Characterization of Colorants Formed by Non-Enzymatic Browning Reactions of Hydroxycinnamic Acid Derivatives
Abstract
1. Introduction
2. Results and Discussion
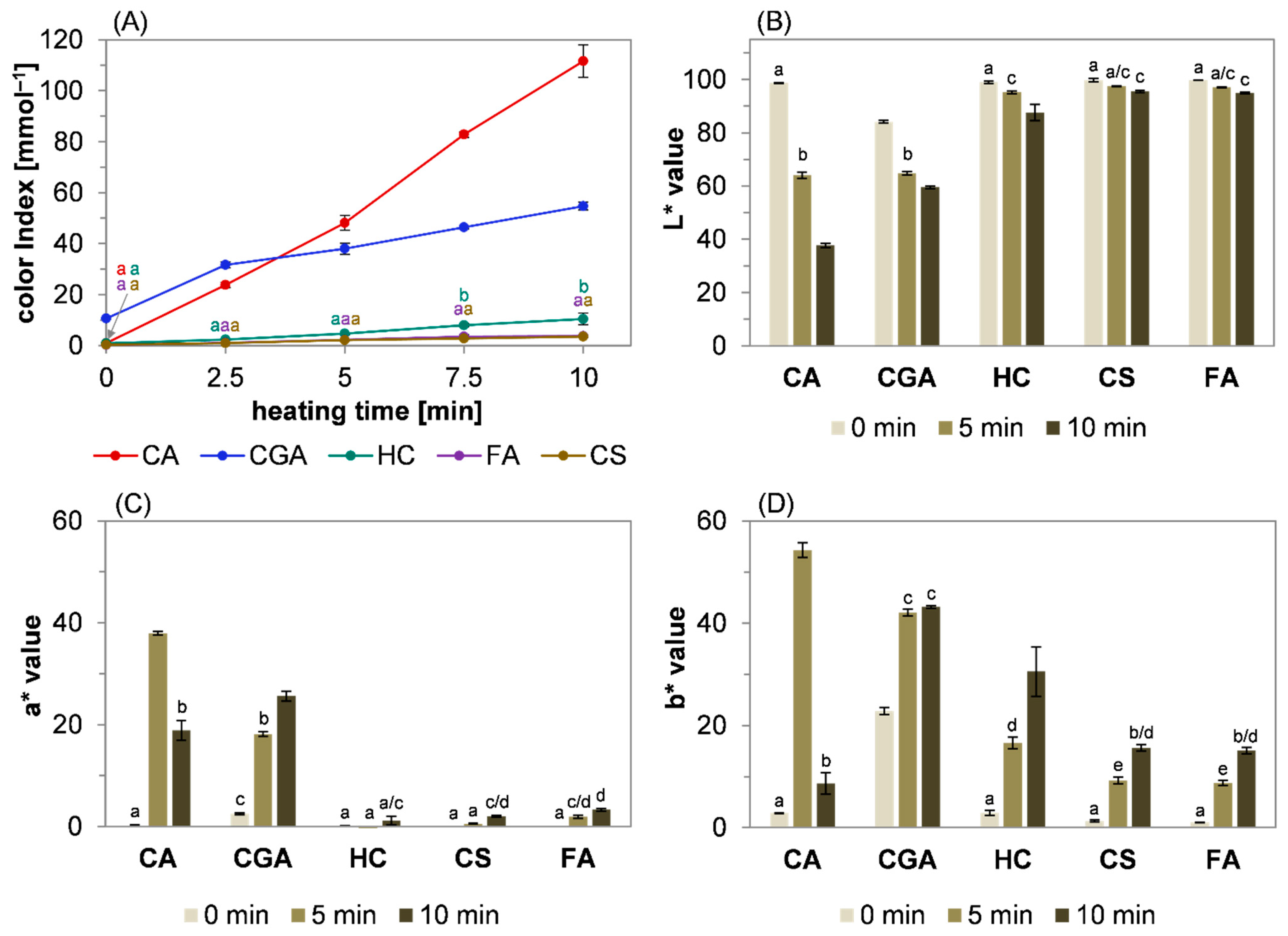
2.1. Browning Potential of Hydroxycinnamic Acid Derivatives under Roasting Conditions
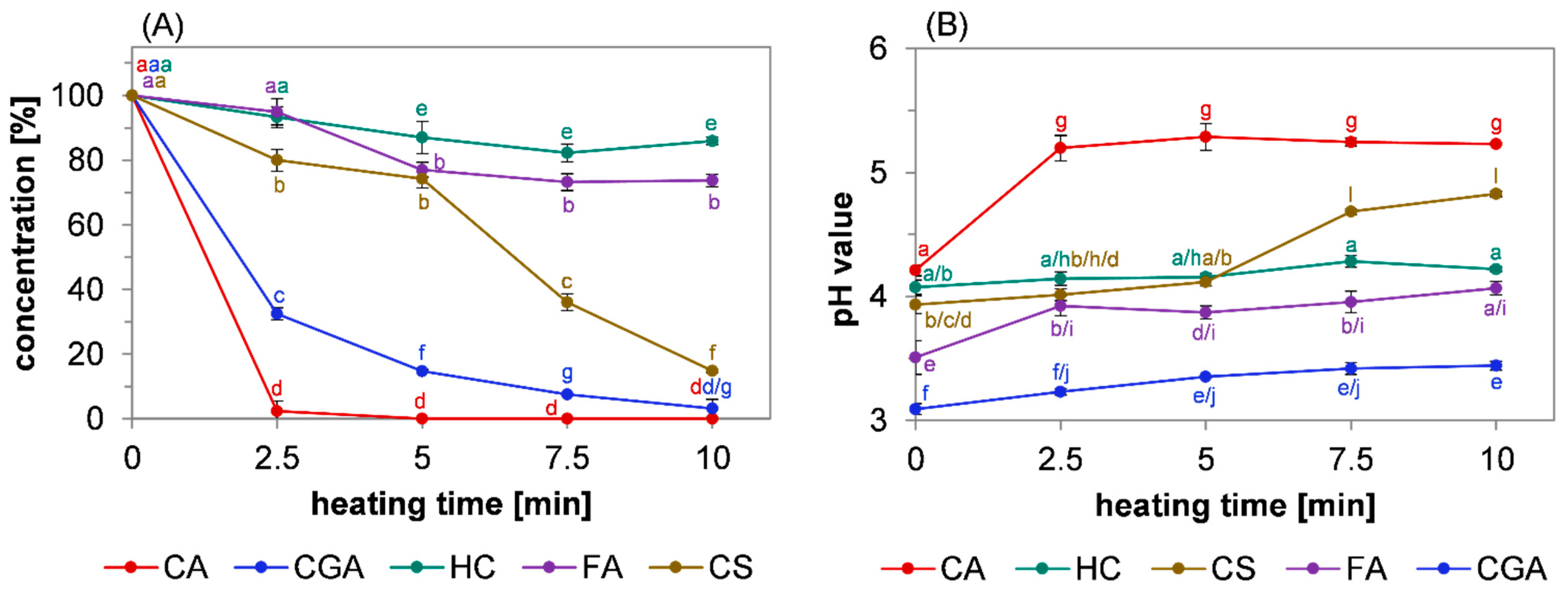
2.2. Thermally Induced Degradation of Hydroxycinnamic Acid Derivatives and Its Influence on pH
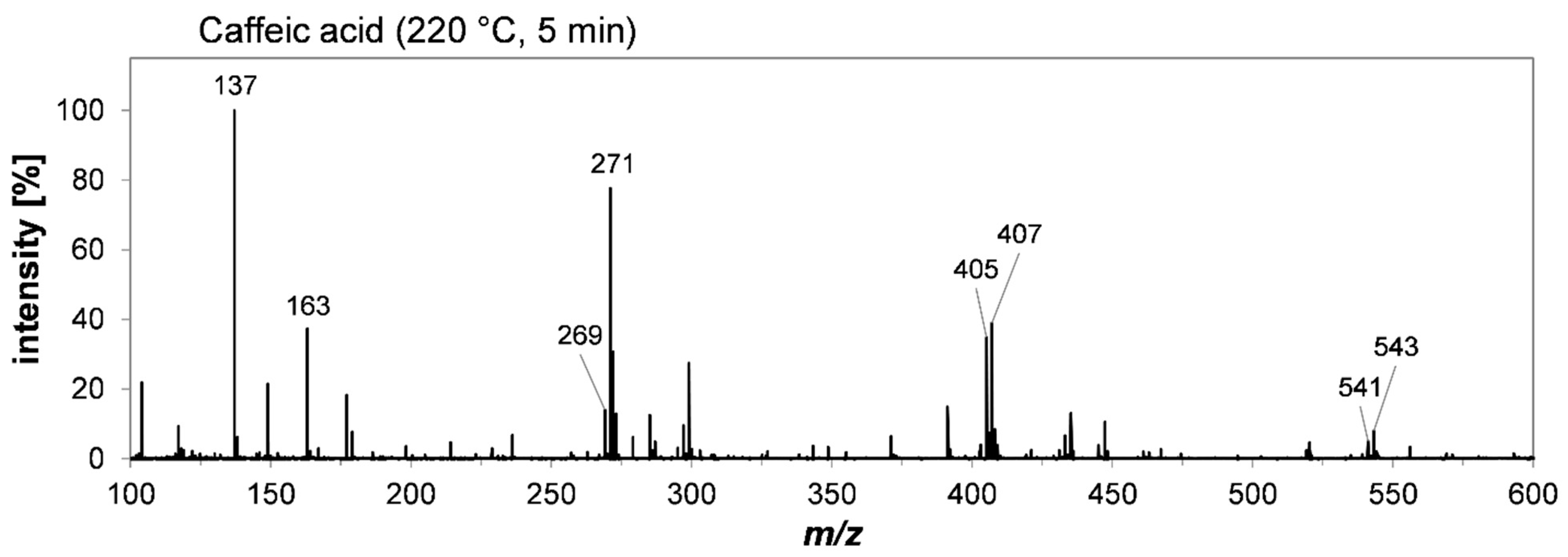
2.3. Structural Characterization of Colored Oligomers Deriving from Hydroxycinnamic Acid Derivatives by High-Resolution Mass Spectrometry
2.4. Isolation, Structural Elucidation, and Formation Mechanism of Colored 4-Vinylcatechol Dimers
2.5. Composition of Novel Colorants by High-Resolution Multiple-Stage Mass Spectrometry
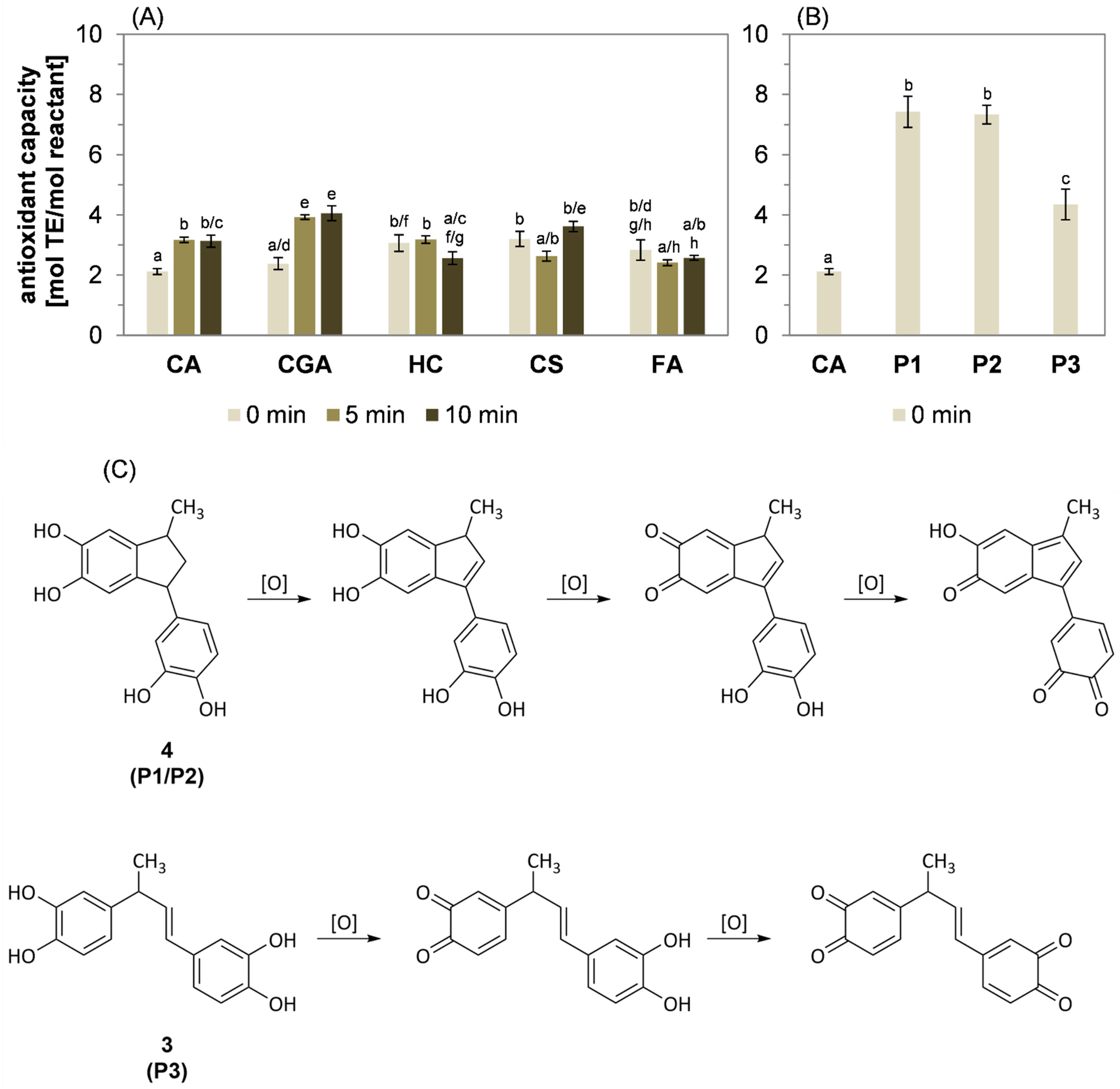
2.6. Impact of Heat-Induced Oligomerization on the Antioxidant Capacity
2.7. Investigation of the Polymerization Mechanism by High-Resolution Mass Spectrometry
3. Materials and Methods
3.1. Chemicals
3.2. Incubation of Phenolic Compounds under Roasting Conditions
3.3. Color Measurements
3.4. HPLC-DAD Analysis of Phenolic Compounds
3.5. Isolation of 1-(3,4-Dihydroxyphenyl)-3-methyl-2,3-dihydro-1H-indene-5,6-diol (P1, P2) and (E)-4,4′-(But-1-ene-1,3-diyl)bis(benzene-1,2-diol) (P3)
3.6. Incubation of Compounds P1, P2, and P3
3.7. Trolox Equivalent Antioxidant Capacity Assay
3.8. APCI(+) and ESI-Orbitrap Multiple-Stage High-Resolution Mass Spectroscopy
3.9. NMR Spectroscopy
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Cerny, C. The aroma side of the Maillard reaction. Ann. N. Y. Acad. Sci. 2008, 1126, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef]
