A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo
Abstract
1. Introduction
2. Results
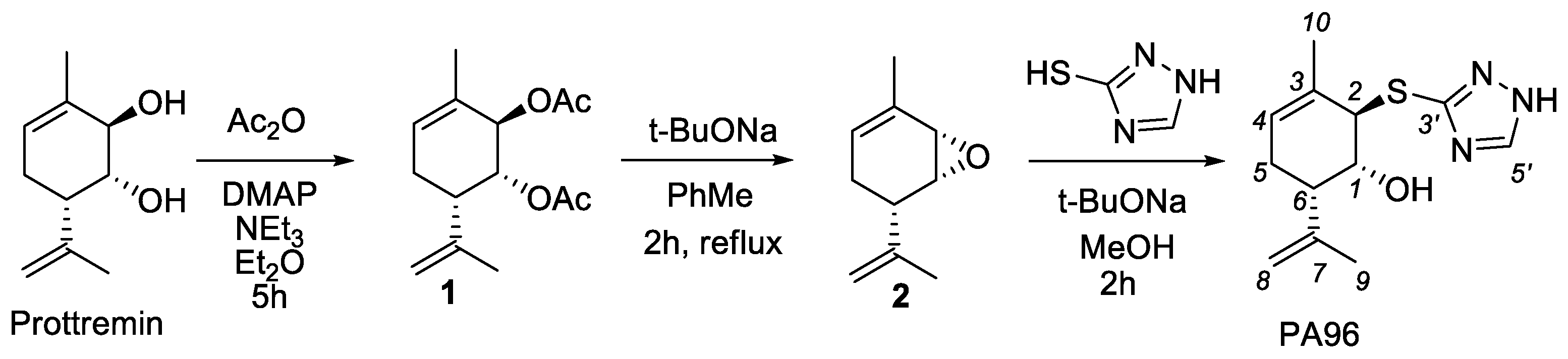
2.1. Synthesis of PA96
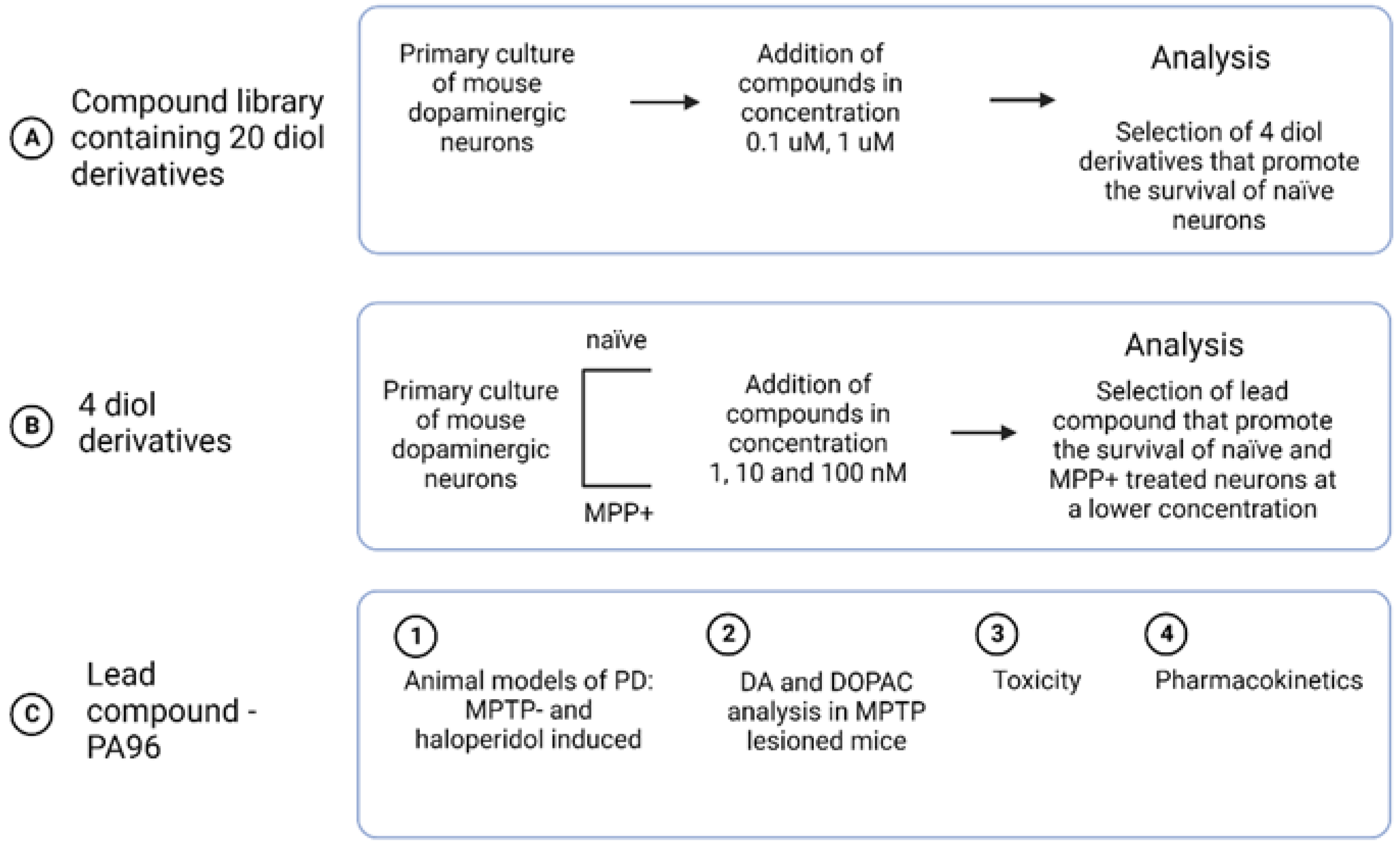
2.2. PA96 Supports the Survival of Naïve and MPP+-Treated Dopamine Neurons
2.3. Antiparkinsonian Activity of PA96 In Vivo in Two Models of PD
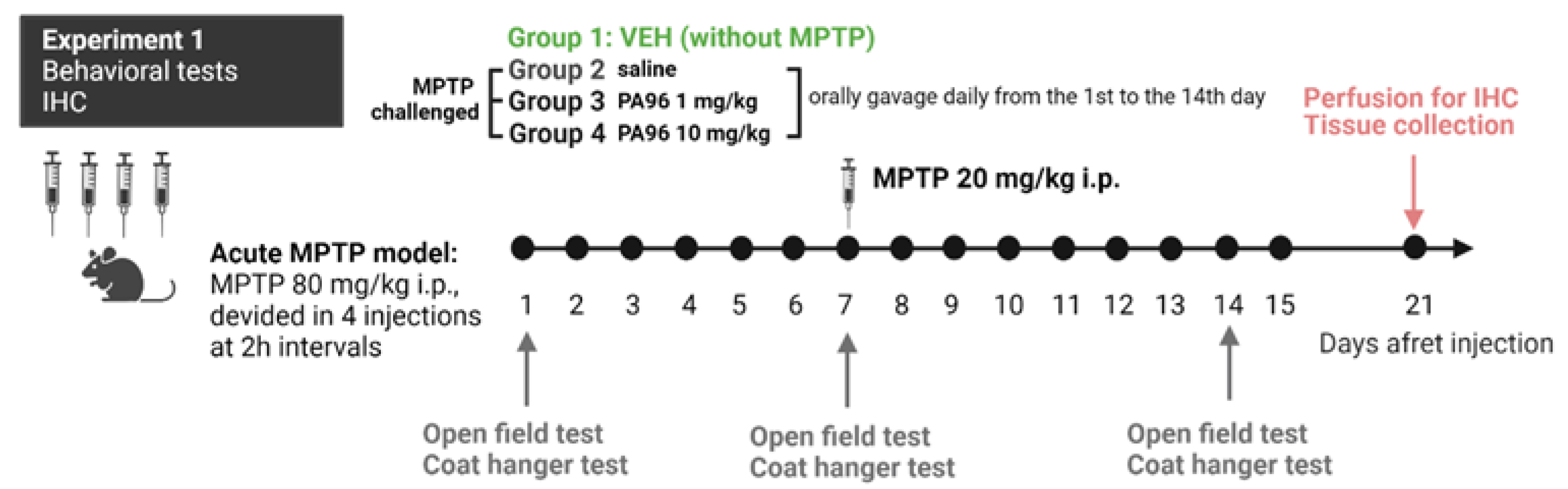
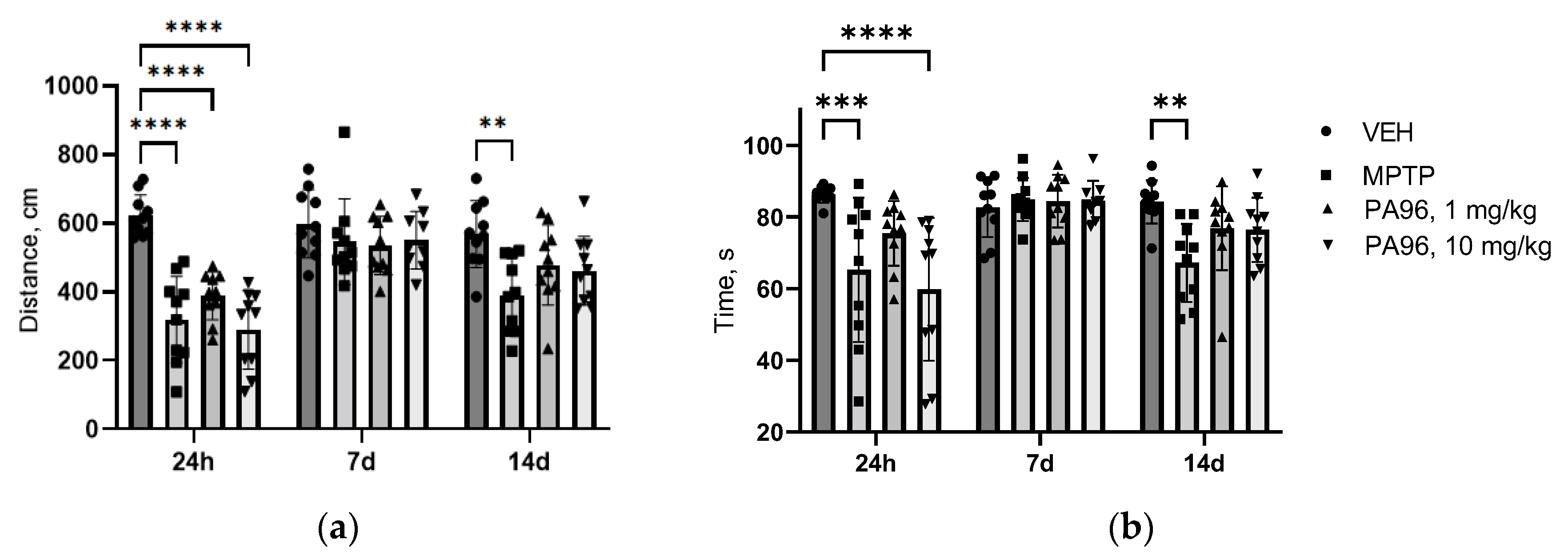
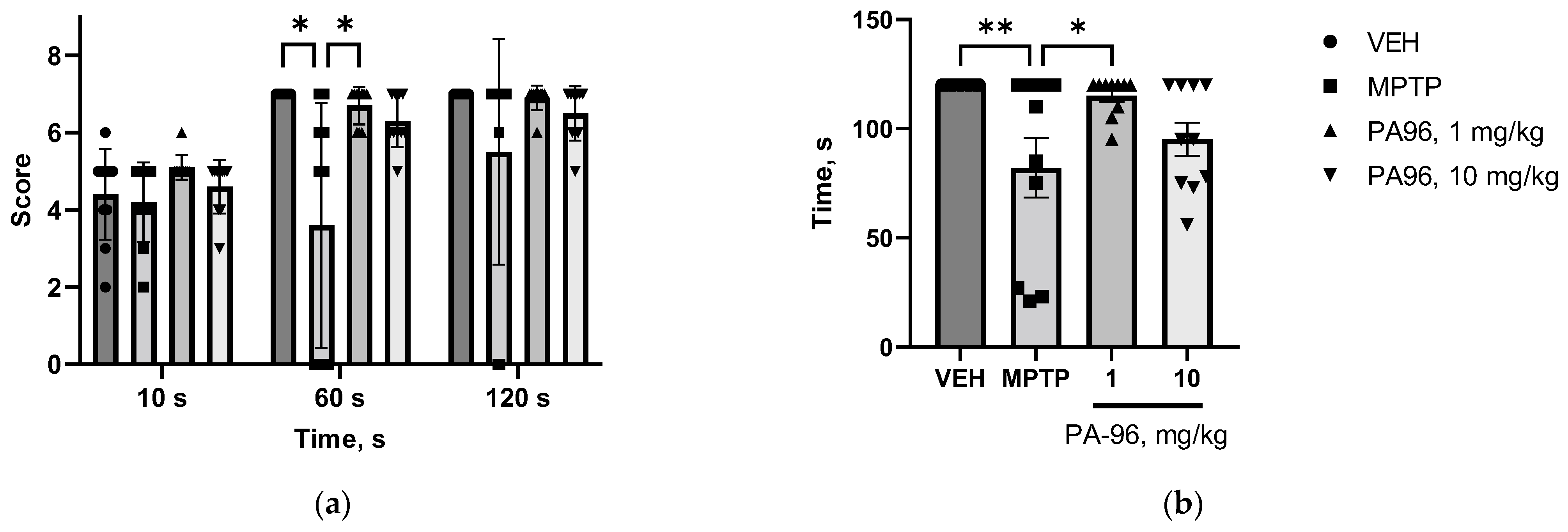
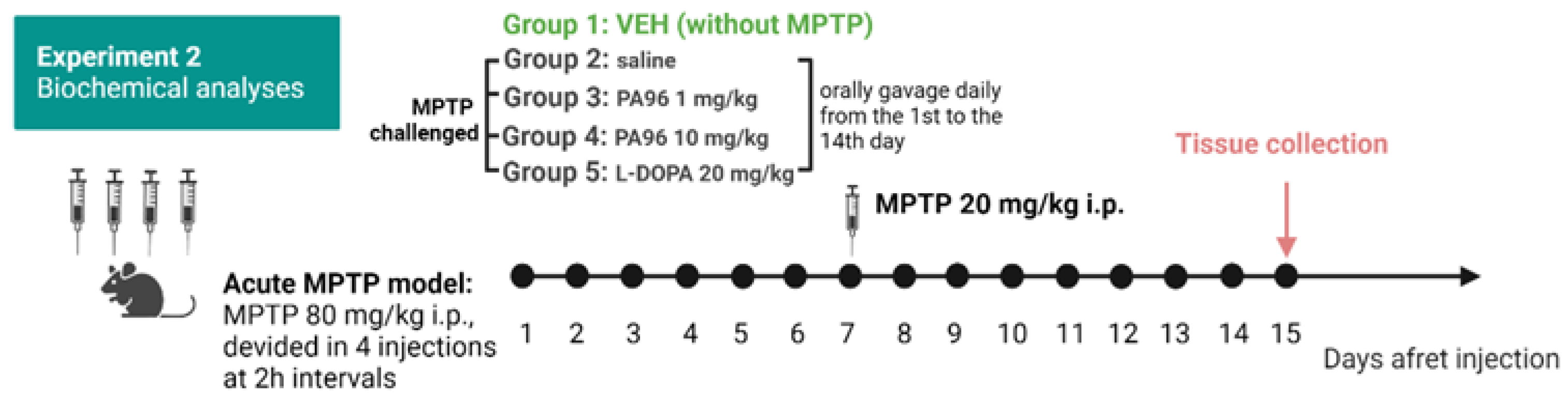
2.3.1. MPTP-Induced Model
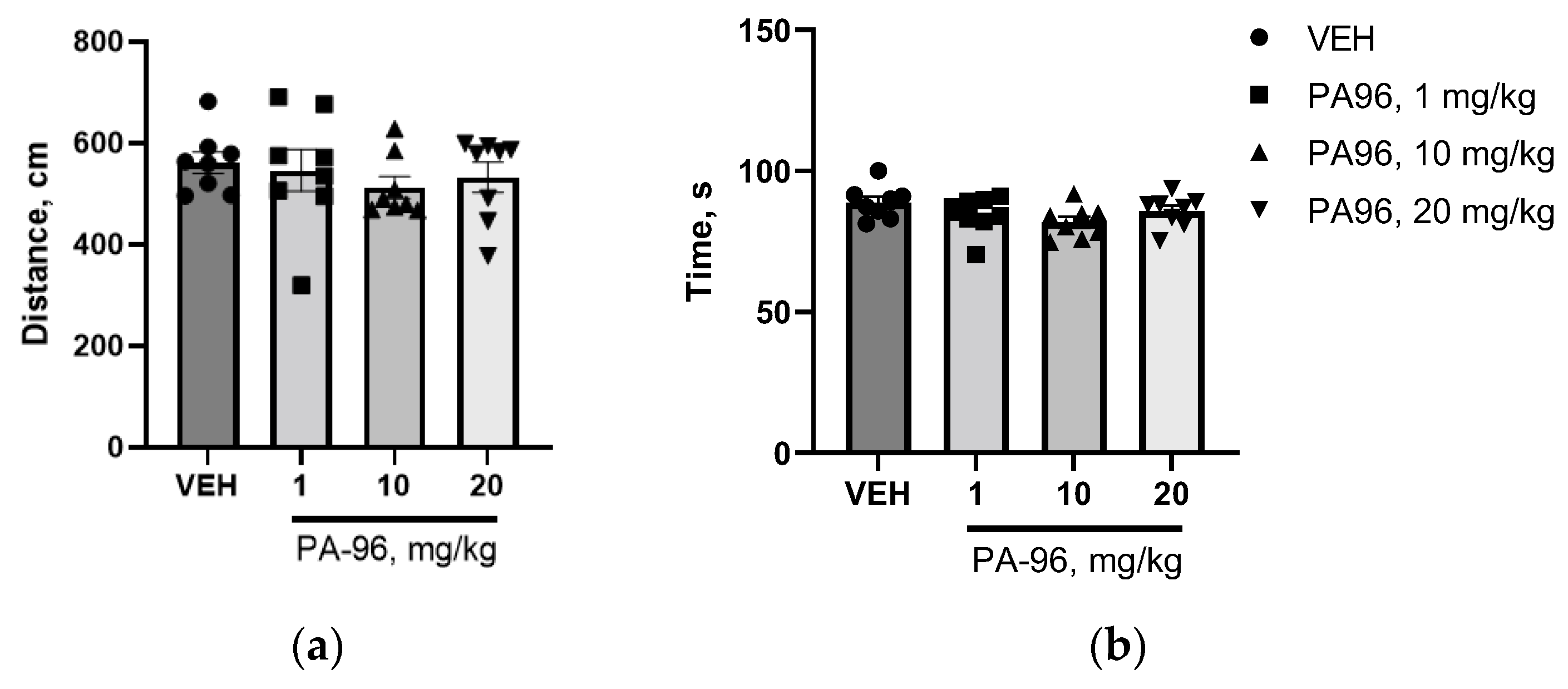
- Behavioral tests (open field, coat hanger)
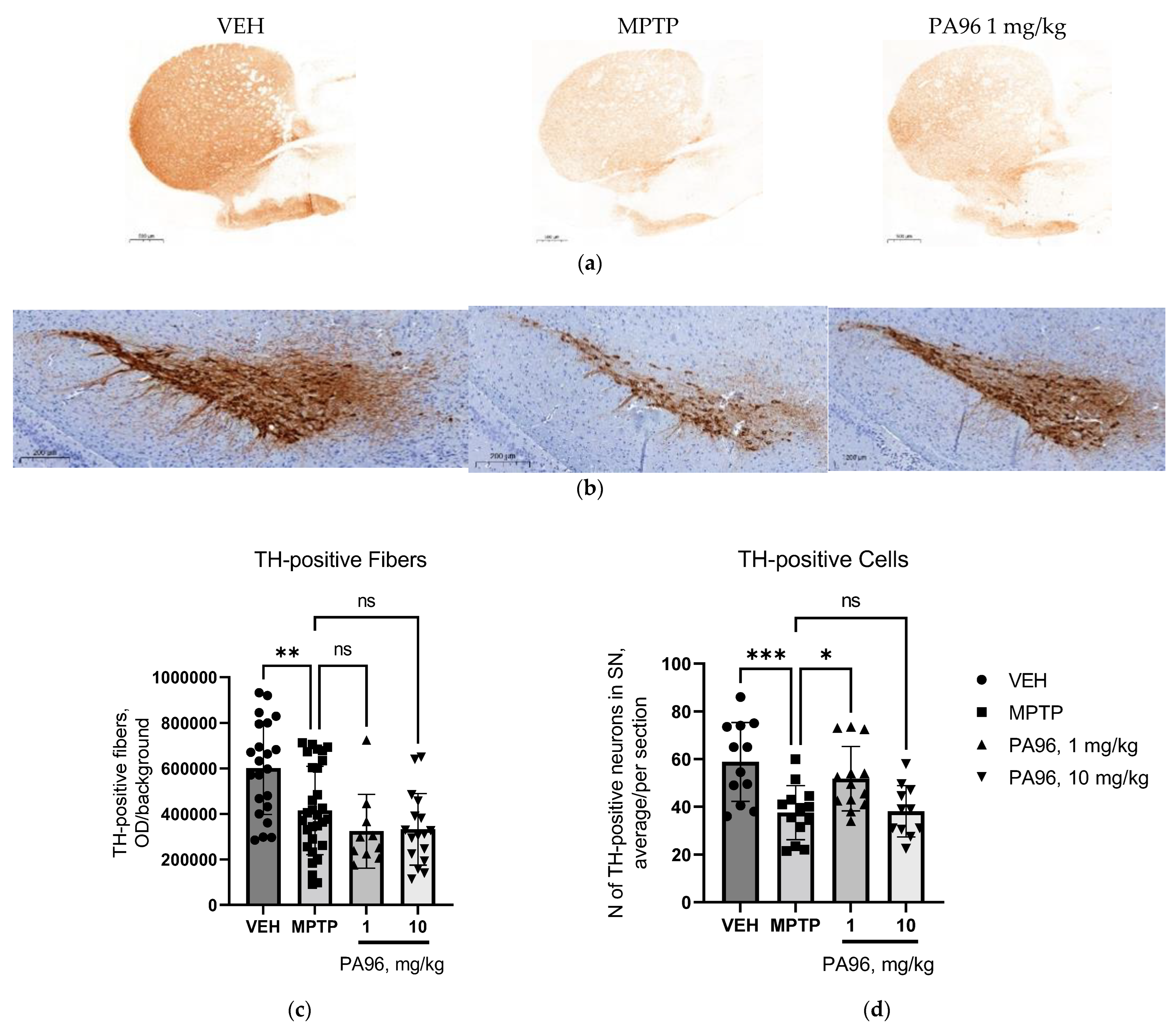
- Evaluation of the neuroprotective properties of PA96 in the nigrostriatal dopamine system
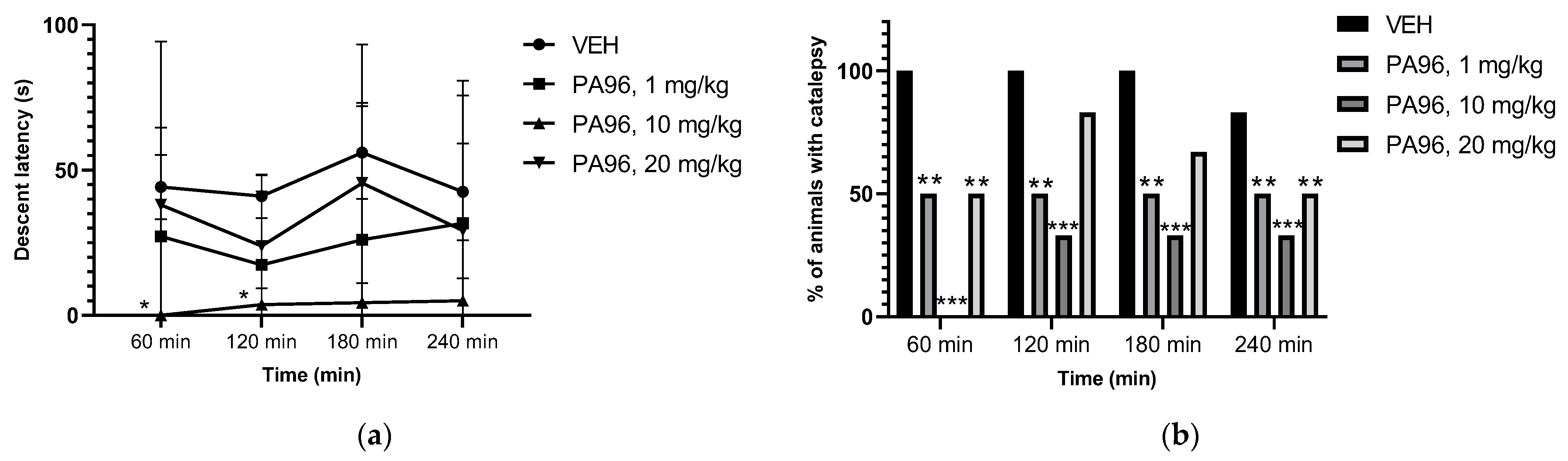
2.3.2. Antiparkinsonian Activity of PA96 in Haloperidol-Induced Catalepsy
2.4. Effects of PA96 on Dopamine and Dopamine Metabolite-DOPAC Levels in MPTP-Lesioned C57BL/6 Mice
2.5. Maximum Tolerated Dose and Toxicity of PA96 in Mice
2.6. Pharmacokinetics Study of PA96
3. Discussion
4. Methods
4.1. General Methods and Materials
4.2. Synthesis of (1R,2R,6S)-2-(1H-1,2,4-triazol-3-ylthio)-3-methyl-6-(prop-1-en-2-yl)cyclohex-3-enol PA96
4.3. Survival of Naïve and MPP+-Challenged Wild-Type Dopamine Neurons
4.4. Experimental Animals
4.5. Antiparkinsonian Activity of PA96 In Vivo
