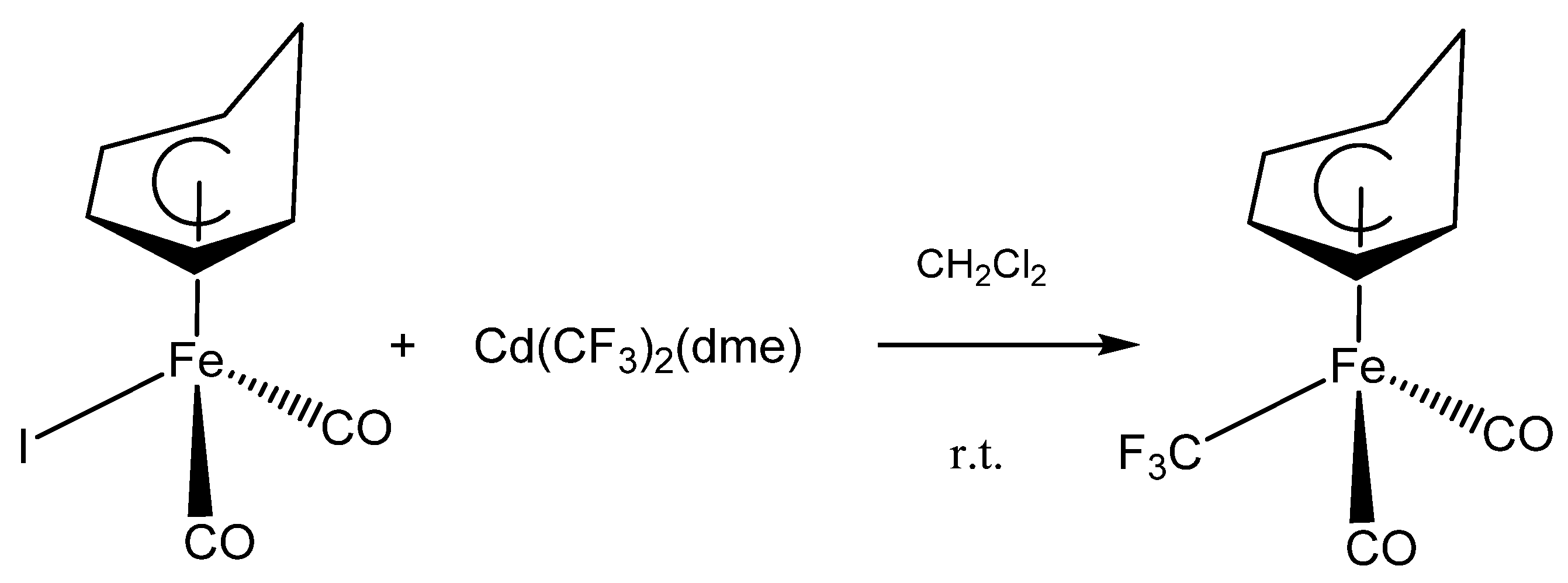Abstract
Fluorochemistry is a field of tremendous developments and advances in several areas of science including materials, pharmaceuticals and agriculture. This makes the design and synthesis of fluorine-containing substances highly desirable research targets. The sub-area of synthetic perfluorinated chemistry proportionately attracts widespread interest by applying to all areas of chemistry including organic and inorganic. Particularly, the latter is much underdeveloped as metal complexes with perfluoroalkyl moieties are scarce, with the vast majority of perfluorinated analogs, of long known, halo and alkylated derivatives never having been synthesized. Focusing on the chemistry of trifluoromethyl group, which is the most important in the class of perfluoroalkyls, we set out to explore the possibility of synthesizing and completely characterizing a cyclohexadienyl metal complex. Upon utilizing a number of trifluorometylating reagents, we only arrived at an efficient preparation by the use of Morrison’s trifluormethylating reagent. As a result, the new, air- and moisture-sensitive complex (η5-C6H7)Fe(CO)2CF3, was prepared in 71% yield, using a nucleophilic iodo-for-trifluoromethyl substitution, and was completely characterized including by X-ray crystallography.
1. Introduction
The chemistry and applications of the trifluoromethyl group have a special place in fluorine chemistry as they continue to intrigue and influence both academic research and industrial laboratories [1,2]. On one hand, academic researchers are attracted by the variety of interesting properties of the CF3 moiety, including its high electronegativity, CF3-induced solubility changes, C-F activation, including C-F oxidative addition and reductive elimination, as well. 19F NMR spectroscopy monitoring of molecules and intermediates [3,4]. On the other hand, the industry is heavily utilizing the trifluoromethyl moiety for the synthesis of a variety of pharmaceuticals and drugs, often replacing a chloride or a methyl group [5,6]. An illustration of this point is the fact that several notable drugs contain trifluoromethyl groups including the antidepressant Prozac, the HIV reverse transcriptase inhibitor Sustiva, and the nonsteroidal anti-inflammatory drug, Celebrex [7].
In organometallic chemistry, despite the high interest on the CF3 moiety, the synthesized trifluoromethylated metal complexes are considerably less than their methylated analogs or the ones that bear halides and hydrides [8,9]. This has implications in the limited application reactions of LnMCF3 (L = ligand) which are much more limited compared to the applications of the aforementioned analogs that include advances in C-H activation, C-C activation, small molecule activation, etc. [10,11]. An example of such a void in the literature of the much-studied dienyl metal complexes, is the fact that there are no cyclohexadienyl metal trifluoromethyl derivatives. This, while there is a plethora of report on methylated, hydride, halide analog complexes as well as interesting applications [10].
