 2-Benzyl-2-[2-(Dimethylamino)ethyl]malonic Acid (4)
A solution of ester 3 (1.16 g, 3.6 mmol) and NaOH (0.504 g, 12.6 mmol) in EtOH (5 mL) was refluxed for 4 h. The resulting suspension was concentrated under reduced pressure, and the residue obtained was dissolved in H2O (5 mL). Acetic acid was added dropwise while cooling in an ice water bath (5 °C) until a white solid was obtained. Then, the solid was filtered, washed with cold water (2 × 3 mL) and EtOH (5 mL), and dried in a vacuum oven at 50 °C to yield product 4 as a white-yellow solid at a 75% yield (0.720 g).
2-Benzyl-2-[2-(dimethylamino)ethyl]malonic acid (4): 1H NMR (600 MHz, D2O) δ 2.18–2.22 (m, 2 H), 2.87 (s, 6 H), 3.08–3.12 (m, 2 H), 3.18 (s, 2 H), 7.21–7.23 (m, 2 H), 7.33–7.38 (m, 3 H); 13C NMR (150 MHz, D2O) δ 29.8, 42.7, 43.1, 54.5, 58.5, 127.3, 128.6, 129.6, 136.3, 178.2; FT-IR (film, cm−1): 3350, 2920, 2850, 1599, 1494, 1243, 1041, 752. HRMS (ESI) m/z calcd for [C14H19NO4-H]−: 264.1241; found: 264.1248.
3.2.3. Step 3: Synthesis and Characterization Data of 2-[2-(Dimethylamino)ethyl]indan-1-one (5a)
Compound 4 (0.72 g, 2.71 mmol) was added to a preheated solution of polyphosphoric acid (0.72 g) in 1 mL of CPME or 2-MeTHF at 90 °C. The resulting brown reaction mixture was then heated under reflux and stirred for 20 min. The reaction was quenched with cold water (1 mL) and basified with an aq. sol. of K2CO3 (2 M). The mixture was extracted with CPME or 2-MeTHF (3 × 1 mL), washed with H2O (5 mL), dried (Na2SO4), and evaporated to produce 5a as a yellow oil at a 50% yield (0.280 g) in CPME and a 55% yield (0.303 g) in 2-MeTHF.
2-[2-(Dimethylamino)ethyl]indan-1-one (5a): 1H NMR (600 MHz, CDCl3) δ 1.54–1.64 (m, 1 H), 2.10–2.19 (m, 1 H), 2.23 (s, 6 H), 2.40–2.47 (m, 2 H), 2.66–2.73 (m, 1 H), 2.81 (dd, J = 17.3 Hz, 4.4 Hz, 1 H), 3.31 (dd, J = 17.3, 9.4 Hz, 1 H), 7.28 (d, J = 7.4 Hz, 1 H), 7.38 (t, J = 7.4 Hz, 1 H), 7.55 (t, J = 7.4 Hz, 1 H), 7.72 (d, J = 7.4 Hz, 1 H); 13C NMR (150 MHz, CDCl3) δ 28.3, 32.8, 45.5, 47.3, 57.6, 125.1, 127.3, 128.6, 132.9, 137.4, 147.8, 205.2; FT-IR (film, cm−1): 2639, 1699, 1598, 1573, 1471, 1434, 1287, 1220, 1147, 500; GC/MS (70 eV) m/z (%): 203 (M+, 100), 120 (11), 109 (15), 108 (32), 96 (78), 95 (36), 80 (12), 79 (24), 78 (43), 67 (37). HRMS (ESI) m/z calcd for [C13H17NO + H]+: 204.1383; found: 204.1384.