4.5.1. The MPTP Mouse Model of Parkinson’s Disease Induced by MPTP Neurotoxin
4.5.2. Behavioral Tests (Open Field and Coat Hunger Tests)
4.6. Assessment of Neurorestorative Properties of PA96 In Vivo
4.6.1. Sample collection and Tissue Processing
4.6.2. Immunohistochemistry
4.6.3. Optical Density and TH-Positive Neurons
4.7. Antiparkinsonian Activity of PA96 in Haloperidol-Induced Catalepsy
4.8. Neurochemical Analyses via HPLC-ED
4.9. Influence of PA96 on Naïve C57bl/6 Mice
4.10. Maximum Tolerated Dose and LD50 of PA96 In Vivo
4.11. Pharmacokinetics of PA96
4.11.1. Pharmacokinetic Study of PA96 in Whole Mouse Blood
- Chemicals and Reagents
- Preparing solutions
- Blood Sample Preparation protocol
4.11.2. Pharmacokinetic Study of PA96 in Brain Tissue Samples
- Chemicals and Reagents
- Preparing solutions
- Brain Tissue Homogenization Protocol
4.11.3. HPLC-MS/MS Conditions for Determining PA96
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gustavsson, A.; Svensson, M.; Jacobi, F.; Allgulander, C.; Alonso, J.; Beghi, E.; Dodel, R.; Ekman, M.; Faravelli, C.; Fratiglioni, L.; et al. Cost of Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 718–779. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; He, X.; Ge, J.; Zhu, J.; Yao, C.; Cai, H.; Ye, X.-Y.; Xie, T.; Bai, R. Discovery of Small-Molecule Compounds and Natural Products against Parkinson’s Disease: Pathological Mechanism and Structural Modification. Eur. J. Med. Chem. 2022, 237, 114378. [Google Scholar] [CrossRef] [PubMed]