- Hellwig, M.; Henle, T. Baking, ageing, diabetes: A short history of the Maillard reaction. Angew. Chem. Int. Ed. Engl. 2014, 53, 10316–10329. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Arnoldi, A. The Maillard reaction as a source of off-flavours. In Taints and Off-Flavours in Foods; Elsevier: Amsterdam, The Netherlands, 2003; pp. 162–175. ISBN 9781855734494. [Google Scholar]
- van Chuyen, N. Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs. Mol. Nutr. Food Res. 2006, 50, 1140–1149. [Google Scholar] [CrossRef]
- Totlani, V.M.; Peterson, D.G. Influence of epicatechin reactions on the mechanisms of Maillard product formation in low moisture model systems. J. Agric. Food Chem. 2007, 55, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Poojary, M.M.; Andersen, M.L.; Lund, M.N. Trapping of Carbonyl Compounds by Epicatechin: Reaction Kinetics and Identification of Epicatechin Adducts in Stored UHT Milk. J. Agric. Food Chem. 2020, 68, 7718–7726. [Google Scholar] [CrossRef]
- Teng, J.; Hu, X.; Tao, N.; Wang, M. Impact and inhibitory mechanism of phenolic compounds on the formation of toxic Maillard reaction products in food. Front. Agric. Sci. Eng. 2018, 5, 321. [Google Scholar] [CrossRef]
- Rizzi, G.P.; Boekley, L.J. Observation of ether-linked phenolic products during thermal degradation of ferulic acid in the presence of alcohols. J. Agric. Food Chem. 1992, 40, 1666–1670. [Google Scholar] [CrossRef]
- Stadler, R.H.; Welti, D.H.; Stämpfli, A.A.; Fay, L.B. Thermal Decomposition of Caffeic Acid in Model Systems: Identification of Novel Tetraoxygenated Phenylindan Isomers and Their Stability in Aqueous Solution. J. Agric. Food Chem. 1996, 44, 898–905. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Liu, H.; Wang, E.; Peng, M.; Deng, T.; Pan, X.; Luo, Z.; Yan, Y.; Yang, L.; et al. Catalyst-free decarboxylation of 4-hydroxycinnamic acids: Efficient synthesis of 4-vinylphenols. R. Soc. Open Sci. 2022, 9, 220014. [Google Scholar] [CrossRef] [PubMed]
- Bork, L.V.; Haase, P.T.; Rohn, S.; Kanzler, C. Formation of melanoidins-Aldol reactions of heterocyclic and short-chain Maillard intermediates. Food Chem. 2022, 380, 131852. [Google Scholar] [CrossRef] [PubMed]
- Bork, L.V.; Haase, P.T.; Rohn, S.; Kanzler, C. Structural Characterization of Polar Melanoidins Deriving from Maillard Reaction Intermediates—A Model Approach. Food Chem. 2022, 395, 133592. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Kanzler, C.; Degenhardt, A.G.; Koch, T.J.; Kroh, L.W. Basic Structure of Melanoidins Formed in the Maillard Reaction of 3-Deoxyglucosone and γ-Aminobutyric Acid. J. Agric. Food Chem. 2019, 67, 5197–5203. [Google Scholar] [CrossRef] [PubMed]
- Kanzler, C.; Wustrack, F.; Rohn, S. High-Resolution Mass Spectrometry Analysis of Melanoidins and Their Precursors Formed in a Model Study of the Maillard Reaction of Methylglyoxal with l-Alanine or l-Lysine. J. Agric. Food Chem. 2021, 69, 11960–11970. [Google Scholar] [CrossRef]
- Maasen, K.; Scheijen, J.L.J.M.; Opperhuizen, A.; Stehouwer, C.D.A.; van Greevenbroek, M.M.; Schalkwijk, C.G. Quantification of dicarbonyl compounds in commonly consumed foods and drinks; presentation of a food composition database for dicarbonyls. Food Chem. 2021, 339, 128063. [Google Scholar] [CrossRef]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.