3.2.4. Step 4: Synthesis and Characterization Data of Dimethyl{2-[5-methyl-3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethyl}amine (Dimethindene) (6)
To a solution of 2-ethylpyridine (0.326 g, 3.04 mmol, 2.2 equiv) in CPME or 2-MeTHF (2 mL), n-BuLi (1.52 mL of a solution in cyclohexanes, 3.04 mmol, 2.2 equiv) was added at RT under nitrogen and stirred for 2 h at this temperature. To the resulting dark red solution, 5a (0.28 g, 1.38 mmol, 1 equiv) was added dropwise, and the mixture was stirred overnight at RT. The reaction was quenched with cold water, washed with saturated NaHCO3 (2 × 2 mL), and extracted with HCl (20%) (2 × 2 mL). The water layer was refluxed for 1 h, cooled to RT, basified with an NH4OH sol., and extracted with CPME or 2-MeTHF (3 × 1 mL). The organic phase was dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure afforded the crude product that was purified by flash-chromatography on silica gel (hexane/EtOAc 8:2) to provide the desired product 6 at a 65% yield (0.262 g) both in CPME and in 2-MeTHF.
Dimethyl{2-[5-methyl-3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethyl}amine (Dimethindene) (6): 1H NMR (600 MHz, CDCl3) δ 1.75 (d, J = 7.2 Hz, 3 H), 2.26 (s, 6 H), 2.37–2.52 (m, 2 H), 2.61–2.74 (m, 2 H), 3.40 (s, 2 H), 4.48 (q, J = 7.2 Hz, 1 H), 6.99–7.11 (m, 4 H), 7.16–7.18 (m, 1 H), 7.35–7.37 (m, 1 H), 7.47–7.51 (m, 1 H), 8.60 (d, J = 4.8 Hz, 1 H); 13C NMR (150 MHz, CDCl3) δ 20.9, 29.2, 39.9, 40.6, 45.4, 59.5, 120.7, 121.7, 122.9, 124.1, 128.2, 137.5, 141.4, 142.6, 143.6, 148.2, 164.7; FT-IR (film, cm−1): 2920, 2750, 1580, 1494, 1253, 1041, 742; GC/MS (70 eV) m/z (%): 292 (M+, 7), 168 (68), 119 (25), 108 (20), 107 (17), 95 (38), 94 (33), 77 (62), 43 (11). HRMS (ESI) m/z calcd for [C20H24O2 + H]+: 293.2012; found: 293.2013.
3.3. Current Industrial Synthesis of Dimethindene (6) in VOCs (Toluene, Et2O)
3.3.1. Step 1: Synthesis of 2-Benzyl-2-[2-(Dimethylamino)ethyl]malonic Acid Diethyl ester (3)
To a refluxing suspension of NaH (0.096 g, 4 mmol, 1 equiv) in toluene (20 mL) 2-benzylmalonic acid diethyl ester 2 (1.0 g, 4 mmol, 1 equiv) was added dropwise. The reaction mixture was refluxed for 1 h to produce a clear yellow solution. 2-chloro-N,N-dimethylethan-1-amine (0.86 g, 8 mmol, 2 equiv) was then added dropwise, and the reaction mixture was refluxed for 6 h. The resulting suspension was extracted with HCl (5%) (3 × 1 mL). The aqueous layers were combined, basified with NH4OH (pH = 11–12), and extracted with Et2O (3 × 4 mL). The combined organic layers were dried (Na2SO4) and concentrated to yield compound 3 as a yellow oil at a 63% yield (0.819 g).
3.3.2. Step 2: Synthesis of 2-Benzyl-2-[2-(Dimethylamino)ethyl]malonic Acid (4)
As described in Section 3.2.2.
3.3.3. Step 3: Synthesis of 2-[2-(Dimethylamino)ethyl]indan-1-one (5a)
Compound 4 (0.72 g, 2.71 mmol) was added to polyphosphoric acid (1.15 g) at 110 °C with overhead stirring. The resulting brown reaction mixture was heated to 140–150 °C and stirred for 20 min. The reaction was finally quenched with the cautious addition of ice chips and then with cold water (1 mL), and it was basified with an aq. sol. of K2CO3 (2 M). The mixture was extracted with Et2O (3 × 2 mL), washed with water (5 mL), dried (Na2SO4), and evaporated under reduced pressure to yield 5a as a yellow oil at a 20% yield (0.110 g).
