Conductometric and Fluorescence Probe Analysis to Investigate the Interaction between Bioactive Peptide and Bile Salts: A Micellar State Study
Abstract
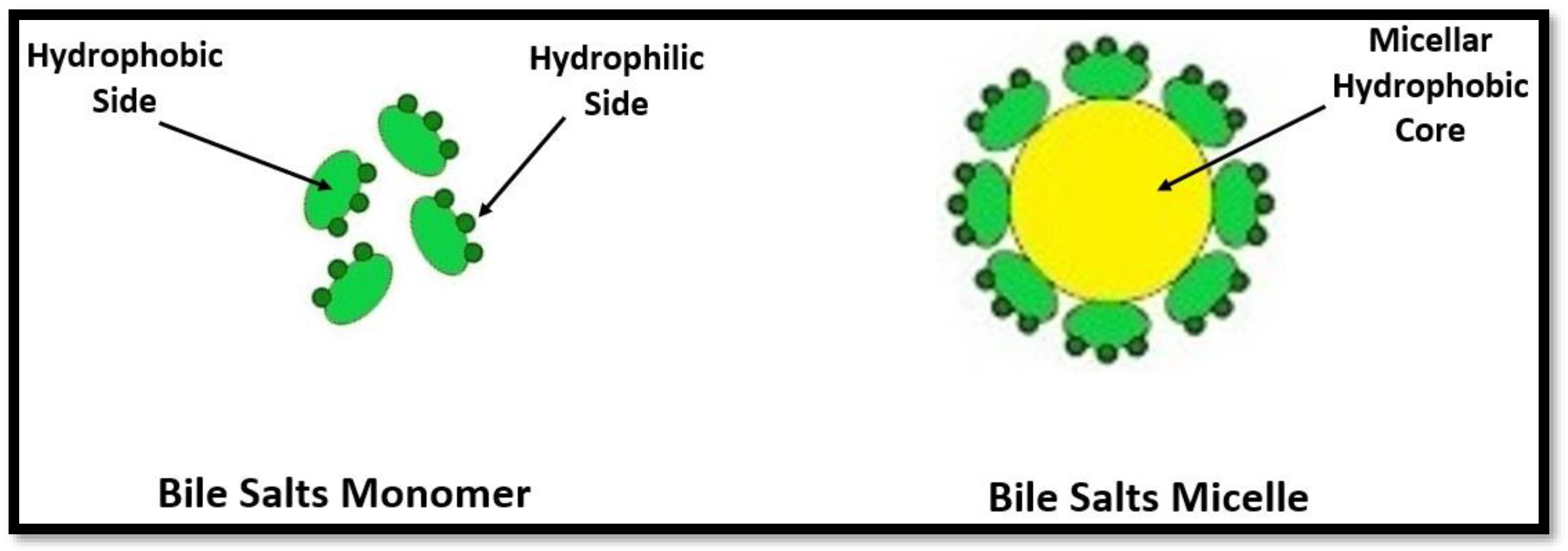
:1. Introduction
2. Experimental Details
2.1. Chemicals
2.2. Experimental Process
2.2.1. Conductivity Measurements
2.2.2. Fluorescence Measurements
3. Results and Discussion
3.1. Conductivity Studies
3.1.1. Micellization of Bile Salts in Aqueous Medium of Glycyl Dipeptide
3.1.2. Critical Micelle Concentration (CMC)
- There is a decrease in the thickness of the solvation layer surrounding the (ionic) head groups of the bile salts.
- The electrostatic repulsive kind of interactions are also lessened amongst the negatively charged part of the bile salts.