As a result, contributing to the synthetic chemistry of trifluoromethyl complexes, we investigated the synthesis of complexes with a cyclohexadienyl ligand. By using Morrison’s trifluoromethylating reagent, Cd(CF3)2(DME), [11] we prepared and fully characterized the (η5-C6H7)Fe(CO)2CF3 and we determined its structure by X-ray crystallography.
2. Experimental
All reactions and manipulations were performed in oven- or flame-dried glassware under Argon atmosphere either in a glovebox or by using standard Schlenk techniques. Column chromatography was carried out using silica gel 62 (60–200 mesh) supplied by Mallinckrodt SilicAR, which had been pre-dried at 250 °C under high vacuum. 1,2-dimethoxyethane (DME) and diethyl ether (Et2O) were distilled from sodium-benzophenone ketyl under nitrogen. Dichloromethane (CH2Cl2) was dried over calcium hydride (CaH2) and distilled under N2 prior to use. Copper bromide (CuBr) was purchased from Aldrich and dried by heating to 45 °C for 12 h under high vacuum. (C6H7)Fe(CO)2I, and Cd(CF3)2(DME) were prepared and purified by literature methods [12,13]. 1H NMR, [13] C NMR, and 19F NMR spectra were recorded on either a Bruker Avance 500, or a Bruker AM-400 spectrometer with Nalorac BB probes. All 1H chemical shifts (δ) are reported in ppm relative to trimethylsilane. 19F NMR chemical shifts are given in ppm downfield from CFCI3. Multiplicities are indicated by s (singlet), d (doublet). Coupling constants are reported in Hertz. Infrared spectra were recorded from KBr pellets on an ATI Mattson 106 Genesis II Series FT-IR spectrometer. Infrared bands are given in cm−1. Uncorrected melting points were determined with a Thomas Hoover capillary melting point apparatus in flame-sealed Pyrex capillaries. Low-resolution electron impact mass spectra (LR/MS-EI) and high-resolution electron impact mass spectra (HR/MS-EI) were obtained on a Finnigan LCQ spectrometer in the APCI mode. LRMS-EI were also obtained on a Hewlett Packard 5987A quadrupole instrument. Elemental combustion analyses were performed by Midwest Microlab, LLC of Indianapolis, IN. For the X-crystallographic analysis, a single crystal of (C6H7)Fe(CO)2CF3 was mounted on the goniometer of a Bruker three circle, single crystal diffractometer with APEX detector and SMART software, using an argon-filled capillary, and all data were collected at room temperature. All the experimental details of the crystallographic study are given in five tables in the Supplementary Material (Tables S1–S5).
Synthesis of (C6H7)Fe(CO)2CF3 without CuBr. C6H7Fe(CO)2l (50.0 mg, 0.157 mmol) and Cd(CF3)2(DME) (75 mg, 0.220 mmol) were placed into a 10 mL flask and the system was attached to a vacuum line. After evacuation, 3 mL of dry CH2Cl2 were distilled onto the reagents and the mixture was magnetically stirred at ambient temperature. After 5 h, the solution started to turn orange from brown. After 20 h, the color had changed to yellow. The solvent and all other volatile materials were removed under vacuum and a yellow solid was obtained. This solid was dissolved in 3 mL of Et2O and then chromatographed on a silica gel column under nitrogen, using Et2O as eluent. Upon removal of the solvent, a yellow solid later identified as (C6H7)Fe(CO)2CF3 (8.5 mg, 0.032 mmol) was obtained in 21% yield. The compound can also be purified by sublimation at ambient temperature under high vacuum (0.1 mm). It melts at 42–43 °C and it is air sensitive. 1H NMR (δ ppm in CDCl3): 2.11 (d, 2JH-H = 14.1 Hz, 1H, C6H7), 2.79 (dt, 2JH-H = 14.1 Hz, 1H, C6H7), 3.10 (t, 3JH-H = 6.2 Hz, 2H, C6H7), 5.36 (t, 3JH-H = 6.2 Hz, 2H, C6H7), 7.02 (t, 3JH-H = 6.2 Hz, 1H, C6H7). 13C{1H} NMR (δ ppm in CDCl3): 23.7 (s, 2C, C6H7), 83.2 (s, 4C, C6H7). 19F NMR (δ ppm in CDCl3): −0.8 (s, 3F, CF3). FT-IR (cm−1, KBr pellet): 3090 (C-H), 2000 (C-O), 1996 (C-O), 1093 (C-F), 1044 (C-F), 991 (C-F). LRMS-EI (Ion, m/e, %): (C6H7)Fe(CO)2CF3 260, 12; (C6H7)Fe(CO)CF3 232, 2; (C6H7)Fe(CO)CF2 213, 2; (C6H7)FeCF2 185, 11; (C6H6)FeCO 162, 39; (C6H7)FeF 154, 36; (C6H6)Fe 134, 100; FeCF3 125, 10. HRMS-EI m/z): found, 259.9738; Calcd. for C9H7F3FeO2, 259.9748 (Δm/m = 3.7 ppm). Elemental Analysis Found: C, 41.68; H, 2.88. Calcd. for C9H7F3FeO2: C, 41.58; H, 2.71.