- Fong, C.W. Permeability of the Blood-Brain Barrier: Molecular Mechanism of Transport of Drugs and Physiologically Important Compounds. J. Membr. Biol. 2015, 248, 651–669. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Volcho, K.P.; Salakhutdinov, N.F. Neuroregeneration in Parkinson’s Disease: From Proteins to Small Molecules. Curr. Neuropharmacol. 2019, 17, 268–287. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.-Y. Current Therapies in Clinical Trials of Parkinson’s Disease: A 2021 Update. Pharmaceuticals 2021, 14, 717. [Google Scholar] [CrossRef]
- Tolstikova, T.G.; Pavlova, A.V.; Morozova, Y.A.; Ardashov, O.V.; Il’ina, I.V.; Volcho, K.P.; Salakhutdinov, N.F.; Tolstikov, G.A. A Highly Effective Antiparkinsonian Drug of a New Structural Type. Dokl. Biol. Sci. 2010, 435, 398–399. [Google Scholar] [CrossRef]
- Ardashov, O.V.; Pavlova, A.V.; Il’ina, I.V.; Morozova, E.A.; Korchagina, D.V.; Karpova, E.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Highly Potent Activity of (1R,2R,6S)-3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol in Animal Models of Parkinson’s Disease. J. Med. Chem. 2011, 54, 3866–3874. [Google Scholar] [CrossRef]