3.1.3. Temperature Dependence of XCMC (or CMC)
3.1.4. Thermodynamics of Micellization of NaC and NaDC in Aqueous Glycyl Dipeptide
| T/K | NaC | NaDC | ||||||
|---|---|---|---|---|---|---|---|---|
(kJ∙mol−1) | (J∙K−1∙mol−1) | (kJ∙mol−1) | (kJ∙mol−1) | (J∙K−1∙mol−1) | (kJ∙mol−1) | |||
| Water | ||||||||
| 293.15 | 0.804 (0.790) a | 6.72 (5.18) a | 0.105 (0.099) a | −24.07 (−24.06) a | 0.677 (0.662) a | 31.32 (22.92) a | 0.207 (0.179) a | −29.40 (−24.06) a |
| 298.15 | 0.803 (0.791) a (0.850) b | 3.17 (1.79) a (0.97) b | 0.093 (0.088) a (0.086) b | −24.59 (−24.50) a (−24.60) b | 0.667 (0.668) a (0.780) b | 18.11 (15.74) a (0.57) b | 0.163 (0.154) a (0.098) b | −30.41 (−30.13) a (−28.50) b |
| 303.15 | 0.799 (0.780) a | −0.65 (−1.86) a | 0.081 (0.077) a | −25.10 (−25.14) a | 0.762 (0.761) a | 03.44 (0.757) a | 0.107 (0.120) a | −28.89 (−28.68) a |
| 308.15 | 0.847 (0.828) a | −4.55 (−5.55) a | 0.065 (0.062) a | −24.46 (24.29) a | 0.774 (0.765) a | −10.76 (0.00) a | 0.059 (0.095) a | −29.02 (−29.12) a |
| 313.15 | 0.861 (0.834) a | −8.63 (−9.50) a | 0.051 (0.048) a | −24.53 (−24.65) a | 0.756 (0.755) a | −26.23 (−08.11) a | 0.012 (0.069) a | −29.86 (−29.76) a |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | ||||||||
| 293.15 | 0.877 | −6.13 | 0.135 | −46.46 | 0.848 | 11.53 | 0.156 | −34.13 |
| 298.15 | 0.919 | −6.35 | 0.135 | −47.17 | 0.821 | 1.40 | 0.122 | −35.02 |
| 303.15 | 0.906 | −6.56 | 0.134 | −47.86 | 0.878 | −9.47 | 0.086 | −35.42 |
| 308.15 | 0.881 | −6.77 | 0.133 | −48.54 | 0.844 | −21.00 | 0.048 | −35.80 |
| 313.15 | 0.925 | −6.98 | 0.132 | −49.13 | 0.891 | −33.75 | 0.010 | −36.79 |
| [Glycyl Dipeptide] = 0.005 mol∙kg−1 | ||||||||
| 293.15 | 0.907 | −7.79 | 0.134 | −47.92 | 0.734 | 14.39 | 0.168 | −34.77 |
| 298.15 | 0.935 | −8.05 | 0.133 | −48.58 | 0.777 | 4.97 | 0.136 | −35.58 |
| 303.15 | 0.939 | −8.33 | 0.132 | −49.28 | 0.796 | −5.16 | 0.102 | −36.07 |
| 308.15 | 0.945 | −8.59 | 0.132 | −49.91 | 0.721 | −15.93 | 0.067 | −36.49 |
| 313.15 | 0.92 | −8.87 | 0.131 | −50.58 | 0.803 | −27.79 | 0.031 | −37.50 |
| [Glycyl Dipeptide] = 0.010 mol∙kg−1 | ||||||||
| 293.15 | 0.947 | −11.37 | 0.123 | −47.98 | 0.765 | 14.98 | 0.173 | −35.72 |
| 298.15 | 0.952 | −11.75 | 0.122 | −48.60 | 0.765 | 6.11 | 0.143 | −36.57 |
| 303.15 | 0.959 | −12.18 | 0.12 | −49.30 | 0.779 | −3.40 | 0.110 | −36.68 |
| 308.15 | 0.966 | −12.6 | 0.119 | −50.01 | 0.779 | −13.49 | 0.077 | −37.23 |
| 313.15 | 0.975 | −12.95 | 0.118 | −50.43 | 0.804 | −24.55 | 0.044 | −38.26 |
3.1.5. Enthalpy–Entropy Compensation for Micelle Formation
3.2. Fluorescence Probe Studies of NaC and NaDC
| a m/mol∙kg−1 | CMC/mmol∙kg−1 | |||
|---|---|---|---|---|
| Fluorescence Probe Study | Conductivity Study | |||
| NaC | NaDC | NaC | NaDC | |
| water | 14.1 | 5.3 | 14 | 5.4 |
| 0.001 | 13.5 | 4.1 | 13.3 | 4 |
| 0.005 | 12.9 | 3.9 | 13.1 | 3.8 |
| 0.010 | 12.6 | 3.8 | 12.5 | 3.6 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badarayani, R.; Kumar, A. Viscometric study of glycine, l-alanine, glycylglycine in aqueous tetra-n-alkylammonium bromide solutions at 298.15 K. J. Chem. Thermodyn. 2004, 36, 983–991. [Google Scholar] [CrossRef]
- Singh, S.K.; Kundu, A.; Kishore, N. Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T=298.15 K: A volumetric approach. J. Chem. Thermodyn. 2004, 36, 7–16. [Google Scholar] [CrossRef]