3.3.4. Step 4: Synthesis of Dimethyl{2-[5-Methyl-3-(1-pyridin-2-ylethyl)-1H-inden-2-yl]ethyl}amine (6)
To a solution of 2-ethylpyridine (0.326 g, 3.04 mmol, 2.2 equiv) in anhydrous Et2O (3 mL), n-BuLi (1.52 mL of a solution in cyclohexanes, 3.04 mmol, 2.2 equiv) was added at −78 °C under nitrogen and stirred for 2 h at this temperature. To the resulting dark red solution, 5a (0.28 g, 1.38 mmol, 1 equiv) was added dropwise, and the resulting mixture was stirred overnight at RT. The reaction was quenched with cold water, washed with saturated NaHCO3 (2 × 2 mL), and extracted with HCl (20%) (2 × 2 mL). The water layer was refluxed for 1 h, cooled to RT, basified with an NH4OH sol., and extracted with Et2O (3 × 1 mL). The organic phase was dried over anhydrous Na2SO4. Evaporation of the solvent under reduced pressure afforded the crude product that was purified by flash-chromatography on silica gel (hexane/EtOAc 8:2) to provide dimethindene (6) at a 60% yield (0.242 g).
4. Conclusions
An environmentally sustainable procedure was developed for the synthesis of the antihistamine drug dimethindene (6) using green solvents 2-MeTHF and CPME belonging to classes 3 and 2, respectively, in agreement with the principles of green chemistry and following the recommendations of the ACS GCI Pharmaceutical Roundtable to redesign the chemical manufacturing of drugs by substituting the use of hazardous chemicals and fossil fuels and/or by reducing the production of waste to a minimum. The old synthetic industrial procedure, consisting of four steps and based on VOCs and harsh reaction conditions, has been now revisited and optimized by employing stoichiometric amounts of reagents and milder conditions, and the outcome of each step was compared when alternatively using 2-MeTHF or CPME as the solvent in place of VOCs. As a result, the overall yield of the process improved from 10% (with VOCs) to 21–22% (with 2-MeTHF or CPME) with a simplified work-up procedure. The decreased environmental impact of the newly reported approach is supported by the calculation of Quantitative Metrics of the CHEM21 Metrics Toolkit. Thus, an old and hazardous industrial process for the synthesis of a drug was usefully transformed into a more environmentally friendly synthetic methodology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217594/s1, typical metrics applied at Zero, First, and Second pass according to the CHEM21 Metrics Toolkit; quantitative metrics of classical and eco-friendly approach for the synthesis of Dimethindene (6); E-factor calculation for the synthesis of Dimethindene (6); Tables S1–S4: various experimental procedures; 1H and 13C NMR spectra of compounds 3, 4, 5a, and 6.
Author Contributions
Conceptualization, J.G.-Á., F.M.P., F.S. and V.C.; methodology, A.F.Q., J.G.-Á., F.M.P., F.S. and V.C.; validation, A.F.Q., F.M.P. and P.V.; formal analysis, A.F.Q., F.M.P. and P.V.; investigation, A.F.Q., C.A. and F.V.R.; resources, J.G.-Á., F.S. and V.C.; data curation, A.F.Q., F.M.P. and P.V.; writing—original draft preparation, A.F.Q., F.M.P. and V.C.; writing—review and editing, A.F.Q., F.V.R., P.V., J.G.-Á., F.M.P., C.A., F.S. and V.C.; visualization, A.F.Q.; supervision, J.G.-Á., F.M.P., F.S. and V.C.; project administration, V.C.; funding acquisition, J.G.-Á. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the framework of the “Programma Operativo Nazionale Ricerca e Innovazione” (PON RI 2014/2020), Axis I “Investments in Human Capital”, Action I.1.—“Innovative PhDs with industrial characterization”, funding FSE-FESR (D.D. n. 1377 on 5 June 2017), and it was financially supported by the Ministero dell’Università e della Ricerca (MUR) through the PRIN project “Unlocking Sustainable Technologies Through Nature-Inspired Solvents” (NATUREChem) (grant number: 2017A5HXFC_002). J.G.