- Valdman, E.; Kapitsa, I.; Ivanova, E.; Voronina, T.I.; Ardashov, O.; Volcho, K.; Khazanov, V.; Salakhutdinov, N. Evolution of Anti-Parkinsonian Activity of Monoterpenoid (1R,2R,6S)-3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol in Various in Vivo Models. Eur. J. Pharmacol. 2017, 815, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, I.; Volcho, K.; Korchagina, D.V.; Barkhash, V.A.; Salakhutdinov, N.F. Reactions of Allyl Alcohols of the Pinane Series and of Their Epoxides in the Presence of Montmorillonite Clay. Helv. Chim. Acta 2007, 90, 353–368. [Google Scholar] [CrossRef]
- Ardashov, O.V.; Pavlova, A.V.; Korchagina, D.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. 3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol: The Importance of Functional Groups for Antiparkinsonian Activity. Med. Chem. 2013, 9, 731–739. [Google Scholar] [CrossRef]
- Ardashov, O.; Pavlova, A.; Korchagina, D.; Volcho, K.; Tolstikova, T.; Salakhutdinov, N. Antiparkinsonian Activity of Some 9-N-, O-, S- and C-Derivatives of 3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol. Bioorg. Med. Chem. 2013, 21, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Ardashov, O.V.; Pavlova, A.V.; Mahato, A.K.; Sidorova, Y.; Morozova, E.A.; Korchagina, D.V.; Salnikov, G.E.; Genaev, A.M.; Patrusheva, O.S.; Li-Zhulanov, N.S.; et al. A Novel Small Molecule Supports the Survival of Cultured Dopamine Neurons and May Restore the Dopaminergic Innervation of the Brain in the MPTP Mouse Model of Parkinson’s Disease. ACS Chem. Neurosci. 2019, 10, 4337–4349. [Google Scholar] [CrossRef]