- Funasaki, N.; Fukuba, M.; Kitagawa, T.; Nomura, M.; Ishikawa, S.; Hirota, S.; Neya, S. Two-Dimensional NMR Study on the Structures of Micelles of Sodium Taurocholate. J. Phy. Chem. B. 2004, 108, 438–443. [Google Scholar] [CrossRef]
- Xie, H.; Xu, S.; Sang, L.; Du, S.; Zhu, F. Detection of Key Proteins in Obstetric Preeclampsia by Nanocomposites Using Gold Nanoparticles/DNA/Methylene Blue. Sci. Adv. Mater. 2021, 13, 321–330. [Google Scholar] [CrossRef]
- Xiong, S.; Guo, W.; Ren, Z.; Fu, Y. Effects and Mechanisms of Drug-Loaded Ulinastatin Nanoparticles on Inflammatory Response in Sepsis. Sci. Adv. Mater. 2021, 13, 483–491. [Google Scholar] [CrossRef]
- Kumar, K.; Chauhan, S. Drug−Amino Acid Interactions in Aqueous Medium: Volumetric, Compressibility, and Viscometric Studies. Thermochim. Acta 2015, 606, 12–24. [Google Scholar] [CrossRef]
- Faustino, C.M.C.; Serafim, C.S.; Ferreira, I.S.N.; Branco, M.A.; Calado, A.R.T.; Rio, L.G. Amino Acids as Raw Material for Biocompatible Surfactants. Ind. Eng. Chem. Res. 2014, 53, 10112–10118. [Google Scholar] [CrossRef]
- Chevalier, Y.; Chachaty, C. NMR investigation of the micellar properties of monoalkylphoshpates. Colloid and Polymer Sci. 1984, 262, 489–496. [Google Scholar] [CrossRef]
- Diaz, A.N.; Sanchez, F.G.; Pareja, A.G. Cholic acid behavior in water and organic solvent: Study of normal and inverted aggregates. Colloids Surf. A 1998, 142, 27–34. [Google Scholar] [CrossRef]
- Weber, L.P.; Lanno, R.P. Effect of bile salts, lipid, and humic acids on absorption of benzo pyrene by isolated channel catfish (ictalurus punctatus) intestine segments. Environm. Toxicol. Chem. 2001, 20, 1117–1124. [Google Scholar] [CrossRef]
- Hildebrand, A.; Neubert, R.; Garidel, P.; Blume, A. Bile Salt Induced Solubilization of Synthetic Phosphatidylcholine Vesicles Studied By Isothermal Titration Calorimetry. Langmuir 2002, 18, 2836–2847. [Google Scholar] [CrossRef]
- Ninomiya, R.; Matsuoka, K.; Moroi, Y. Micelle formation of sodium chenodeoxycholate and solubilization into the micelles: Comparison with other unconjugated bile salts. Biochim. Biophys. Acta 2003, 1634, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, J.; Carpena, P.; Bolívar, J.A.M.; Ruiz, C.C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Matsuoka, K.; Moroi, Y. Micelle formation of sodium deoxycholate and sodium ursodeoxycholate (Part 1). Biochim. Biophys. Acta-Mol. Cell Bio Lip. 2002, 1580, 189–199. [Google Scholar] [CrossRef]
- Matsuokaa, K.; Nakazawaa, T.; Nakamuraa, A.; Hondaa, C.; Endoa, K.; Tsukadab, M. Study of thermodynamic parameters for solubilization of plant sterol and stanol in bile salt micelles. Chem. Phys. Lipids 2008, 154, 87–93. [Google Scholar] [CrossRef]
- Enhsen, A.; Kramer, W.; Wess, G. Bile acids in drug discovery. Drug Discov. Today 1998, 3, 409–418. [Google Scholar] [CrossRef]
- Ravichandran, G.; Lakshiminarayanan, G.; Ragouramane, D. Apparent molar volume and ultrasonic studies on some bile salts in water–aprotic solvent mixtures. Fluid Phase Equilib. 2013, 356, 256–263. [Google Scholar] [CrossRef]