-T.; Sang, S. Essential Structural Requirements and Additive Effects for Flavonoids to Scavenge Methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Yu, J.; Cui, H.; Zhang, Q.; Hayat, K.; Zhan, H.; Yu, J.; Jia, C.; Zhang, X.; Ho, C.-T. Adducts Derived from (-)-Epigallocatechin Gallate-Amadori Rearrangement Products in Aqueous Reaction Systems: Characterization, Formation, and Thermolysis. J. Agric. Food Chem. 2020, 68, 10902–10911. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Carbonyl–Phenol Adducts: An Alternative Sink for Reactive and Potentially Toxic Lipid Oxidation Products. J. Agric. Food Chem. 2018, 66, 1320–1324. [Google Scholar] [CrossRef]
- Lederer, M.O.; Klaiber, R.G. Cross-linking of proteins by maillard processes: Characterization and detection of lysine–Arginine cross-links derived from glyoxal and methylglyoxal. Bioorg. Med. Chem. 1999, 7, 2499–2507. [Google Scholar] [CrossRef]
- Ma, J.; Peng, X.; Ng, K.-M.; Che, C.-M.; Wang, M. Impact of phloretin and phloridzin on the formation of Maillard reaction products in aqueous models composed of glucose and L-lysine or its derivatives. Food Funct. 2012, 3, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.; Ribeiro, M.; Cruz, A.C.S.; Domingues, M.R.M.; Coimbra, M.A.; Bunzel, M.; Nunes, F.M. Nature of phenolic compounds in coffee melanoidins. J. Agric. Food Chem. 2014, 62, 7843–7853. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, L.; Baltes, W. Vorkommen von Phenolen in Kaffee-Melanoidinen. Z. Lebensm. Unters. Forsch. 1987, 185, 366–370. [Google Scholar] [CrossRef]