Synthesis of (C6H7)Fe(CO)2CF3 with CuBr. C6H7Fe(CO)2l (150.0 mg, 0.472 mmol), Cd(CF3)2(DME) (200 mg, 0.588 mmol) and CuBr (200 mg, 1.394 mmol) were placed into a 25 mL flask and the system was attached to a vacuum line and cooled to −78 °C. After evacuation, 10 mL of dry dichloromethane were distilled onto the reagents and the mixture was warmed with stirring to room temperature. After 1 h, the solution started to turn orange from brown. Finally, after 3 h, the color had changed to yellow. The solvent and all other volatile materials were removed under vacuum and the yellow solid was obtained. This solid was dissolved in 5 mL of diethyl ether and then chromatographed on a silica gel column under nitrogen, using diethyl ether as eluent. Upon removal of the solvent, (C6H7)Fe(CO)2CF3 (87.1 mg, 0.335 mmol) was obtained in 71% yield.
Supplementary data CCDC <2196136> contains the supplementary crystallographic data for the (C6H7)Fe(CO)2CF3 complex. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, accessed on 22 October 2022, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk.
3. Results and Discussion
The main cause of the imbalance between the number of synthetic and application reports on trifluoromethylated metal complexes, LnM-CF3, and the reports on analogous complexes, LnM-CH3, LnM-H, and LnM-X (X = halide), is the lack of effective trifluoromethylating reagents as compared to the potency of analogous reagents for the other derivatives, which include, among others, alkyllithiums, Grignard in methylation reactions, sodium borohydride and lithium borohydride in hydride yielding reactions, and metal halides in preparing halide reactions [14]. This problem makes the introduction of the CF3 moiety to metal substrates complicated, especially when challenging substrates are subjected to trifluoromethylation [15]. An illustrative example is the organometallic chemistry of Fe, in which there is currently only one Fe-CF3 complex known in the literature in which the Fe center is connected to a organocyclic ligand. In contrast, there are hundreds of Fe-CH3, Fe-H and Fe-X counterparts reported. Moreover, X-ray analysis data on Fe-CF3 complexes is even more scarce in the literature with only a handful studied and published [16].
In our efforts to explore the synthetic chemistry; enrich the knowledge; and provide access to pathways that lead to trifluoromethyl compounds; this work has targeted the synthesis of the first trifluoromethylated cyclohexadienyl metal complex. Part of the interest in metal complexes bearing the cyclohexadienyl ligand is their potential in catalytic applications [17]. An iodo Fe precursor was selected as a substrate for nucleophilic trifluromethylation reactions. Upon surveying the literature of the limited number of previously synthesized Fe-CF3 containing complexes; we realized that apart of Morrison’s trifluoromethylating reagent; Cd(CF3)2(DME), which was utilized once in the past; only reactions with CF3I and decarboxylation reactions of a Fe-COCF3 bond; have been reported to result in formation of Fe-CF3 bonds [16,18]. A number of reactions were attempted utilizing milder nucleophilic trifluoromethylating reagents; but they did not result in Fe-CF3 bond formation at the various temperature and solvent conditions. In particular; as monitoring by 19F spectroscopy; the precursor (C6H7)Fe(CO)2I did not react for an iodo to trifluoromethyl exchange for all of the following previously utilized combinations at ambient and elevated temperatures: i. CF3I in CH2Cl2, [19] ii. AgCF3 prepared in situ from AgF and Ruppert’s reagent, [20] iii. Me3SiCF3 in THF, [21] and iv. CuCF3 prepared in situ from CF3CO2Na/CuI in NMP [22]. During these reactions; a variety of 19F NMR resonances; outside the M-CF3 region were observed, [23] most likely due to the nucleophilic attach of the CF3-moiety to the carbocylic ligand; consistent with reports of nucleophile; metal complex-assisted arene functionalization [24]. Attempts to isolate pure products on these reactions were unsuccessful.
Upon treating (C6H7)Fe(CO)2I with Cd(CF3)2(DME) in anhydrous dichloromethane at ambient temperature; a slow reaction occurred that turned the brown solution yellow; converting the iodide to the new (C6H7)Fe(CO)2CF3 as shown in Figure 1. The progress of the reaction was monitored by 19F NMR spectroscopy which indicated the disappearance of the peak at −36.8 ppm (2JF-Cd = 454 Hz); due to Cd(CF3)2(dme), [15] and the appearance of new signals at −0.8 and −0.2 ppm for (C6H7)Fe(CO)2CF3. These signals are consistent with the region previously reported for Fe-CF3 bonds [16,18]. As it has been observed for similar reactions; the yield of the yellow air-sensitive solids was enhanced by the addition of CuBr to the reaction mixture [15].
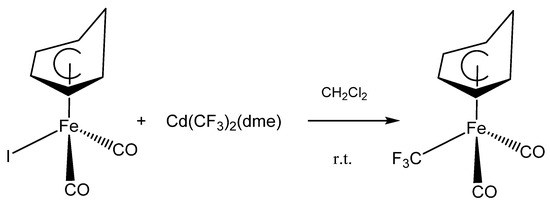
Figure 1.
The reaction of (C6H7)Fe(CO)2CF3 with Morrison’s reagent, Cd(CF3)2(dme).