- Bonincontro, A.; Archivio, A.D.; Galantini, L.; Giglio, E.; Punzo, F. Electrolytic conductance, and dielectric studies of bile salt micellar aggregates. Langmuir 2000, 16, 10436–10443. [Google Scholar] [CrossRef]
- Patel, V.; Bharatiya, B.; Ray, D.; Aswal, V.K.; Bahadur, P. Investigations on microstructural changes in phresponsive mixed micelles of Triton X-100 and bile salt. J. Colloid Interface Sci. 2015, 441, 106–112. [Google Scholar] [CrossRef]
- Lin, C.E.; Lin, W.C.; Chiou, W.C. Migration behaviour and selectivity of dichlorophenols in micellar electrokinetic capillary chromatography Influence of micelle concentration and buffer pH. J. Chromatogr. A 1996, 722, 333–343. [Google Scholar] [CrossRef]
- Abbas, M.; Alqahtani, M.S.; Murayah, A.; Algahtani, A.; Kessentini, A.; Loukil, H.; Parayangat, M.; Ijyas, T.; Mohammed, A.W. Novel Nanoelectromechanical System Pressure Biosensing Method for Early Detection of Cholesterol Accumulation in Blood Vessels. Sci. Adv. Mater. 2021, 13, 966–980. [Google Scholar] [CrossRef]
- Fuguet, E.; Rafols, C.; Roses, M.; Bosch, E. Critical micelle concentration of surfactants in aqueous buffered and unbuffered system. Anal. Acta 2005, 548, 95–100. [Google Scholar] [CrossRef]
- Singh, K.; Chauhan, S. Temperature dependent micellar behaviour of sodium cholate and sodium deoxycholate in the presence of ceftriaxone sodium: A physicochemical study. J. Mol. Liq. 2020, 316, 113833. [Google Scholar] [CrossRef]
- Claffey, W.J.; Holzbach, R.T. Dimorphism in bile salt/lecithin mixed micelles. Biochemistry 1981, 20, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Coello, A.; Meijide, F.; Nunez, E.R.; Tato, J.V. Aggregation behavior of bile salts in aqueous solution. J. Pharm. Sci. 1996, 85, 9–15. [Google Scholar] [CrossRef]
- Hammad, M.A.; Muller, B.W. Increasing drug solubility by means of bile salt–phosphatidylcholine-based mixed micelles. J. Pharma. Biopharma 1998, 46, 361–367. [Google Scholar] [CrossRef]
- Natalini, B.; Sardella, R.; Gioiello, A.; Ianni, F.; di Michele, A.; Marinozzi, M. Determination of bile salts critical micellization concentration on the road of drug discovery. J. Pharma. Biomed. Anal. 2014, 87, 62–81. [Google Scholar] [CrossRef]
- Chauhan, S.; Kaur, M.; Kumar, K.; Chauhan, M.S. Study of the effect of electrolyte and temperature on the critical micelle concentration of dodecyltrimethylammonium bromide in aqueous medium. J. Chem. Thermodyn. 2014, 78, 175–181. [Google Scholar] [CrossRef]
- Chauhan, S.; Kumar, K.; Singh, K.; Jyoti, J. Volumetric, compressibility, and surface tension studies on micellization behavior of sds in aqueous medium: Effect of sugars. J. Surfactants Deterg. 2014, 17, 169–175. [Google Scholar] [CrossRef]
- Elbehairi, S.E.I.; Alfaifi, M.Y.; Shati, A.A.; Fahmy, U.A.; Gorain, B.; Md, S. Encapsulation of Ellagic Acid in Di-Block Copolymeric Micelle for Non-Small Cell Lung Cancer Therapy. Sci. Adv. Mater. 2021, 13, 66–72. [Google Scholar] [CrossRef]
- Tan, G.; Li, B.; Zhang, Y.J.; Zhang, R.Y.; Wang, L.; Liu, F.J.; Shan, F. Targeted Fluorescence Imaging and Anti-Tumor Effects of QDs-NGR Nanoparticles on Pancreatic Cancer Cells. Sci. Adv. Mater. 2021, 13, 2475–2482. [Google Scholar] [CrossRef]
- Badache, L.; Lehanine, Z.; Abderrahmane, W.N. Synthesis and Surface Properties Study of a Series of Cationic Surfactants with Different Hydrophobic Chain Lengths. J. Surfactants Deterg. 2012, 15, 715–720. [Google Scholar] [CrossRef]