- Nunes, F.M.; Coimbra, M.A. Role of hydroxycinnamates in coffee melanoidin formation. Phytochem. Rev. 2010, 9, 171–185. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M. Influence of coffee roasting on the incorporation of phenolic compounds into melanoidins and their relationship with antioxidant activity of the brew. J. Agric. Food Chem. 2012, 60, 4265–4275. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Moreira, A.S.P.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Coffee melanoidins: Structures, mechanisms of formation and potential health impacts. Food Funct. 2012, 3, 903–915. [Google Scholar] [CrossRef]
- Fogliano, V.; Morales, F.J. Estimation of dietary intake of melanoidins from coffee and bread. Food Funct. 2011, 2, 117–123. [Google Scholar] [CrossRef]
- Cilliers, J.J.L.; Singleton, V.L. Nonenzymic autoxidative phenolic browning reactions in a caffeic acid model system. J. Agric. Food Chem. 1989, 37, 890–896. [Google Scholar] [CrossRef]
- Frank, O.; Blumberg, S.; Kunert, C.; Zehentbauer, G.; Hofmann, T. Structure determination and sensory analysis of bitter-tasting 4-vinylcatechol oligomers and their identification in roasted coffee by means of LC-MS/MS. J. Agric. Food Chem. 2007, 55, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, S.; Frank, O.; Hofmann, T. Quantitative studies on the influence of the bean roasting parameters and hot water percolation on the concentrations of bitter compounds in coffee brew. J. Agric. Food Chem. 2010, 58, 3720–3728. [Google Scholar] [CrossRef] [PubMed]
- Uranga, J.G.; Podio, N.S.; Wunderlin, D.A.; Santiago, A.N. Theoretical and Experimental Study of the Antioxidant Behaviors of 5-O-Caffeoylquinic, Quinic and Caffeic Acids Based on Electronic and Structural Properties. ChemistrySelect 2016, 1, 4113–4120. [Google Scholar] [CrossRef]