After purification the product were sublimed, to give highly pure (C6H7)Fe(CO)2CF3 as a yellow solid in 71% yield. The new complex is air sensitive decomposing in the air withing 48 h to a brown solid that has no 19F NMR signal. Moreover, the complex is moisture sensitive, decomposing upon exposure to wet solvent again to a solid that has no 19F NMR signal. The decomposition products were not investigated further. Finally, the new complex is not affected by light and it stays indefinitely stable in a flame sealed NMR tube.
The spectral properties of the new complex reveal five different protons in the 1H NMR with two distinct protons at 2.11 and 2.79 ppm for the perturbed saturated carbon, and three resonances for the remaining five protons of the five coplanar unsaturated carbons at 3.10, 5.36 and 7.02 ppm. The two saturated and unsaturated carbons also show two distinct resonances in the 13C NMR at 23.7 and 83.2 respectively. A singlet at −0.8 ppm is registered in the 19F NMR for the three equivalent fluorine atoms of the trifluoromethyl group, which in the same region as the −7.1 ppm of the Fe[(CF3)(CONiPr2)(CO2)(PPh3)] and +10.2 ppm for the CpFe(CO)2CF3. Finally, the IR spectra of the new complex contained weak C-H stretches around 3100 cm−1 and two strong absorptions at 2000 and 1996 cm−1 from the symmetric and asymmetric C-O stretches of the carbonyl groups. The C-F stretches were observed as strong absorptions at 1093, 1044 and 991 cm−1. Comparison of the C-F stretching frequencies of the new complex with the ones at 1010 and 1055 cm−1 of the corresponding CpFe(CO)2CF3 may suggest that the cyclohexadienyl group is less electron donating than the cyclopentadienyl group, overall making the backbonding and the Fe-CF3 bond of the former weaker.
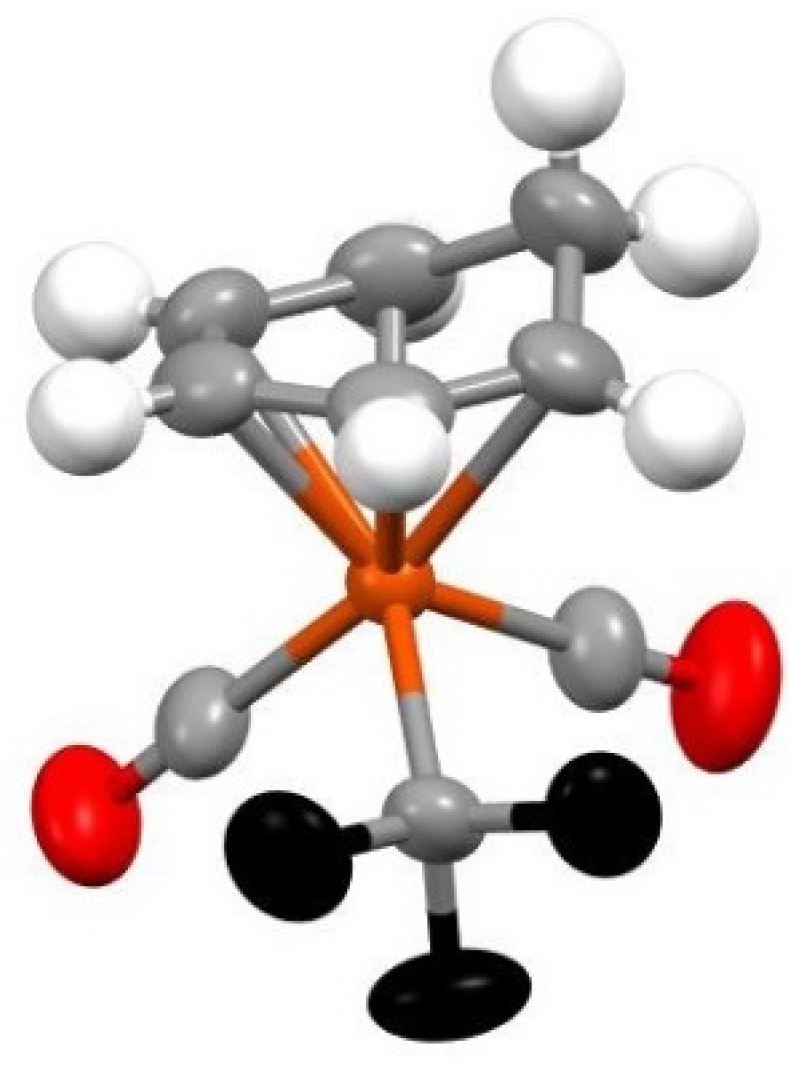
Yellow crystals of the product were grown by layering anhydrous dichloromethane solution with anhydrous hexanes and keeping undisturbed for 14 days in a sealed NMR tube. This were analyzed by X-ray crystallography to give the structure of (C6H7)Fe(CO)2CF3 as shown in Figure 2.
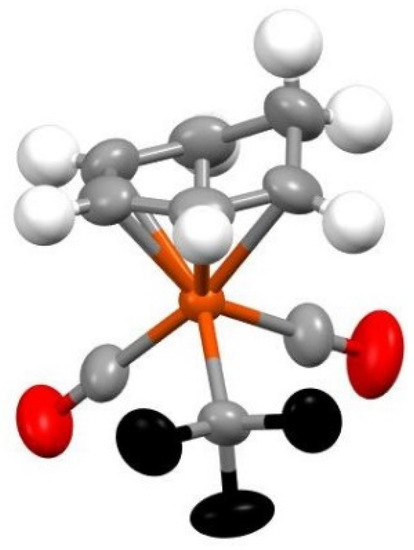
Figure 2.