- Gupta, B.S.; Shen, C.R.; Lee, M.J. Effect of biological buffers on the colloidal behavior of sodium dodecyl sulfate (SDS). Colloids Surf. A. 2017, 529, 64–72. [Google Scholar] [CrossRef]
- Kuperkar, K.; Modi, J.; Patel, K. Surface-active properties and antimicrobial study of conventional cationic and synthesized symmetrical gemini surfactants. J. Surfactants Deterg. 2012, 15, 107–115. [Google Scholar] [CrossRef]
- Kumar, K.; Patial, B.S.; Chauhan, S. Conductivity and fluorescence studies on the micellization properties of sodium cholate and sodium deoxycholate in aqueous medium at different temperatures: Effect of selected amino acids. J. Chem. Thermodyn. 2015, 82, 25–33. [Google Scholar] [CrossRef]
- Liu, J.; Li, M. Preparation of Neuropeptide Nanoparticle and Its Mechanism in Corneal Nerve Regeneration in Substance P-Neurokinin 1 Receptor Signaling Pathway. Sci. Adv. Mater. 2021, 13, 254–263. [Google Scholar] [CrossRef]
- Shaw, R.; Elliott, W.H.; Barisas, B.G. Estimation of critical micelle concentrations of bile acids by reversed-phase high performance liquid chromatography. Mikrochim. Acta 1991, 105, 137–145. [Google Scholar]
- Cirin, D.M.; Posa, M.M.; Krstonosic, V.S. Interactions between sodium cholate or sodium deoxycholate and nonionic surfactant (tween 20 or tween 60) in aqueous solution. Ind. Eng. Chem. Res. 2012, 51, 3670–3676. [Google Scholar] [CrossRef]
- Subuddhi, U.; Mishra, A.K. Micellization of bile salts in aqueous medium: A fluorescence study. Colloids Surf. B Biointerfaces 2007, 57, 102–107. [Google Scholar] [CrossRef]
- Akhtar, F.; Hoque, M.A.; Khan, M.A. Interaction of cefadroxyl monohydrate with hexadecyltrimethyl ammonium bromide and sodium dodecyl sulfate. J. Chem. Thermodyn. 2008, 40, 1082–1086. [Google Scholar] [CrossRef]
- Hoque, A.; Khan, M.A.; Hossain, M.D. Interaction of cefalexin monohydrate with cetyldimethylethylammonium bromide. J. Chem. Thermodyn. 2013, 60, 71–75. [Google Scholar] [CrossRef]
- Mehta, S.K.; Bhasin, K.K.; Chauhan, R.; Dham, S. Effect of temperature on critical micelle concentration and thermodynamic behavior of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloid Surf. A 2005, 255, 153–157. [Google Scholar] [CrossRef]
- Chauhan, S.; Singh, K.; Sundaresan, C.N. Physico-chemical characterization of drug–bio-surfactant micellar system: A road for developing better pharmaceutical formulations. J. Mol. Liq. 2018, 266, 692–702. [Google Scholar] [CrossRef]
- Huo, J.-C.; Yang, H.-X.; Ma, Y.; Bai, J. Lightweight, Flexible and Hydrophobic Cotton Fiber/Silica Aerogel Composite by Freeze-Drying for Organic Solvent/Water Separation and Thermal Insulation. Sci. Adv. Mater. 2021, 13, 1820–1824. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Sar, S.K. Micellization of some bile salts in binary aqueous solvent mixtures. J. Surfact. Deterg. 2014, 17, 143–150. [Google Scholar] [CrossRef]
- Nusselder, J.J.H.; Engberts, J.B. Toward a better understanding of the driving force for micelle formation and micellar growth. J. Colloid Interface Sci. 1992, 148, 353–361. [Google Scholar] [CrossRef]