- Ardashov, O.; Zarubaev, V.; Shtro, A.; Korchagina, D.; Volcho, K.; Salakhutdinov, N.; Kiselev, O. Antiviral Activity of 3-Methyl-6-(Prop-1-En-2-Yl) Cyclohex-3-Ene-1,2-Diol and Its Derivatives Against Influenza A(H1N1)2009 Virus. Lett. Drug Des. Discov. 2011, 8, 375–380. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, P. Advancement in the Modelling and Therapeutics of Parkinson’s Disease. J. Chem. Neuroanat. 2020, 104, 101752. [Google Scholar] [CrossRef] [PubMed]
- Airavaara, M.; Parkkinen, I.; Konovalova, J.; Albert, K.; Chmielarz, P.; Domanskyi, A. Back and to the Future: From Neurotoxin-Induced to Human Parkinson’s Disease Models. Curr. Protoc. Neurosci. 2020, 91, e88. [Google Scholar] [CrossRef]
- Sidorova, Y.A.; Saarma, M. Can Growth Factors Cure Parkinson’s Disease? Trends Pharmacol. Sci. 2020, 41, 909–922. [Google Scholar] [CrossRef]
- Przedborski, S.; Jackson-Lewis, V.; Naini, A.B.; Jakowec, M.; Petzinger, G.; Miller, R.; Akram, M. The Parkinsonian Toxin 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP): A Technical Review of Its Utility and Safety. J. Neurochem. 2001, 76, 1265–1274. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP Mouse Model of Parkinson’s Disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef]