X-ray structure of (C6H7)Fe(CO)2CF3 at the 50% probability level.
In the analyzed structure and in line with what it has been reported before for structures of metal coordinated cyclohexadienyl organic rings [25], the six carbons are highly nonplanar, with one of them, the one and only saturated carbon being out of the plane that the remaining five carbon atoms form. Specifically, the methylene carbon lies ~0.63 Å above the unsaturated ring, and the Fe atom is 1.60 Å away from the plane of this ring, analogously to the corresponding values (~0.65 and 1.66 Å) reported by Shirin, et al. for a Ru-coordinated cyclohexadienyl ring [25]. The dihedral angle between the plane of the five carbons and the second plane of the three carbons that contains the saturated carbon is 45°, which is slightly larger but analogous to the 40° recorded for the (2-methoxycyclohexadienyl)Fe(CO)3+ species [26]. The Fe-CF3 bond of 1.968(3) Å, which very similar to the Fe-CF3 of one of the few iron trifluoromethyl structures Fe[(CF3)(CONiPr2)(CO2)(PPh3)], which is 1.979(4) Å [16]. In general the trifluoromethyl group takes up almost identical positions with that of the Fe[(CF3)(CONiPr2)(CO2)(PPh3)] complex, with the C-F bonds averaging 1.355(4) Å in the new complex vs. 1.353 Å in the old one and F-C-F angle 103.2° vs. 102.1°. Finally, the C-O bonds average is 1.225(4) Å, which is consistent with other Fe-CO bonds.
Hirshfeld surface analysis serves as a powerful tool for gaining additional insight into the intermolecular interaction of molecular crystals. The size and shape of Hirshfeld surface allows the qualitative and quantitative investigation and visualization of intermolecular close contacts in molecular crystals [27]. The Hirshfeld surface enclosing a molecule is defined by a set of points in 3D space where the contribution to the electron density from the molecule of interest is equal to the contribution from all other molecules.
Molecular Hirshfeld surfaces are constructed based on electron distribution calculated as the sum of spherical atom electron densities [28]. Thus, an isosurface is obtained, and for each point of the isosurface two distances can be defined: de, the distance from the point to the nearest atom outside to the surface, and di, the distance to the nearest atom inside to the surface. Moreover, the identification of the regions of particular importance to intermolecular interactions is obtained by mapping normalized contact distance (dnorm), expressed as:
where rivdw and revdw are the van der Waals radii of the atoms [29]. The value of dnorm is negative or positive when intermolecular contacts are shorter or longer than rvdw, respectively. Graphical plots of the molecular Hirshfeld surfaces mapped with dnorm employ the red–white–blue color scheme where red color indicates the shorter intermolecular contacts, white color shows the contacts around the rvdW separation, and blue color is used to indicate the longer contact distances. Because of the symmetry between de and di in the expression for dnorm, where two Hirshfeld surfaces touch, both will display a red spot identical in color intensity as well as size and shape [30].