- Wagle, V.B.; Kothari, P.S.; Gaikar, V.G. Effect of temperature on aggregation behavi.or of aqueous solutions of sodium cumene sulfonate. J. Mol. Liq. 2007, 133, 68–76. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Philip, P.R. Can. Enthalpy–entropy compensation for micellization and other hydrophobic interactions in aqueous solutions. J. Chem. 1974, 52, 1834–1839. [Google Scholar]
- Lindmann, B.; Wennerstrom, H. Topics in Current Chemistry; Dewar, M.J.S., Hafner, K., Heilbronner, E., Ito, S., Lehn, J.M., Niedenzu, K., Rees, C.W., Schafer, K., Wittig, G., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1980. [Google Scholar]
- Kang, K.H.; Kim, H.U.; Lim, K.H. Effect of temperature on critical micelle concentration and thermodynamic potentials of micellization of anionic ammonium dodecyl sulfate and cationic octadecyl. Colloid Surf. A 2001, 189, 113–121. [Google Scholar] [CrossRef]
- Vold, R.D.; Vold, M.J. Colloid Interf. Chem; Addison–Wesley: Reading, MA, USA, 1983. [Google Scholar]
- Lumry, R.; Rajender, S. Enthalpy-entropy compensation phenomena in water solutions of proteins and small molecules: A ubiquitous property of water. Biopolymers 1970, 9, 1125–1127. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, S.Y.; Huang, C.C.; Chen, E.M. Temperature dependence of critical micelle concentration of polyoxyethylenated non-ionic surfactants. Colloids Surf. A 1998, 135, 175–181. [Google Scholar] [CrossRef]
- Sharp, K. Entropy-enthalpy compensation: Fact or artifact. Protein Sci. 2001, 10, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Sulthana, S.B.; Bhat, S.G.T.; Rakshit, A.K. Determination of critical micelle concentration (cmc) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of cmc with hydrophile-lipophile balance and other parameters of the surfactants. Colloids Surf. A 1996, 111, 57–65. [Google Scholar] [CrossRef]
- Chen, L.J.; Lin, S.Y.; Huang, C.C. Effect of hydrophobic chain length of surfactants on enthalpy−entropy compensation of micellization. J. Phys. Chem. B 1998, 102, 4350–4356. [Google Scholar] [CrossRef]
- Chauhan, M.S.; Rajni; Chauhan, S.; Rana, D.S.; Umar, A. Effect of temperature on micellar properties of sodium dodecyl sulfate in aqueous solutions of some amino acids (glycine, alanine, valine and leucine). Adv. Sci. Lett. 2012, 5, 43–51. [Google Scholar] [CrossRef]
- Vasilescu, M.; Angelescu, D.; Almgren, A.; Valstar, A. Interactions of globular proteins with surfactants studied with fluorescence probe methods, Langmuir 1999, 15, 2635–2643. Langmuir 1999, 15, 2635–2643. [Google Scholar] [CrossRef]
- Nivaggioli, T.; Alexandridis, P.; Hatton, T.A.; Yekta, A.; Winnik, M.A. Fluorescence probe studies of pluronic copolymer solutions as a function of temperature. Langmuir 1995, 11, 730–737. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: A fluorescence probe study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Ray, G.B.; Chakraborty, I.; Moulik, S.P. Pyrene absorption can be a convenient method for probing critical micellar concentration (cmc) and indexing micellar polarity. J. Colloid Interface Sci. 2006, 294, 248–254. [Google Scholar] [CrossRef]
- Dixit, S.B.; Bhasin, R.; Rajasekaran, E.; Jayaram, B. Solvation thermodynamics of amino acids: Assessment of the electrostatic contribution and force-field dependence. J. Chem. Soc. Faraday Trans. 1997, 93, 1105–1113. [Google Scholar] [CrossRef]