- Zeeb, D.J.; Nelson, B.C.; Albert, K.; Dalluge, J.J. Separation and identification of twelve catechins in tea using liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry. Anal. Chem. 2000, 72, 5020–5026. [Google Scholar] [CrossRef] [PubMed]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef]
- Robertson, A.R. The CIE 1976 Color-Difference Formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Kanzler, C.; Schestkowa, H.; Haase, P.T.; Kroh, L.W. Formation of Reactive Intermediates, Color, and Antioxidant Activity in the Maillard Reaction of Maltose in Comparison to d-Glucose. J. Agric. Food Chem. 2017, 65, 8957–8965. [Google Scholar] [CrossRef]
- Kanzler, C.; Haase, P.T. Melanoidins Formed by Heterocyclic Maillard Reaction Intermediates via Aldol Reaction and Michael Addition. J. Agric. Food Chem. 2020, 68, 332–339. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bork, L.V.; Rohn, S.; Kanzler, C. Characterization of Colorants Formed by Non-Enzymatic Browning Reactions of Hydroxycinnamic Acid Derivatives. Molecules 2022, 27, 7564. https://doi.org/10.3390/molecules27217564
Bork LV, Rohn S, Kanzler C. Characterization of Colorants Formed by Non-Enzymatic Browning Reactions of Hydroxycinnamic Acid Derivatives. Molecules. 2022; 27(21):7564. https://doi.org/10.3390/molecules27217564
Chicago/Turabian StyleBork, Leon Valentin, Sascha Rohn, and Clemens Kanzler. 2022. "Characterization of Colorants Formed by Non-Enzymatic Browning Reactions of Hydroxycinnamic Acid Derivatives" Molecules 27, no. 21: 7564. https://doi.org/10.3390/molecules27217564
APA StyleBork, L. V., Rohn, S., & Kanzler, C. (2022). Characterization of Colorants Formed by Non-Enzymatic Browning Reactions of Hydroxycinnamic Acid Derivatives. Molecules, 27(21), 7564. https://doi.org/10.3390/molecules27217564