- Xu, Z.; Cawthon, D.; McCastlain, K.A.; Slikker, W.; Ali, S.F. Selective Alterations of Gene Expression in Mice Induced by MPTP. Synapse 2005, 55, 45–51. [Google Scholar] [CrossRef]
- Jîtcă, G.; Gall, Z.; Vari, C.-E.; Bianca, O.; Tero-Vescan, A.; Groșan, A.; Dogaru, M. Beneficial Effects of Metformin on Haloperidol-Induced Motor Deficits in Rats. A Behavioral Assessment. Acta Marisiensis Ser. Med. 2021, 67, 115–121. [Google Scholar] [CrossRef]
- Balijepalli, S.; Boyd, M.R.; Ravindranath, V. Inhibition of Mitochondrial Complex I by Haloperidol: The Role of Thiol Oxidation. Neuropharmacology 1999, 38, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, A.; Razzaq, B.; Rasheed, A.; Falah, I. Anti-Angiogenic Activity of Annona Reticulata n-Hexane Seeds Extract: In Vivo Study. Biochem. Cell. Arch. 2021, 21, 4327–4335. [Google Scholar]
- Mahato, A.K.; Sidorova, Y.A. Glial Cell Line-Derived Neurotrophic Factors (GFLs) and Small Molecules Targeting RET Receptor for the Treatment of Pain and Parkinson’s Disease. Cell Tissue Res. 2020, 382, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, P.; Saarma, M. Novel CDNF/MANF Family of Neurotrophic Factors. Dev. Neurobiol. 2010, 70, 360–371. [Google Scholar] [CrossRef]
- Sidorova, Y.A. Neurotrophic Factors to Combat Neurodegeneration. In Frontiers in Clinical Drug Research CNS and Neurological Disorders; Bentham Books: Potomac, MD, USA, 2022; Volume 10, pp. 132–187. ISBN 978-981-5040-68-5. [Google Scholar]