- Zhao, H. Viscosity b-coefficients and standard partial molar volumes of amino acids, and their roles in interpreting the protein (enzyme) stabilization. Biophys. Chem. 2006, 122, 157–183. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Ahluwalia, J.C. Partial molar heat capacities and volumes of transfer of some amino acids and peptides from water to aqueous sodium chloride solutions at 298.15k. J. Phys. Chem. 1985, 89, 1099–1105. [Google Scholar] [CrossRef]






| Chemical Name | Source | Mol.Wt./kg∙mol−1 | Purification Method | Mass Fraction Purity a |
|---|---|---|---|---|
| Glycylglycine (C4H8N2O3) | Spectrochem Pvt. Ltd. | 0.132 | None | 0.98 |
| Sodium cholate (C24H39O5Na) | Himedia Pvt. Ltd. | 0.431 | Recrystallization | 0.98 |
| Sodium deoxycholate (C24H39O4Na) | Himedia Pvt. Ltd. | 0.415 | Recrystallization | 0.98 |
| Pyrene (C16H10) | Merck | 0.202 | None | 0.96 |
| T (K) | CMC, 103 | |||||||
|---|---|---|---|---|---|---|---|---|
| NaC, mmol∙kg−1 | NaDC, mmol∙kg−1 | |||||||
| Water | 0.001 | 0.005 | 0.010 | Water | 0.001 | 0.005 | 0.010 | |
| 293.15 | 14.4 (14.4) a | 13.9 | 13.5 | 12.9 | 5.1 | 4.5 | 4.1 | 3.9 |
| 298.15 | 14.0 (14.1) a (13.8) b (12.9) c (16.0) d | 13.3 | 13.1 | 12.5 | 5.4 | 4.0 | 3.8 | 3.6 |
| 303.15 | 13.9 (14.0) a | 13.5 | 13.3 | 12.8 | 5.7 | 4.5 | 4.2 | 4.0 |
| 308.15 | 14.1 (14.1) a | 13.8 | 13.6 | 13.2 | 5.9 | 5.0 | 4.7 | 4.4 |
| 313.15 | 14.2 (14.3) a | 14.1 | 13.9 | 13.5 | 6.1 | 5.4 | 5.1 | 4.8 |
| T (K) | XCMC, 104 | |||||||
|---|---|---|---|---|---|---|---|---|
| NaC, mmol∙kg−1 | NaDC, mmol∙kg−1 | |||||||
| Water | 0.001 | 0.005 | 0.010 | Water | 0.001 | 0.005 | 0.010 | |
| 293.15 | 2.59 | 2.48 | 2.43 | 2.32 | 10.44 | 8.09 | 7.37 | 7.01 |
| 298.15 | 2.52 | 2.41 | 2.36 | 2.25 | 9.72 | 7.19 | 6.83 | 6.47 |
| 303.15 | 2.48 | 2.45 | 2.39 | 2.3 | 9.9 | 8.09 | 7.55 | 7.19 |
| 308.15 | 2.54 | 2.5 | 2.45 | 2.37 | 10.44 | 8.99 | 8.45 | 7.91 |
| 313.15 | 2.56 | 2.55 | 2.5 | 2.43 | 10.98 | 9.71 | 9.17 | 8.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Chauhan, S.; Umar, A.; Fouad, H.; Akhtar, M.S. Conductometric and Fluorescence Probe Analysis to Investigate the Interaction between Bioactive Peptide and Bile Salts: A Micellar State Study. Molecules 2022, 27, 7561. https://doi.org/10.3390/molecules27217561
Kumari S, Chauhan S, Umar A, Fouad H, Akhtar MS. Conductometric and Fluorescence Probe Analysis to Investigate the Interaction between Bioactive Peptide and Bile Salts: A Micellar State Study. Molecules. 2022; 27(21):7561. https://doi.org/10.3390/molecules27217561
Chicago/Turabian StyleKumari, Santosh, Suvarcha Chauhan, Ahmad Umar, Hassan Fouad, and Mohammad Shaheer Akhtar. 2022. "Conductometric and Fluorescence Probe Analysis to Investigate the Interaction between Bioactive Peptide and Bile Salts: A Micellar State Study" Molecules 27, no. 21: 7561. https://doi.org/10.3390/molecules27217561
APA StyleKumari, S., Chauhan, S., Umar, A., Fouad, H., & Akhtar, M. S. (2022). Conductometric and Fluorescence Probe Analysis to Investigate the Interaction between Bioactive Peptide and Bile Salts: A Micellar State Study. Molecules, 27(21), 7561. https://doi.org/10.3390/molecules27217561