-Á thanks MCIN/AEI/10.13039/501100011033 (Project numbers CTQ2016-75986-P, RED2018-102387-T, and PID2020-113473GB-I00) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Laboratori Alchemia for supporting this project at every stage.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Nidumolu, R.; Prahalad, C.K.; Rangaswami, M.R. Why Sustainability is Now the Key Driver of Innovation. Harv. Bus. Rev. 2009, 87, 56–64. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000; pp. 1–148. [Google Scholar]
- Tang, S.Y.; Bourne, R.A.; Smith, R.L.; Poliakoff, M. The 24 Principles of Green Engineering and Green Chemistry: “IMPROVEMENTS PRODUCTIVELY”. Green Chem. 2008, 10, 268–269. [Google Scholar] [CrossRef]
- Allen, D.T.; Shonnard, D.R. Green Engineering: Environmentally Conscious Design of Chemical Processes; Prentice Hall: New York, NY, USA, 2002; pp. 475–489. [Google Scholar]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.; Overcash, M.R. How do you select the “greenest” technology? Development of guidance for the pharmaceutical industry. Clean Prod. Process. 2001, 3, 35–41. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green solvents for sustainable organic synthesis: State of the art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Quivelli, A.F.; D’Addato, G.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Expeditious and practical synthesis of tertiary alcohols from esters enabled by highly polarized organo-metallic compounds under aerobic conditions in Deep Eutectic Solvents or bulk water. Tetrahedron 2021, 81, 131898. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Marino, M.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Ligand-free Copper-Catalyzed Ullmann-type C-O Bond Formation in Non-innocent Deep Eutectic Solvents under Aerobic Conditions. ChemSusChem 2022, 15, e2021022111. [Google Scholar] [CrossRef]
- Laboratori Alchemia. Available online: http://www.laboratorialchemia.com (accessed on 8 October 2022).
- Hubner, C.F. Certain 1-[(2-pyridyl)-lower alkyl]-2-(tertamino-lower alkyl)-indan-1-ols, and Acid Addition Salts. U.S. Patent 297 0149, 03 November, 1958. [Google Scholar]
- Bohme, T.M.; Keim, C.; Kreutzmann, K.; Linder, M.; Dingermann, T.; Dannhardt, G.; Mutschler, E.; Lambrecht, G. Structure-Activity Relationships of Dimethindene Derivatives as New M2-Selective Muscarinic Receptor Antagonists. J. Med. Chem. 2003, 46, 856–867. [Google Scholar] [CrossRef]
- Moree, W.J.; Li, B.F.; Jovic, F.; Coon, T.; Yu, J.; Gross, R.S.; Tucci, F.; Marinkovic, D.; Zamani-Kord, S.; Malany, S.; et al. Characterization of Novel Selective H1-Antihistamines for Clinical Evaluation in the Treatment of Insomnia. J. Med. Chem. 2009, 52, 5307–5310. [Google Scholar] [CrossRef]
- Li, B.F.; Moree, W.J.; Yu, J.; Coon, T.; Zamani-Kord, S.; Malany, S.; Jalali, K.; Wen, J.; Wang, H.; Yang, C.; et al. Selectivity profiling of novel indene H1-antihistamines for the treatment of insomnia. Bioorg. Med. Chem. Lett. 2010, 20, 2629–2633. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, T.; Gosar, A.; Sayyed, H. Nitrosamine Impurities in Drug Substances and Drug Products. J. Adv. Pharm. Pract. 2020, 2, 50–59. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Azzena, U.; Carraro, M.; Pisano, L.; Monticelli, S.; Bartolotta, R.; Pace, V. Cyclopentyl Methyl Ether: An Elective Ecofriendly Ethereal Solvent in Classical and Modern Organic Chemistry. ChemSusChem 2019, 20, 40–70. [Google Scholar] [CrossRef] [PubMed]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Ramón, D.J.; Guillena, G. Deep Eutectic Solvents: Synthesis, Properties, and Applications; Wiley-VCH: Weinheim, German, 2019; pp. 1–365. [Google Scholar]