- Meredith, G.E.; Kang, U.J. Behavioral Models of Parkinson’s Disease in Rodents: A New Look at an Old Problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Shao, D.; Song, C. Behavior, Neurotransmitters and Inflammation in Three Regimens of the MPTP Mouse Model of Parkinson’s Disease. Physiol. Behav. 2009, 98, 130–138. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Marazziti, D.; Golini, E.; Mandillo, S.; Magrelli, A.; Witke, W.; Matteoni, R.; Tocchini-Valentini, G.P. Altered Dopamine Signaling and MPTP Resistance in Mice Lacking the Parkinson’s Disease-Associated GPR37/Parkin-Associated Endothelin-like Receptor. Proc. Natl. Acad. Sci. USA 2004, 101, 10189–10194. [Google Scholar] [CrossRef]
- Huebner, E.A.; Strittmatter, S.M. Axon Regeneration in the Peripheral and Central Nervous Systems. Results Probl. Cell Differ. 2009, 48, 339–351. [Google Scholar] [CrossRef]
- Tolstikova, T.G.; Pavlova, A.V.; Dolgikh, M.P.; Il’ina, I.V.; Ardashov, O.V.; Volcho, K.P.; Salakhutdinov, N.F.; Tolstikov, G.A. (4S,5R,6R)-Para-Mentha-1,8-Dien-5,6-Diol Is a New Highly Effective Anticonvulsant Agent. Dokl. Biol. Sci. 2009, 429, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Hazardous Drug Exposures in Health Care | NIOSH | CDC. Available online: https://www.cdc.gov/niosh/topics/hazdrug/default.html (accessed on 29 September 2022).