dnorm = (di − rivdw)/rivdw + (de − revdw)/revdw
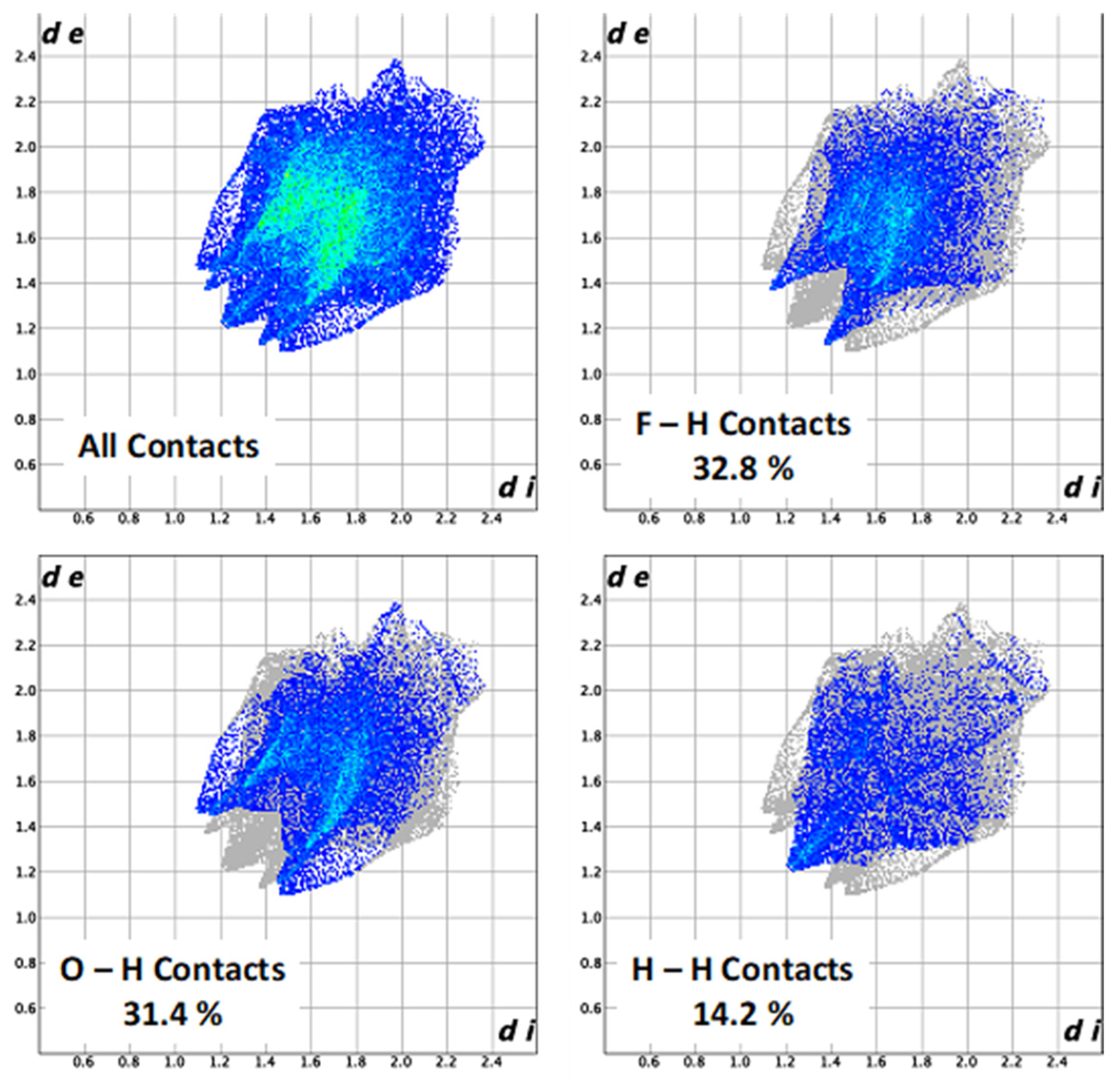
The combination of de and di in the form of a 2D fingerprint plot provides summary of intermolecular contacts in the crystal and are in complement to the Hirshfeld surfaces [31]. Such plots provide information about the intermolecular interactions in the immediate environment of each molecule in the asymmetric unit. Moreover, the close contacts between particular atom types can be highlighted in so-called resolved fingerprint plots, [29] which allows the facile assignment of an intermolecular contact to a certain type of interaction and quantitatively summarize the nature and type of intermolecular contacts. The Hirshfeld surfaces are mapped with dnorm, de, and 2D fingerprint plots presented were generated using Crystal-Explorer 17.5 [32,33,34,35,36]. As can be seen from the results of the contact analysis, presented in Figure 3 and summarized in Table 1, H-X interactions account for 84.8% of all contacts, signifying a highly compact solid-state structure.
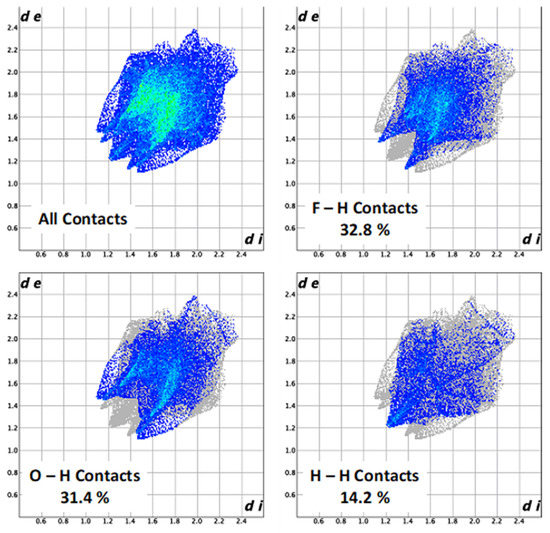
Figure 3.
Fingerprint plots (de vs. di) for the Hirshfeld surface of (C6H7)Fe(CO)2CF3. (top left) Full fingerprint plot; (top right) resolved fingerprint plot for the F–H contacts, accounting for 32.8% of the contacts; (bottom left) resolved fingerprint plot for the O–H contacts, accounting for 31.4% of the contacts; (bottom right) resolved fingerprint plot for the H–H contacts, accounting for 14.2% of the contacts.

Table 1.
Hirshfeld surface analysis results for complex (C6H7)Fe(CO)2CF3.
4. Conclusions
Filling a void in the chemistry literature, and facilitating access to novel trifluoromethyl metal complexes, the synthesis and full characterization, including X-ray diffraction of the first cyclohexadienyl metal complexes (C6H7)Fe(CO)2CF3 in high yields is reported by the reaction of the iron precursor with Morrison’s trifluoromethylating reagent, Cd(CF3)2(DME) at room temperature. The new complex is an air- and moisture-sensitive complex species that was prepared in 71% yield. X-ray crystallography of the new complex revealed that the six carbons are highly nonplanar, with one of them, the one and only saturated carbon being out of the plane that the remaining five carbon atoms form, while the Fe-CF3 bond is very similar to Fe-CF3 bonds reported in the past. Finally, Hirshfeld surface analysis was applied to gain additional understanding into the intermolecular interaction of iron crystal. According to the results of the analysis H-X interactions account for 84.8% of all contacts, indicating a highly compact solid-state structure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217595/s1. Figure S1: 19F NMR of (C6H7)Fe(CO)2CF3; Table S1: Crystal data and structure refinement; Table S2: Bond Lengths [Å]; Table S3: Bond Angles (°); Table S4: Anisotropic displacement parameters (Å2 × 103) The anisotropic displacement factor exponent takes the form: -2p2[h2 a*2U11 + … + 2 h k a* b* U12]; Table S5: Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for cdwjm81m. U(eq) is defined as one third of the trace of the orthogonalized Uij tensor.