- Cicco, L.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V. Advances in deep eutectic solvents and water: Applications in metal- and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds. Org. Biom. Chem. 2021, 19, 2558–2577. [Google Scholar] [CrossRef]
- Vitale, P.; Cicco, L.; Perna, F.M.; Capriati, V. Introducing deep eutectic solvents in enolate chemistry: Synthesis of 1-arylpropan-2-ones under aerobic conditions. React. Chem. Eng. 2021, 6, 1796–1800. [Google Scholar] [CrossRef]
- Stålsmeden, A.S.; Belmonte Vázquez, J.L.; van Weerdenburg, K.; Rae, R.; Norrby, P.-O.; Kann, N. Glycerol Upgrading via Hydrogen Borrowing: Direct Ruthenium-Catalyzed Amination of the Glycerol Derivative Solketal. ACS Sustain. Chem. Eng. 2016, 4, 5730–5736. [Google Scholar] [CrossRef]
- Cicco, L.; Salomone, A.; Vitale, P.; Ríos-Lombardía, N.; González-Sabín, J.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Addition of Highly Polarized Organometallic Compounds to N-tert-Butanesulfinyl Imines in Deep Eutectic Solvents under Air: Preparation of Chiral Amines of Pharmaceutical Interest. ChemSusChem 2020, 13, 3583–3588. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Curzons, A.D.; Constable, D.J.; Cunningham, V.L. Cradle-to-gate life cycle inventory and assessment of pharmaceutical compounds. Int. J. Life Cycle Assess. 2004, 9, 114–121. [Google Scholar] [CrossRef]
- Curzons, A.D.; Jimenez-Gonzalez, C.; Duncan, A.L.; Constable, D.J.C.; Cunningham, V.L. Fast life cycle assessment of synthetic chemistry (FLASC™) tool. Int. J. Life Cycle Assess. 2007, 12, 272–280. [Google Scholar] [CrossRef]
- Cue, B.W.; Zhang, J. Green process chemistry in the pharmaceutical industry. Green Chem. Lett. Rev. 2009, 2, 193–211. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline, Q3C (R8) Impurities: Guideline for Residual Solvents, Step 2b; European Medicines Agency: Amsterdam, The Netherlands, 2020.
- Antonucci, V.; Coleman, J.; Ferry, J.B.; Johnson, N.; Mathe, M.; Scott, J.P.; Xu, J. Toxicological Assessment of 2-Methyltetrahydrofuran and Cyclopentyl Methyl Ether in Support of Their Use in Pharmaceutical Chemical Process Development. Org. Process Res. Dev. 2011, 15, 939–941. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- A Solvent Selection Guide Integrating Green Chemistry and Engineering Principles Is under Development. Available online: www.acs.org/gcipharmaroundtable (accessed on 19 September 2022).
- Giubellina, N.; Stabile, P.; Laval, G.; Perboni, A.D.; Cimarosti, Z.; Westerduin, P.; Cooke, J.W.B. Development of an Efficient Large-Scale Synthesis for a 4H-imidazo [5,1-c][1,4]benzoxazine-3-carboxamide Derivative for Depression and Anxiety. Org. Process Res. Dev. 2010, 14, 859–867. [Google Scholar] [CrossRef]
- Houpis, I.N.; Shilds, D.; Nettekoven, U.; Schnyder, A.; Bappert, E.; Weerts, K.; Canters, M.; Vermuelen, W. Utilization of Sequential Palladium-Catalyzed Cross-Coupling Reactions in the Stereospecific Synthesis of Trisubstituted Olefins. Org. Process Res.Dev. 2009, 13, 598–606. [Google Scholar] [CrossRef]
- McElroy, C.R.; Constantinou, A.; Jones, L.C.; Summerton, L.; Clark, J.H. Towards a holistic approach to metrics for the 21th century pharmaceutical industry. Green Chem. 2015, 17, 3111–3121. [Google Scholar] [CrossRef]
- Quivelli, A.F.; Rossi, F.V.; Vitale, P.; García-Álvarez, J.; Perna, F.M.; Capriati, V. Sustainable and Scalable Two-Step Synthesis of Thenfadil and Some Analogs in Deep Eutectic Solvents: From Laboratory to Industry. ACS Sustain. Chem. Eng. 2022, 10, 4065–4072. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry To Drive More Sustainable Processes. Org. Process Res. Dev. 2011, 15, 912. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).