- Sidorova, Y.A.; Bespalov, M.M.; Wong, A.W.; Kambur, O.; Jokinen, V.; Lilius, T.O.; Suleymanova, I.; Karelson, G.; Rauhala, P.V.; Karelson, M.; et al. A Novel Small Molecule GDNF Receptor RET Agonist, BT13, Promotes Neurite Growth from Sensory Neurons in Vitro and Attenuates Experimental Neuropathy in the Rat. Front. Pharmacol. 2017, 8, 365. [Google Scholar] [CrossRef]
- Mätlik, K.; Võikar, V.; Vilenius, C.; Kulesskaya, N.; Andressoo, J.-O. Two-Fold Elevation of Endogenous GDNF Levels in Mice Improves Motor Coordination without Causing Side-Effects. Sci. Rep. 2018, 8, 11861. [Google Scholar] [CrossRef]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole Animal Perfusion Fixation for Rodents. JoVE J. Vis. Exp. 2012, 65, e3564. [Google Scholar] [CrossRef] [PubMed]
- Penttinen, A.-M.; Suleymanova, I.; Albert, K.; Anttila, J.; Voutilainen, M.H.; Airavaara, M. Characterization of a New Low-Dose 6-Hydroxydopamine Model of Parkinson’s Disease in Rat. J. Neurosci. Res. 2016, 94, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Sinyakova, N.A.; Bazhenova, E.Y.; Kulikova, E.A.; Fursenko, D.V.; Kulikov, A.V. Effect of the C1473G Polymorphic Variant of the Tryptophan Hydroxylase 2 Gene and Photoperiod Length on the Dopamine System of the Mouse Brain. Mol. Biol. 2020, 54, 60–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]




| Dose Level (mg/kg/day) | Mortality, % |
|---|---|
| 500 | 100 |
| 200 | 60 |
| 150 | 50 |
| 100 | 0 |
| Parameter | Blood | Units | Brain | Units |
|---|---|---|---|---|
| t1/2 | 22.8 ± 1.5 | min | 18.8 ± 0.3 | min |
| Tmax | 5 | min | 5 | min |
| Cmax | (5.5 ± 0.5) × 102 | ng/mL | (1.25 ± 0.04) × 102 | ng/g |
| AUC0-t | (16.7 ± 1.5) × 103 | ng/mL∗min | (1.99 ± 0.09) × 103 | ng/g∗min |
| AUC0-inf_obs | (17.4 ± 1.8) × 103 | ng/mL∗min | (2.03 ± 0.09) × 103 | ng/g∗min |
| MRT0-inf_obs | 29.7 ± 2.2 | min | 21.5 ± 0.7 | min |
| Vz/F_obs | (18.9 ± 0.6) × 10−3 | (ml/kg)/(ng/mL) | (133 ± 4) × 10−3 | (mg/kg)/(ng/g) |
| Cl/F_obs | (5.8 ± 0.6) × 10−4 | (mg/kg)/(ng/mL)/min | (50 ± 1) × 10−4 | (mg/kg)/(ng/g)/min |
| Analyte and Precursor Ion (m/z) | Product Ion (m/z) | DP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|
| PA96 252.2 | 102.1 (quantifier) | 21 | 23 | 4 |
| 133.2 (qualifier) | 21 | 21 | 4 | |
| 151.3 (qualifier) | 21 | 17 | 4 | |
| IS 152.3 | 93.1 (qualifier) | 16 | 35 | 14 |
| 107.2 (qualifier) | 21 | 37 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotliarova, A.; Podturkina, A.V.; Pavlova, A.V.; Gorina, D.S.; Lastovka, A.V.; Ardashov, O.V.; Rogachev, A.D.; Izyurov, A.E.; Arefieva, A.B.; Kulikov, A.V.; et al. A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo. Molecules 2022, 27, 8286. https://doi.org/10.3390/molecules27238286
Kotliarova A, Podturkina AV, Pavlova AV, Gorina DS, Lastovka AV, Ardashov OV, Rogachev AD, Izyurov AE, Arefieva AB, Kulikov AV, et al. A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo. Molecules. 2022; 27(23):8286. https://doi.org/10.3390/molecules27238286
Chicago/Turabian StyleKotliarova, Anastasiia, Alexandra V. Podturkina, Alla V. Pavlova, Daria S. Gorina, Anastasiya V. Lastovka, Oleg V. Ardashov, Artem D. Rogachev, Arseniy E. Izyurov, Alla B. Arefieva, Alexander V. Kulikov, and et al. 2022. "A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo" Molecules 27, no. 23: 8286. https://doi.org/10.3390/molecules27238286
APA StyleKotliarova, A., Podturkina, A. V., Pavlova, A. V., Gorina, D. S., Lastovka, A. V., Ardashov, O. V., Rogachev, A. D., Izyurov, A. E., Arefieva, A. B., Kulikov, A. V., Tolstikova, T. G., Volcho, K. P., Salakhutdinov, N. F., & Sidorova, Y. (2022). A Newly Identified Monoterpenoid-Based Small Molecule Able to Support the Survival of Primary Cultured Dopamine Neurons and Alleviate MPTP-Induced Toxicity In Vivo. Molecules, 27(23), 8286. https://doi.org/10.3390/molecules27238286