Author Contributions
Conceptualization, C.D.; methodology, C.D.; formal analysis, C.D., D.M., D.J.W. and C.L.; investigation, C.D., D.M., C.L. and D.J.W.; writing—original draft preparation, C.D., C.L. and D.B.; writing—review and editing, C.D., D.B. and C.L.; supervision, C.D.; project administration, C.D.; funding acquisition, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the New York Institute of Technology.
Institutional Review Board Statement
This is not applicable.
Informed Consent Statement
This is not applicable.
Data Availability Statement
This is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taw, F.L.; Mueller, A.E.; Janicke, A.H.; Cantat, M.T.; Scott, B.L.; Hay, P.J. Hughes, R.P.; Kiplinger, J.L. Titanium(IV) Trifluoromethyl Complexes: New Perspectives on Bonding from Organometallic Fluorocarbon Chemistry. Organometallics 2012, 31, 1484–1499. [Google Scholar] [CrossRef]
- Alonso, C.N.; Martínez De Marigorta, E.; Rubiales, G.; Palacios, F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A.; Chambers, R.D.; Prakash, G.K.S. Synthetic Fluorine Chemistry; John Wiley: Hoboken, NJ, USA, 1992. [Google Scholar]
- Douvris, C. Synthesis and characterization of the first η6-arene trifluoromethyl transition-metal complex. J. Fluor. Chem. 2021, 245, 109755. [Google Scholar] [CrossRef]
- Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W. Akber Aisa. H. Substitution Effect of the Trifluoromethyl Group on the Bioactivity in Medicinal Chemistry: Statistical Analysis and Energy Calculations. J. Chem. Inf. Model. 2020, 60, 6242–6250. [Google Scholar] [CrossRef]
- Mei, H.; Ibrahim, F.J. Two New Diazonium Bis(perfluoroalkyl)arylsulfonyl Imide Zwitterionic Monomers from Perfluoro(3-oxa-4-pentene)sulfonyl Fluoride for Proton Exchange Membrane Fuel Cells. Fluor. Chem. 2017, 199, 46–51. [Google Scholar] [CrossRef]
- McCormack, P.L. Celecoxib. Drugs 2011, 71, 2457–2489. [Google Scholar] [CrossRef]
- Morrison, J.A. Trifluoromethyl-Containing Transition Metal Complexes. Adv. Organomet. Chem. 1993, 35, 211. [Google Scholar]
- Lampropoulos, C.; Rashad, G.; Douvris, C. Syntheses and Characterization of the First Cycloheptatrienyl Transition-Metal Complexes with a M-CF3 Bond. Molecules 2021, 26, 6838. [Google Scholar] [CrossRef]
- Kwan, A.L.; Morris, R.H. A Plausible Mechanism for the Iridium-Catalyzed Hydrogenation of a Bulky N-Aryl Imine in the (S)-Metolachlor Process. Molecules 2022, 27, 5106. [Google Scholar] [CrossRef]
- Huang, Z.; Dong, G. Catalytic Csingle bondC bond forming transformations via direct β-Csingle bondH functionalization of carbonyl compounds. Tetrahedron Lett. 2014, 55, 5869–5889. [Google Scholar] [CrossRef]
- Campos, J.; Lopez-Serrano, J.; Peloso, R.; Carmona, E. Methyl complexes of the transition metals. Chem. Eur. J. 2016, 22, 6432. [Google Scholar] [CrossRef] [PubMed]
- Douvris, C.; Lampropoulos, C.; Matatov, D.; Wink, D.J.; Kuznetsov, A.E.; Bussan, D. Synthesis and structural characterization of the first stable cycloheptatrienyl metal complexes bearing a CF3 moiety. DFT investigations of structures, energetics, NBO charges, and frontier MOs of W-CF3 and Mo-CF3 with η7-C7H7 and η5-C5H5. Polyhedron 2022, 221, 115875. [Google Scholar] [CrossRef]
- Shapovalov, S.S.; Gordienko, A.V.; Pasynskii, A.A.; Torubaev, Y.V.; Skabitskii, I.V.; Aleksandrov, G.G. (Dicarbonyl)(π-cyclohexadienyl)iron iodide, trichlorostannate, and tris(cymantrenecarboxylato)stannate: Synthesis and molecular structures. Russ. J. Coord. Chem. 2011, 37, 447–451. [Google Scholar] [CrossRef]
- Krause, L.J.; Morrison, J.A. Trifluoromethyl group 2B compounds: bis(trifluoromethyl)cadmium.base. New, more powerful ligand-exchange reagents and low-temperature difluorocarbene sources. J. Am. Chem. Soc. 1981, 103, 2995. [Google Scholar] [CrossRef]
- Hartwig, J.F. Organotransition Metal Chemistry: From Bonding to Catalysis, 1st ed.; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Loizou, D.C.; Castillo, J.; Oki, A.R.; Hosmane, N.S.; Morrison, J.A. Synthersis of cyclopentadienyldinitrosyl(trifluoromethyl)chromium(0), CpCr(NO)2CF3, and cyclopentadienyldinitrosyl(trifluoromethyl)molybdenum(0), CpMo(NO)2CF3. Crystal Structure of CpMo(NO)2CF3. Organometallics 1992, 11, 4189–4193. [Google Scholar] [CrossRef]
- Anderson, S.; Hill, A.F.; Clark, G.R. Reaction of [Fe[C(:O)NPr-iso2](CO)4]- with trifluoroacetic anhydride: Molecular structure of [Fe(CF3)[.eta.2-C(:O)NPr-iso2](CO)2(PPh3). Organometallics 1992, 11, 1988–1990. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Perekalin, D.S.; Loskutova, N.L.; Nelyubina, Y.V.; Kudinov, A.R. Synthesis of cyclohexadienyl ruthenium complexes by replacement of the naphthalene ligand in [(η5-C6H3Me4)Ru(η6-C10H8)]+. J. Organomet. Chem. 2015, 785, 106–111. [Google Scholar] [CrossRef]
- McDonald, R.N.; Schell, P.L.; McGhee, W.D. Oxidative-addition processes in the reactions of (OC)4Fe.cntdot. with XCY3 molecules. Organometallics 1984, 3, 182–184. [Google Scholar] [CrossRef]
- Sanner, R.D.; Satcher, J.H.; Droege, M.W. Synthesis and characterization of (trifluoromethyl) gold complexes. Organometallics 1989, 8, 1498–1506. [Google Scholar] [CrossRef]
- Tyrra, W.E.J. Oxidative perfluoroorganylation methods in group 12–16 chemistry: The reactions of haloperfluoroorganics and In and InBr, a convenient new route to AgRf (Rf = CF3, C6F5) and reactions of AgRf with group 12–16 elements. Fluor. Chem. 2001, 112, 149. [Google Scholar] [CrossRef]
- Hughes, R.P.; Laritchev, R.B.; Yuan, J.; Golen, J.A.; Rucker, A.N.; Rheingold, A.L. A Simple Route to Difluorocarbene and Perfluoroalkylidene Complexes of Iridium. J. Am. Chem. Soc. 2005, 127, 15020. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Tobita, E.; Ando, M.; Kondo, K. A Convenient Trifluoromethylation of Aromatic Halides with Sodium Trifluoroacetate. Chem. Lett. 1981, 10, 1719. [Google Scholar] [CrossRef]
- Daniels, A.L.; Da Gama, J.G.; Edjoc, R.; Gabidullin, B.M.; Baker, R.T. Synthesis and Reactivity of Mn–CF3 Complexes. Inorganics 2019, 7, 3. [Google Scholar] [CrossRef]
- Chae, H.S.; Burkey, D.J. Spirocyclic and Bicyclic Cyclohexadienyl Complexes from Intramolecular Nucleophilic Addition Reactions in Dicationic Arene Complexes. Organometallics 2003, 22, 1761–1765. [Google Scholar] [CrossRef]
- Shirin, Z.; Pramanik, A.; Ghosh, P.; Mukherjee, R. Stable Cyclohexadienyl Complexes of Ruthenium in a Piano Stool Geometry Containing a Tridentate Nitrogen Donor Ligand. First Structural Characterization of the (η5-Cyanocyclohexadienyl)ruthenium(II) Complex and Spectroelectrochemical Correlation. Inorg. Chem. 1996, 35, 3431–3433. [Google Scholar] [CrossRef]
- Hoffmann, R.; Hofmann, P. The electronic origin of geometrical deformations in cyclohexadienyl and cyclobutenyl transition metal complexes. J. Am. Chem. Soc. 1976, 98, 598–604. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Mitchell, A.S.; Spackman, M.A. Hirshfeld Surfaces: A New Tool for Visualising and Exploring Molecular Crystals. Chem. Eur. J. 1998, 4, 2136–2141. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J.; Jayatilaka, D. Electrostatic Potentials mapped on Hirshfeld surfaces provide direct Insight into intermolecular interactions in Crystals. Cryst. Eng.Commun. 2008, 10, 377–388. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A novel definition of a molecule in a crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef]
- Madura, I.D.; Zachara, J.; Hajmowicz, H.; Synoradzki, L. Interplay of carbonyl–carbonyl, Csingle bondH⋯O and Csingle bondH⋯π interactions in hierarchical supramolecular assembly of tartaric anhydrides – Tartaric acid and its O-acyl derivatives: Part 11. J. Mol. Struct. 2012, 1017, 98–105. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. Cryst. Eng. Commun. 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Garnero, M.; Corrales, S.A.; Williams, E.R.; Mowson, A.; Ozarowski, A.; Wernsdorfer, W.; Christou, G.; Lampropoulos, C. Controlled Dimerization of Mn12 Single-Molecule Magnets. Inorg. Chem. 2017, 56, 14755–14758. [Google Scholar] [CrossRef] [PubMed]
- Laos, R.; Lampropoulos, C.; Benner, S.A. The surprising pairing of 2-amino-imidazo[1,2-a][1,3,5]triazin-4-one, a component of an expanded DNA alphabet. Acta Cryst. 2019, C75, 22–28. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).